Highlights
-
•
Color changes were less occurred in germinated grain after ultrasound treatment.
-
•
Total flavonoid contents were increased (up to 3 times) in germinated buckwheat.
-
•
Catechin and epicatechin significantly decreased with alkali treatment.
-
•
Ultrasound was the most effective pre-treatment to decrease phytic acid in quinoa.
-
•
The highest TPC and AOA were measured in germinated samples by ultrasound treatment.
Keywords: Anti-nutrient, Bioactive compounds, Germination, Pseudocereal, Thermo-alkaline hydrolysis, Ultrasound
Abstract
This study evaluated the effects of pre-germination treatments on the nutritional and anti-nutritional values of buckwheat and quinoa during germination. Pre-germination method was effective on the chemical composition and phenolic profile of buckwheat and quinoa samples (p < 0.05). During the germination, color changes were notable, particularly in the alkali-treated samples. The decrease in tannin content reached the highest rate in germinated buckwheat (83 %) and quinoa (20 %) by alkali treatment. The highest antioxidant and total phenolic content were measured in germinated pseudocereals treated by ultrasound. However, the lowest phytic acid content was determined after germination in the quinoa sample treated by ultrasound. Rutin was the major flavonoid in buckwheat while quercetin, galangin, ellagic, syringic, and p-coumaric acids were only synthesized after 72 h of germination. Catechin and epicatechin were decreased only in the alkali-treated buckwheat sample. Controlled germination processes can enhance the antioxidant activity and development of functional foods from whole grains.
Introduction
Buckwheat (Fagopyrum esculentum L.) and quinoa (Chenopodium quinoa L.) are among the most widely consumed pseudocereals and can be used in the production of gluten-free goods particularly for celiac disease patients due to their gluten-free structure (Alvarez-Jubete et al., 2010, Thakur and Kumar, 2019, Niro et al., 2019). Buckwheat and quinoa are an important part of nutritious diets with a high proportion of carbohydrates, high-quality protein, dietary fiber, and lipid contents. The protein quantity of buckwheat (13 %) and quinoa (14.5 %) is higher than rice (6.5 %) and maize (9.5 %) (Hernández-Ledesma, 2019, Filho et al., 2017). Buckwheat is characterized by the high amount of essential amino acid content such as leucine (2.8 – 6.1 g/100 g protein), phenylalanine (2.0 – 4.4 g/100 g protein), lysine (4.9 – 6.7 g/100 g protein), and threonine (1.9 – 4.0 g/100 g protein), methionine (0.1 – 2.3 g/100 g protein) (Tömösközi & Langó, 2017). Quinoa also contains a significant amount of lysine (5.5 g/100 g protein), arginine (7.8 g/100 g protein), tryptophan (1.25 g/100 g protein), and methionine (2.24 g/100 g protein) (Rodríguez et al., 2020). Buckwheat and quinoa, like maize and soybean, are noteworthy sources of high-quality lipids, characterized by a high proportion (up to 90 g/100 g total fatty acids) of unsaturated fatty acid (Agregán et al., 2023). In addition to their macronutrient profile, buckwheat gaining great attention due to their rich bioactive content (G. Zhang et al., 2015). Buckwheat has high nutritional value due to the bioactive compounds such as flavonoids (rutin and quercetin) Nurul Huda et al., (2020). Xiang et al. (2023) showed that germination improved phenolic profiles and antioxidant capacity of proso millets. Germinated buckwheat contains more flavonoids, including orientin, isoorientin, vitexin, isovitexin, quercetin-3-O-robinobioside (Q3R), and rutin, than buckwheat seeds (Rois Mansur et al., 2019, Terpinc et al., 2016). Germinated quinoa contains greater amounts of hydroxybenzoic acids (vanillic and p-OH benzoic acid), hydroxycinnamic acids (p-coumaric and ferulic acids), and flavonoids (quercetin, kaempferol and their derivatives) than quinoa seeds (Alvarez-Jubete et al., 2010, Carciochi et al., 2014). Nevertheless, it should be noted that buckwheat and quinoa contain significant amounts of anti-nutrients, including phytic acid, tannins, and saponins, which may limit their nutritional quality (Demir, 2016; Z. L. Zhang et al., 2012).
Overall, germination is an effective process to improve the nutritional and nutraceutical quality of whole grains (Y. Zhang et al., 2023). Germination time has a great impact on aroma variations in grain during malting (Gu et al., 2023). In previous studies, soaking was used as a traditional method for the germination of buckwheat and quinoa seeds (Shreeja et al., 2021, Thakur et al., 2021). In recent years, pre-germination treatment has been used to enhance the quality of germinated whole grains. However, each pre-treatment might have a unique impact on the quality parameters of the grain (Li et al., 2022). Ultrasound pre-treatment is a novel method for stimulating the accumulation of bioactive chemicals in seed as well as increasing sprout length (Ding et al., 2018). However, the ultrasound-stimulated pre-germination processes were only recently studied for brown rice (Xia et al., 2020) and wheat (Ding et al., 2018). Beta et al. (2000) used sodium hydroxide (NaOH) as an alkali pre-treatment to reduce tannin content in sorghum and increase the malt quality. Besides NaOH, sodium bicarbonate (NaHCO3) and lime (Ca(OH)2) solutions can be used in various alkaline treatments to evaluate changes in phenolic content and antioxidant capacity of sorghum (Díaz González et al., 2019, Gaytán-Martínez et al., 2017). However, the effects of ultrasound and alkali pre-treatment on enhancing antioxidant properties and mitigating tannin and phytic acid content in germinated buckwheat and quinoa have not been studied.
The current study aimed to evaluate the effects of soaking, ultrasound, and thermo-alkaline hydrolysis pretreatment on the germination of buckwheat and quinoa. This was the first study that compared the changes of both nutritional and antinutritional properties in two major pseudocereals (buckwheat and quinoa) during different controlled germination processes. The main objective of this study was to determine the impact of pretreatment on germinated whole grains to improve their nutritional and functional properties.
Materials and methods
Materials and reagents
Buckwheat (Fagopyrum esculentum Moench.) and quinoa (Chenopodium quinoa) were obtained from the commercial market (İngro, Turkiye) in 2022. All chemicals were of analytical grade and were purchased from Merck (Darmstadt, Germany). Folin − Ciocalteu reagent, 2,2- diphenyl-1-picrylhydrazyl (DPPH), Trolox, and all chemicals (all with purity ≥ 95 %) used as standards in LC-MS/MS analysis including apigenin, pinobanksin, ferulic acid, p-coumaric acid, rutin, naringin, (+)-catechin, caffeic acid, chlorogenic acid, syringic aldehyde, chrysin, hesperidin, syringic acid, kaempferol, naringenin, (−)-epicatechin, pinocembrin, protocatechuic acid, ellagic acid, gentisic acid, galangin, salicylic acid, rosmarinic acid, resveratrol, quercetin, gallic acid, benzoic acid, luteolin-7-glucoside, and luteolin were purchased from Sigma Chemical (St. Louis, Missouri). Buckwheat and quinoa grains were separated from impurities and kept under + 4 °C until further applications.
Pretreatment and germination processes
100 g of each whole grain was measured and washed with distilled water. Water was drained and pretreatments including soaking, ultrasound, and thermo-alkaline hydrolysis were applied. The seeds of each group were steeped in deionized (DI) water (1:3 w/v) for 30 min at room temperature for soaking pre-germination treatment. For ultrasound pre-germination treatment, the seeds were steeped in DI water (1:3 w/v) under ultrasonic power during 30 min at 25 ± 2 °C. Ultrasound 37 kHz and 100 % amplitude generated by an ultrasonic bath (Sonorex DigiPlus DL 255H, BANDELIN, Germany). Thermo-alkali hydrolysis was done %1 (w/v) NaOH at 30 ± 2 °C with the 1:3 (w/v) solid solvent ratio. The seeds were treated with alkali solution during 30 min at 600 rpm. Pre-treated buckwheat and quinoa grains were germinated in quartz jar method at 25 ± 2 °C under dark condition and in each treatment were incubated at different times (12, 24, 48, 72 h). Germinated and control samples (ungerminated grains) were dried (Memmert, UN 55, Germany) at 40 ± 2 °C for 48 h. Two independent germination replications were used for each treatment condition. Germinated and control samples were ground to 500 μm particle size for further analysis. The germination percentage (G%) was calculated using the following equation (Guardianelli et al., 2022).
| (1) |
Chemical composition and color changes
The moisture contents of whole grains and germinated grains were measured using by Pfeuffer He 50 (Pfeuffer GMBH, Kitzingen, Germany) moisture analyzer. The crude protein, lipid, starch, and ash contents were measured using a near-infrared reflectance (NIR) spectroscopy (Perten DA 7250, PerkinElmer, Inc., Waltham, MA, ABD). The measurement was carried out between 950 and 1650 nm in 5 nm intervals according to described before by Peiris et al. (2019).
The CIE color parameters were measured using a Minolta CM-3600d (Minolta, Osaka, Japan) colorimeter as described by Güzel (2021). The measurements were recorded L* (Lightness), +a* (redness), +b* (yellowness), C* (Chroma), and h° (hue angle) color coordinates. The total color changes (ΔE) were calculated using L*, a* and b* coordinates according to the following equations (Güzel et al., 2022).
| ΔΕ = [(Δa)2 + (Δb)2 + (ΔL)2]0.5 |
Phenolic content and antioxidant activity
Buckwheat and quinoa flour were extracted using ultrasonic power (37 kHz and 100 % amplitude) at 25 ± 2 °C. Extraction was carried out with 1:10 g/mL solid solvent ratio and AcOH:MeOH:H2O (0.5:80:19.5; v/v/v) was used as the extraction solvent (Paucar-Menacho et al., 2017). The extract was centrifuged (Sigma, 3–30 K, Germany) at 5000 x g for 10 min. The Folin-Ciocalteu colorimetric method was used to determine the total phenolic content (TPC), as described by Güzel et al. (2020) with minor modifications. The buckwheat and quinoa extract (500 and 100 μL, respectively) was mixed with 500 μL of Folin’s reagent (0.2 N) and 1.0 mL of sodium carbonate solution (7.5 %). The solution was reached 5 mL with distilled water and mixed. The mixture was incubated at room temperature for 1 h in the dark. Measurements were performed at 720 nm (Shimadzu UV-1800, Japan), and the total phenolic content of buckwheat and quinoa were expressed as mg Gallic Acid Equivalents (GAE)/100 g dry weight (R2 = 0.9993 and R2 = 0,9986, respectively).
The DPPH (2.2-diphenyl-1-picrylhydrazyl) method was performed according to described by Brand-Williams et al. (1995) with minor modifications. A DPPH solution (0.1 mM) of 1900 μL was mixed with 50 μL of buckwheat and quinoa extract that was allowed to stand for 30 min at room temperature in the dark. The absorbance was measured at 515 nm. The results were expressed as μm Trolox Equivalents (TE)/g (R2 = 0.9918).
Identification and quantifications of phenolic profile by LC-MS/MS
Phenolic extraction
The extraction was performed by ultrasonication for 1 h described by Zhang et al. (2015) with a slight modification. A 3 g of buckwheat and quinoa flour was extracted with 50 mL of extraction solution (AcOH:MeOH:H2O; 0.5:80:19.5) and then centrifuged (8000 x g, 15 min) to collect the supernatants. The solvent from the phenolic extract fraction was removed in a vacuum rotary evaporator (Heidolph Laborota 4003) set at 35 °C. The dry residue was dissolved in 5 mL of 80 % methanol. The samples were filtered through a 0.22 μm filter into an amber-colored vial and immediately used for LC-MS/MS analysis.
Instrumentation and chromatography
The separation and quantification of phenolic acids and flavonoids were performed LC-MS/MS (liquid chromatography tandem mass spectrometry, Thermo Scientific). The mass spectrometer measurements were conducted triple-quadrupole mass spectrometer (TSQ quantum access max, Thermo Scientific) with ESI (electrospray ionization). LC separation was performed in ODS Hypersil C18 column (4.6 × 250 mm, 5-μm particle size, 120-Å pore size, fully porous type a silica analytical column). Separation was performed with gradient elution using the elution profile described by Çakır and Güzel (2003). The flow rate was 0.7 mL/min and the column temperature was 30 °C. The eluent used was 0.1 % formic acid in water (solvent A) and 100 % methanol (solvent B). A 20 μL injection volume was used for each sample with the following gradient elution: 0 – 22 min 100 % A; 22 – 25 min linear increase to 95 % B; 25 – 30 min linear decrease to 0 % A; 25 – 30 min 100 % B. Mass spectrometry detection conditions were the following: capillary temperature: 300 °C, sheath gas pressure: 30 psi, and spray voltage: 4.0 kV when the positive scan mode was used and 2.5 kV for negative scan mode.
Condensed tannin
Tannin content was measured using the vanillin assay as described by Güzel (2021). The vanillin (1 %, w/v) and sulfuric acid (70 %, w/w) solutions were used to measure color changes at 500 nm. Catechin (0,5–30 mg/L) was used as the external standard for quantification of tannin content. The results were expressed as mg CE (catechin equivalent)/g.
Phytic acid
Phytic acid (PA) content was measured by the colorimetric method as previously described in (Haug & Lantzsch, 1983). PA was extracted from 0.3 g of sample with the HCl solution (0.2 N) at room temperature for 2 h. The extracts were treated with Fe III solution and centrifuged at 8000 x g for 10 min (Sigma, 3–30 K, Germany). The absorbance of the supernatant was measured at 519 nm against distilled water using a spectrophotometer (Shimadzu UV-1800, Japan). The PA concentration was calculated by calibration curve of standard phytic acid solution (R2 = 0,9909) and expressed as mg/100 g.
Statistical analysis
The results were analyzed by analysis of variance using the General Linear Model procedure in Minitab for Windows (ver. 17). All measurements were performed in triplicate and results were expressed as mean ± standard deviation. The difference between mean values was measured by Tukey’s test with the p ≤ 0.05 significance.
Result and discussion
The germination percentage of whole grain increased significantly in the first 48 h of germination (91.5 % and 64.0 % for buckwheat and quinoa, respectively). After 72 h of germination, G% was reached at 100 % for buckwheat and quinoa grains.
Changes in chemical composition
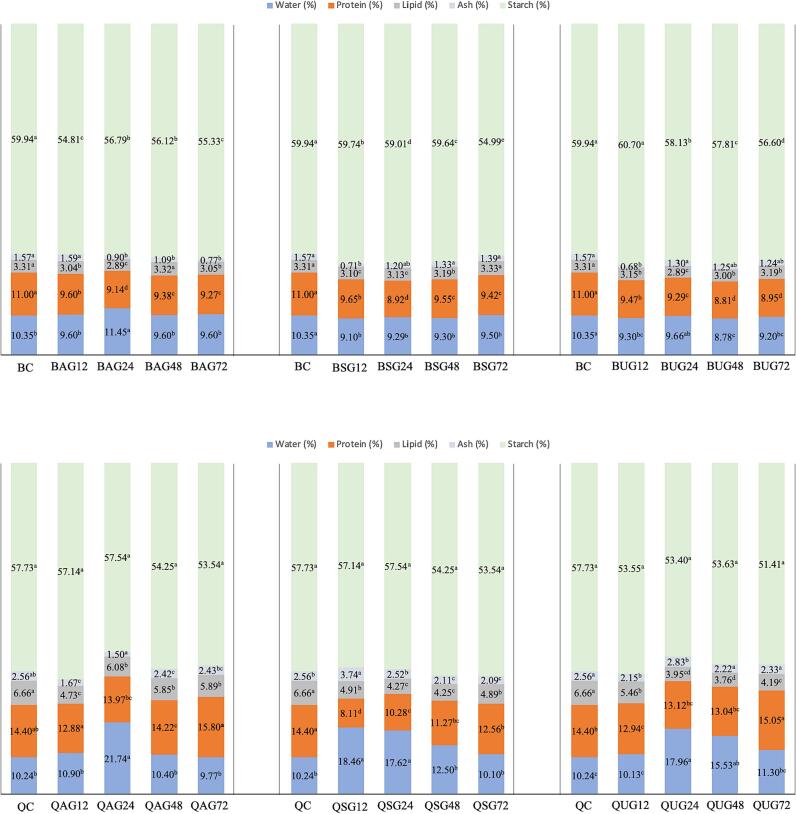
Buckwheat and quinoa are good sources of carbohydrates, high-quality protein, lipids, and fiber. Starch, protein, and lipids are the main macronutrients in these grains. The reported amount of starch was in the range between 60 and 70 % in buckwheat and 55 – 65 % in quinoa (Hernández-Ledesma, 2019, Tömösközi and Langó, 2017). Considering the varietal differences, the average protein and lipid content has been reported to be 13 % and 3 %, respectively in buckwheat and 14.5 % and 6 %, respectively in quinoa (Agregán et al., 2023). The chemical compositions of raw and germinated buckwheat and quinoa samples are shown in Fig. 1. The initial moisture content of buckwheat (10.35 %) and quinoa (10.24 %) was higher than that of germinated samples. The ash contents of ungerminated buckwheat (1.57 g/100 g) and quinoa (2.56 g/100 g) samples were measured similarly to a previous study (Thakur et al., 2021). After 72 h of germination, the significant percentage decrease in ash content was measured only in alkali-treated buckwheat (51 %) and quinoa sample treated by soaking (18 %) samples (p < 0.05) while a slight decrease was observed in all the other germinated buckwheat and quinoa sample (p > 0.05). Thakur et al. (2021)measured a decrease in the ash content of quinoa from 2.15 g/100 g to 1.90 g/100 g after germination. However, Guardianelli et al. (2022) observed that ash content remained consistent in quinoa after germination. Bhinder et al. (2022) found a slight reduction in the ash content of germinated buckwheat. During germination, the grain seeds supply sprouting energy by degradation of lipids and carbohydrates, which decreases their content (G. Zhang et al., 2015). Due to increasing lipase activity, lipid content was decreased in germinated grain (Bewley et al., 2013, Bhinder et al., 2022, Omary et al., 2012, Shreeja et al., 2021; G. Zhang et al., 2015). In our study, the lipid content of quinoa decreased from the initial content (6.66 g/100 g) to 5.89 g/100 g, 4.89 g/100 g, and 4.19 g/100 g in germinated quinoa sample treated by alkali, soaking and ultrasound, respectively (p < 0.05). The highest percentage decrease in lipid content of buckwheat was measured in thermo-alkaline hydrolysis (7.8 %) and ultrasound (3.6 %) treated samples (p < 0.05). Germination time and pretreatment method were also effective on the starch content of buckwheat (p < 0.05) while a slight decrease was measured in the starch content of quinoa samples. The highest reduction from the initial starch content of buckwheat (59.94 g/100 g) was observed in the sample treated by soaking (54.99 g/100 g) after 72 h of germination, followed by alkali-treated (55.33 g/100 g) and ultrasound treated samples (56.60 g/100 g). During germination, the arrangement of starch becomes more accessible to hydrolytic enzymes, while increased protease activity can cause protein degradation (Bhinder et al., 2022, Guardianelli et al., 2022). In our study, a significant decrease in protein content was measured in all germinated buckwheat samples (p < 0.05). The highest percent decrease from initial protein content (11.00 ± 0.01 g/100 g) in buckwheat was measured in the germinated sample treated by ultrasound (18.6 %) followed by treated with alkali (15.7 %) and soaking (14.4 %). A similar decrease in protein content of Tartary buckwheat was reported by (Bhinder et al., 2022). A significant decrease in protein content was measured in germinated quinoa samples treated by soaking (12.56 ± 0.04 g/100 g) after 72 h of germination. However, the protein contents remained consistent in germinated quinoa samples treated by ultrasound and alkali hydrolyzes (15.05 ± 018, 15.80 ± 0.00 g/100 g, respectively) when compared to the initial protein content (p > 0.05). After 48 h of germination, the protein contents were slightly decreased from 14.40 ± 0.00 g/100 g in control to 13.04 ± 0.24 g/100 g and 14.22 ± 0.0 g/100 g in the quinoa sample treated by ultrasound and alkali hydrolyzes, respectively while the protein content in these samples after 72 h of germination were measured similar to the control sample. Guardianelli et al. (2022) observed an increase in the protein content of white quinoa after 48 h of germination from 14.4 % to 15.5 %. A similar result was reported by Bhinder et al. (2021). The increment was related to the mobilization of protein reserves in the cotyledons, together with the synthesis of new proteins, necessary for shoot growth. Furthermore, it was pointed out that the amino acids produced by the hydrolysis of storing proteins were not only used to synthesize new components but also can be used as an energy source, especially in the early stages of germination. During gemination, several proteins might be hydrolyzed with protease while some other proteins were synthesized by a series of biochemical reactions. Protein content is determined by the effects of proteolysis and protein synthesis (G. Zhang et al., 2015). As a result, changes in protein content during germination are a dynamic regulation process.
Fig. 1.
Changes in the chemical composition of buckwheat and quinoa during the germination. BC, buckwheat control; BAG12/24/48/72, thermo alkaline pre-treated, buckwheat germinated 12 h, 24 h, 48 h, and 72 h; BSG12/24/48/72, soaking pre-treated, buckwheat germinated 12 h, 24 h, 48 h, and 72 h; BUG12/24/48/72, ultrasound pre-treated, buckwheat germinated 12 h, 24 h, 48 h, and 72 h; QC, quinoa control; QAG12/24/48/72, thermo alkaline pre-treated, quinoa germinated 12 h, 24 h, 48 h, and 72 h; QSG12/24/48/72, soaking pre-treated, quinoa germinated 12 h, 24 h, 48 h, and 72 h; QUG12/24/48/72, ultrasound pre-treated, quinoa germinated 12 h, 24 h, 48 h, and 72 h.
Color changes
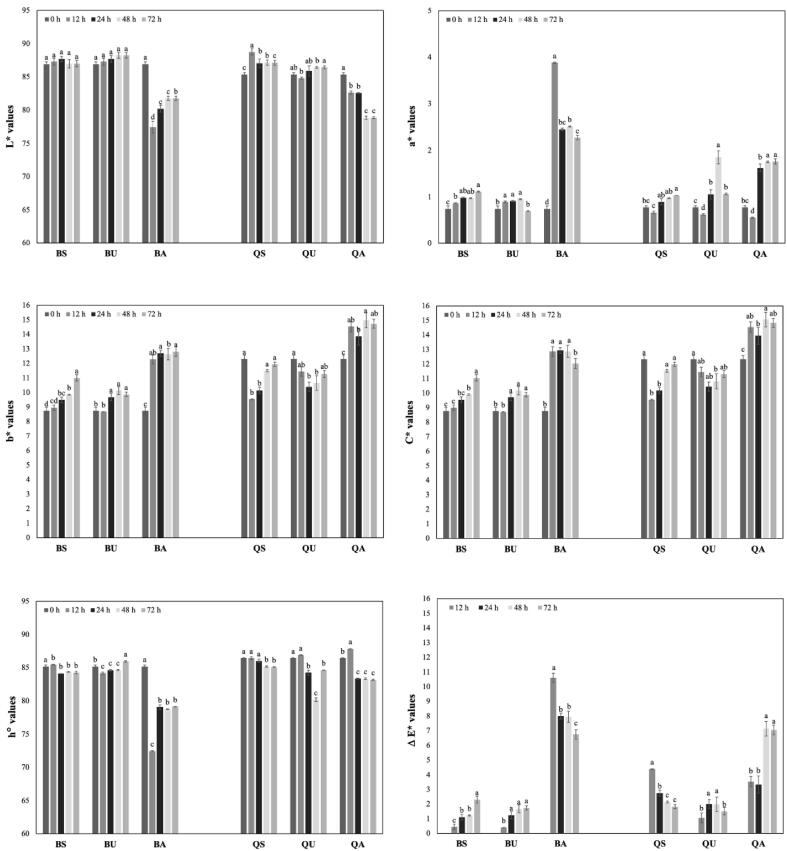
The whole grain color affects the color of end-products besides reactions that occurred before and during baking processes such as malting, fermentation, sprouting, and Maillard. The color changes during the germination and effects of pretreatment are given in Fig. 2. The brightness values (L* value) are closer to 100, which suggests the flour color is white and more desirable for many baking goods (Olojede et al., 2020). L* values in ungerminated buckwheat and quinoa samples were measured 86.90 ± 0.34 and 85.36 ± 0.27, respectively. L* values were found similar in germinated and ungerminated buckwheat and quinoa samples (p > 0.05) except for the samples that were pretreated with thermo-alkaline hydrolysis (p < 0.05). In our study, after 72 h of germination, L* values decreased by 5.9 % (buckwheat) and 7.6 % (quinoa) in alkali-treated samples. Sharma et al. (2018) measured higher L* values (59.13 ± 2.45) in ungerminated millet flour when compared to germinated sample (50.01 ± 1.99) and this reduction was associated with increased protein content due to germination. The redness value (+a*) was significantly increased after germination. The highest increase in a* value was observed in alkali-pretreated quinoa (from 0.77 ± 0.04 to 1.76 ± 0.06) and buckwheat (0.74 ± 0.06 to 2.27 ± 0.05) samples after 72 h of germination. Similarly, alkali pretreatment increased the yellowness (+b*) of quinoa samples (p < 0.05) from initial 12.31 ± 0.25 to 14.73 ± 0.31 in germinated quinoa by alkali treatment. However, b* values were not significantly changed after 72 h of germination in soaking (11.94 ± 0.14) and ultrasound (11.27 ± 0.25) pretreated samples (p > 0.05). Germination time and pretreatment methods had a significant effect on b* values in buckwheat (p < 0.05). The highest increase in b* values in germinated buckwheat was observed in the alkali treated (35 %) sample while the lowest was in ultrasound treated (13 %). Total color changes (ΔE) confirm that the color changes of buckwheat and quinoa samples during the germination mostly occurred in thermo-alkaline pretreated samples. However, the color changes less occurred in ultrasonic pretreated samples (Fig. 2). After 72 h of germination, ΔE in alkali treated buckwheat and quinoa samples was found 6.74 and 7.03, respectively. However, ΔE values in soaking and ultrasound treated samples were below 3.0 during the germination processes. (Gu et al., (2023) stated that human eyes can clearly identify distinct colors when ΔE is greater than 3. However, when ΔE is between 1 and 3, human eyes might not be able to distinguish color differences. When ΔE is less than one, human eyes cannot perceive the difference. In germinated buckwheat and quinoa samples treated by ultrasound and soaking, the overall color changes might not be detectable with human eyes, whereas the alkali-treated sample might be noticeable.
Fig. 2.
Changes in color values of pseudocereals during germination. BA, thermo alkaline pre-treated buckwheat; BS, soaking pre-treated; BU, ultrasound pre-treated buckwheat; QA, thermo alkaline pre-treated quinoa; QS, soaking pre-treated quinoa; QU, ultrasound pre-treated quinoa.
Changes in phenolic content and antioxidant activity
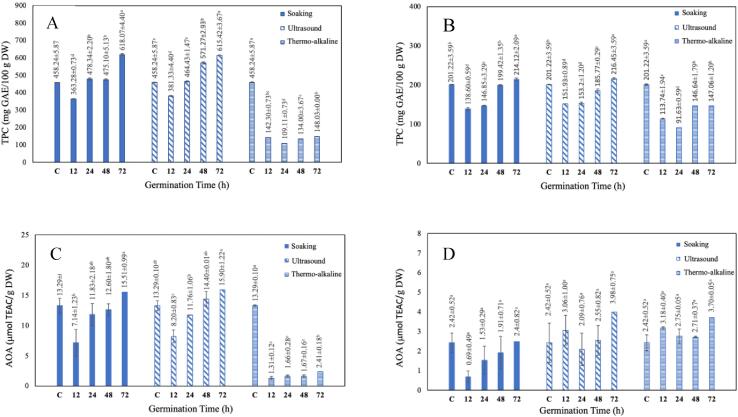
Total phenolic content (TPC) and antioxidant activity (AOA) in ungerminated buckwheat were 458.54 ± 5.87 mg GAE/100 g and 13.29 ± 0.10 μmol TEAC/g, respectively. In comparison, TPC was 201.22 ± 3.59 mg GAE/100 g and AOA was 2.42 ± 0.52 μmol TEAC/g in ungerminated quinoa (Fig. 3). Previously, similar TPC (303 mg GAE/100 g) and AOA (12,56 μmol TEAC/g) were reported for Tartary buckwheat (Bhinder et al., 2022; G. Zhang et al., 2015). Guardianelli et al. (2022) found 94.3 ± 12.4 mg GAE/100 g flour total phenolic content in ungerminated white quinoa, while Bhinder et al. (2021) reported 3.61 ± 0.46 μmol/g AOA.
Fig. 3.
Changes in phenolic content and antioxidant activity of buckwheat and quinoa during the germination. A, changes in TPC of germinated buckwheat; B, changes in TPC of germinated quinoa; C, changes in AOA of germinated buckwheat; D, changes in AOA of germinated quinoa.
Germination time and pre-treatment methods caused significant changes in TPC and AOA of buckwheat and quinoa samples (p < 0.005, supplementary Table 1). Phenolic contents in buckwheat samples treated by ultrasound and soaking were significantly increased after 24 h of germination. The TPC in these samples increased by 35 % (soaking) and 34 % (ultrasound) while TPC decreased by 68 % in the germinated buckwheat sample treated by alkali hydrolyzes. However, there was a slight increase of 6 % and 8 % in the total phenolic content of quinoa samples treated by soaking and ultrasound, respectively. As in buckwheat, thermo-alkaline germination caused a significant decrease in the TPC of quinoa samples after 24 h of germination (p < 0.05). The decrease in total phenolic content could be because of the leaching of free polyphenols in steeping water and/or decumulation (Kim et al., 2016). Although TPC tended to increase as germination process, buckwheat and quinoa samples treated by alkali hydrolyzes had the lowest total phenolic content. Alvarez-Jubete et al. (2010) and Kaur et al. (2016) reported an increase in the phenolic content of quinoa and buckwheat after germination. In a previous study, the increase in phenolic content was related to the biosynthesis of polyphenolic compounds through the shikimic acid-phenylpropanoid pathway (Kim et al., 2016). In cereals, polyphenols can be found either free or attached to cell wall components. Free phenolics are generally found in the pericarp and can be extracted using organic solvents while bound phenolics are bound to lignin or arabinoxylans (Hübner & Arendt, 2013). During germination, free phenolic accumulation was found higher in buckwheat when compared to bound phenolics. This increase in free phenolic compounds was associated with the hydrolysis action of enzyme esterase and glucosidase on polymeric unextractable polyphenolic compounds by Bhinder et al. (2022).
Table 1.
Characteristics of the phenolic compounds in germinated buckwheat that were analyzed by LC–MS/MS. Data are means ± standard deviation. NI, not identified. Different letters in the same row are significantly different (p < 0.05) for buckwheat samples. aParent Ion (m/z). bRT = Retention time. cControl = Ungerminated buckwheat.
| Phenolic compounds | [M − H]−(m/z)a | RTb (min) | Phenolic content (μg/g DW) | |||
|---|---|---|---|---|---|---|
| Flavonoids | Controlc | Soaking | Ultrasound | Thermo-Alkali | ||
| Rutin | 609 | 18.03 | 6.97 ± 0.18C | 25.67 ± 0.66A | 23.73 ± 0.61A | 9.59 ± 0.25B |
| Quercetin | 301 | 20.29 | NI | 0.05 ± 0.00B | 0.11 ± 0.00 A | NI |
| Kaempferol | 285 | 21.55 | NI | NI | NI | NI |
| Galangin | 269 | 23.44 | NI | 0.09 ± 0.00A | 0.03 ± 0.00C | 0.05 ± 0.00B |
| Luteolin | 285 | 20.78 | NI | NI | NI | NI |
| Luteolin-7-glucoside | 447 | 17.78 | 0.003 ± 0.00C | 0.21 ± 0.01A | 0.19 ± 0.01B | 0.002 ± 0.00C |
| Chrysin | 254 | 21.91 | 0.02 ± 0.00C | 0.03 ± 0.00B | 0.02 ± 0.00B | 0.06 ± 0.00A |
| Apigenin | 269 | 21.79 | NI | NI | NI | NI |
| Hesperidin | 609 | 17.34 | 0.04 ± 0.00D | 0.07 ± 0.00A | 0.06 ± 0.00B | 0.05 ± 0.00C |
| Naringenin | 271 | 19.71 | NI | NI | NI | NI |
| Catechin | 289 | 12.98 | 4.30 ± 0.11C | 19.31 ± 0.50A | 16.81 ± 0.43B | 2.84 ± 0.00D |
| Epicatechin | 289 | 14.38 | 5.00 ± 0.13C | 9.27 ± 0.24A | 8.26 ± 0.21B | 0.12 ± 0.00D |
| Phenolic acids and their derivatives | ||||||
| P-hydroxybenzoic acid | 137 | 17.28 | 0.42 ± 0.01C | 0.07 ± 0.00D | 0.95 ± 0.02B | 1.54 ± 0.04A |
| Gallic acid | 169 | 9.68 | NI | NI | NI | NI |
| Protocatechuic acid | 153 | 11.77 | NI | NI | NI | NI |
| Syringic acid | 197 | 15.77 | NI | NI | NI | NI |
| Salicylic acid | 137 | 17.19 | 0.35 ± 0.01C | NI | 0.81 ± 0.02B | 1.51 ± 0.04A |
| Ellagic acid | 301 | 19.16 | NI | 0.56 ± 0.01 | 0.39 ± 0.01 | NI |
| Gentisic acid | 155 | 12.91 | NI | NI | NI | NI |
| Syringic aldehyde | 181 | 15.72 | NI | 0.12 ± 0.00 | NI | NI |
| P-coumaric acid | 163 | 16.42 | NI | NI | NI | 0.82 ± 0.02 |
| Chlorogenic acid | 353 | 14.00 | 0.04 ± 0.00C | 2.91 ± 0.08A | 4.02 ± 0.10A | 0.17 ± 0.00C |
| Caffeic acid | 179 | 14.76 | NI | NI | NI | NI |
| Ferulic acid | 193 | 17.34 | NI | NI | NI | NI |
| Rosmarinic acid | 359 | 17.41 | NI | NI | NI | NI |
| Resveratrol | 227 | 18.05 | NI | NI | NI | NI |
The antioxidant activity in cereals is primarily related to their phenolic content, as well as vitamins, sterols, and phytic acid (Hübner & Arendt, 2013). AOA in buckwheat increased by 20 % after ultrasonic germination while decreased by 82 % in alkali treated sample after 72 h of germination (Fig. 3). AOA in quinoa was increased after 72 h of germination and the highest increase was measured in ultrasound-treated quinoa (64 %) followed by alkali-treated (53 %) and soaking (2 %). Carciochi et al. (2016) reported a similar increase in antioxidant capacity in quinoa after germination of 72 h. The increase in antioxidant activity was related to the accumulation and biosynthesis of flavonoids and phenolic acids in germinated grain (Bhinder et al., 2022). The effects of ultrasound, microwave, and thermal stress on the germination of Tartary buckwheat were evaluated by (Wang et al., 2020). The AOA (ABTS) of the ultrasound-treated buckwheat sample was increased and significantly higher than that of control and microwave and thermal-treated samples (p < 0.05). As a result, controlled germination is an effective method to enhance bioactive characteristics in grain. Furthermore, in a controlled germination process, pre-germination treatment is as critical as germination time to increase bioactive content.
Profiles and changes of phenolic acid and flavonoid content
Rutin was found the most abundant flavonoid compound in ungerminated (6.97 ± 0.18 μg/g) buckwheat, representing about 42.7 % of total flavonoids measured by LC-MS/MS. Other major (epicatechin and catechin) and minor flavonoids (hesperidin, chrysin, luteolin-7-glucoside, quercetin, and galangin) were also identified in buckwheat samples during germination. However, apigenin, kaempferol, and naringenin flavonoids were not detected in buckwheat samples (Table 1). After 72 h of germination, the flavonoid content significantly increased in buckwheat samples treated by ultrasound and soaking (49.213 ± 1.273 and 54.691 ± 1.415 μg/g dw, respectively) which is in agreement with the TPC data. Quercetin and galangin were also measured in germinated buckwheat while these flavonoids were not determined in ungerminated buckwheat. In our studies, a considerable increase was measured in rutin concentration, as well as chrysin and hesperidin, in all germinated buckwheat samples. Similarly, Luteolin-7-glucoside concentration significantly increased in germinated buckwheat samples treated by ultrasound (0.19 ± 0.01 μg/g dw) and soaking (0.21 ± 0.01 μg/g dw). The total flavonoid contents were increased 3 times in germinated buckwheat sample treated by soaking (54.69 ± 3.55 μg/g dw) and ultrasound (49.21 ± 6.16 μg/g dw) when compared the initial (16.33 ± 0.42 μg/g dw) total flavonoid content. During the germination process, flavonoids accumulate due to increasing PAL (phenylalanine ammonia lyase) activity. Meanwhile, glycosylases such as flavonoid 3-O-glucosyltransferase were activated, which led to flavonoid glycoside formation (G. Zhang et al., 2015).
Previous studies showed that rutin, quercetin, and epicatechin contents were increased after 72 h of germination (Bhinder et al., 2021; G. Zhang et al., 2015). Although an increase in catechin and epicatechin contents was measured in germinated buckwheat samples treated with ultrasound and soaking, the catechin and epicatechin content significantly decreased (34 % and 98 %, respectively) in the alkali treated buckwheat sample after germination in our studies. Kim et al. (2016) reported that catechin concentration was related to decreasing tannin content. In germinated buckwheat, a high correlation was found between tannin and epicatechin content (r = 0.8195) as well as catechin content (r = 0.7961). After 72 h of germination thermo-alkali pretreated buckwheat sample has the lowest catechin and epicatechin concentration as well as the total tannin concentration. The flavonoid content depends on several factors such as cultivar, growth stage, growing region, and season (Nurul Huda et al., 2020). In our study, kaempferol, apigenin, naringenin, and some phenolic acids such as gallic, caffeic, and ferulic acids in buckwheat were not detected unlike previous studies (Terpinc et al., 2016; G. Zhang et al., 2015). In the ungerminated buckwheat sample, p- hydroxybenzoic, salicylic, and chlorogenic acid were only determined phenolic acids in the ungerminated buckwheat, and the total amount was significantly lower (0.81 ± 0.01 μg/g dw) than germinated samples. In addition to the significant increase in these phenolic acids, ellagic acid, syringic aldehyde, and p-coumaric acid were also determined in germinated buckwheat samples. The total phenolic acid content was the highest concentration (6.16 ± 0.16 μg/g dw) in the germinated buckwheat sample that ultrasound-pretreated. When comparing alkali and soaking treatment, total phenolic acid content was higher in alkali-treated buckwheat (4.04 ± 0.11 μg/g dw) than in germinated samples after soaking (3.55 ± 0.09 μg/g dw). The phenolic acid profile also was different in germinated buckwheat that applied different pretreatment methods. For example, ellagic acid was found in both germinated samples (soaking and ultrasound treated) while syringic aldehyde was determined only in germinated buckwheat treated by soaking, and p-coumeric acid was measured in the alkali-treated buckwheat sample.
Hesperidin, rutin, and chlorogenic acid are the most abundant phenolic compounds in the ungerminated quinoa sample and these phenolics represent almost 92 % of total phenolic compounds measured by LS-MS/MS (Table 2). The individual flavonoid content was increased significantly after 72 h of germination except for rutin content in quinoa sample treated by soaking. Chlorogenic acid was the most abundant phenolic acid followed by ferulic and gentisic acid. In a previous study, ferulic acid has been reported as the most abundant phenolic acid. However, chlorogenic acid has not been detected in raw and malted quinoa (Bhinder et al., 2021). In our study, a decreasing trend was also observed in the chlorogenic acid content after germination, unlike other phenolic acids. The highest rutin (3.78 ± 0.10 μg/g dw), quercetin (0.68 ± 0.02 μg/g dw), and gentisic acid (0.91 ± 0.02 μg/g dw) were measured in germinated quinoa samples treated by ultrasound. Previously, de Bock et al. (2021) found that rutin ranged between 0.033 ± 0.065 and 0.110 ± 0.025 mg/g dm in quinoa. After germination, the highest increase (58 %) in hesperidin content was determined in the quinoa sample treated by soaking. Kaempferol was only determined in germinated quinoa samples, mostly in treated with alkali (0.53 ± 0.01 μg/g dw) and soaking (0.52 ± 0.00 μg/g dw). Alkali-treated quinoa sample was rich in chrisin and ferulic acid as well as kaempferol, after 72 h of germination. Tang et al. (2015) have also reported that ferulic acid and their glucoside form was the most prominent phenolic acid while quercetin and kaempferol were the main flavonoids. In summary, the total phenolic acid content in quinoa samples increased by 21 % after germination, and the highest increase was determined in the sample treated with soaking. The total flavonoid content also increased by 43 % and the highest amount (8.93 ± 0.23 μg/g dw) was measured in the germinated quinoa sample treated by ultrasound. The results showed that the phenolic profile was significantly dependent on pre-germination methods. Compared with the quinoa samples, the contents of flavonoids and phenolic acids were much higher in buckwheat. The flavonoid content also was much higher than phenolic acids in both pseudocereals. Overall, the high content of phenolic acids and flavonoids has been attributed to PAL activity during germination. Phenylalanine ammonia lyase (PAL) is a crucial enzyme in the shikimate and phenylpropanoid biosynthesis pathways. Many phenolics are synthesized in this metabolic pathway and can be also transformed into flavonoids, tannins, and other compounds (G. Zhang et al., 2015). In this study, the changes in TPC, AOA, and tannin content during germination were accordant with the flavonoid and phenolic acid content. After germination, especially after ultrasound and soaking pretreatment, the increase of flavonoids led to excellent enhancement of antioxidant activities (rbuckwheat = 0.7526; Pearson correlation coefficient between AOA and total flavonoid content). Germinated buckwheat and quinoa might be good alternatives to natural sources of phenolics, especially rutin and chlorogenic acid. Therefore, germinated buckwheat and quinoa has great potential as functional ingredient, especially for a gluten-free diet and food product.
Table 2.
Characteristics of the phenolic compounds in germinated quinoa that were analyzed by LC–MS/MS.
| Phenolic compounds | [M − H]−(m/z)a | RT (min)b | Phenolic content (μg/g DW) | |||
|---|---|---|---|---|---|---|
| Flavonoids | Controlc | Soaking | Ultrasound | Thermo-Alkali | ||
| Rutin | 609 | 18.03 | 2.50 ± 0.06B | 0.79 ± 0.02D | 3.78 ± 0.10A | 1.55 ± 0.04C |
| Quercetin | 301 | 20.29 | 0.26 ± 0.01C | 0.51 ± 0.01B | 0.68 ± 0.02A | 0.21 ± 0.01C |
| Kaempferol | 285 | 21.55 | NI | 0.52 ± 0.00A | 0.25 ± 0.01B | 0.53 ± 0.01A |
| Galangin | 269 | 23.44 | NI | NI | NI | NI |
| Luteolin | 285 | 20.78 | NI | NI | NI | NI |
| Luteolin-7-glucoside | 447 | 17.78 | NI | NI | NI | NI |
| Chrysin | 254 | 21.91 | 0.017 ± 0.00C | 0.023 ± 0.00B | 0.022 ± 0.00B | 0.048 ± 0.00A |
| Apigenin | 269 | 21.79 | NI | NI | NI | NI |
| Hesperidin | 609 | 17.34 | 3.48 ± 0.09C | 5.51 ± 0.14A | 4.21 ± 0.11B | 3.42 ± 0.09C |
| Naringenin | 271 | 19.71 | NI | NI | NI | NI |
| Catechin | 289 | 12.98 | NI | NI | NI | NI |
| Epicatechin | 289 | 14.38 | NI | NI | NI | NI |
| Phenolic acids and their derivatives | ||||||
| P-hydroxybenzoic acid | 137 | 17.28 | NI | NI | NI | NI |
| Gallic acid | 169 | 9.68 | NI | NI | NI | NI |
| Protocatechuic acid | 153 | 11.77 | NI | NI | NI | NI |
| Syringic acid | 197 | 15.77 | NI | NI | NI | NI |
| Salicylic acid | 137 | 17.19 | NI | NI | NI | NI |
| Ellagic acid | 301 | 19.16 | NI | NI | NI | NI |
| Gentisic acid | 155 | 12.91 | 0.15 ± 0.00C | 0.35 ± 0.01B | 0.91 ± 0.02A | 0.09 ± 0.00D |
| Syringic aldehyde | 181 | 15.72 | NI | NI | NI | NI |
| P-coumaric acid | 163 | 16.42 | NI | NI | NI | NI |
| Chlorogenic acid | 353 | 14.00 | 2.88 ± 0.07A | 2.63 ± 0.07B | 1.64 ± 0.04D | 1.90 ± 0.05C |
| Caffeic acid | 179 | 14.76 | NI | NI | NI | NI |
| Ferulic acid | 193 | 17.34 | 0.29 ± 0.01D | 1.06 ± 0.03B | 0.62 ± 0.02C | 1.37 ± 0.04A |
| Rosmarinic acid | 359 | 17.41 | NI | NI | NI | NI |
| Resveratrol | 227 | 18.05 | NI | NI | NI | NI |
Data are means ± standard deviation. NI, not identified. Different letters in the same row are significantly different (p < 0.05) for quinoa samples.
aParent Ion (m/z). bRT = Retention time. cControl = Ungerminated quinoa.
Changes in antinutritional content
The changes in the antinutritional content of buckwheat and quinoa samples during germination are given in Table 3. Phytic acid and tannin are among the main anti-nutrients that limit the bioavailability of grains (Demir, 2016). Phytic acid is a saturated cyclic acid with six reactive phosphate groups. As a chelating agent, phytic acid can bind positively charged functional groups or minerals, limiting their bioavailability during food digestion (Bhinder et al., 2021). Phytic acid was found 10.41 mg/g and 10.85 mg/g in ungerminated buckwheat and quinoa, respectively while tannin contents were higher in ungerminated quinoa (2.11 mg CE/g) than that in buckwheat (0.40 mg CE/g). Alkali pretreatment has no significant effect on the phytic acid content of germinated buckwheat and quinoa (p < 0.05). However, the phytic acid content of germinated quinoa decreased significantly by ultrasonic (85.5 %) and soaking treatments (80.6 %) compared to the initial content. When compared to quinoa, a slight reduction (9.4 – 9.8 %) was observed in germinated buckwheat (p > 0.05). Similarly, Egli et al. (2002) found that germination had no significant effect on phytic acid content in buckwheat. However, some previous studies showed that there is a negative and significant correlation between the phytic acid content of buckwheat and germination time (Bhinder et al., 2022; G. Zhang et al., 2015). Similar to our finding, Bhinder et al. (2021) also found a significant reduction (59 – 64 %) in the phytic acid content of black and white quinoa after 96 h of germination.
Table 3.
Effects of pretreatments on anti-nutritional content in buckwheat and quinoa grains during germination.
| Phytic acid (mg/g) |
Tannin (mg CE/g) |
||||
|---|---|---|---|---|---|
| Pretreatment | Germination Time (h) |
Buckwheat | Quinoa | Buckwheat | Quinoa |
| Soaking (S) | 0 | 10.41 ± 0.56AB | 10.85 ± 0.50A | 0.40 ± 0.01A | 2.11 ± 0.23B |
| 12 | 11.38 ± 0.78A | 3.60 ± 0.04B | 0.21 ± 0.01B | 2.63 ± 0.15AB | |
| 24 | 10.91 ± 0.02AB | 1.78 ± 0.84C | 0.40 ± 0.03A | 2.77 ± 0.53A | |
| 48 | 10.90 ± 0.80AB | 3.03 ± 1.11BC | 0.25 ± 0.01B | 2.77 ± 0.24A | |
| 72 | 9.39 ± 0.26B | 2.10 ± 0.04BC | 0.21 ± 0.00B | 2.58 ± 0.08AB | |
| Ultrasound (U) | 0 | 10.41 ± 0.56C | 10.85 ± 0.50A | 0.40 ± 0.01A | 2.11 ± 0.23B |
| 12 | 13.18 ± 0.12A | 8.54 ± 0.02B | 0.16 ± 0.02C | 1.79 ± 0.35B | |
| 24 | 12.22 ± 0.21AB | 1.63 ± 0.19C | 0.25 ± 0.02BC | 3.17 ± 0.46A | |
| 48 | 10.70 ± 1.13BC | 1.69 ± 0.62C | 0.29 ± 0.05B | 3.00 ± 0.28A | |
| 72 | 9.43 ± 0.88C | 1.57 ± 0.36C | 0.34 ± 0.02AB | 2.45 ± 0.30AB | |
| Thermo-Alkaline Hydrolysis (A) | 0 | 10.41 ± 0.56AB | 10.85 ± 0.50A | 0.40 ± 0.01A | 2.11 ± 0.23AB |
| 12 | 10.44 ± 0.58AB | 7.93 ± 0.31C | 0.08 ± 0.01C | 1.54 ± 0.05C | |
| 24 | 9.16 ± 0.14B | 9.00 ± 1.02BC | 0.18 ± 0.02B | 2.60 ± 0.39A | |
| 48 | 11.82 ± 0.19A | 9.96 ± 0.36AB | 0.16 ± 0.00B | 2.02 ± 0.12BC | |
| 72 | 10.63 ± 0.02AB | 9.78 ± 0.09AB | 0.07 ± 0.00C | 1.88 ± 0.26BC | |
Data are means ± standard deviation. Data values with the same alphabetic superscript in a row do not vary significantly (p < 0.05). CE = Catechin equivalents.
Tannins are polyphenols that are complex oligomeric or polymeric forms of flavan-3-ol (Güzel, 2021). Besides considerable antioxidant properties, tannins impart astringency in foods and limit the absorption of vitamin B12, Fe+2, and the bioavailability of proteins (Bhinder et al., 2021). In our study, tannin content in ungerminated quinoa was measured 2.11 mg CE/g while it was 0.40 mg CE/g in ungerminated buckwheat. Tannin content was reported 0.322 ± 0.08 and 0.051 ± 0.01 % at dry basis for raw buckwheat and quinoa, respectively (Gorinstein et al., 2008). After 72 h of germination, the tannin content of buckwheat was significantly decreased from the initial content (p < 0.05). Thermo-alkaline pre-germination treatment was the most effective method to mitigate the tannin content in buckwheat (83 %) as well as quinoa (20 %). The reduction in tannin content also significant was in germinated (72 h) buckwheat sample treated by ultrasound (15 %) and soaking (48 %) samples (p < 0.05) while tannin contents in germinated (72 h) quinoa sample after ultrasound and soaking treatment were similar to initial content (p > 0.05). Bhinder et al. (2021) also did not find a steady decrease during germination in quinoa. The change in tannin concentration could be related to the formation of macromolecular molecules from phenolic compounds such as catechins (Kim et al., 2016). Kumari et al. (2023)found that tannin content in buckwheat was decreased (55.5 %) after germination. Similar tannin content reduction (60 %) in buckwheat was reported after germination by (Thakur et al., 2021). Overall, these findings confirmed that an alkali pre-germination treatment might be advantageous to achieve the highest reduction rate in buckwheat and quinoa tannin concentration.
Conclusions
Our study indicated that germination time and pre-germination methods had significant importance on nutritional and anti-nutritional factors in buckwheat and quinoa (p < 0.05). In general, a significant reduction was revealed in starch, lipid, and ash content during germination of buckwheat and quinoa. The protein content generally decreased in buckwheat during germination while the changes in protein content of quinoa varied differently by pre-treatment method. Pre-germination treatment had a notable effect on color changes, particularly in samples treated with thermo-alkaline hydrolysis. Total phenolic content and antioxidant activity significantly increased in germinated samples, especially in ultrasound treated. Pre-germination treatment was also effective on the phenolic profile for both grains. In alkali-pretreated buckwheat, catechin and epicatechin content was significantly decreased after germination. This decrement was in parallel with the tannin content and antioxidant activity of this sample. Although, these results indicate that the enhanced antioxidant activity in germinated grains might be primarily attributed to flavonoid content, other phenolic content and their synergistic effects can also cause this increment. Furthermore, the results also highlighted the reduction in anti-nutritional factors such as phytic acid and tannins. These findings emphasize the potential of controlled germination processes, mostly ultrasonic pretreatment, as effective strategies to enhance the nutritional and antioxidant properties of buckwheat and quinoa while reducing antinutritional factors. These insights contribute to the improvement of the overall nutritional quality and potential applications of buckwheat and quinoa in food production. Also, these products can become valuable alternatives for celiac disease patients due to their gluten-free nature.
CRediT authorship contribution statement
Ebrar Altıkardeş: Investigation, Formal analysis. Nihal Güzel: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101182.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Agregán R., Guzel N., Guzel M., Bangar S.P., Zengin G., Kumar M., Lorenzo J.M. The Effects of processing technologies on nutritional and anti-nutritional properties of pseudocereals and minor cereal. Food and Bioprocess Technology. 2023;16(5):961–986. doi: 10.1007/s11947-022-02936-8. [DOI] [Google Scholar]
- Alvarez-Jubete L., Arendt E.K., Gallagher E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends in Food Science and Technology. 2010;21(2):106–113. doi: 10.1016/j.tifs.2009.10.014. [DOI] [Google Scholar]
- Alvarez-Jubete L., Wijngaard H., Arendt E.K., Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chemistry. 2010;119(2):770–778. doi: 10.1016/J.FOODCHEM.2009.07.032. [DOI] [Google Scholar]
- Beta T., Rooney L.W., Marovatsanga L.T., Taylor J.R.N. Effect of chemical treatments on polyphenols and malt quality in sorghum. Journal of Cereal Science. 2000;31(3):295–302. doi: 10.1006/jcrs.2000.0310. [DOI] [Google Scholar]
- Bewley J.D., Bradford K.J., Hilhorst H.W.M., Nonogaki H. Seeds. 2013 [Google Scholar]
- Bhinder S., Kumari S., Singh B., Kaur A., Singh N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chemistry. 2021;346 doi: 10.1016/j.foodchem.2020.128915. [DOI] [PubMed] [Google Scholar]
- Bhinder S., Singh N., Kaur A. Impact of germination on nutraceutical, functional and gluten free muffin making properties of Tartary buckwheat (Fagopyrum tataricum) Food Hydrocolloids. 2022;124 doi: 10.1016/j.foodhyd.2021.107268. [DOI] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a Free Radical Method to Evaluate Antioxidant Activity. 1995;28 [Google Scholar]
- Carciochi R.A., Galván-D’Alessandro L., Vandendriessche P., Chollet S. Effect of germination and fermentation process on the antioxidant compounds of quinoa seeds. Plant Foods for Human Nutrition. 2016;71(4):361–367. doi: 10.1007/s11130-016-0567-0. [DOI] [PubMed] [Google Scholar]
- Carciochi R.A., Manrique G.D., Dimitrov K. Changes_in_phenolic_compositio. International Food Research Journal. 2014;21(2):767–773. [Google Scholar]
- de Bock P., Daelemans L., Selis L., Raes K., Vermeir P., Eeckhout M., Van Bockstaele F. Comparison of the chemical and technological characteristics of wholemeal flours obtained from amaranth (Amaranthus sp.), quinoa (chenopodium quinoa) and buckwheat (fagopyrum sp.) seeds. Foods. 2021;10(3):651. doi: 10.3390/foods10030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir M.K. Quinoa: Nutritional and anti-nutritional characteristics. Journal of Food and Health Science. 2016;104–111 doi: 10.3153/jfhs16011. [DOI] [Google Scholar]
- Díaz González D., Morawicki R., Mauromoustakos A. Effect of nixtamalization treatment of three varieties of grain sorghum on the reduction of total phenolics and their subsequent enzymatic hydrolysis. Journal of Food Processing and Preservation. 2019;43(9) doi: 10.1111/jfpp.14067. [DOI] [Google Scholar]
- Ding J., Hou G.G., Nemzer B.V., Xiong S., Dubat A., Feng H. Effects of controlled germination on selected physicochemical and functional properties of whole-wheat flour and enhanced γ-aminobutyric acid accumulation by ultrasonication. Food Chemistry. 2018;243:214–221. doi: 10.1016/j.foodchem.2017.09.128. [DOI] [PubMed] [Google Scholar]
- Egli I., Davidsson L., Juillerat M.A., Barclay D., Hurrell R.F. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. 3484 Journal of Food Science. 2002;67 [Google Scholar]
- Gaytán-Martínez M., Cabrera-Ramírez Á.H., Morales-Sánchez E., Ramírez-Jiménez A.K., Cruz-Ramírez J., Campos-Vega R., Velazquez G., Loarca-Piña G., Mendoza S. Effect of nixtamalization process on the content and composition of phenolic compounds and antioxidant activity of two sorghums varieties. Journal of Cereal Science. 2017;77:1–8. doi: 10.1016/j.jcs.2017.06.014. [DOI] [Google Scholar]
- Gorinstein S., Lojek A., Číž M., Pawelzik E., Delgado-Licon E., Medina O.J., Moreno M., Salas I.A., Goshev I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. International Journal of Food Science and Technology. 2008;43(4):629–637. doi: 10.1111/j.1365-2621.2007.01498.x. [DOI] [Google Scholar]
- Gu Z., Jin Z., Schwarz P., Rao J., Chen B. Unraveling the role of germination days on the aroma variations of roasted barley malts via gas chromatography-mass spectrometry based untargeted and targeted flavoromics. Food Chemistry. 2023;426 doi: 10.1016/j.foodchem.2023.136563. [DOI] [PubMed] [Google Scholar]
- Guardianelli L.M., Salinas M.V., Brites C., Puppo M.C. Germination of white and red quinoa seeds: improvement of nutritional and functional quality of flours. Foods. 2022;11(20) doi: 10.3390/foods11203272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güzel N. Morphometric and Physico-chemical Properties of Cornelian Cherry (Cornus mas L.) Grown in Çorum, Turkey. Akademik Gıda. 2021:373–380. doi: 10.24323/akademik-gida.1050750. [DOI] [Google Scholar]
- Güzel N., Kahraman O., Feng H. Solid-liquid extraction by manothermosonication: recapturing the value of pomegranate peels and nanocomplexation of extracts with pea protein. ACS Sustainable Chemistry & Engineering. 2020;8(44):16671–16679. doi: 10.1021/acssuschemeng.0c06316. [DOI] [Google Scholar]
- Güzel N., Taği Ş., Özkan M. Effects of moisture contents and storage temperatures on the physical, chemical and microbiological qualities of non-sulfitted dried apricots. Tarim Bilimleri Dergisi. 2022;28(4):691–703. doi: 10.15832/ankutbd.959820. [DOI] [Google Scholar]
- Haug W., Lantzsch H.-J. Sensitive method for the rapid determination of phytate in cereals and cereal products. Journal of the Science of Food and Agriculture. 1983;34(12):1423–1426. doi: 10.1002/jsfa.2740341217. [DOI] [Google Scholar]
- Hernández-Ledesma B. Bioactive Compounds in Health and Disease. Functional Food Institute; 2019. Quinoa (Chenopodium quinoa Willd.) as a source of nutrients and bioactive compounds: A review. (Vol. 2, Issue 3, pp. 27–47) https://doi.org/10.31989/bchd.v2i3.556. [Google Scholar]
- Hübner F., Arendt E.K. Germination of cereal grains as a way to improve the nutritional value: A review. Critical Reviews in Food Science and Nutrition. 2013;53(8):853–861. doi: 10.1080/10408398.2011.562060. [DOI] [PubMed] [Google Scholar]
- Kaur I., Tanwar B., Reddy M., Chauhan A. Vitamin C, total polyphenols and antioxidant activity in raw, domestically processed and industrially processed Indian Chenopodium quinoa seeds. Journal of Applied Pharmaceutical Science. 2016;6(4):139–145. doi: 10.7324/japs.2016.60419. [DOI] [Google Scholar]
- Kim M.Y., Jang G.Y., Lee Y., Li M., Ji Y.M., Yoon N., Lee S.H., Kim K.M., Lee J., Jeong H.S. Free and bound form bioactive compound profiles in germinated black soybean (Glycine max L.) Food Science and Biotechnology. 2016;25(6):1551–1559. doi: 10.1007/s10068-016-0240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S., Bhinder S., Singh B., Kaur A. Physicochemical properties, non-nutrients and phenolic composition of germinated freeze-dried flours of foxtail millet, proso millet and common buckwheat. Journal of Food Composition and Analysis. 2023;115 doi: 10.1016/j.jfca.2022.105043. [DOI] [Google Scholar]
- Li R., Li Z.J., Wu N.N., Tan B. Vol. 99(2. John Wiley and Sons Inc.; 2022. Effect of pre-treatment on the functional properties of germinated whole grains: A review; pp. 253–269. (Cereal Chemistry). [DOI] [Google Scholar]
- Manoel Maradini Filho A., Ribeiro Pirozi M., Da Silva Tomaz, Borges J., Maria Pinheiro Sant H., Benício Paes Chaves J., Dos Reis Sélia, Coimbra J., Sélia Dos Reis J., Pirozi Ribeiro, onica, Tomaz Da Silva Borges, ao, Ben ıcio Paes Chaves J., elia Dos Reis Coimbra J.S. Quinoa: Nutritional, functional, and antinutritional aspects. Critical Reviews in Food Science and Nutrition. 2017;57(8):1618–1630. doi: 10.1080/10408398.2014.1001811. [DOI] [PubMed] [Google Scholar]
- Niro S., D’Agostino A., Fratianni A., Cinquanta L., Panfili G. Gluten-free alternative grains: Nutritional evaluation and bioactive compounds. Foods. 2019;8(6):208. doi: 10.3390/foods8060208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurul Huda, M., Lu, S., Jahan, T., Ding, M., Jha, R., Zhang, K., Zhang, W., Georgiev, M. I., Park, S. U., & Zhou, M. (2020). Treasure from garden: Bioactive compounds of buckwheat. https://doi.org/10.1016/j.foodchem.2020.127653. [DOI] [PMC free article] [PubMed]
- Olojede A.O., Sanni A.I., Banwo K. Effect of legume addition on the physiochemical and sensorial attributes of sorghum-based sourdough bread. LWT. 2020;118 doi: 10.1016/J.LWT.2019.108769. [DOI] [Google Scholar]
- Omary M.B., Fong C., Rothschild J., Finney P. Effects of germination on the nutritional profile of gluten-free cereals and pseudocereals: A review. Cereal Chemistry. 2012;89(1):1–14. doi: 10.1094/CCHEM-01-11-0008. [DOI] [Google Scholar]
- Paucar-Menacho L.M., Peñas E., Dueñas M., Frias J., Martínez-Villaluenga C. Optimizing germination conditions to enhance the accumulation of bioactive compounds and the antioxidant activity of kiwicha (Amaranthus caudatus) using response surface methodology. LWT. 2017;76:245–252. doi: 10.1016/j.lwt.2016.07.038. [DOI] [Google Scholar]
- Peiris K.H.S., Bean S.R., Chiluwal A., Perumal R., Jagadish S.V.K. Moisture effects on robustness of sorghum grain protein near-infrared spectroscopy calibration. Cereal Chemistry. 2019;96(4):678–688. doi: 10.1002/cche.10164. [DOI] [Google Scholar]
- Rodríguez J.P., Rahman H., Thushar S., Singh R.K. Healthy and resilient cereals and pseudo-cereals for marginal agriculture: Molecular advances for improving nutrient bioavailability. Frontiers in Genetics. 2020;Vol. 11 doi: 10.3389/fgene.2020.00049. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rois Mansur, A., Song, N.-E., Jang, H. W., Lim, T.-G., Yoo, M., & Nam, G. (2019). Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. https://doi.org/10.1016/j.foodchem.2019.05.003. [DOI] [PubMed]
- Sharma S., Saxena D.C., Riar C.S. Changes in the GABA and polyphenols contents of foxtail millet on germination and their relationship with in vitro antioxidant activity. Food Chemistry. 2018;245:863–870. doi: 10.1016/j.foodchem.2017.11.093. [DOI] [PubMed] [Google Scholar]
- Shreeja K., Devi S.S., Suneetha W.J., Prabhakar B.N. Effect of Germination on Nutritional Composition of Common Buckwheat (Fagopyrum esculentum Moench) International Research Journal of Pure and Applied Chemistry. 2021;1–7 doi: 10.9734/irjpac/2021/v22i130350. [DOI] [Google Scholar]
- Tang Y., Li X., Zhang B., Chen P.X., Liu R., Tsao R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chemistry. 2015;166:380–388. doi: 10.1016/j.foodchem.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Terpinc, P., Cigić, B. C., Polak, T., Hribar, J., & Požrl, T. (2016). LC-MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting. https://doi.org/10.1016/j.foodchem.2016.04.030. [DOI] [PubMed]
- Thakur P., Kumar K. Nutritional Importance and Processing Aspects of Pseudo-cereals. Journal of Agricultural Engineering and Food Technology. 2019;6(2) http://www.krishisanskriti.org/Publication.html [Google Scholar]
- Thakur P., Kumar K., Ahmed N., Chauhan D., Eain Hyder Rizvi Q.U., Jan S., Singh T.P., Dhaliwal H.S. Effect of soaking and germination treatments on nutritional, anti-nutritional, and bioactive properties of amaranth (Amaranthus hypochondriacus L.), quinoa (Chenopodium quinoa L.), and buckwheat (Fagopyrum esculentum L.) Current Research in Food Science. 2021;4:917–925. doi: 10.1016/j.crfs.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tömösközi S., Langó B. In: Gluten-Free Ancient Grains. Taylor J.R.N., Awika J.M., editors. Woodhead Publishing; 2017. Chapter 7 - Buckwheat: Its unique nutritional and health-promoting attributes; pp. 161–177. [Google Scholar]
- Wang J., Bian Z., Wang S., Zhang L. Effects of ultrasonic waves, microwaves, and thermal stress treatment on the germination of Tartary buckwheat seeds. Journal of Food Process Engineering. 2020;43(10) doi: 10.1111/jfpe.13494. [DOI] [Google Scholar]
- Xia Q., Tao H., Li Y., Pan D., Cao J., Liu L., Zhou X., Barba F.J. Characterizing physicochemical, nutritional and quality attributes of wholegrain Oryza sativa L. subjected to high intensity ultrasound-stimulated pre-germination. Food Control. 2020;108 doi: 10.1016/j.foodcont.2019.106827. [DOI] [Google Scholar]
- Xiang J., Yuan Y., Du L., Zhang Y., Li C. Modification on phenolic profiles and enhancement of antioxidant activity of proso millets during germination. 2023 doi: 10.1016/j.fochx.2023.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Xu Z., Gao Y., Huang X., Zou Y., Yang T. Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat. Journal of Food Science. 2015;80(5):H1111–H1119. doi: 10.1111/1750-3841.12830. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Zhang X., Li T., Wang L. Impact of microwave and germination on physicochemical, functional properties, and solubility of black rice powder. Cereal Chemistry. 2023 doi: 10.1002/cche.10664. [DOI] [Google Scholar]
- Zhang Z.L., Zhou M.L., Tang Y., Li F.L., Tang Y.X., Shao J.R., Xue W.T., Wu Y.M. Bioactive compounds in functional buckwheat food. Food Research International. 2012;49(1):389–395. doi: 10.1016/j.foodres.2012.07.035. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.