Abstract
Although the strong protease activity of Porphyromonas gingivalis appears to be an important virulence property of these organisms, little information is currently available regarding the regulation of expression of the multiple protease genes. Utilizing the lacZ reporter gene strategy, the environmental factors which regulate the expression of the Arg-gingipain gene rgpA and the prtT protease gene were investigated. These two genes are reciprocally regulated since factors which retarded growth (iron depletion and nutrient limitation) appeared to upregulate rgpA expression while down-regulating prtT expression. However, inactivation of the major rgpA gene resulted in increased transcription of the prtT and tpr protease genes while decreasing expression of the Lys-gingipain kgp gene as detected by Northern blot analysis. By contrast, inactivation of the prtT gene did not significantly affect kgp expression but moderately decreased rgpA mRNA levels. These results indicate that the protease genes of P. gingivalis are not coordinately regulated and suggest that some of these enzymes play specific roles in the physiology and/or virulence of these organisms.
Anaerobic gram-negative bacteria, including Porphyromonas gingivalis, have been implicated in the etiology of human periodontal diseases (8, 9). P. gingivalis has been demonstrated to express a variety of factors which may play a role in pathogenicity, including adhesins, proteases, endotoxins, and cytotoxins (10). The strong protease activity of these organisms appears to be an important virulence factor because of the multiple effects which can be produced by these enzymes (13, 24). Among these are the abilities to degrade immunoglobulins, inactivate cytokines and their receptors, attenuate the antibacterial activities of neutrophils, degrade host tissue, activate host proenzymes, increase vascular permeability, and promote bleeding. Moreover, the construction of a P. gingivalis W83 mutant defective in the major rgpA cysteine protease gene (7) has demonstrated attenuation of virulence in the mouse abscess model. However, the effects of the loss of the RgpA enzyme on the multiple virulence properties of P. gingivalis (29) have not allowed a clear delineation of the role of this enzyme in periodontitis.
The genes for five distinct proteases have been isolated from and characterized for several different strains of P. gingivalis (23). Two of the genes, rgpA and rgpB (these genes have been named differently in several laboratories [23]), express arginine-specific cysteine proteases, while a third, kgp, encodes a lysine-specific cysteine protease (19, 22). The latter gene appears to be identical to the prtP gene recently characterized as a lysine- and arginine-specific protease (3). In addition, two other protease genes, prtT (20) and tpr (4) have also been identified as a result of gene cloning, but the corresponding proteins have not yet been purified from the homologous organisms. Although some specific functions have now been assigned to two of the enzymes, RgpA and Kgp (24), the roles of the PrtT and Tpr enzymes in the physiology of P. gingivalis remain unclear.
Although the genes for multiple proteases have been identified in various strains of P. gingivalis, little information is available regarding the mechanisms involved in regulating their expression. This information would be important in assessing the function of the individual enzymes relative to periodontal diseases. In addition, it is not clear how expression of these enzymes is controlled under the environmental conditions existing in the gingival crevice. Furthermore, it has been suggested that individual proteases effect other proteases at a posttranscriptional level (1, 2). Therefore, this investigation was initiated to examine the regulation of expression of several of the proteases and to determine if these enzymes are coordinately regulated.
MATERIALS AND METHODS
Bacterial strains and plasmids.
P. gingivalis 381 and its mutants were maintained anerobically on blood agar plates containing tryptic soy broth (TSB) (Difco Laboratories, Detroit, Mich.) supplemented with 1.5% agar, 10% sheep blood, hemin (5 μg/ml), and menadione (1.0 μg/ml). Escherichia coli S17.1 (12) and plasmid pKDCMZ (15) were used for conjugation with P. gingivalis. Plasmids pKS (20) and pKpL (6) served as the sources of the prtT and rgpA genes, respectively. Plasmid pResKm (26) was used for construction of the prtT:lacZ fusion plasmid. Plasmid pMC1871 (25), containing the lacZ gene, was obtained from Pharmacia (Uppsala, Sweden). Plasmids pKK51, pKK52, pKK53, pKpL:lacZ, and pRgp:lacZ were constructed in the present study. Plasmids pNH5 (3) and pYS307 (21) were utilized to construct probes for the kgp and tpr genes. E. coli JM109 was used for maintenance of these plasmids in Luria-Bertani broth (Bethesda Research Laboratory, Gaithersburg, Md.). Antibiotics were added for selection at the following concentrations: for E. coli, 50 μg of ampicillin per ml, 20 μg of tetracycline per ml, and 25 μg of chloramphenicol per ml; and for P. gingivalis, 100 μg of gentamicin per ml and 10 μg of erythromycin per ml.
Construction of lacZ fusion strains of P. gingivalis.
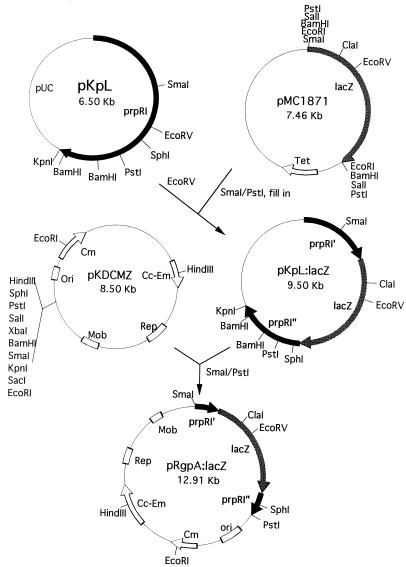
The rgpA:lacZ fusion gene (Fig. 1) was constructed by initially isolating the lacZ gene from plasmid pMC1871 as a SmaI/PstI fragment and blunt ending this fragment following Klenow treatment. The fragment was then ligated into the EcoRV site of plasmid pKpL containing the rgpA gene to yield pKpL:lacZ. A 4.4-kb DNA fragment containing the fusion gene was then removed following SmaI and PstI digestion and ligated into plasmid pKDCMZ to produce plasmid pRgp:lacZ.
FIG. 1.
Construction of the rgpA:lacZ fusion plasmid. Plasmid pKpL containing the 5′ end of the prpRI (rgpA) gene from P. gingivalis W50 was cleaved with EcoRV and ligated with the blunt-ended 3.0-kb SmaI/PstI lacZ fragment from pMC1871. This fragment was then introduced into plasmid pKDCMZ and transformed into E. coli S17.1 for conjugation into P. gingivalis 381.
In order to construct a prtT:lacZ gene fusion (Fig. 2), a 3-kb PstI fragment containing the lacZ gene was excised from plasmid pMC1871 and ligated into the PstI site of plasmid pKS containing the prtT gene, yielding plasmid pKK51. A KpnI/HindIII fragment containing the prtT:lacZ fusion gene was then removed from pKK51 and ligated into similarly cleaved pResKm to produce plasmid pKK52. Finally, a NotI fragment of plasmid pKK52 was ligated into the NotI site of plasmid pKDCMZ to yield plasmid pKK53.
FIG. 2.
Construction of the prtT:lacZ fusion plasmid. The 3.0-kb PstI fragment from pMC1871 containing the lacZ fragment was ligated into the PstI site of plasmid pKS containing the prtT gene to produce pKK51. A KpnI/HindIII fragment from this plasmid was then ligated into pResKm (pKK52). The NotI fragment from pKK52 was finally ligated into pKDCMZ, and the resulting plasmid was transformed into E. coli S17.1 for conjugation with P. gingivalis 381.
Each plasmid containing the lacZ chimeric genes was then transformed into E. coli S17.1 with selection on Luria-Bertani agar plates containing chloramphenicol. Conjugation between these constructs and P. gingivalis 381 was carried out as previously described (21). Briefly, the E. coli strains and strain 381 were grown to the mid-log phase (A600 = 0.6 to 0.8) and 2.0 ml of each was mixed and harvested together by centrifugation at 9,600 × g for 5 min. The cells were then suspended in 0.2 ml of TSB and spotted onto TSB agar plates containing sheep blood. The plates were incubated aerobically at 37°C for 4 h and then anaerobically for 48 h at this temperature. Next, the cells were harvested by scraping the plates and were resuspended in TSB (1.0 ml). The cell suspension was finally added to TSB-blood agar plates containing erythromycin plus gentamicin and incubated anaerobically for approximately 10 days. Colonies were picked, purified, and transferred to TSB containing gentamicin and erythromycin for further characterization.
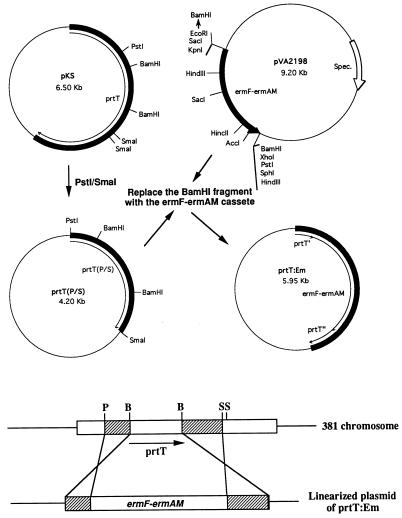
Construction of the prtT mutant.
The PstI/SmaI fragment containing a portion of the prtT gene was ligated into plasmid pUC19 to produce prtT(P/S) (see Fig. 6). The Erm cassette (7) was removed as a BamHI fragment from pVA2198 and ligated into the BamHI site of plasmid prtT(P/S), replacing the internal BamHI fragment of prtT to yield plasmid prtT:Em. The latter plasmid was then linearized with PstI and electroporated into strain 381 as recently described (29).
FIG. 6.
Strategy for constructing a prtT mutant. The 1.5-kb PstI/SmaI fragment from the prtT gene on plasmid pKS was recloned into plasmid pUC119. The BamHI fragment within prtT was then replaced by the 2.0-kb BamHI fragment containing the Erm cassette. The resulting plasmid was then linearized with SmaI, and the fragment was transformed into strain 381 by electroporation. Double-crossover recombination would result in inactivation of the chromosomal copy of the prtT gene. Spec., spectinomycin resistance gene.
Parameters affecting expression of protease genes.
For determination of the effects of growth stage on the expression of the proteases in strain 381, cells inoculated into TSB were harvested at early log (A600 = 0.3 to 0.4), mid-log (A600 = 0.5 to 0.6), late log (A600 = 0.8 to 1.0), or stationary (A600 = 1.2 to 1.5) phase. In order to produce an iron limitation, strain 381 was grown in TSB containing various concentrations of the iron chelator 2,2′-bipyridine (2,2′-BPD) to the mid-log phase. Protein-limited cultures were obtained by diluting the TSB medium as indicated.
Measurement of β-galactosidase activities.
The β-galactosidase activities of the cultures were determined as previously described (12). Briefly, the cells were cultured as indicated, aliquots were harvested and suspended in Z buffer (14) to an A600 of 1.0, and the cells were treated with toluene. The permeabilized cells were then incubated with o-nitrophenyl-β-galactosidase, and activity was measured in Miller units as previously described (14). Assays were carried out in triplicate for each sample, and results are presented as averages ± standard deviations.
Northern blot analysis.
Total RNA was isolated from P. gingivalis cells grown to the mid-log phase as recently described (5). The indicated concentrations of RNA (20 to 120 μg) were loaded onto 1.0% agarose–2.2 M formaldehyde gels and electrophoresed in MOPS (morpholine propanesulfonic acid) buffer, and the RNA fragments were transferred to Hybond N membranes (Amersham, Arlington Heights, Ill.) following capillary transfer. The indicated probes were labeled with the enhanced chemiluminescence system (Amersham) according to the directions of the supplier, and analysis of the gels was carried out as previously described (12).
RESULTS
Regulation of expression of rgpA and prtT proteases in strain 381.
In order to examine the regulation of expression of the rgpA and prtT genes, lacZ fusions were constructed for each gene. The rgpA gene (also named prpRI [6]) was fused in frame to the lacZ cartridge from plasmid pMC1871, and the fused gene was ligated into plasmid pKDCMZ (Fig. 1). The resultant plasmid, pRgpA:lacZ, was conjugated into strain 381, and Ermr transconjugants were isolated. Southern blot analysis confirmed the integration of the plasmid into the chromosome of strain 381 following a single crossover integration event (data not shown).
Likewise, the P. gingivalis prtT gene in plasmid pKS (20) was fused in frame to the lacZ cartridge and the chimeric gene was ligated into pKDCMZ to produce plasmid pKK53. The latter plasmid was then conjugated into strain 381, and Ermr colonies were purified for confirmation of the expected single-crossover integration event by Southern blotting and β-galactosidase activity determinations (data not shown).
Growth of both the prtT:lacZ and rgpA:lacZ constructs in TSB revealed that maximum expression of the prtT gene occurred at the mid-log phase of growth while rgpA expression was relatively constant from the mid-log phase into the stationary phase (Table 1). These results suggest that at least for these two proteases, expression was not coordinately regulated. Furthermore, under iron-limited conditions produced by addition of the chelator 2,2′-BPD to the growth medium, rgpA expression appeared to be upregulated while prtT expression was reduced. This regulation appears to occur at the level of transcription for rgpA, since Northern blot analysis revealed that rgpA transcription is elevated in the presence of the iron chelator (Fig. 3).
TABLE 1.
Expression of rgpA and prtT genes as lacZ fusions
| Culture condition | Fusion expressed (U)a
|
|
|---|---|---|
| prtT:lacZ | rgpA:lacZ | |
| Growth stage | ||
| Early log | 32.765 ± 0.125 | NDc |
| Mid-log | 89.475 ± 0.465 | 36.845 ± 1.005 |
| Late log | 67.515 ± 1.525 | 29.312 ± 5.118 |
| Stationary | 44.805 ± 3.385 | 30.668 ± 0.555 |
| Iron restriction (concn)b | ||
| 2,2′-BPD (none) | 102.950 ± 0.515 | 36.738 ± 4.895 |
| 2,2′-BPD (50 μg/ml) | 56.440 ± 3.225 | 48.317 ± 1.701 |
| 2,2′-BPD (100 μg/ml) | 41.850 ± 3.135 | 70.798 ± 2.550 |
| Medium (dilution)b | ||
| TSB (1×) | 114.715 ± 5.340 | 38.107 ± 1.953 |
| TSB (0.75×) | 76.925 ± 3.713 | 63.819 ± 2.530 |
| TSB (0.5×) | 49.820 ± 9.052 | 80.512 ± 9.365 |
Each value indicates average units ± standard deviation (determined in triplicate assays). The units are calculated as Miller units (14).
Samples were harvested at mid-log phase for analysis.
ND, not determined.
FIG. 3.
Northern blot analysis of rgpA expression under iron-limited conditions. RNA (50 μg) from strain 381 grown in the presence of no chelator (lanes 1), 15 μM 2,2′-BPD (lanes 2), or 30 μM 2,2′-BPD (lanes 3) was subjected to Northern blot analysis with the 0.7-kb EcoRV/SmaI rgpA probe (A) or the 0.9-kb MscI/BglII sod gene fragment.
Since iron limitation also slowed the growth of these organisms under these conditions, it was of interest to determine if these effects were due to growth inhibition rather than a specific iron requirement. Growth of both constructs in the presence of several dilutions of the growth media indicated that rgpA expression was indeed elevated under poor growth conditions while prtT expression was downregulated. Therefore, it was not clear whether these changes were the result of a specific iron limitation.
Effects of rgpA on expression of the kgp gene.
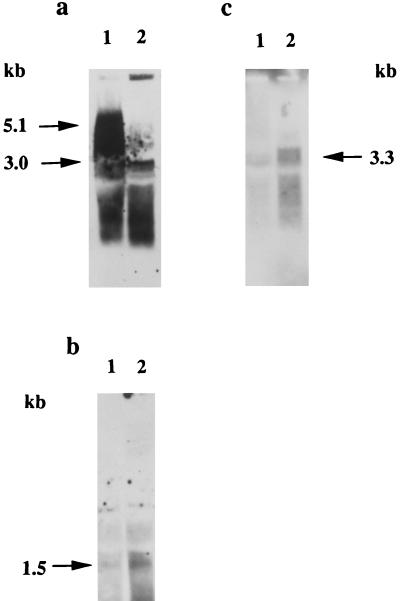
Recently, we have isolated a strain 381 mutant which is defective in the major Arg-specific protease gene rgpA (29). Since it was observed that similar mutants in strain 33277 expressed lower Lys-gingipain activity (1) it was of interest to determine if the loss of Arg-gingipain activity affected the expression of the kgp gene. Northern blot analysis of strain 381 compared to the rgpA mutant MT10 utilizing a kgp probe indicated that the mutant expressed lower levels of kgp mRNA relative to the parental strain (Fig. 4). This appears to be a specific effect, since expression of the sod gene was equivalent for both the parental and MT10 strains (data not shown). Furthermore, mutant MT10 expressed significantly lower levels of Lys-gingipain activity relative to the parental strain (data not shown). Therefore, inactivation of a major Arg-specific protease appeared to decrease the expression of the kgp gene.
FIG. 4.
Northern blot analysis of kgp expression in parental and rgpA mutant MT10. The 1.1-kb HindIII fragment of the kgp gene was used as a probe. The molecular size markers are shown on the left, and the arrow indicates the position of the 5.2-kb kgp mRNA. Lanes: 1, 40 μg of RNA from strain 381; 2, 40 μg of RNA from MT10; 3, 80 μg of RNA from MT10; 4, 120 μg of RNA from MT10.
Expression of other protease genes in mutant MT10.
In order to determine if the loss of RgpA activity had a general effect on protease expression in P. gingivalis, Northern blot analysis was used to compare rgpB, prtT, and tpr expression in the parental and MT10 mutant strains (Fig. 5). Inactivation of the rgpA gene appeared to increase somewhat the expression of the rgpB gene and markedly increased the expression of both the prtT and tpr genes. Therefore, the loss of the RgpA activity did not have the same effects on the expression of all of the proteases of P. gingivalis.
FIG. 5.
Northern blot analysis of rgpB, tpr, and prtT protease expression in an rgpA mutant. Equal amounts of RNA (20 μg) from strains 381 (lanes 1) and MT10 (lanes 2) were loaded onto agarose gels for analysis. (a) rgpA EcoRV/SmaI probe; (b) tpr probe (3.2-kb HindIII fragment); (c) prtT probe (1.4-kb PstI/SmaI fragment). The arrows indicate the positions of the relevant genes. The probes are described in the text.
Construction of a prtT mutant.
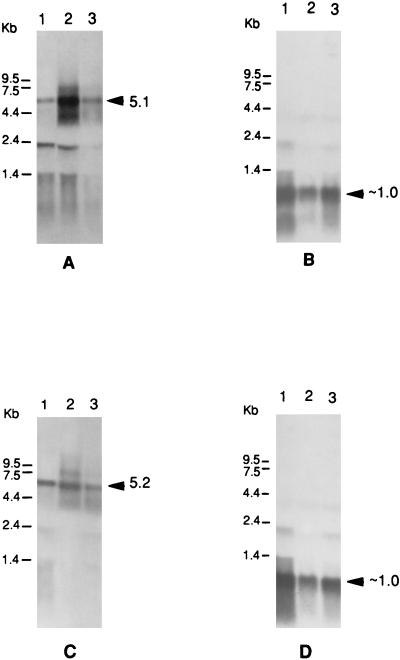
In order to examine the effects of a prtT mutation on the expression of the major gingipain proteases of P. gingivalis, insertional inactivation of the prtT gene was initially carried out with an Ermr cassette (Fig. 6). Electroporation of strain 381 with the linearized prtT:Em gene resulted in Ermr transformants following a double-crossover recombination event. This was confirmed by Southern blot analysis (Fig. 7), which indicated that the Erm probe reacted with an ∼7-kb PstI fragment in one of the transformants while no positive band was observed in parental strain 381. The 5.6-kb PstI fragment hybridizing with the prtT probe in strain 381 was replaced by a fragment slightly larger than 7 kb in the mutant as is expected from the size, 2.1 kb, of the Ermr cassette (7).
FIG. 7.
Southern blot analysis of prtT mutants. Chromosomal DNA from strain 381 (lanes 1) and the prtT mutants (lanes 2) were digested with PstI and loaded onto agarose gels. (a) analysis with the prtT SmaI/PstI fragment as a probe; (b) use of the SstI/PstI fragment of pVA2198 containing the Erm cassette as a probe. The sizes of the predicted bands are indicated by the arrows.
Effects of prtT inactivation on expression of the rgpA and kgp genes.
A comparison of rgpA expression in two randomly isolated prtT mutants relative to parental strain 381 by Northern blot analysis (Fig. 8A) revealed that the mutation moderately decreased rgpA mRNA levels. In addition, the loss of PrtT activity did not appear to significantly affect kgp expression (Fig. 8C). Therefore, prtT inactivation did not appear to markedly affect expression of the two major gingipain proteases of strain 381, RgpA and Kgp. Likewise, Arg-gingipain activity in the prtT mutants was not significantly different from that in parental strain 381 (data not shown) as measured by BAPNA (N-α-benzoyl-dl-arginine-p-nitroanilide) hydrolysis (20). Therefore, the PrtT protease appears to be a minor protease in strain 381 under normal laboratory growth conditions.
FIG. 8.
Northern blot analysis of prtT mutants. Equal amounts of RNA (40 μg) were loaded onto agarose gels and subjected to analysis. (A) rgpA fragment (0.7 kb) as probe; (B) same blot as in panel A, probed with the sod gene probe; (C) kgp fragment (1.1 kb) as probe; (D) same blot as in panel C, with the sod gene probe. The sizes of expected positive fragments are indicated next to the arrowheads. Lanes 1 and 3, prtT mutants WK1 and WK2; 2, strain 381.
DISCUSSION
Based upon the properties of mutants MT10 (29) and G102 (28), it appears that RgpA represents the major Arg-specific cysteine protease expressed by strain 381 while RgpB accounts for a lower proportion of such activity. However, the expression of other Arg-specific cysteine proteases (PrtT) could complicate such assumptions. Interestingly, it was observed that inactivation of either the rgpA or rgpB genes from strain 33277 produced marked reductions in Arg-specific protease activity more pronounced than the comparable mutants in strain 381 (17). It is not clear if such differences are the result of strain differences or the distinct strategies used to construct the mutants. Therefore, the present investigation was initiated to begin to examine the regulation of expression of some of the multiple proteases of P. gingivalis.
Since at least three enzymes which display Arg-specific cysteine-activated protease activity have been detected in strain 381 (1, 11, 20), it was necessary to construct lacZ reporter fusions with individual protease genes to evaluate the expression of specific enzymes. The utilization of the rgpA:lacZ construct indicated that rgpA expression appeared to be relatively constant throughout growth of strain 381. However, under iron limitation or nutrient deprivation, the expression of rgpA appeared to be induced. This would be consistent with its role as a major protease in P. gingivalis. Since iron limitation also retarded the growth of strain 381, it is not clear if iron itself directly regulates the expression of this gene. No sequences characteristic of iron-regulated Fur boxes (18) were detected upstream of the rgpA sequence from strain W50.
The Kgp protease appears to be the major Lys-specific protease expressed in P. gingivalis (16). Therefore, it may be significant that elimination of the major Arg-specific protease, RgpA, in strain 381 also resulted in decreased expression of the kgp gene. It will be of interest to determine the molecular basis for such regulation. However, increased expression of the rgpA gene by incubation of the cultures in the presence of 2,2′-BDP did not significantly alter kgp expression (data not shown). Therefore, the relatively slow growth of the rgpA mutant (29) is not responsible for down-regulating kgp expression. It is also of interest that the rgpA mutant was recently demonstrated to be defective in the expression of the major fimbrillin protein, FimA, in this strain. Thus, inactivation of the rgpA gene can markedly effect the expression of multiple potential virulence factors in P. gingivalis as recently indicated (29). It is possible that the RgpA protease is required for processing a regulatory protein which is required for expression of several genes in the organism.
By contrast, the expression of the minor PrtT protease of P. gingivalis indicated that the enzyme was maximally expressed at the mid-log phase and declined in specific activity during growth into the stationary phase. This result is compatible with the recent suggestion that this enzyme plays a role in the early log growth phase of P. gingivalis (30). Expression of the prtT gene also appeared to be repressed during growth in enriched medium but appeared to be induced when RgpA expression was inhibited. Likewise, the expression of the Tpr protease was also induced in the rgpA mutant. Previously, it was demonstrated that the construction of a tpr mutant in strain W83 did not decrease protease activity as much as the Arg-gingipain mutations (21). These results suggest that expression of the two relatively minor proteases was upregulated under conditions of protease deficiency. It is not clear if this is a general effect of amino acid limitation or the result of specific effects of RgpA on expression of these genes. The relatively weak expression of both the prtT and tpr genes during normal growth of strain 381 may explain why neither of these two enzymes has been detected during purification of the protease activities from culture fluids of P. gingivalis strains. It will be of interest to attempt to isolate and characterize these enzymes from the rgpA mutant or multiple protease mutants.
A comparison of the β-galactosidase activities from the PrtT and RgpA fusion constructs during normal growth appeared to indicate that the former is expressed at higher levels than the latter. However, such an interpretation appears not to be correct since Northern blot analysis suggested that prtT expression is relatively weak during normal growth of strain 381. Furthermore, the relative β-galactosidase activities of the two constructs can be affected by the different conformations of the two protein fusions as well as differences in the relative cellular distribution of the fusion proteins in the cells (27).
The present results suggest that the multiple proteases of P. gingivalis are not all coordinately regulated. The two relatively minor proteases, Tpr and PrtT, appear to be weakly expressed during normal growth of P. gingivalis and are induced under conditions of amino acid limitation or perhaps other nutrient stresses. By contrast, inactivation of the prtT gene did not have major effects on the expression of the major proteases, RgpA and Kgp. The roles of the PrtT and Tpr proteases in the physiology of P. gingivalis still remain to be determined, although recent preliminary results (30) suggest that these two enzymes are important for cellular growth under certain conditions. These results taken together suggest that individual proteases play specific functions in the growth of these organisms. It will be of great interest to now determine the molecular basis for the regulation of the proteases described as well as monitor the expression of these genes under the in vivo conditions found in the subgingival margin.
Since hemin levels in the gingival margin may vary depending upon the extent of bleeding, P. gingivalis may be subjected to both iron-limiting as well as iron-replete conditions. The present results suggest that the major Arg-specific protease, RgpA, would be induced under iron-poor conditions. By contrast, under iron-replete conditions the enzyme would be down-regulated. Since this enzyme is a likely virulence factor in P. gingivalis (7, 24), iron-limited cells would be predicted to be more virulent. This has been demonstrated in the case of one, but not all, studies relating iron availability and virulence (10). In addition, since the present results suggest that an RgpA deficiency induces potential compensating protease activities, PrtT and/or Tpr, as well as down-regulating Kgp expression, it is not clear how these proteases in toto contribute to virulence in the periodontal pocket. Such an understanding would be of direct clinical relevance, since proteases appear to be major virulence factors for these organisms (13, 24).
ACKNOWLEDGMENTS
We gratefully acknowledge the provision of plasmids to us by Michael Curtis, Barry McBride, and Marilyn Lantz.
This investigation was supported in part by National Institutes of Health grant DE08293.
REFERENCES
- 1.Abe N, Kadowaki T, Okamoto K, Nakayama K, Ohishi M, Yamamoto K. Biochemical and functional properties of lysine-specific cysteine proteinase (Lys-gingipain) as a virulence factor of Porphyromonas gingivalis in periodontal disease. J Biochem. 1998;123:305–312. doi: 10.1093/oxfordjournals.jbchem.a021937. [DOI] [PubMed] [Google Scholar]
- 2.Aduse-Opoku J, Rangarajan M, Young K A, Curtis M A. Maturation of the arginine-specific proteases of Porphyromonas gingivalis W50 is dependent on a functional prR2 protease gene. Infect Immun. 1998;66:1594–1600. doi: 10.1128/iai.66.4.1594-1600.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkocy-Gallagher G A, Han N, Patti J M, Whitlock J, Progulske-Fox A, Lantz M S. Analysis of the prtP gene encoding porphypain, a cysteine proteinase of Porphyromonas gingivalis. J Bacteriol. 1996;178:2734–2741. doi: 10.1128/jb.178.10.2734-2741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgeau G, Lapointe H, Peloquin P, Mayrand D. Cloning, expression, and sequencing of a protease gene (tpr) from Porphyromonas gingivalis W83 in Escherichia coli. Infect Immun. 1992;60:421–446. doi: 10.1128/iai.60.8.3186-3192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Curtis M A, Aduse-Opoku J, Slaney J M, Rangarajan M, Booth W, Cridland J, Shepherd P. Characterization of an adherence and antigenic determinant of the ArgI protease of Porphyromonas gingivalis which is present in multiple gene products. Infect Immun. 1996;64:2532–2539. doi: 10.1128/iai.64.7.2532-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher H M, Schenkein H A, Morgan R M, Bailey K A, Berry C R, Macrina F L. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossi S G, Zambon J J, Ho A W, Koch G, Dunford R G, Machtei E E, Norderyd O M, Genco R J. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260–267. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 9.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 10.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki T, Yoneda M, Okamoto K, Maeda K, Yamamoto K. Purification and characterization of a novel arginine-specific cysteine protease (Argingipain) involved in the pathogenesis of periodontal disease from the culture supernatant of Porphyromonas gingivalis. J Biol Chem. 1994;269:21371–21378. [PubMed] [Google Scholar]
- 12.Karunakaran T, Madden T, Kuramitsu H. Isolation and characterization of a hemin-regulated gene, hemR, from Porphyromonas gingivalis. J Bacteriol. 1997;179:1898–1908. doi: 10.1128/jb.179.6.1898-1908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuramitsu, H. K. Proteases of Porphyromonas gingivalis: what don’t they do? Oral Microbiol. Immunol., in press. [DOI] [PubMed]
- 14.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 15.Nakayama K. Rapid viability loss on exposure to air in a superoxide dismutase-deficient mutant of Porphyromonas gingivalis. J Bacteriol. 1994;176:1939–1943. doi: 10.1128/jb.176.7.1939-1943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama, K. Personal communication.
- 17.Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. J Biol Chem. 1995;270:23619–23626. doi: 10.1074/jbc.270.40.23619. [DOI] [PubMed] [Google Scholar]
- 18.Niederhoffer E C, Naranjo C M, Bradley K L, Fee J A. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J Bacteriol. 1990;172:1930–1938. doi: 10.1128/jb.172.4.1930-1938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto K, Kadowaki T, Nakayama K, Yamamoto K. Cloning and sequencing of the gene encoding a novel lysine-specific cysteine proteinase (Lys-gingipain) in Porphyromonas gingivalis: structural relationship with the arginine-specific cysteine proteinase (Arg-gingipain) J Biochem. 1996;120:398–406. doi: 10.1093/oxfordjournals.jbchem.a021426. [DOI] [PubMed] [Google Scholar]
- 20.Otogoto J-I, Kuramitsu H K. Isolation and characterization of the Porphyromonas gingivalis prtT gene, coding for protease activity. Infect Immun. 1993;61:117–123. doi: 10.1128/iai.61.1.117-123.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park Y, McBride B C. Characterization of the tpr gene product and isolation of a specific protease-deficient mutant of Porphyromonas gingivalis W83. Infect Immun. 1993;61:4139–4146. doi: 10.1128/iai.61.10.4139-4146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavloff N, Pemberton P A, Potempa J, Chen W-C A, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and characterization of Porphyromonas gingivalis lysine-specific gingipain. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 23.Potempa J, Pavloff N, Travis J. Porphyromonas gingivalis: a proteinase/gene accounting audit. Trends Microbiol. 1995;3:430–434. doi: 10.1016/s0966-842x(00)88996-9. [DOI] [PubMed] [Google Scholar]
- 24.Potempa J, Pike R, Travis J. Host and Porphyromonas gingivalis proteinases in periodontitis: a biochemical model for infection and tissue destruction. Perspect Drug Discov Des. 1995;2:445–458. [Google Scholar]
- 25.Shapira S K, Chou J, Richaud F V, Casadaban M J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ sequences encoding enzymatically active carboxyl-terminal portions of β-galactosidase. Gene. 1983;25:71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- 26.Shiroza T, Kuramitsu H K. Construction of a model secretion system for oral streptococci. Infect Immun. 1993;61:3745–3755. doi: 10.1128/iai.61.9.3745-3755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silhavy T J, Beckwith J R. Uses of lac fusions for the study of biological problems. Microbiol Rev. 1985;49:398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokuda M, Duncan M, Cho M-I, Kuramitsu H K. Role of Porphyromonas gingivalis protease activity in colonization of oral surfaces. Infect Immun. 1996;64:4067–4073. doi: 10.1128/iai.64.10.4067-4073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokuda M, Karunakaran T, Duncan M, Hamada N, Kuramitsu H. Role of Arg-gingipain A in virulence of Porphyromonas gingivalis. Infect Immun. 1998;66:1159–1166. doi: 10.1128/iai.66.3.1159-1166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan S, Lu B, Park Y, McBride B C. Characterization of Tpr and PrtT single and double mutants of Porphyromonas gingivalis. J Dent Res. 1997;76:225. [Google Scholar]