Highlights
-
•
A comprehensive approach was used to uncover potential flavor-contributing factors.
-
•
Highly correlated modules were identified, including blue, black, pink and turquoise.
-
•
Potential flavor constituents strongly associated with sensory attributes were given.
-
•
The influence of taste factors on olfactory characteristics were displayed.
-
•
This study provides a novel approach and valuable insights into flavor perception.
Keywords: Pyrus spp., Potential flavor factors, Metabolomics, Sensory attributes, Weighted correlation network analysis
Abstract
Flavor profiles of various Pyrus spp. cultivars exhibit significant variations, yet the underlying flavor-contributing factors remain elusive. In this investigation, a comprehensive approach encompassing metabolomics analysis, volatile fingerprint analysis, and descriptive sensory analysis was employed to elucidate the flavor disparities among Nanguoli, Korla fragrant pear, and Qiuyueli cultivars and uncover potential flavor contributor. The study comprehensively characterized the categories and concentrations of nonvolatile and volatile metabolites, and 925 metabolites were identified. Flavonoids and esters dominated the highest cumulative response, respectively. Utilizing weighted correlation network analysis (WGCNA), seven highly correlated modules were identified, yielding 407 pivotal metabolites. Further correlation analysis of the differential substances provided potential flavor constituents strongly associated with various sensory attributes; taste factors had a certain association with olfactory characteristics. Our findings demonstrated the manifestation of flavor was a result of the synergistic effect of various compounds; evaluation olfactory flavor necessitated a comprehensive consideration of taste substances.
1. Introduction
Pyrus spp., a deciduous perennial fruit tree belonging to the Rosaceae family, holds a distinguished position as one of the earliest cultivated fruit trees. Due to its refreshing, succulent, sweet, and tangy flavor, it is often referred to as the “Natural Mineral Water” among fruits. Ancient historical documents have attested to the superior quality of pears and their potential in treating ailments (Zhang, Zhang, & Gao, 2012). Contemporary research has revealed the nutritional richness of pears, which contain abundant carbohydrates, proteins, fats, an array of vitamins, polyphenols, and other essential nutrients and bioactive compounds (Sun, Hao, Tang, Wang, & Zhang, 2020). These substances contribute to the positive impact on human health, aiding lung moisturization, fever reduction, cough relief, and overall well-being (Zhang, Zhang, & Gao, 2012). These attributes make pears a beloved and widely consumed fruit, and they are gaining popularity among individuals with diverse tastes and preferences.
In China, an impressive diversity of pears has been reported, encompassing 13 distinct species and a remarkable collection of over 1600 varieties (Zhang et al., 2021) belonging to different botanical categorizations, such as Pyrus bretschneideri Rehd., Pyrus pyrifolia Nakai., Pyrus ussuriensis Max., and Pyrus sinkiangensis Yu. Moreover, pears are extensively cultivated in China. These give rise to a diverse array of pear varieties characterized by their distinctive and captivating flavors (Chen et al., 2018). The flavor of fruit serves as an objective indicator of ripeness and a crucial determinant of both fruit quality and processed products, thereby exerting a substantial influence on market competitiveness (Zhang et al., 2023). Therefore, several scholars have conducted research on the identification and exploration of aroma differences in pears, and 300 individual volatile compounds, including hexyl acetate, ethyl 2-methylbutyrate, hexanal, (E)-2-hexenal, hexanol, (E)-2-hexen-1-ol, 6-methyl-5-hepten-2-one, 2-butanone, α-farnesene, (Z, E)-α-farnesene, decane, propyl cyclopropane, and ethyl 3-methyl thiopropionate, were identified (Qin et al., 2012). By conducting a comprehensive examination of aroma traits, various Chinese pear varieties have been classified into separate aromatic groups, each distinguished by its own distinct fragrance (Zhang et al., 2023). Scholars have employed the “ABC” value method to investigate the aromatic characteristics of various pear cultivars, revealing that esters are the predominant aroma type in Korla fragrant pear, Hongnanguo and Bartlett after ripening (Chen et al., 2018). Furthermore, during the early and middle developmental stages of pears, the aroma profile is characterized primarily by ester scent; however, the aroma profile undergoes a transition to a more pronounced fruity aroma during the maturity and storage phases (Chen et al., 2018). The above studies provide crucial foundational insights into deciphering the flavor attributes of Pyrus spp., yet the specific compounds responsible for contributing to flavor have not been explored in previous investigations. In addition to aromas, flavor encompasses a complex interplay of taste factors, which have been found to be intricately linked with metabolites such as sugars, organic acids, amino acids, flavonoids, and tannins (Ikegaya et al., 2019, Fan et al., 2021). Previous literature has demonstrated that polyphenols, alkaloids, amino acids, and other chemical constituents play pivotal roles in shaping umami and astringent taste perceptions (Ye et al., 2022). Catechins and flavonoid glycosides are closely linked to astringency, increasing the complexity of the flavor profile (Zuo et al., 2021). The main sugars, acids and phenolic substances in pears have been reported in the literature (Sun, Hao, Tang, Wang, & Zhang, 2020). However, the number of known metabolites suitable for correlation analysis with sensory characteristics remains relatively limited. The interplay of both volatile and nonvolatile compounds contributes to the distinctive taste experiences of various foods (Tian, Zheng, Yu, Chen, & Lou, 2023). Olfactory stimuli triggered by odors can modify the perception of all five basic tastes including sweet, salty, bitter, umami, and sour (Xiao, Chen, Niu, & Zhu, 2021). This olfactory-taste interaction occurs not only through the positive nasal pathway in response to aroma compounds but also through the modulation of taste perception itself (Tian, Zheng, Yu, Chen, & Lou, 2023). This demonstrates the complexity of pear flavor study.
Widely targeted metabolomics, a cutting-edge method within this domain, integrates the broad scope of nontargeted metabolomics with the precision of targeted metabolomics (Zou et al., 2020). Due to its capacity for the identification and quantification of a vast array of metabolites, it has been successfully used for the ultrasensitive detection of constituents in various fruits, such as Eriobotrya japonica (Thunb.) Lindl. and Litchi chinensis Sonn. (Zou et al., 2020, Yin et al., 2022). Two-dimensional gas chromatography/time-of-flight mass spectrometry (GC × GC-TOFMS) technology possesses exceptional peak capacity, high sensitivity, superior resolution, and rapid analysis speed (Zhao et al., 2022), which make it particularly valuable for the identifying volatile and semivolatile compounds, and providing a comprehensive volatile fingerprint with increased data collection frequency. Owing to these advantages, GC × GC-TOFMS has gained widespread application in wine (Welke, Zanus, Lazzarotto, Pulgati, & Zini, 2014) and green tea (Zhu et al., 2018). Recently, it has also been employed for analyzing aromatic substances in different varieties of Pyrus communis Linn. and Pyrus ussuriensis Max. (Zhang et al., 2023). The association between metabolites and sensory traits could be demonstrated through the combination of sensory evaluation and metabolite analysis (Sung, Suh, Chambers, Crane, & Wang, 2019), which also provided theoretical support for the identification of key flavor factors. However, to date, there is no existing report, which investigates the correlation between metabolic compounds and the sensory characteristics of pears. An extensive approach encompassing wide metabolomics analysis, GC × GC-TOFMS analysis, and descriptive sensory assessment of representative pear varieties was employed to elucidate the flavor diversity among different pear cultivars in this study. Through the application of WGCNA to establish correlations between metabolites and sensory attributes, modules highly related to sensory traits were obtained; by combination with visual network analysis, potential key contributors with the highest correlations to sensory traits were ultimately identified; and the intricate interplay between fundamental metabolic components and variations in pear flavor could be explained. Furthermore, the influence of taste factors on olfactory perception was be explored. This investigation aims to reveal the flavor disparities in pears caused by metabolism, confirm key flavor factors through a new comprehensive analysis method, and provide useful analytical data for identifying the key flavor factors of pear. This study has significant implications for advancing pear flavor research, confirming pivotal flavor factors, perfecting quality evaluation based on flavor factors and ensuring high-quality development in the pear industry.
2. Materials and methods
2.1. Materials
Nanguoli (NGL), Korla fragrant pear (KFP), and Qiuyueli (QYL) cultivars were collected from distinct regions: Anshan city (40°52′53″N, 122°41′04″E/120 m) of Liaoning Province, Korla city (41°43′33″N, 86°10′29″E/934 m) of Xinjiang Province, and Yantai city (36°58′48″N, 120°42′38″E/87 m) of Shandong Province. For each variety, samples were meticulously chosen from five different trees, ensuring intact and undamaged whole fruits for the experiment. Traditional metrics such as the growing period (measured in days after flowering), external morphology, and skin color were employed to ascertain the maturity of each cultivar. The designated harvesting dates for the aforementioned pears were October 5th, 2021; September 27th, 2021; and September 23rd, 2021. The physical appearance of these three pear cultivars is depicted in Fig. 1. All harvested fruits were promptly preserved at low temperatures and expeditiously transported to the Institute of Quality Standard and Testing Technology for Agro-Products, Shandong Academy of Agricultural Sciences, China. The samples were collected consecutively in triplicate.
Fig. 1.
Physical appearance of three pear cultivars. Note: The lengths of the bars represent 5 cm. A, B, and C are QYL, KFP, and NGL, respectively; E, D, and F refer to the profiles of QYL, KFP, and NGL, respectively.
2.2. Widely targeted metabolomics analysis
Upon immediate return to the laboratory, the three pear cultivars underwent analysis in their fresh state. Be applying the quartic method, each pear in each sample was cross-sectioned along its growth axis into four sections. Then a quartered pear was randomly selected, cored and cut into small pieces. The next step involved the treatment of the three pear cultivars with liquid nitrogen. This was followed by processing through a specialized external service provider (Genedenovo Biotechnology Co., Ltd., Guangzhou, China). Briefly, the pear samples were freeze-dried using a Scientz-100F vacuum freeze-dryer (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China). Subsequently, the dried samples were pulverized utilizing an MM 400 mixer mill (Retsch GmbH, Haan, Germany) equipped with zirconia beads, employing a 1.5 min cycle at a frequency of 30 Hz. The lyophilized powder (100 mg) was then mixed with 1.2 mL of a 70 % methanol solution, vortexed for 30 s at 30 min intervals for a total of six iterations, and then left in a refrigerator at 4 °C overnight. The obtained mixture was centrifuged at 12000 rpm for 10 min, and the resultant extracts were filtered through 0.22 μm SCAA-104 filter membranes (ANPEL, Shanghai, China) prior to subsequent ultra-performance liquid chromatography-tandem mass spectrometry (UPLC–MS/MS) analysis.
We used a UPLC system (UPLC, SHIMADZU Nexera X2; Kyoto, Japan) coupled with an MS (Allen-Bradley Applied Biosystems 4500 Q TRAP; Milwaukee, Wisconsin, USA) for the analysis of nonvolatile sample extracts. The analytical conditions were configured as follows: The column used was an Agilent SB-C18 (1.8 µm, 2.1 mm × 100 mm) (San Jose, California, USA). The mobile phase consisted of solvent A, which was pure water containing 0.1 % formic acid, and solvent B, which was composed of acetonitrile with 0.1 % formic acid. The sample analysis was conducted using a gradient elution procedure, commencing with 95 % A and 5 % B. Mobile phase A was linearly decreased from 95 % to 5 % within the time frame of 0 to 9 min, followed by a one-minute hold at 5 %. Subsequently, the volume of mobile phase A was rapidly increased from 5 % to an initial composition of 95 % within a duration of 1.1 min, and maintained at this level for an additional period of 2.9 min. The flow rate was set at 0.35 mL/min, the column temperature was 40 °C, and the injection volume was 4 μL. The effluent was alternately connected to a Q TRAP-MS instrument. The UPLC/MS/MS system, equipped with an electrospray ionization (ESI) Turbo Ion-Spray interface, was operated in both positive and negative ion modes and managed using Analyst 1.6.3 AB Scitex software (Milwaukee, Wisconsin, USA). The ion source was operated under the following conditions: turbo spray for the ion source, source temperature 550 °C, ion spray voltage (IS) 5500 V (positive ion mode) and − 4500 V (negative ion mode), and gas settings of ion source gas I (GSI), gas II (GSII), and curtain gas (CUR) at 50, 60, and 25.0 psi, respectively. Collision-activated dissociation (CAD) was set to high. The acquisition of triple quadrupole (QQQ) scans was conducted as multiple reaction monitoring (MRM) experiments, using nitrogen as the collision gas at medium settings. Distinct declustering potential (DP) and collision energy (CE) parameters were established for each MRM transition, with further optimization. A specific set of MRM transitions was monitored for each analysis period, tailored to the elution profiles of metabolites within that timeframe.
Qualitative analysis of metabolites was performed based on a self-built metware database (MWDB, Genedenovo Biotechnology Co., Ltd., Guangzhou, China) utilizing secondary spectrum information. The quantitative determination of metabolites was achieved by selecting characteristic fragment ions using triple quadrupole mass spectrometry in multi-reaction monitoring mode, followed by chromatographic peak integration and correction using AB Scitex software.
2.3. Analysis of volatile compounds
Volatile compounds were extracted using the headspace solid phase microextraction (HS-SPME) method. A Supelco 50/30 µm DVB/CAR/PDMS SPME fiber (Bellefonte, Pennsylvania, USA) was selected based on prior validation and established literature (Zhang et al., 2023). Upon immediate return to the laboratory, the three pear cultivars underwent analysis in their fresh state. Employing the quartic method, each pear in each sample was cross-sectioned along its growth axis into four sections; one section of each pear was randomly selected, and 0.5 cm thick slices without cores were cut. These slices were subsequently cut into 0.5 cm × 0.5 cm pieces. Subsequently, 6.0 g of the mixed sample was placed into a 15 mL headspace bottle. Prior to sealing the vials, 5 µL of 10 µg/mL 2-nonanone (Dr. Ehrensorfer, Augsburg, Bavaria, Germany) was added as an internal standard. After standing for 10 min, the vials were then placed in a thermostatic controller set at 40 °C for 40 min. Finally, the SPME fiber was promptly inserted into the GC injector for desorption at 270 °C for 2 min with a split mode of 10:1.
Volatile compound analysis was conducted using an Agilent 7890B gas chromatograph (San Jose, California, USA) equipped with a LECO Pegasus 4D-C time-of-flight mass spectrometric detector (Saint Joseph, California, USA). A Shimadzu Rxi-5MS column (Kyoto, Japan) (30 m × 250 µm × 0.25 µm) served as the first-dimensional (1D) column, while a Shimadzu Rxi-17Sil MS column (Kyoto, Japan) (2 m × 250 µm × 0.25 µm) functioned as the second-dimensional (2D) column. Helium was employed as the carrier gas, and was allowed to flow steadily at 1.4 mL/min. The front inlet and transfer line temperatures were set to 270 °C and 280 °C, respectively. The temperature program for the oven was as follows: an initial temperature of 40 °C for 2 min, increasing at a rate of 5 °C/min up to 200 °C, further elevated to 280 °C at a rate of 20 °C/min and held for 2 min. The secondary oven temperature remained 5 °C higher than the GC oven temperature throughout the chromatographic run. The modulator temperature was set 15 °C higher than the secondary oven temperature. Modulation occurred every 3 s with a 0.6 s hot pulse. The mass spectrometry parameters were set as follows: acquisition delay 60 s; acquisition rate 100 spectra/s; acquisition voltage 1450 V; electron energy − 70 V; and ion source temperature 250 °C. Mass spectra were captured within the m/z range of 35–––550 amu.
The mass spectrometry fragments corresponding to each chromatographic peak were initially searched using NIST2017, and volatile aroma compounds with matching scores exceeding 700 were identified. Moreover the retention index for each aroma component was calculated to further identification using the established formula (Zhang et al., 2023). The quantification of aroma constituents was accomplished by assessing peak areas through the internal standard method (Zhang et al., 2023).
2.4. Sensory evaluation
A quantitative descriptive analysis was conducted to assess the flavor profiles of distinct pear varieties, encompassing seven aroma attributes (green grass, fresh, sweet, apple-like, floral, woody, ferment, and bouquet) and four taste attributes (sweetness, sourness, fruit wine, and astringency).
A panel of ten expert assessors (four females and six males) with professional training was recruited. A 0–5 intensity scale was adopted for rating the flavor attributes, and Table S1 displays the definition, reference solution, and intensity for each flavor attribute. The sensory evaluation took place within 24 h of sample acquisition in an odor-free room maintained at 22 ± 1 °C. For each cultivar, 12 to 20 pear fruits were randomly selected; according to the four-point method, samples were prepared 30 min before the experiment. The samples were peeled, pitted, and cut into 2 × 2 cm pieces, ensuring even mixing. A total of 10 samples, each comprising 15–––30 g of cut pieces, were randomly prepared and presented to the evaluators in a random sequence. The assessors subsequently rated the samples and recorded the intensity of the sensory attributes. Adequate resting periods were required between each sample to alleviate sensory fatigue. The experiment was conducted in triplicate.
This study was approved by the ethics committee of the Shandong Academy of Agricultural Sciences.
2.5. Association of metabolic materials and sensory attributes via WGCNA
This investigation aimed to establish correlations between sensory attributes and metabolic compounds. Sensory attributes, including seven aroma attributes and five taste attributes, were used to establish a metabolite co-expression network with metabolic materials utilizing the WGCNA R package (v 1.47). The correlation matrix soft-thresholding power β was 18. After calculating the topological overlap measure (TOM) using the adjacency matrix, a dendrogram was generated using the dissimilarity TOM. The modules were established using the dynamic tree cut algorithm and assigned different colors, and each module contained highly correlated metabolites.
2.6. Data processing and statistical analysis
Principal component analysis (PCA) was conducted using SIMCA 14.1 to group pear varieties according to the detected volatile components and nonvolatile metabolites. For visual representation of compound content intensity, a heatmap and clustering analysis (HCA) were conducted utilizing TBtools. The data were subjected to variance analysis via SPSS Statistical 19.0, and the mean values were compared utilizing Tukey’s test at a significance level of P < 0.05. Radar plots and histograms were drawn using Originlab 2022. The application of partial least squares regression (PLSR) was carried out by Unscrambler X 10.4. The visual network analysis was conducted with Cytoscape 3.7.2.
3. Results and discussion
3.1. Descriptive sensory analysis of three pear cultivars
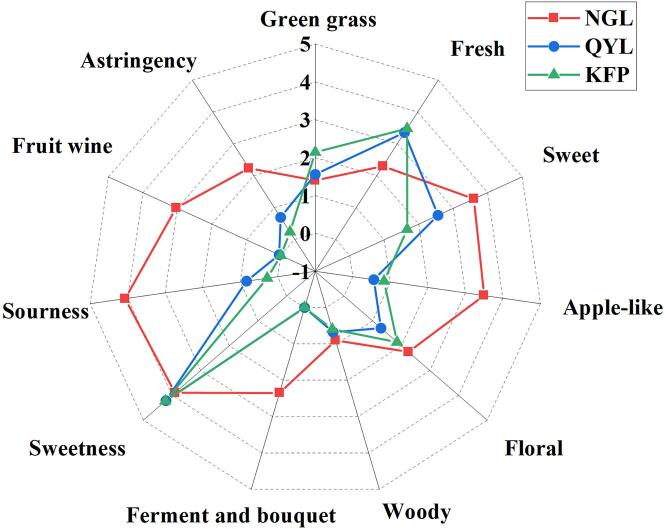
We evaluated the flavor characteristics of three distinct pear cultivars by descriptive sensory analysis. Except for ferment and bouquet and fruit wine, the other flavors were common among all three pear types, but there were still great differences in intensity among the different varieties of pear. A radar diagram (Fig. 2) provided insight into these differences. Notably, Nanguoli exhibited a robust flavor profile, with significantly elevated intensities of attributes such as apple-like (3.50), sweet (3.60), floral (2.25), ferment and bouquet (2.35), sourness (4.07), fruit wine (3.03), and astringency (2.23) compared to Qiuyueli and Korla fragrant pear. While the sensory attribute profiles of Korla fragrant pear and Qiuyueli displayed some similarities, there were certain differences between them. Qiuyueli, categorized as a sweet and fresh pear, displayed pronounced sweet (2.56), mild sourness (0.83), and astringency (0.69), surpassing those of Korla fragrant pear. Moreover, the fresh (3.35), sweetness (4.21), and green grass aromas (1.56) of Qiuyueli were more pronounced than those of Nanguoli. Korla fragrant pear stands as a variety bearing both green grass and floral notes, with its green grass (2.14) and fresh scent (3.48) being more prominent than those of Qiuyueli and Nanguoli. Moreover, the floral (1.86) and apple-like (0.83) attributes of Korla fragrant pear surpassed those of Qiuyueli.
Fig. 2.
Radar diagram of the sensory characteristics of the three pear cultivars.
3.2. Analysis of nonvolatile and volatile metabolite profiles
To gain deeper insights into the differences in flavor among the pear cultivars, we performed a comprehensive analysis involving widely targeted metabolite profiling of Nanguoli, Korla fragrant pear, and Qiuyueli, and their UPLC–MS/MS ion mass spectra was displayed in Fig. S1, Fig. S2 and Fig. S3, respectively. Simultaneously, we employed GC × GC-TOFMS to conduct an exhaustive comparison of the aroma profiles of the three pear species, and their mass spectra was displayed in Fig. S4.
In total, we successfully identified 844 nonvolatile metabolites, encompassing a diverse array of categories, including 81 amino acids and their derivatives, 162 phenolic acids, 39 nucleotides and their derivatives, 169 flavonoids, 29 lignans and coumarins, 70 sugar alcohols, 32 vitamins and stilbenes, 17 tannins, 34 alkaloids, 40 terpenoids, 62 organic acids, and 109 lipids, as detailed in Table S2. Additionally, our analysis identified 81 distinct aroma components, including 54 esters, 7 alcohols, 5 alkenes, 4 aldehydes, 2 ketones, 7 alkanes, 2 aromatics, and other compounds, as presented in Table S3.
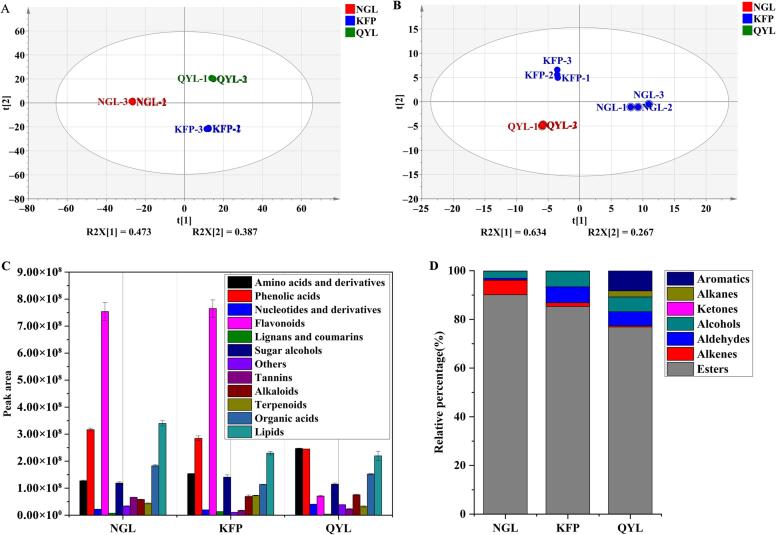
PCA clearly separated the three pear varieties for both nonvolatile and volatile components, as illustrated in Fig. 3A and 3B. In the case of nonvolatile constituents, the first two principal components (PCs) accounted for 86 % of the total variance (PC1 = 47.3 %, PC2 = 38.7 %). For volatile components, the first PC collected 90.1 % of the total variation (PC1 = 63.4 %, PC2 = 26.7 %). These findings indicated that substantial disparities existed in the nonvolatile and volatile substances of the three pear cultivars. Moreover, the discrimination achieved by the first two principal components for both nonvolatile and volatile compounds was effective in representing the overall sample profiles.
Fig. 3.
Analysis of nonvolatile and volatile metabolite profiles. A: Principal component analysis of nonvolatile compounds in three pear varieties; B: Principal component analysis of volatile compounds in three pear cultivars; C: Responses of different types of metabolites in three pear species; D: Relative proportions of different volatile compounds in three pear cultivars.
Upon analyzing the relative contents of volatile and nonvolatile substances (Fig. 3C and Fig. S5), along with the relative proportions of diverse substance types (Fig. S6 and Fig. 3D), we observed significant variations in the contents of different substance categories. Concerning nonvolatile components, the cumulative responses of amino acids and their derivatives, phenolic acids, flavonoids, sugars and alcohols, organic acids, and lipids were all greater, reflecting their significance as key contributors to food flavor, as corroborated by prior literature (Zou et al., 2020, Ye et al., 2022). As shown in Fig. 3C, flavonoids exhibited the highest cumulative response, and their content in Qiuyueli was lower than that in both Nanguoli and Korla fragrant pear. Phenolic acids and lipids showed similar cumulative responses as the second-highest content group; Qiuyueli had the highest phenolic acid content, while Nanguoli had the highest lipid content. Amino acids, organic acids, sugars and alcohols followed in terms of cumulative response levels with Qiuyueli having the highest content of amino acids, sugars and alcohols while Nanguoli had the highest content of organic acids. In contrast, the tannin and alkaloid contents were relatively low across the different varieties of pears; Nanguoli exhibited a significantly higher tannin response value compared to other varieties whereas the Qiuyue pear displayed the highest alkaloid response. Although tannins and alkaloids constitute a relatively small portion of pear fruits, they significantly contribute to astringency and bitterness in food contexts (Frost, Harbertson, & Heymann, 2017). Our astringency aligned with the alterations in tannin content among the three pear cultivars (Fig. 2 and Table S2), offering partial insight into the shifts in astringency across the pear species. Generally, fluctuations in sugar content reflect the sensory sweetness of pear fruits, whereas shifts in sweetness do not correspond directly with the changes in sugar content in the three pear cultivars, possibly due to intricate interactions between various flavor attributes (Ye et al., 2022).
For volatile components, the ratio between aroma scents determines the specific aroma characteristics of the matrix (Chen et al., 2018, Zhang et al., 2023). Consequently, we primarily concentrated on analyzing the relative percentages of aroma substances to elucidate the aroma attributes of each pear cultivar. Notably, ester aroma compounds, which generally emit a sweet smell and fruity taste (Qin et al., 2012, Chen et al., 2018), constituted a relative percentage of 76.9 % to 90.1 %, which made them the primary aroma constituents across the three pear varieties. Nanguoli possessed an exceptionally high proportion of 90.1 %, while Qiuyueli had the lowest proportion. As depicted in Fig. 3D, the substances accounting for the second-highest relative percentage of Nanguoli species were alkenes (6.0 %) which were typically associated with floral notes (Qin et al., 2012, Chen et al., 2018), followed by alcohols; thus, Nanguoli species had a complex fragrance characterized by both fruity and floral traits. This finding aligned with the aroma intensity observed in the sensory evaluation. In contrast, the percentages of aldehydes and alcohols which typically contribute to fresh and grass fragrances (Qin et al., 2012, Chen et al., 2018) in Korla fragrant pear and Qiuyueli were comparable, and these percentages were notably higher than those in Nanguoli (Fig. 3D). Among the two, Korla fragrant pear possessed greater relative percentage of alkenes (1.6 %) than Qiuyueli did (0.5 %), while Qiuyueli had higher proportions of alkanes (8.3 %) and aromatics (2.4 %) than Korla fragrant pear did. This was consistent with the facts that the grass and fresh properties of Korla fragrant pear and Qiuyueli were greater than those of Nanguoli, and the floral properties of Korla fragrant pear were greater than those of Qiuyueli. Moreover, Nanguoli also emitted discernible fermentation and wine aromas.
Although the content or relative percentage of the above different kinds of substances are consistent with the changes in some flavor attributes or reflect some flavor characteristics, further analysis and exploration are imperative to pinpoint the key contributing substances.
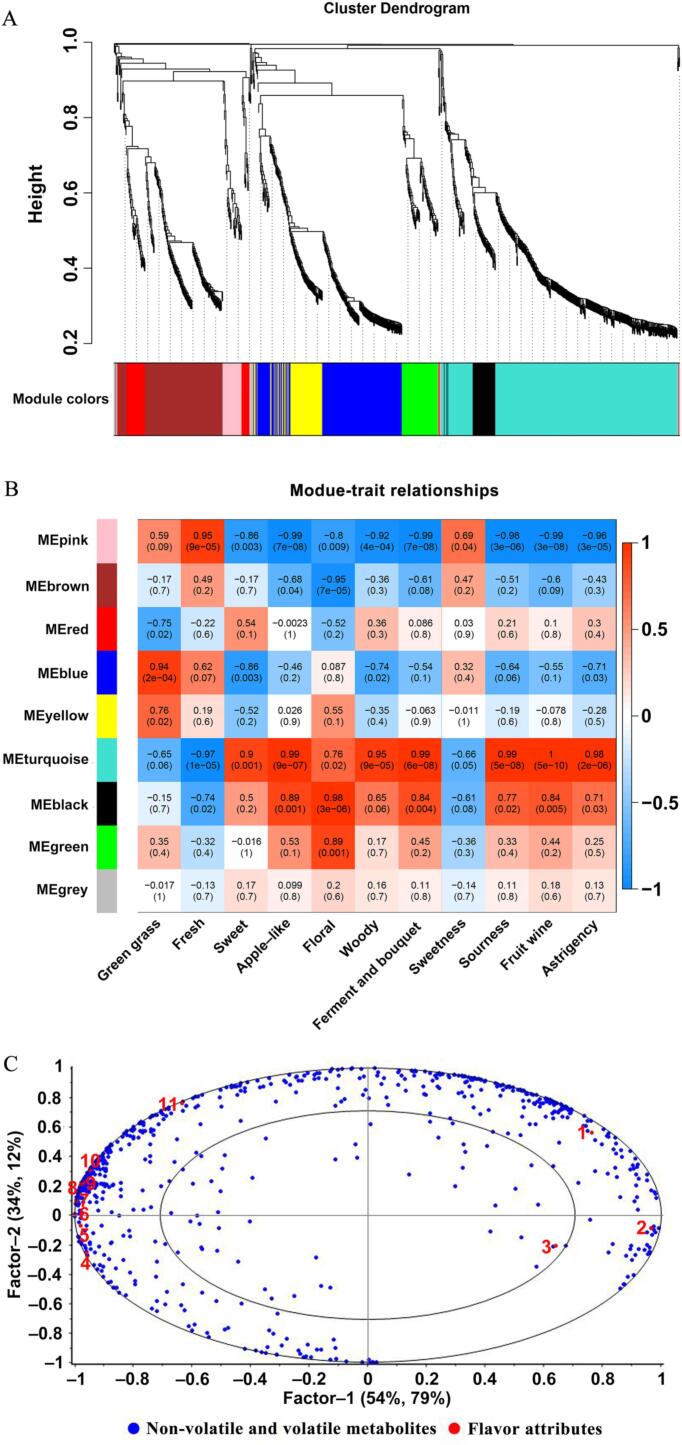
3.3. Association of metabolite profiles and important sensory traits via WGCNA
In this investigation, we employed the WGCNA approach to construct a co-expression network, aiming to explore correlations between 925 metabolites and specific flavor attributes encompassing seven aroma traits (green grass, fresh, sweet, apple-like, floral, woody, ferment, and bouquet) and four taste qualities (sweetness, sourness, fruit wine, and astringency). Nine co-expression modules were obtained; these were grouped into three main branches, each of which contained different modules (Fig. 4A and B). According to the module-trait relationship correlation heatmap (Fig. 4B), the pink and red modules demonstrated a positive correlation with the attributes of fresh, juiciness, and sweetness (r > 0.69); the blue, yellow, and green modules exhibited positive correlations with the green grass and floral traits (r > 0.69). Moreover, the turquoise and black modules displayed positive correlations with various attributes, including sweetness, apple-like, woody, etc. (r > 0.71). A total of 766 metabolites were highly correlated with specific modules (pink, red, blue, yellow, green, black, and turquoise), including 136 phenolic acids, 164 flavonoids, 81 lipids, 69 volatile aroma compounds, 43 organic acids, 47 amino acids and derivatives, 27 lignans and coumarins, 37 terpenoids, 33 nucleotides and derivatives, 27 alkaloids, 17 tannins, 85 sugar alcohols and others.
Fig. 4.
Correlations of metabolites with taste attributes based on WGCNA and PLSR. A: Clustering dendrogram of the average network adjacency for the identification of metabolite co-expression modules; B: Module-trait relationship correlation heatmap between modules and flavor attributes; C: Correlation loadings of metabolites achieved from the key modules. Note: 1: Green grass; 2: Fresh; 3: Sweetness; 4: Sweet; 5: Woody; 6: Astringency; 7: Sourness; 8: Fruit wine; 9: Ferment and bouquet; 10: Apple-like; 11: Floral. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Furthermore, PLSR was applied to explore the relationships between the 766 metabolites from the eight key modules and sensory attributes and to further verify the reliability of the WGCNA correlation network. As depicted in Fig. 4C, the PLSR model accounted for 88 % of the X variance and 91 % of the Y variance. The majority of the metabolic substances clustered between the two ovals were closely related to sensory attributes. The PLSR model indicated that sensory attributes could be effectively predicted by the high-correlation analysis modules. Remarkably, characteristics such as sweetness, apple-like, floral, woody, ferment and bouquet, sourness, fruit wine, and astringency were associated with the negative direction of Factor − 1, while green grass, fresh, and sweetness traits were associated with the positive direction of Factor − 1. This finding concurred with the trends observed in sensory traits via WGCNA, further reinforcing the reliability of the identified key modules and their association with metabolites.
3.4. Selection of key sensory trait-related metabolites
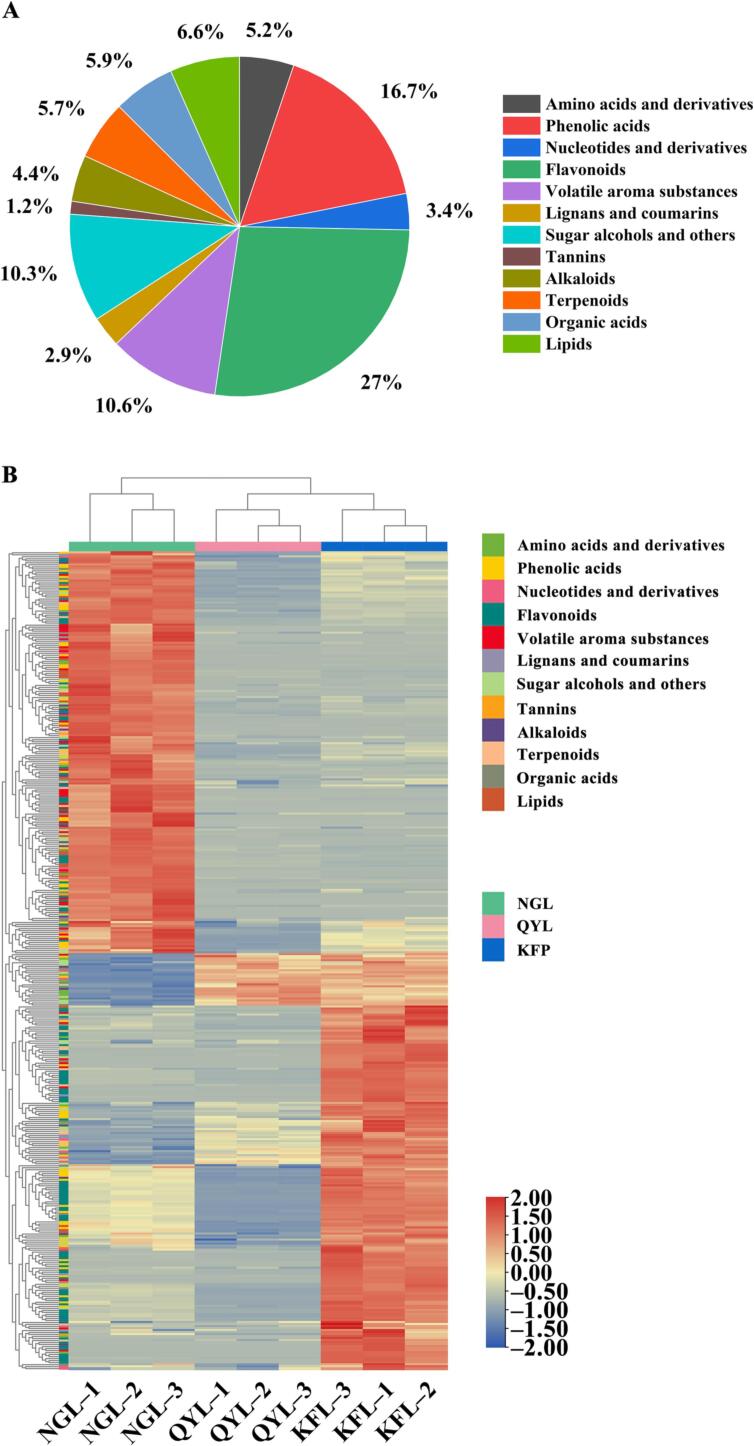
To further identify pivotal metabolites influencing pear flavor, we selected metabolites within the highly correlated module based on a gene significance (GS) > 0.6 and a module membership (MM) > 0.8. A total of 407 compounds were confirmed (Table S2 and Table S3), including 68 phenolic acids, 110 flavonoids, 27 lipids, 43 volatile aroma substances, 24 organic acids, 21 amino acids and derivatives, 12 kinds of lignans and coumarins, 23 terpenoids, 14 nucleotides and derivatives, 18 alkaloids, 5 tannins, 42 sugar alcohols, and other compounds. Remarkably, flavonoids constituted the largest group, accounting for 27 % of the total, and followed by phenolic acids, volatile substances, and sugar alcohols, with proportions greater than 10 %. Terpenoids, organic acids, lipids, and amino acids comprise constitute 5 to 10 % of the total aggregates, while the remaining substances constitute less than 5 % of the total aggregates (Fig. 5A). At present, comprehensive exploration of flavor traits in pear varieties has not been thoroughly performed. The significant metabolites elucidated through the association analysis of metabolomics and sensory characteristics in this study will provide foundational information for unraveling the intricacies of pear flavor variations.
Fig. 5.
Types and proportions of the significant metabolites in three pear cultivars (A) and heatmaps of the key metabolites in three pear species (B).
According to the heatmap (Fig. 5B), there were significant differences in the levels of these key substances among the different pear cultivars. A substantial portion of metabolites displayed higher levels in Nanguoli, while the remaining compounds were more abundant in Korla fragrant pear. Comparatively, most substances in Qiuyueli exhibited lower contents than those in the other pear cultivars. The levels of most volatile aroma substances, tannins, alkaloids, terpenoids and lipids were greater in Nanguoli, while the concentrations of most amino acids and derivatives, flavonoids, lignans and coumarins, nucleotides and derivatives were graeter in Korla fragrant pear and Qiuyueli. The responses of half phenolic acids, organic acids, sugar alcohols and others were greater in Nanguoli, and the remaining were greater in Korla fragrant pear and Qiuyueli (Table S2 and Table S3). Notably, the substances present in Nanguoli predominantly belonged to the turquoise module, while those in the other two pear varieties predominantly resided within the blue, pink, and yellow modules (Table S2 and Table S3).
Sugar alcohols play a primary role in providing sweetness to pears (Ikegaya et al., 2019). The identified pivotal compounds existed in the blue, pink, and turquoise modules, in which the pink module had a stronger correlation with sweetness (r = 0.69). Erythrose, d-threitol, and d-ribose had high responses in the pink module, thus indicating their potential significance in contributing to the inherent sweetness of pears. Apart from these sugar alcohols, d-sorbitol, allitol, d-mannitol, lactobiose, isomaltulose, d-maltose, d-trehalose, d-glucose, d-sucrose, and sedoheptulose had elevated concentrations in Table S2; among these, most of the substances exhibited higher concentrations in Korla fragrant pear. However, lactobiose and d-sucrose exhibited concentrations of 1.86 × 107 and 3.52 × 106, respectively, in Qiuyueli, surpassing the levels found in other pear varieties. Substances such as d-sorbitol (ranging from 7.35 × 106 to 2.00 × 107), d-xylonic acid (ranging from 5.44 × 106 to 1.60 × 107), allitol (ranging from 5.91 × 106 to 1.20 × 107), d-mannose (ranging from 5.20 × 106 to 1.07 × 107), and erythrose (ranging from 6.73 × 106 to 9.72 × 106) exhibited noteworthy concentrations across all three pear types. The cumulative content of these substances was highest in Korla fragrant pear, while Nanguoli had a marginally higher sugar content performed Qiuyueli. However, these findings were inconsistent with the variations in the sweetness score shown in Fig. 2. Several studies have shown that total flavonoids, caffeine and tea polyphenols are negatively correlated with the sweetness of tea, particularly tea polyphenols (Oh & Kim, 2021). Moreover, organic acids significantly affect and restrain citrus sweetness (Pu et al., 2021). In our study, the number and content of flavonoids, polyphenols and organic acids in Nanguoli were generally higher than those of the other two kinds of pears (Table S2). Possibly, the above high content of flavonoids and other compounds in Nanguoli might decrease the sweetness of Nanguoli.
Fruit sourness is often closely associated with organic acids. Several prominent organic acids, including γ-aminobutyric acid, methylenesuccinic acid, 4-hydroxy-2-oxopentanoic acid, 2-hydroxy-2-methyl-3-oxobutanoic acid, dimethylmalonic acid, 2-methylsuccinic acid, α-ketoglutaric acid, adipic acid, sodium valproate, and citric acid, were identified. Notably, among these compounds, citric acid emerged as the predominant organic acid in the analyzed pear cultivars.
Tannins account for less than 5 % of the total number of selected key substances, but they played an important role in the contribution of astringency. Procyanidin A2, procyanidin B4, procyanidin A6, and galloylprocyanidin B4 were identified in the turquoise module. In terms of tannin content, Nanguoli possessed the highest overall concentration, closely followed by Qiuyueli (as shown in Table S2). This trend aligned with the sensory evaluations. These results indicated a direct correlation between the intensity and tannin content. In the context of tea, catechins make up a substantial portion of the total polyphenols, ranging from 27 % to 80 %, and play a direct role in influencing bitterness and astringency (Ye et al., 2022). Flavonoid glycosides, possessing mild astringency, can augment the bitterness of caffeine (Zuo et al., 2021). Phenolic acids such as gallic acid, tannic acid, and chlorogenic acid are closely associated with the bitterness and astringency of tea (Chen et al., 2022). Hence, the potential impact of compounds containing phenolic acids, flavanols, anthocyanins, flavonols, and flavonoids on the astringency of pears was also considered in this investigation. Table S2 clearly showed that apart from flavonoids, which exhibited the highest content in Korla fragrant pear, the collective contents of other types of substances were most abundant in Nanguoli, followed by Korla fragrant pear. Among the phenolic acids, certain compounds, such as arbutin, 1-butyl-2-isobutyl phthalate, dibutyl phthalate, diisobutyl phthalate, cryptochlorogenic acid (4-O-cafeoylquinic acid), chlorogenic acid (3-O-cafeoylquinic acid), 3-isopentenyl-4-O-glucoxy-4-hydroxybenzoic acid, 2-acetyl-3-hydroxyphenyl-1-O-glucoside, p-coumaryl malic acid, bis (2-ethylhexyl) phthalate, isochlorogenic acid B, isochlorogenic acid A, isochlorogenic acid C, 6′-p-coumaryl arbutin, and 6′-caffeoyl arbutin, had the highest contents in Nanguoli. In contrast, the concentrations of isochlorogenic acid B, isochlorogenic acid A, and isochlorogenic acid C were highest in Qiuyueli. Furthermore, 6′-p-coumaryl arbutin and 6′-caffeoyl arbutin were most abundant in Korla fragrant pear. Catechins, as well as the epicatechin catechins (7,8-BC)-4β- (3,4-dihydroxy phenyl)-dihydro-2-(3 h)-ketone and catechins (7,8-BC)-4α (3,4-dihydroxy phenyl)-dihydro-2-(3 h)-ketone, were the prominent constituents within the flavanol group; among them, catechins were the most abundant substances and were highest in Nanguoli. The primary anthocyanin compound identified was centaurin-3-O-glucoside, which had the highest concentration in Nanguoli. The flavonols with notable content in Nanguoli included isorhamnein-3-O-glucoside, isorhamnein-7-O-glucoside (cranioside), quercetin-3-O-galactoside (hypericin), isorhamnein-3-O-(6″-malonyl) glucoside, quercetin-4′-O-glucoside (spirametin), quercetin-7-O-glucoside, isorhamnein-3-O-(6″-acetyl) glucoside, quercetin-7-O-(6″-malonyl) glucoside, and quercetin-3-O-(“-malonyl) galactoside. Notably, among the flavonoids, 6-methylkaemphenol-3-O-glucoside, homoplantine-7-O-glucoside, apigenin-3′-O-α-d-glucopyranoside, apigenin-7-O-glucoside, luteinin-3′-O-glucoside, and luteinin-4′-O-glucoside were the predominant components in Korla fragrant pear, followed by Nanguoli. However, the changes in catechins, phenolic acids, flavonoids, and flavonols contents were not perfectly consistent with the results of the sensory evaluations. Thus, the complexity of pear astringency is evident from this observation. Some studies suggest that the levels of original bitter compounds and aroma can influence the perception of bitterness in food (Yin, Zhang, Wu, Chen, & Deng, 2022). Dietary lipids were found to decrease the perception of astringency in grape tannin solutions (Saad, Bousquet, Fernandez-Castro, Loquet, & Géan, 2021). A study by Saenz-Nabajas et al. revealed that the fruity aroma compounds present in white wine could effectively mitigate the bitterness and astringency of recombinant model solution in deflavored wine, subsequently intensifying perceptions of sweetness (Sáenz-Navajas, Campo, Fernández-Zurbano, Valentin, & Ferreira, 2010). Moreover, sweet substances at moderate to high concentrations have been demonstrated to counteract the bitterness and astringency of such compounds (Brannan, Setser, & Kemp, 2007). By analyzing the data in Table S2 and Table S3, it becomes evident that although the overall concentration of astringency-contributing compounds in Korla fragrant pear surpasses that in Qiuyueli, the levels of sugar, volatile components responsible for sweet and fruity notes, and lipids were greater in Korla fragrant pear. Though literature we know that these substances exhibit superior inhibition of bitterness compared to Qiuyueli. Consequently, we inferred that the presence of sugar alcohols, volatile aromatic compounds, and lipids in Korla fragrant pear might play a role in tempering the expression of these bitter and astringent characteristics.
The flavor profile of fruit wine is a harmonious interplay of both taste and postnasal aroma perception (Escudero, Campo, Fariña, Cacho, & Ferreira, 2007). Postnasal aroma perception is, in fact, an integral facet of overall flavor sensation (Ramirez, Du, & Wallace, 2020). A proper “sugar-acid ratio” is the key to the sweet and sour taste of fruit wine (Ikegaya et al., 2019). The ester compounds present in fruits contribute significantly to the fruity aroma, evoking a sense of delight and playing a vital role in enhancing the presentation of a mellower and more pleasurable fruity sweetness (Yu et al., 2021). Bitter substances play a noteworthy role in enriching the overall taste profile; a touch of bitter substances could make the sweetness mellow, not thin, rich and layered (Arvisenet, Guichard, & Ballester, 2016). Good-tasting wine is mostly determined by the balance among sweet and sour, bitter and astringent. Skillful modulation of these four dimensions can profoundly influence the wine characteristics, leading to a multifaceted taste experience marked by mellowness, fullness, refreshment, suppleness, a gentle sweetness and other tastes (Li, 2006). Research has also shown that varying combinations of flavors could result in different effects, including enhancement, inhibition, and synergy (Oh & Kim, 2021). The intricate interplay between volatile and nonvolatile substances plays a significant role in shaping the presentation of a rich taste (Tian, Zheng, Yu, Chen, & Lou, 2023). Therefore, it became apparent that the presentation of the fruit wine taste was the result of the interaction of a large number of substances and various sensory characteristics of pears. According to the metabolic profiling results, Nangguoli contained relatively high levels of volatile esters, phenolic acids, flavonoids, organic acids, tannins, key sugar alcohols, lipid substances, etc. These substances played an important role in shaping the rich taste and postnasal flavor of Nanguoli, and were identified as the key substances that form the taste of fruit Nanguoli wine. Notably, the substances selected as pivotal components within the turquoise module had the highest concentrations in Nanguoli (as labelled in Table S2 and Table S3).
Fig. 4B reveals a pronounced association between the blue module and the green grass attribute present in the pear samples. Given that the sensory perception of green grass primarily emanates from olfaction, we commenced our analysis by focusing on the volatile compounds within the blue module. Among these compounds, (2Z)-hexenyl acetate, with fresh green apple and pear scent (Liu, Wei, Li, & Zang, 2008), emerged as a prominent ester displaying the highest concentration within the blue module and found predominantly in Korla fragrant pear. Likewise, compounds such as methyl (3Z)-3-hexenoate, featuring an earthy sweetness with subtle fruity undertones (The Good Scents Company Information System, 1980), and (E, E)-2,4-hexadienal, imparting a green fruity aroma, as well as (E)-2-hexenol, emitting fresh and green aroma of foliage (The Good Scents Company Information System, 1980) were also identified. Notably, these compounds collectively contributed to the green grass aroma and were chiefly detected in Korla fragrant pear. C6 − C9 alcohols and aldehydes can yield green grass fragrances (Qin et al., 2012). Table S3 displays the presence of these compounds, including hexanal and hexanol, which were notably more abundant in Korla fragrant pear. All of the above identified substances collaboratively contributed to the green grass aroma in the pear samples.
The fresh attribute was strongly correlated with the pink module (r = 0.95) (Fig. 4B), but the only volatile substance screened in this module was dodecane, which has a high odor threshold and is usually not a key aroma substance of fruits (Qin et al., 2012). Nevertheless, some scholars have reported that Echa 10 green tea has a fresh and mellow flavor with an obvious honeysuckle fragrance, and this fragrance was mainly related to key components, such as dodecane, octadecane, phenylethanol and jasmonone (Li et al., 2022). Thus, it is conceivable that the dodecane in our study might play a role in contributing to the fresh aroma of pear fruits.
The attribute of fresh aroma, distinct from being rich or intense, is an overall evaluation attribute (Zhang et al., 2017). It may not only be related to the selected aroma substances but also be affected by other aromas that can emit a variety of aromas, including fresh scents and metabolites in pears. By analyzing the aroma characteristics of volatile compounds, we found that certain volatile substances, while contributing to other flavor aspects, also carry a fresh aroma. For instance, compounds such as 2-ethylhexanol (7.53 ng/g) and (E)-2-hexenol (2.03 ng/g) exhibited a fresh pear-like aroma, contributing to the freshness of Korla fragrant pear. Hexyl acetate, with a content of 467.70 ng/g in Nanguoli and 129.97 ng/g in Korla fragrant pear, imparted a fresh fruit scent. Another noteworthy instance was the compound (2Z)-hexenyl acetate, marked by its aroma of fresh green apple and pear, with higher levels (66.26 ng/g) in Korla fragrant pear. Furthermore, α-farnesene exuded a refreshing plant-like essence, while heptyl acetate, ethyl 2-methylbutyrate, and hexyl 2-methylbutyrate emitted fruity freshness, and hexyl hexanoate carried herbal freshness, with contents of 76.61 ng/g, 12.92 ng/g, 30.10 ng/g, 0.74 ng/g, and 0.79 ng/g, respectively, and had higher contents in Nanguoli. The cumulative content of these substances was highest in Nanguoli, followed by Korla fragrant pear, and lowest in Qiuyueli. However, intriguingly, the perceived intensity of the fresh aroma in Korla fragrant pear and Qiuyueli was greater than that in Nanguoli (Fig. 2), which might be attributed to the relatively higher presence of other flavor components in Nanguoli potentially diluting the perception of the fresh attribute (Zhang et al.,2023). Consequently, it became evident that the fresh aroma attribute was complicated and needed further comprehensive investigation.
The turquoise module also displayed strong correlations with sweet, apple-like, woody, ferment and bouquet, and floral attributes, which were primarily assessed through olfaction. Upon analyzing the aromatic profiles of the volatile compounds, we noted that roughly half of the aromatic substances within this module exhibited fruity, apple-like, and tropical fruit aromas (The Good Scents Company Information System, 1980). The identified key contributors included isoamyl acetate, ethyl (2E,4Z)-decadienoate, ethyl 2-octenoate, methyl (E)-2-octenoate, ethyl (E)-3-hexenoate, hexyl acetate, ethyl 2-methylbutyrate, amyl acetate, hexyl 2-methylbutyrate, methyl 2-methylbutyrate, methyl butyrate, ethyl tiglate, methyl valerate, and ethyl propionate. The concentrations of these compounds were highest in Nanguoli, followed by Korla fragrant pear, and lowest in Qiuyueli (Table S2). Substances such as isoamyl acetate, hexyl acetate, amyl acetate, ethyl butyrate, ethyl tiglate, ethyl hexanoate, methyl valerate, and ethyl propionate predominantly contributed to the sweet scent, which had the highest content in Nanguoli (Table S2). Compounds such as methyl (E)-2-octenoate, amyl acetate, octyl acetate, methyl octanoate, and α-farnesene were instrumental in generating a woody aroma, and the last three substances exuded mainly fruit and fresh herb scents along with a woody aroma (The Good Scents Company Information System, 1980). Notably, octyl acetate, ethyl butyrate, ethyl isobutyrate, ethyl propionate, methyl butyrate, methyl valerate, and ethyl phenylacetate, contributing to the ferment and bouquet aroma, were predominantly present in Nanguoli (Table S2). Among these, the first four substances exhibited flavors of rum, cognac, and alcohol, while the latter three emitted sweet, fruity, and floral aromas with hints of cheese and fermentation (The Good Scents Company Information System, 1980). The floral aroma characteristics of the samples were attributed to compounds such as α-farnesene, methyl (E)-2-octenoate, ethyl phenylacetate, methyl 2-methylbutyrate, and methyl octanoate (The Good Scents Company Information System, 1980), among which α-farnesene was the main substance with highest content, and was found mainly in Nanguoli (Table S2).
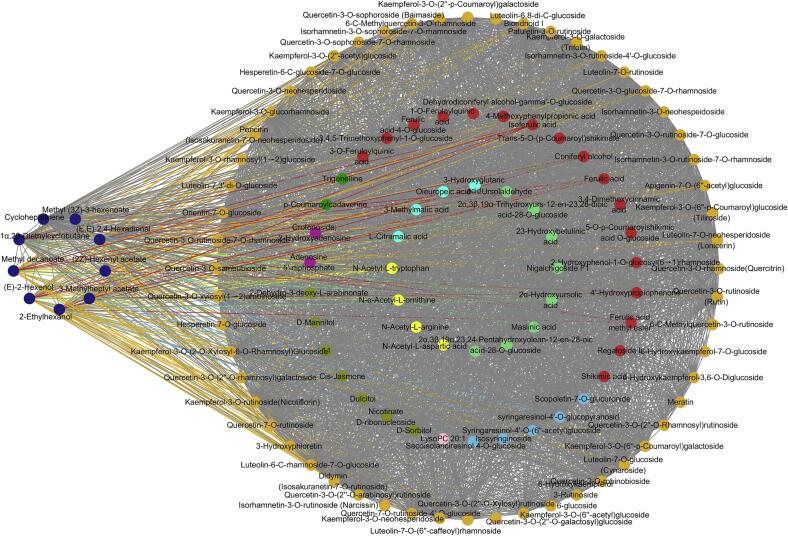
3.5. Visualization analysis of the effect of taste factors on aroma attributes
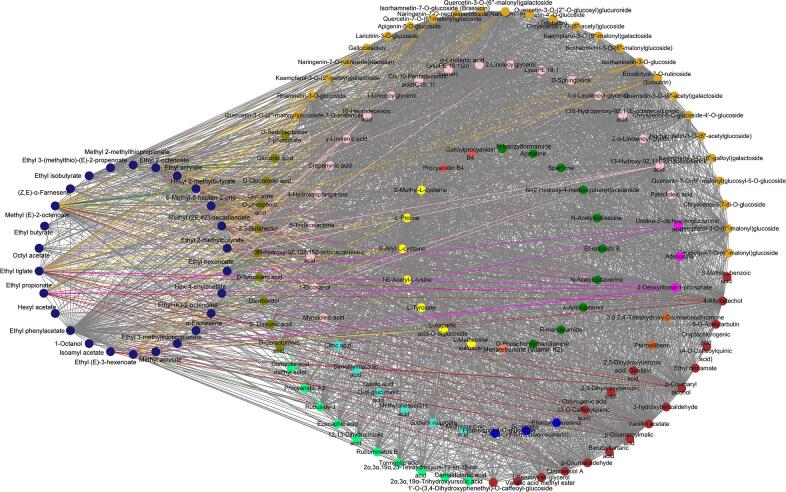
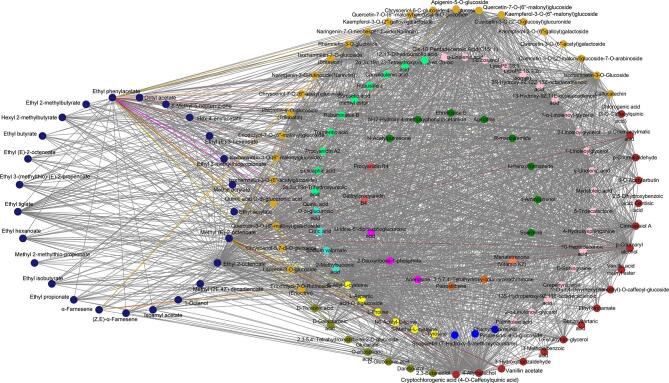
In addition to the volatile aroma substances, the blue module encompasses a variety of nonvolatile compounds, including flavonoids (56), phenolic acids (18), organic acids (7), terpenoids (10), amino acids (6), alkaloids (3), lignans and coumarins (7), tannins (1), sugar alcohols and other substances (13) (Table S2). Notably, olfactory perception can also be influenced by taste perception, in addition to the impact of aroma compounds via the positive nasal pathway (Xiao et al., 2021, Tian et al., 2023). As a result, a network analysis was conducted for substances with a weight > 0.7 within the blue module (Fig. 6). Notably, this analysis revealed that 32 flavonoids, 5 phenolic acids, 3 terpenoids, 3 kinds of lignans and coumarins, and 1 amino acid exhibited close correlations with volatile compounds responsible for imparting the green grass aroma. The relationships between these substances were visually categorized using distinct colors, as shown in Fig. 6. Through association analysis, it was discovered that 50 % of the aromatic compounds, including (E)-2-hexenol, (E, E)-2,4-hexadienal, 2-ethylhexanol, 3-methylheptyl acetate, and methyl decanoate, exhibited strong correlations with 21 nonvolatile substances. Notably, flavonoids such as kaempferol-3-O-galactoside (trifolin), luteolin-7-O-neohesperidoside (lonicerin), kaempferol-3-O-glucorhamnoside, and kaempferol-3-O-rutinoside (nicotiflorin) exhibited a high cumulative response and were closely associated with the aforementioned aromatic compounds (Table S2). Additionally, one terpenoid and two lignans and coumarins namely 2α-hydroxyursolic acid, syringaresinol-4′-O-glucopyranosid, and secoisolariciresinol 4-O-glucoside (Table S2) also demonstrated significant correlations with the aforementioned aromatic compounds while exhibiting a high cumulative response.
Fig. 6.
Interaction of metabolites contributing to green grass in the blue module. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Notably, the yellow module also had a higher correlation with the green grass attribute (r = 0.76) than with the other flavor attributes in Fig. 4B. It primarily contained phenolic acids (12), flavonoids (17), alkaloids (2), organic acids (2), lipids (3), amino acids and derivatives (1), and sugar alcohols and others (3), as labelled in Table S2. In terms of flavor, these substances may have bitter and astringent properties (Ye et al., 2022), and are known to have a significant effect on green grass perception (Tian, Zheng, Yu, Chen, & Lou, 2023). Consequently, we considered that these compounds could potentially contribute to green grass characteristics.
These above nonvolatile compounds contributing to green grass scent all had greater abundances in Korla fragrant pear, and their changes in content in the three pear varieties were consistent with the sensory evaluation scores (Table S2, Table S3). These compounds likely played a combined role with volatile compounds in imparting the grassy fragrance of pears.
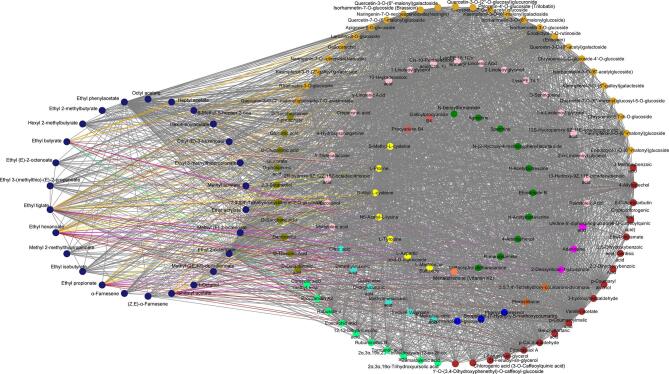
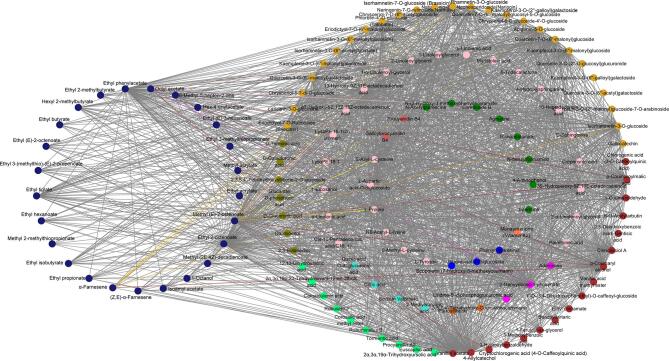
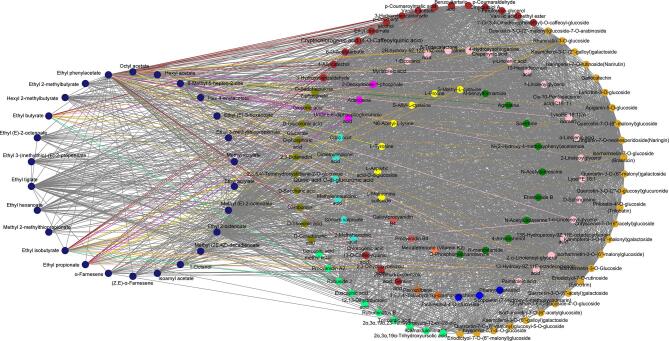
The turquoise module, with strong correlations with attributes such as sweet, apple-like, woody, ferment and bouquet, and floral, also contained a large number of nonvolatile substances, including flavonoids (37), phenolic acids (36), organic acids (11), terpenoids (13), amino acids (7), alkaloids (11), lignans and coumarins (5), tannins (4), volatile aroma substances (33), lipids (22), sugar alcohols and others (17) (Table S2). Considering the interplay between taste and olfactory sensory attributes as reported in the literature (Tian, Zheng, Yu, Chen, & Lou, 2023), our approach for selecting key nonvolatile compounds related to apple-like, sweet, ferment and bouquet, woody, and floral aromas involved the implementation of correlation network analyses. Our findings identified a set of closely linked compounds, including flavonoids, phenolic acids, terpenoids, organic acids, alkaloids, amino acids and derivatives, nucleotides and derivatives, lipids, and sugar alcohols, with a weight threshold exceeding 0.7; the relationships between these substances were visually categorized using distinct colors, as shown in Fig. S7, Fig. S8, Fig. S9, Fig. S10, and Fig. S11. Fig. S7 illustrated the aroma compounds with significant contribution to apple flavor, which were closely associated with 15 nonvolatile compounds. Among these, 10-heptadecenoic acid, p-coumaroylmalic acid, p-coumaryl alcohol, quercetin-7-O-(6′'-malonyl) glucoside and rhamnetin-3-O-glucoside with higher contents were found to be closely related to isoamyl acetate, methyl (2E, 4Z)-decadienoate, ethyl (E)-3-hexenoate, ethyl 2-methylbutyrate, hexyl 2-methylbutyrate and methyl valerate, respectively (Fig. S7 and Table S2). Eriodictyol-7-O-rutinoside (eriocitrin), isorhamnetin-3-O-glucoside and isorhamnetin-7-O-glucoside (brassicin) with higher cumulative responses were also found to be closely related to aroma compounds such as methyl (2E, 4Z)-decadienoate, ethyl 2-octenoate, methyl (E)-2-octenoate, ethyl (E)-3-hexenoate, hexyl 2-methylbutyrate, and ethyl tiglate (Fig. S7 and Table S2). The correlations between the compounds with sweet scent, such as isoamyl acetate, ethyl butyrate, ethyl tiglate, ethyl hexanoate, methyl valerate and ethyl propionate, and nonvolatile substances was depicted in Fig. S8. Notably, p-coumaroylmalic acid, p-coumaryl alcohol and rhamnetin-3-O-glucoside exhibited close associations with the aforementioned aromas and were presented in significant concentrations (Fig. S8; Table S2). The correlations between aroma compounds, such as octyl acetate, ethyl phenylacetate, ethyl butyrate, methyl valerate, ethyl isobutyrate and ethyl propionate associated, which are associated with ferment and bouquet, and nonvolatile substances were illustrated in Fig. S9. Quercetin-7-O-(6′'-malonyl) glucoside, p-coumaroylmalic acid, rhamnetin-3-O-glucoside, and p-coumaryl alcohol were closely associated with the aforementioned aromas and exhibited high concentrations (Fig. S9; Table S2). The number of compounds contributing to floral and woody aromas was relatively low in the turquoise module. Fig. S10 illustrated the correlations between methyl (E)-2-octenoate, ethyl phenylacetate, α-farnesene (contributing to floral aroma) and nonvolatile compounds. Notably, isorhamnetin-3-O-glucoside, p-coumaroylmalic acid, l-proline, rhamnetin-3-O-glucoside, and apigenin-5-O-glucoside exhibited close relationships and were present in high abundance (Fig. S10; Table S2). Additionally, Fig. S11 demonstrated the correlations between methyl (E)-2-octenoate, octyl acetate, α-farnesene (contributing to woody aroma) and nonvolatile compounds. Among these correlations, p-coumaryl alcohol, rhamnetin-3-O-glucoside, isorhamnetin-7-O-glucoside (brassicin), isorhamnetin-3-O-glucoside, and p-coumaroylmalic acid displayed strong associations and were found in significant quantities (Fig.S11; Table S2).
In conclusion, the identified nonvolite compounds contributing to the perception of apple-like scent also exhibited strong interconnectedness with other sensory attributes within the turquoise module, suggesting that these compounds might enhance various characteristics within this module. Moreover, 10 lipid compounds closely associated with sensory properties in the turquoise module were also screened (Table S2 and Table S3); among them, 1-linoleoylglycerol and 2-linoleoylglycerol were the most abundant.
The interaction mechanism between taste substances and aroma substances is intricate, and taste substances with a higher contents, which closely interact with aromas, are more likely to elicit perceptual awareness (Zhang et al., 2022, Winstel et al., 2020). In this study, flavonoids and phenolic acids were found to be the substances most closely related to different flavor characteristics of pears; these two kinds of compounds exhibited the highest contents in Nanguoli and Korla fragrant pear (Table S2). The impact of taste substances on aroma characteristics varies with their structure and concentration (Esteban-Fernández, Muñoz-González, Jiménez-Girón, Pérez-Jiménez, & Pozo-Bayón, 2018), which may be attributed to the different molecular forces and intensities between taste compounds and aroma substances (Jung, Ropp, & Ebeler, 2000). In this study, the correlation between aroma compounds and taste components varied. Additionally, the aroma characteristics are also influenced by sugars (Baldwin, Goodner, & Plotto, 2008), organic acids (Baldwin, Goodner, & Plotto, 2008), and amino acids (Genovese, Yang, Linforth, Sacchi, & Fisk, 2018). In this study, the sugar alcohols exhibiting strong associations with aroma substances (with a weight greater than 0.7) included 2,3,5,4′-tetrahydroxystilbene-2-O-glucoside, 2,3-butanediol, glucarate O-phosphoric acid, and d-galacturonic acid in turquoise module. Among them, 2,3,5,4′-tetrahydroxystilbene-2-O-glucoside was significantly correlated with ethyl 2-octenoate, and glucarate O-phosphoric acid was significantly correlated with ethyl 2-octenoate and methyl (E)-2-octenoate, enhancing apple-like aroma (Fig. S7); d-galacturonic acid and glucarate O-phosphoric acid were only related to ethyl phenylacetate, contributing to ferment and bouquet (Fig. S9); d-galacturonic acid was significantly associated with ethyl phenylacetate, and glucarate O-phosphoric acid was significantly correlated with methyl (E)-2-octenoate, contributing to floral aroma (Fig. S10); moreover, glucarate O-phosphoric acid was significantly related to methyl (E)-2-octenoate, contributing to woody aroma (Fig. S11). The organic acids in turquoise module including 2-methylsuccinic acid and dimethylmalonic acid were only significantly correlated with ethyl 2-octenoate, contributing to apple-like aroma (Fig. S7). The contents of these sugar alcohols and organic acids were lower than those of the above-mentioned flavonoids and phenolic acids (Table S2). The amino acids in turquoise module mainly including l-aspartic acid-O-diglucoside, l-proline, l-tyrosine, and S-allyl-l-cysteine, were closely related to apple-like aroma, ferment and bouquet, sweet aroma, floral aroma, and woody aroma; among them, l-aspartic acid-O-diglucoside had the highest content in pears, and the number of substances closely related to l-proline were most. Currently, there is a lack of research on the interplay among flavor factors in pear fruit. In this study, we constructed a co-expression network to identify taste substances that were closely associated with aroma compounds, thereby providing fundamental data for further investigations into the interactions of flavor components in pear fruits.
4. Conclusions
Widespread metabolomics analysis and volatile substance fingerprint analysis revealed that there were many kinds of metabolites in pears, and their contents and categories differed significantly. Descriptive sensory analysis indicated that, except for ferment and bouquet and fruit wine, the other flavors were shared among all three pear types; nevertheless, great discrepancies in intensity among different varieties of pears existed. After association analysis, a large number of compounds were found to be closely related to sensory properties, mainly flavonoids, phenolic acids, volatile substances, sugar alcohols, organic acids, terpenoids, lipids, and tannins. Through visual network analysis and comparative assessment of differential substances, potential flavor contributors were confirmed. These compounds not only contributed to the taste of pear fruits, but also influenced the olfactory characteristics. Based on the aforementioned findings, this study elucidated the metabolic underpinnings responsible for the varietal distinctions in flavor among Nanguoli, Korla fragrant pear, and Qiuyueli. In addition, the complexity of the network associations revealed that each flavor attribute was closely associated with numerous distinct key compounds, indicating that the expression of pear aroma resulted from a synergistic effect of multiple substances and evaluation olfactory flavor necessitated a comprehensive consideration of taste substances. This study provides a new research framework for the perception of potential flavor factors through the application of comprehensive analysis and provides available data support for the in-depth study of flavor factor confirmation and flavor perception.
CRediT authorship contribution statement
Wenjun Zhang: Writing – original draft, Project administration, Data curation. Bo Bai: Resources, Data curation. Hongxia Du: Methodology, Investigation. Qian Hao: Data curation, Software. Lulu Zhang: Investigation, Methodology. Zilei Chen: Funding acquisition, Resources. Jiangsheng Mao: Data curation, Formal analysis. Chao Zhu: Conceptualization, Resources. Mengmeng Yan: Funding acquisition, Software. Hongwei Qin: Formal analysis, Validation. A.M. Abd El-Aty: Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32102088), the Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2018E05, CXGC2021B14 and CXGC2022E05) and the National Pear Industry Technology System (CARS-28-23).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101189.
Contributor Information
Mengmeng Yan, Email: ynky202@163.com.
Hongwei Qin, Email: qhw01@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
Supplementary figure 4.
Supplementary figure 5.
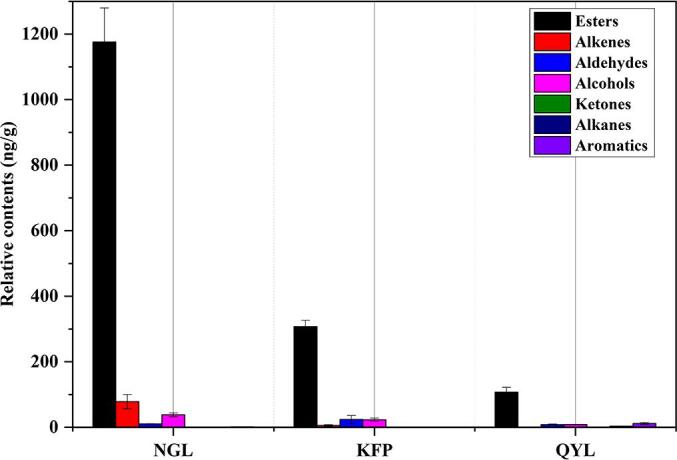
Supplementary figure 6.
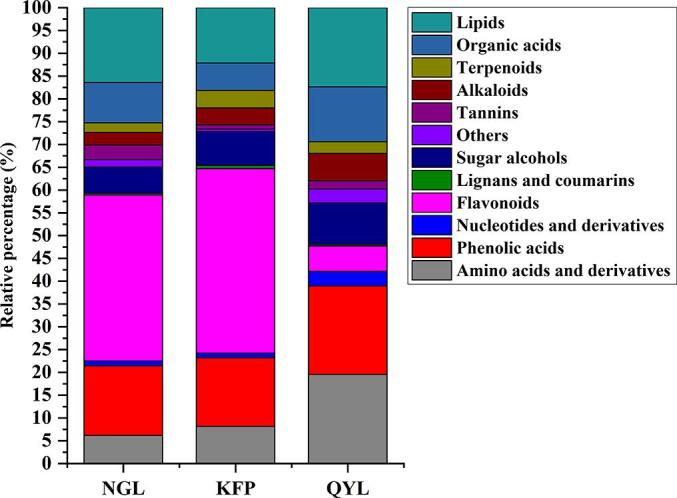
Supplementary figure 7.
Supplementary figure 8.
Supplementary figure 9.
Supplementary figure 10.
Supplementary figure 11.
Data availability
The authors do not have permission to share data.
References
- Arvisenet G., Guichard E., Ballester J. Taste-aroma interaction in model wines: Effect of training and expertise. Food Quality and Preference. 2016;52:211–221. [Google Scholar]
- Baldwin E.A., Goodner K., Plotto A. Interaction of Volatiles, Sugars, and Acids on Perception of Tomato Aroma and Flavor Descriptors. Journal of Food Science. 2008;73(6):294–307. doi: 10.1111/j.1750-3841.2008.00825.x. [DOI] [PubMed] [Google Scholar]
- Brannan G.D., Setser C.S., Kemp K.E. Interaction of astringency and taste characteristics. Journal of Sensory Studies. 2007;16(2):179–197. [Google Scholar]
- Chen Y.H., Zhang Y.H., Chen G.S., Yin J.F., Chen J.X., Wang F., Xu Y.Q. Effects of phenolic acids and quercetin-3-O-rutinoside on the bitterness and astringency of green tea infusion. NPJ Science of Food. 2022;6:8. doi: 10.1038/s41538-022-00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.Y., Yin H., Wu X., Shi X.J., Qi K.J., Zhang S.L. Comparative analysis of the volatile organic compounds in mature fruits of 12 Occidental pear (Pyrus communis L.) cultivars. Scientia Horticulturae. 2018;240:239–248. [Google Scholar]
- Escudero A., Campo E., Fariña L., Cacho J., Ferreira V. Analytical characterization of the aroma of five premium red wines. Insights in to the role of odor families and the concept of fruitiness of wines. Journal of Agricultural and Food Chemistry. 2007;11:4501–4510. doi: 10.1021/jf0636418. [DOI] [PubMed] [Google Scholar]
- Esteban-Fernández A., Muñoz-González C., Jiménez-Girón A., Pérez-Jiménez M., Pozo-Bayón M.Á. Aroma release in the oral cavity after wine intake is influenced by wine matrix composition. Food Chemistry. 2018;243:125–133. doi: 10.1016/j.foodchem.2017.09.101. [DOI] [PubMed] [Google Scholar]
- Fan F.Y., Huang C.S., Tong Y.L., Guo H.W., Zhou S.J., Ye J.H., Gong S.Y. Widely targeted metabolomics analysis of white peony teas with different storage time and association with sensory attributes. Food Chemistry. 2021;362 doi: 10.1016/j.foodchem.2021.130257. [DOI] [PubMed] [Google Scholar]
- Frost S.C., Harbertson J.F., Heymann H. A full factorial study on the effect of tannins, acidity, and ethanol on the temporal perception of taste and mouthfeel in red wine. Food Quality and Preference. 2017;62:1–7. [Google Scholar]
- Genovese A., Yang N., Linforth R., Sacchi R., Fisk I. The role of phenolic compounds on olive oil aroma release Author links. Food Research International. 2018;112:319–327. doi: 10.1016/j.foodres.2018.06.054. [DOI] [PubMed] [Google Scholar]
- Ikegaya A., Toyoizumi T., Ohba S., Nakajima T., Kawata T., Ito S., Arai E. Effects of distribution of sugars and organic acids on the taste of strawberries. Food Science & Nutrition. 2019;7:2419–2426. doi: 10.1002/fsn3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D.M., Ropp J.S.D., Ebeler S.E. Study of interactions between food phenolics and aromatic flavors using one- and two-dimensional (1)H NMR spectroscopy. Journal of Agricultural and Food Chemistry. 2000;48(2):407–412. doi: 10.1021/jf9906883. [DOI] [PubMed] [Google Scholar]
- Li H. Science Press; Wine Taste: 2006. pp 91. [Google Scholar]
- Liu S.H., Wei C.B., Li W.C., Zang X.P. Analysis of the aromatic constituents in 3 starfruit cultivars. Journal of Fruit Science. 2008;25(1):119–121. [Google Scholar]
- Li Y.C., Wei R., He C., Zhou J.T., Chen Y.Q., Yu Z., Ni D.J. Effects of different tea tree varieties on the color, aroma, and taste of Chinese Enshi green tea. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Kim M.K. The roles of sucrose on the retronasal thresholds of tea catechins and polyphenols in water-based system. Journal of Sensory Studies. 2021;36(3):12653. [Google Scholar]
- Pu D.D., Zhang Y.Y., Sun B.G., Ren F.Z., Zhang H.Y., Chen H.T., Tang Y.Z. Characterization of the key taste compounds during bread oral processing by instrumental analysis and dynamic sensory evaluation. LWT - Food Science and Technology. 2021;138 [Google Scholar]
- Qin G.H., Tao S.T., Cao Y.F., Wu J.Y., Zhang H.P., Huang W.J., Zhang S.L. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chemistry. 2012;134:2367–2382. doi: 10.1016/j.foodchem.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Ramirez J.L., Du X.F., Wallace R.W. Investigating sensory properties of seven watermelon varieties and factors impacting refreshing perception using quantitative descriptive analysis. Food Research International. 2020;138 doi: 10.1016/j.foodres.2020.109681. [DOI] [PubMed] [Google Scholar]
- Saad A., Bousquet J., Fernandez-Castro N., Loquet A., Géan J. New Insights into Wine Taste: Impact of Dietary Lipids on Sensory Perceptions of Grape Tannins. Journal of Agricultural and Food Chemistry. 2021;69(10):3165–3174. doi: 10.1021/acs.jafc.0c06589. [DOI] [PubMed] [Google Scholar]
- Sáenz-Navajas M.P., Campo E., Fernández-Zurbano P., Valentin D., Ferreira V. An assessment of the effects of wine volatiles on the perception of taste and astringency in wine. Food Chemistry. 2010;121(4):1139–1149. [Google Scholar]
- Sun L.Q., Hao W.J., Tang X.Q., Wang K.C., Zhang S.L. Analysis of characteristic polyphenols and triterpenic acids in ripe pears of 36 cultivars by UPLC-MS/MS. Food Science. 2020;41(22):206–214. [Google Scholar]
- Sung J., Suh J.H., Chambers A.H., Crane J., Wang Y. Relationship between sensory attributes and chemical composition of different mango cultivars. Journal of Agricultural and Food Chemistry. 2019;67:5177–5188. doi: 10.1021/acs.jafc.9b01018. [DOI] [PubMed] [Google Scholar]
- The Good Scents Company Information System. (1980). Providing information for the Flavor, Fragrance, Food and Cosmetic industries. Retrieved from http://www.thegoodscentscompany.com/search. Accessed August 1, 2023.
- Tian H.X., Zheng G.M., Yu H.Y., Chen C., Lou X.M. Research progress on the influence of the interaction between odour and taste on food flavor perception. Food Science. 2023;44(9):259–269. [Google Scholar]
- Welke J.E., Zanus M., Lazzarotto M., Pulgati F.H., Zini C.A. Main differences between volatiles of sparkling and base wines accessed through comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection and chemometric tools. Food Chemistry. 2014;164:427–437. doi: 10.1016/j.foodchem.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Winstel D., Gautier E., Marchal A. Role of Oak Coumarins in the Taste of Wines and Spirits: Identification, Quantitation, and Sensory Contribution through Perceptive Interactions. Journal of Agricultural and Food Chemistry. 2020;68(28):7434–7443. doi: 10.1021/acs.jafc.0c02619. [DOI] [PubMed] [Google Scholar]
- Xiao Z.B., Chen H.T., Niu Y.W., Zhu J.C. Characterization of the aroma-active compounds in banana (Musa AAA Red green) and their contributions to the enhancement of Sweetness perception. Journal of Agricultural and Food Chemistry. 2021;69(50):15301–15313. doi: 10.1021/acs.jafc.1c06434. [DOI] [PubMed] [Google Scholar]
- Ye J.H., Ye Y., Yin J.F., Jin J., Liang Y.R., Liu R.Y., Xu Y.Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends in Food Science & Technology. 2022;123:130–143. [Google Scholar]
- Yin Q.C., Zhang R.H., Wu G., Chen Z., Deng H. Comparative metabolomics analysis reveals the taste variations among three selected wampee cultivars. Plant Foods for Human Nutrition. 2022;77(2):250–257. doi: 10.1007/s11130-022-00973-4. [DOI] [PubMed] [Google Scholar]
- Yu H.Y., Xie T., Xie J.G., Chen C., Ai L.Z., Tian H.X. Aroma perceptual interactions among ester compounds and their effects on sensory attributes of Huangjiu. Food Science. 2021;42(14):218–225. [Google Scholar]
- Zhang B., Shi X., Zhang Y., Wang Q., Zhou P.P., Li Y.K., Tao Y.S. The implication of phenolic acid matrix effect on the volatility of ethyl acetate in alcohol-free wine model: Investigations with experimental and theoretical methods. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2022.132114. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang J.Y., Gao W.Y. Advances in studies on chemical constituents in medicinal plants of Pyrus L. and their pharmacological activities. Chinese Traditional and Herbal Drugs. 2012;43(10):2077–2082. [Google Scholar]
- Zhang Q., Yang J.G., Ao Z.H., Guo J.X., Su C., Shen C.H. Research Progress on the Aromatic Components of Fen-flavor Liquor (Baijiu) Advance. Journal of Food Science and Technology. 2017;13(5):190–195. [Google Scholar]
- Zhang W.J., Li H.D., Mao J.S., Fang L.P., Ding R.Y., Guo C.Y., Chen Z.L. Difference analysis of aroma fingerprint of Cuiguanli from different places of origin by GC-IMS. Quality and Safety of Agricultural Products. 2021;5:23–28. [Google Scholar]
- Zhang W.J., Yan M.M., Zheng X.X., Chen Z.L., Li H.D., Mao J.S., Abd El-Aty A.M. Exploring the aroma fingerprint of various Chinese pear cultivars through qualitative and analysis of volatile compounds using HS-SPME and GC×GC-TOFMS. Molecules. 2023;28(12):4794. doi: 10.3390/molecules28124794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Li T., Yang F., Cui X.Y., Zou T.T., Song H.L., Liu Y. Characterization of key aroma-active compounds in Hanyuan Zanthoxylum bungeanum by GC-O-MS and switchable GC×GC-O-MS. Food Chemistry. 2022;385 doi: 10.1016/j.foodchem.2022.132659. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Lv H.P., Shao C.Y., Kang S.Y., Zhang Y., Guo L., Lin Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Research International. 2018;108:74–82. doi: 10.1016/j.foodres.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Zou S.C., Wu J.C., Shahid M.Q., He Y.H., Lin S.Q., Liu Z.H., Yang X.H. Identification of key taste components in loquat using widely targeted metabolomics. Food Chemistry. 2020;323 doi: 10.1016/j.foodchem.2020.126822. [DOI] [PubMed] [Google Scholar]
- Zuo Y.M., Tan G.H., Xiang D., Chen L., Wang J., Zhang S.S., Wu Q. Development of a novel green tea quality roadmap and the complex sensory-associated characteristics exploration using rapid near-infrared spectroscopy technology. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Supports open access. 2021;258 doi: 10.1016/j.saa.2021.119847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.