Abstract
Staphylococcus aureus was recently shown to be internalized by and to induce apoptosis in a bovine mammary epithelial cell line, suggesting that these processes could be involved in staphylococcal pathogenesis or persistence. To examine the role of virulence factor regulators during internalization, mutant agr and sar strains of S. aureus were analyzed for their abilities to enter and induce apoptosis in epithelial cells. Like a previously characterized bovine mastitis isolate, the standard laboratory strain, RN6390 (wild type), entered the epithelial cells and subsequently induced apoptosis. In contrast, the mutant strains RN6911 (agr), ALC136 (sar), and ALC135 (agr sar) were internalized by the cultured cells at levels reproducibly greater than that for RN6390 but failed to induce apoptosis. The internalization of S. aureus was affected by growth phase, suggesting a role for agr-regulated surface proteins in this process. Furthermore, the ability to induce apoptosis required metabolically active intracellular bacteria. These data indicate that the ability of S. aureus to enter mammalian cells and induce apoptosis is dependent on factors regulated by Agr and Sar. Since transcriptional control by these global regulators is mediated by quorum-sensing and environmental factors, staphylococci may have the potential to induce several alternative effects on cells from an intracellular environment. A model for the function of the agr locus in the context of internalization, intracellular persistence, and dissemination is proposed.
Staphylococcus aureus has remained a persistent pathogen that causes serious community-acquired and nosocomial infections throughout the world. The diseases associated with S. aureus are diverse, ranging from minor wound infections to more serious diseases, including endocarditis, osteomyelitis, and septic shock. Although some S. aureus infections are communicable, persistence of the organism on mucosal surfaces (e.g., vagina or upper respiratory tract) in a high percentage of the population provides a substantial source for endogenously acquired infections. In such cases, the organism is largely opportunistic but may be capable of inducing inflammatory (abscess) or toxigenic (toxic shock syndrome) infections. This threat is compounded by the appearance of methicillin-resistant S. aureus (MRSA) strains in the 1970s and the rapidly increasing frequency of MRSA strains in recent years. Because of the propensity of MRSA strains to be resistant to multiple antibiotics, the last line of defense has been the use of the glycopeptide antibiotic vancomycin. However, the recent emergence of S. aureus isolates that exhibit intermediate-level vancomycin resistance has underscored the urgent need to understand the regulation of events that occur during persistence compared to events required for the development of staphylococcal disease.
Hamill et al. (4) established that S. aureus internalizes and survives within nonprofessional phagocytes such as bovine aortic endothelial cells. Several reports subsequent to this confirmed that S. aureus can internalize and survive in a wide variety of mammalian cells (1, 2, 5, 19). Based on these results, it was proposed that the intracellular survival of S. aureus plays an important role in staphylococcal persistence and chronic staphylococcal disease (2, 5, 19). Furthermore, the ability to form small colony variants (SCVs), metabolically inactive forms of S. aureus, may be an important aspect of intracellular survival that promotes the development of persistent staphylococcal infections and resistance to antibiotics (16).
Recently, much progress has been made in our understanding of the regulation of staphylococcal virulence factors through an extensive study of the virulence gene regulators Agr and Sar (17). The agr locus encodes an elaborate quorum-sensing system that was hypothesized to differentially regulate the expression of cell wall-associated proteins and secreted exoproteins in response to the density of the bacterial population (7). The proposed function of this regulatory system is to enhance the production of cell wall-associated attachment (fibronectin- and collagen-binding proteins) and potential defensive factors (protein A) during the early stages of infection, followed by the expression of invasive factors (hemolysins, proteases, and lipases, etc.) once the infection is established. The sar locus, which is required for the expression of agr, provides an additional level of virulence factor regulation in response to signals that have yet to be defined.
In this study, the effects of the agr and sar regulatory loci on the ability of S. aureus to be internalized, survive, and induce apoptosis in cultured bovine mammary epithelial cells was examined. Although the agr and sar mutant strains reached higher intracellular concentrations compared to those of the wild-type cells, S. aureus-induced apoptosis was undetectable in MAC-T cells infected with these mutant strains. These data indicate that the ability of S. aureus to induce apoptosis is mediated by agr- and sar-dependent factors.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. aureus strains RN6390 (wild type), RN6911 (agr) (8), ALC136 (sar), and ALC135 (agr sar) (3) used in this study were kindly provided by Ambrose Cheung (Rockefeller University, New York, N.Y.). Prior to each experiment, bacteria in a single colony on a Todd Hewitt (TH; Difco Laboratories, Detroit, Mich.) agar plate were inoculated into 4 ml of TH broth (containing appropriate antibiotics for the mutant strains) and incubated at 37°C with vigorous shaking for 6 to 8 h. From these cultures, 100 μl was transferred into 10 ml of TH broth and typically incubated overnight (16 h) at 37°C with vigorous shaking. The bacterial cells were collected by centrifugation, washed three times with sterile phosphate-buffered saline (PBS [pH 7.2]), and resuspended in 10 ml of invasion medium (see below) to give stock cell suspensions with a density of approximately 1010 CFU/ml. Dilutions of these stock suspensions were used to inoculate the MAC-T cell monolayers at a multiplicity of infection ranging from approximately 0.1 to 100.
In some experiments, properties of exponential- and stationary-phase S. aureus cultures were compared. To prepare exponential phase cells, 100 μl of the overnight culture was transferred into 10 ml of TH broth and incubated until mid-exponential growth was achieved (125 Klett units; approximately 2 h at 37°C with vigorous shaking). Exponential-phase and stationary-phase cells were then centrifuged at 4°C, and the pellets were washed three times in ice-cold sterile PBS. The pellets of both cultures were adjusted to 125 Klett units by resuspending in ice-cold invasion medium. Cultures were kept on ice for a short period of time prior to use in experiments. Bacterial cell surface proteins were maintained under conditions representative of their growth phase (exponential or stationary) during the initial 2-h incubation of the invasion assay (see below) by adding rifampin to the media (5 μg/ml).
Cell culture.
An established bovine mammary epithelial cell line, designated MAC-T (6), was used for all experiments. The MAC-T cell growth medium was Dulbecco’s modified Eagle’s medium (Gibco BRL, Grand Island, N.Y.) with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 5 μg of insulin/ml, 1 μg of hydrocortisone/ml, 44 mM NaHCO3 (Sigma, St. Louis, Mo.), 100 U of penicillin G/ml, and 100 μg of streptomycin sulfate (Gibco BRL)/ml. Prior to each experiment, cells were seeded at 6 × 104 cells/well in 24-well tissue culture plates (Costar, Cambridge, Mass.) and grown for 3 days at 37°C with 6% CO2.
Invasion assay.
Approximately 16 h prior to each experiment, the MAC-T cell growth medium was replaced with 1 ml of invasion medium (growth medium without antibiotics or fetal bovine serum). On the day of the experiment, MAC-T cell monolayers were washed once and then infected by coculturing with washed S. aureus cells resuspended in fresh invasion medium (1 ml). After 2 h (37°C with 6% CO2), supernatants of the cocultures were replaced with 1 ml of invasion medium containing 100 μg of gentamicin (Sigma). Incubation of cocultures with gentamicin proceeded for an additional 3 h at 37°C with 6% CO2. In control experiments, no bacteria survived treatment with gentamicin in the absence of MAC-T cells. Supernatants of the cocultures were then discarded, and the monolayers were washed three times with sterile PBS, treated for 5 min at 37°C with 100 μl of 0.25% trypsin (Sigma) in Hanks balanced salt solution (Gibco BRL), and lysed by the addition of 900 μl of 0.025% Triton X-100 (U.S. Biochemical, Cleveland, Ohio) in sterile distilled water. Cell lysates were serially diluted 10-fold and plated in triplicate on TH agar plates to quantify intracellular bacteria. Time course experiments were performed in the same manner, except that the length of incubation after the addition of gentamicin varied.
Rifampin was used to assess the requirement of metabolically active intracellular bacteria to induce apoptosis in MAC-T cells. Rifampin is a bacteriostatic antibiotic that inhibits prokaryotic RNA transcription and readily penetrates eukaryotic membranes (9, 12). After the 2-h invasion period described above, supernatants of the cocultures were replaced with 1 ml of invasion medium containing 5.0 μg of rifampin (Sigma) and 100 μg of gentamicin (Sigma). Incubation of the infected MAC-T cells continued for 22 h at 37°C with 6% CO2, and samples were collected for DNA extraction as described below.
Assessment of apoptosis.
Apoptosis in MAC-T cells was monitored by two methods: (i) DNA laddering and (ii) an assessment of morphological changes in the cells. Evaluation of DNA laddering in infected MAC-T cells was done by collecting coculture supernatants and corresponding monolayers into a single sample. The DNA was extracted twice with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol/vol]), followed by extraction with an equal volume of chloroform-isoamyl alcohol (24:1 [vol/vol]). DNA in the aqueous phase was precipitated overnight at −20°C with a 1/10 volume of 3 M sodium acetate (pH 5.1), MgCl2 to 2 mM, and 2 volumes of 100% ethanol. DNA was harvested by centrifugation, and the pellet was washed once with 70% ethanol, dried, resuspended in sterile water, and treated with 20 μg of DNase-free RNase A (Sigma) per ml for 30 min at 37°C. DNA samples were resolved by electrophoresis at 5 V/cm in 1.8% agarose, stained with ethidium bromide, and visualized with UV light. A 100-bp DNA ladder (Gibco BRL) was used to size the DNA fragments.
Morphological changes to MAC-T cell monolayers during internalization by S. aureus were assessed visually with an inverted microscope (model IM; Olympus America Inc., Melville, N.Y.) equipped with a C-35AD camera (Olympus America Inc.).
RESULTS
Internalization of regulatory mutant strains.
A recent study (2) demonstrated that an S. aureus bovine mastitis isolate was internalized by and induced apoptosis in an established bovine mammary epithelial cell line, designated MAC-T. The ability to induce apoptosis was hypothesized (2) to be mediated by one or more of the agr and/or sar-regulated virulence factors produced by S. aureus. To study the potential effects of agr and/or sar on staphylococcal internalization and apoptosis, the degrees of internalization by MAC-T cells of S. aureus RN6390 (wild type), RN6911 (agr), ALC136 (sar), and ALC135 (agr sar) were compared. MAC-T cell monolayers were infected with increasing doses of each strain; after 2 h, monolayers were washed and incubation of cocultures with gentamicin proceeded for an additional 3 h. As shown in Fig. 1, each strain was able to enter the MAC-T cells in a dose-dependent manner, demonstrating that these common laboratory strains, like the previously characterized Novel strain (2), were also capable of being internalized. However, each of the mutant strains reproducibly yielded two- to threefold higher numbers of viable cells recovered from the MAC-T cells compared to the wild type at all dosages tested, suggesting either that they were internalized in greater numbers or that they had increased intracellular survival compared to the parental strain, RN6390.
FIG. 1.
Effects of agr and sar on invasion. A dose response invasion assay was performed by infecting MAC-T cell monolayers for 2 h with increasing numbers of S. aureus cells so that the multiplicity of infection (MOI) was altered within the ranges indicated. The monolayers were then washed, and incubation of cocultures proceeded in the presence of gentamicin to kill extracellular bacteria. Strains RN6390 (wild type [wt]), RN6911 (agr), ALC136 (sar), and ALC135 (agr sar) were used. Error bars represent the means ± the standard errors of the means.
To examine the ability of the different strains to persist within the MAC-T cells, the intracellular viability of the bacteria was determined over a period of 72 h. Interestingly, the viability of these bacteria within MAC-T cells was also shown to be strain dependent (Fig. 2). Although the number of viable RN6390 cells initially increased almost twofold within the first 10 h (from 4.8 × 105 to 7.6 × 105), the viability of this strain eventually (after 72 h) declined to approximately 0.001% of the original inoculum. This was presumably the result of initial growth of the organism and then subsequent killing due to entry of gentamicin through hemolysin-damaged MAC-T cell membranes. Unlike RN6390, the mutant strains did not exhibit an early increase in viable cell numbers but began to decline in cell numbers immediately after infection. Despite a lack of any apparent intracellular multiplication, the MAC-T cells infected with RN6911 (agr) and ALC135 (agr sar) yielded higher overall S. aureus survival rates (1.7 and 0.16%, respectively) than RN6390 (wild type) after 72 h. In contrast, although the number of viable ALC136 cells did not initially increase, the low level persistence of ALC136 (sar) in the MAC-T cells was similar to that of RN6390 (0.002% survival after 72 h).
FIG. 2.
Persistence of S. aureus within MAC-T cells. MAC-T cells were incubated in the presence of S. aureus RN6390 (MOI, 27), RN6911 (MOI, 13), ALC136 (MOI, 15), and ALC135 (MOI, 18) for 2 h prior to the addition of gentamicin and were then incubated in the presence of gentamicin for the times indicated. Error bars represent the means ± the standard errors of the means. wt, wild type.
Growth-phase-dependent internalization.
Previous studies have demonstrated that S. aureus cell surface proteins are required for tissue-specific adherence (14). Most genes encoding these surface proteins are expressed maximally in the early to mid-exponential phase of growth and are down-regulated by Agr late in the growth cycle (17). Thus, given the effect of the agr mutation on internalization shown in Fig. 1, we hypothesized that the stage of growth could have a dramatic effect on the ability of S. aureus to adhere to and therefore to enter MAC-T cells. To test this hypothesis, standard invasion assays were performed to compare exponentially growing and stationary-phase S. aureus RN6390 cells. To maintain bacterial cell surface proteins in a static state representative of their current phase of growth (exponential or stationary), rifampin and bacteria were added simultaneously to some of the culture wells and then cocultured for 2 h. In the presence of rifampin, the exponential-phase cells yielded 1.7 × 106 intracellular survivors compared to 4.7 × 102 with the stationary-phase cells (Fig. 3), a 3,600-fold difference. In the absence of rifampin, exponential- and stationary-phase cells yielded similar numbers of survivors (8.4 × 105 and 5.6 × 105, respectively), indicating that the stationary-phase cells had probably reestablished exponential growth and expression of cell surface proteins after dilution into tissue culture medium. These data indicate that the stage of growth is crucial for optimal internalization of the bacteria.
FIG. 3.
Growth phase-dependent invasion of MAC-T cells. An invasion assay was performed by exposing MAC-T monolayers to exponential- or stationary-phase S. aureus RN6390 cells for 2 h in culture medium with or without 5.0 μg of rifampin per ml followed by an additional 3 h in culture medium containing only gentamicin. Error bars represent the means ± the standard errors of the means.
Light microscopy.
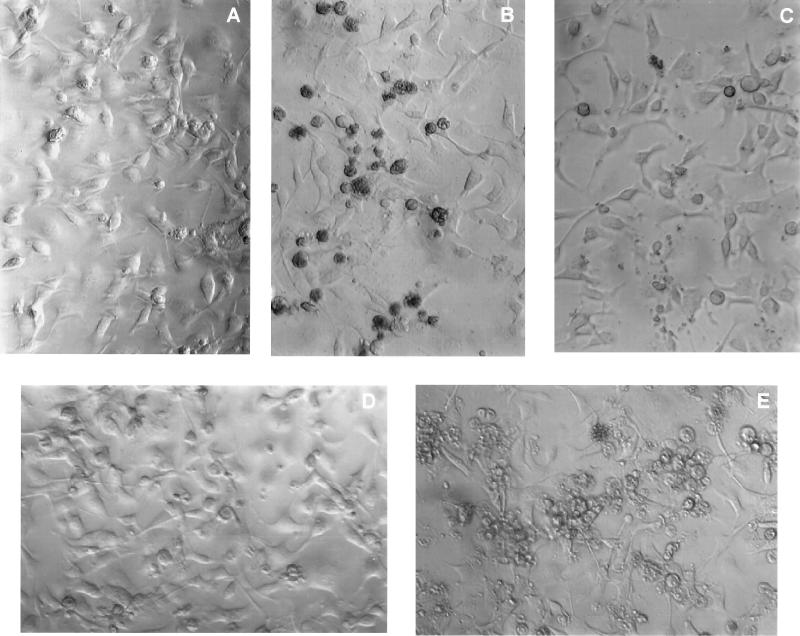
In addition to differences in the numbers of bacteria recovered in the invasion assays, dramatic differences in the abilities of these strains to induce morphological changes in the MAC-T cells were also observed (Fig. 4). While the parental strain, RN6390 (Fig. 4B), induced morphological changes in the MAC-T cells after 24 h (shrinking, rounding and detachment of the cells, and densely mottled cell membranes), MAC-T cells infected with RN6911 or ALC135 (Fig. 4C and D, respectively) appeared similar to the uninfected control (Fig. 4A). The sar mutant strain, ALC136, induced dramatic morphological changes in which the infected MAC-T cells appeared larger and exhibited less densely mottled cell membranes than the parental strain (Fig. 4E). All of our observations indicated that there was a gradual change in cell morphology, with approximately 24 h being the optimal time to observe the morphological features induced by S. aureus infection. These visual differences could reflect the fact that the agr and agr sar mutant strains do not express hemolysins, enzymes, and other cytotoxic molecules, whereas the sar mutant expresses various levels of these proteins (reference 3; see Discussion). The apparent cytotoxic differences observed in the MAC-T cell morphology are consistent with the differences in persistence shown in Fig. 2.
FIG. 4.
Effects of S. aureus strains on MAC-T cell morphology. MAC-T cell monolayers were either left untreated (A) or were incubated in the presence of S. aureus RN6390 (wild type) (B), RN6911 (agr) (C), ALC135 (agr sar) (D), or ALC136 (sar) (E) for 2 h prior to the addition of gentamicin. Photomicrographs were taken at 22 h following the addition of gentamicin to uninfected and infected cultures.
Induction of apoptosis.
The morphological changes observed in MAC-T cells infected with RN6390 and ALC136 were similar to features that were previously found to be associated with apoptosis (2). To assess whether internalization of the wild-type and mutant S. aureus strains induced apoptosis, DNA was isolated from the infected MAC-T cells and resolved in a 1.8% agarose gel. As shown in Fig. 5, DNA isolated from the RN6390-infected MAC-T cells (Fig. 5, lane 3) exhibited chromosomal DNA laddering in increments of 180 bp, which is characteristic of apoptotic cells (10, 15, 20). In contrast, genomic DNA from MAC-T cells infected with any of the three mutant strains appeared intact and migrated as a single large-molecular-weight band with no evidence of the 180-bp DNA laddering. Thus, the ability of S. aureus to induce apoptosis is a characteristic of the common laboratory strain, RN6390, and is dependent on an Agr- and Sar-regulated factor(s).
FIG. 5.
(A) Effects of agr and sar on the induction of apoptosis. DNA was extracted from MAC-T cells infected with RN6390 (wild type [lane 3]), RN6911 (agr [lane 4]), ALC135 (agr sar [lane 5]), or ALC136 (sar [lane 6]). MAC-T cells were exposed to bacteria for 2 h prior to the addition of gentamicin and then incubated for an additional 22 h prior to the DNA extraction. Electrophoretic separation of the DNA was performed with a 1.8% agarose gel. DNA in lane 2 was extracted from the uninfected control MAC-T cells treated with gentamicin for 22 h. Lanes 1 and 7 contain a 100-bp DNA ladder. Data are derived from a representative experiment repeated three times. (B) Agarose gel (1.8%) electrophoretic separation of DNA extracted from MAC-T cells to demonstrate the effect of inhibition of RNA transcription on apoptosis. Lanes 1 and 6, 100-bp ladder; lane 2, uninfected control MAC-T cells treated with gentamicin for 22 h; lane 3, uninfected control MAC-T cells treated with gentamicin and rifampin for 22 h; lane 4, MAC-T cells infected with S. aureus RN6390 for 2 h prior to the addition of gentamicin and then incubated for an additional 22 h; lane 5, MAC-T cells infected with S. aureus RN6390 for 2 h prior to the addition of gentamicin and rifampin and then incubated for an additional 22 h.
To determine whether the ability of S. aureus to induce apoptosis was dependent on growth and/or gene expression once the bacteria had been internalized, rifampin was added to RN6390-infected MAC-T cells after the 2-h internalization period to inhibit intracellular bacterial RNA transcription. As shown in Fig. 5B, treatment with rifampin inhibited the induction of apoptosis, as evidenced by the lack of MAC-T cell DNA laddering (Fig. 5B, lane 5) and the observation that the MAC-T cells remained adherent and appeared normal (data not shown). Although treatment of infected monolayers with rifampin resulted in a fourfold reduction of surviving intracellular bacteria, this number of bacteria is normally sufficient to induce apoptosis in the absence of antibiotic (data not shown). Also, lack of apoptosis was not due to an effect of rifampin on the MAC-T cells, since these cells treated simultaneously with S. aureus overnight culture supernatants and rifampin exhibited characteristic DNA laddering (data not shown). These data illustrate that the ability of intracellular S. aureus to induce apoptosis in MAC-T cells requires that the bacteria are metabolically active.
DISCUSSION
The data presented in this report extend the recent finding that S. aureus internalizes within and induces apoptosis in bovine mammary epithelial cells (2). Our previous investigation demonstrated that once S. aureus contacts the surface of MAC-T cells, the bacteria are engulfed by the cell, escape from the endosome, and possibly multiply within the cytoplasm. During this process, the MAC-T cells undergo apoptosis.
The strains employed in this study behaved similarly in invasion assays to the bovine mastitis isolate previously studied (2), suggesting that RN6390 and its derivatives express the factor(s) necessary for internalization by the MAC-T cells. The results of invasion assays demonstrate that the accumulation of the agr and sar mutant strains within MAC-T cells was reproducibly greater than that with the parental strain. The increased intracellular accumulation of the mutant strains could be a result of two factors. First, decreased production of hemolysins or other membrane-damaging cytotoxins by the internalized mutant strains could promote staphylococcal survival because gentamicin cannot cross intact MAC-T cell membranes. Second, the increased production of cell surface proteins (e.g., fibronectin-binding protein) by the agr mutant (17) could promote greater initial binding and subsequently greater internalization by the cultured MAC-T cells.
High-affinity binding by cell wall-associated proteins is a prerequisite for internalization of intracellular pathogens (13). Our data are entirely consistent with a similar requirement by S. aureus. The agr and/or sar mutants, which are known to express increased levels of surface proteins (17), were internalized better than the wild-type strain. In addition, staphylococcal cells displayed a highly significant growth phase dependence on the ability to be internalized by MAC-T cells, with 3,600-fold more exponential cells internalized compared to stationary-phase cells. It is well-established that the cell surface proteins of S. aureus are expressed maximally during the exponential phase of growth and are down-regulated as cells enter stationary phase (17), thus providing an explanation for these results. In fact, experiments to identify microbial surface components recognizing adhesive matrix molecules (MSCRAMMs [14]) crucial to internalization have been ongoing in our laboratory and have indicated that fibronectin-binding protein plays a critical role in adherence of S. aureus to MAC-T cells (unpublished data).
The relatively high levels of intracellular bacteria achieved in monolayers infected with agr and sar mutants apparently were not due to their replication inside the MAC-T cells; only RN6390 appeared capable of intracellular growth. While the number of viable bacteria increased from 4.6 × 105 to 7.6 × 105 after the first 10 h of infection with the wild-type cells, the number of viable bacteria decreased during this time period following infection with any of the regulatory mutant strains. The requirements for intracellular growth are probably complex. The fact that the Agr and Sar global regulators are required may suggest a need of the bacteria to sense the intracellular environment. An alternative possibility is that agr and/or sar-regulated products may be required to escape the endosome and enter the cytoplasm, which presumably provides an environment more conducive to growth of S. aureus. Studies to compare the intracellular fates of the staphylococcal mutants are ongoing in our laboratories.
Our data indicate that metabolically active intracellular S. aureus strains are required for induction of apoptosis. The addition of rifampin after the 2-h invasion period completely inhibited the ability of the bacteria to induce detectable apoptosis. These results are consistent with our hypothesis that a secreted protein is involved in this process. Induction of apoptosis in macrophages by secreted proteins has been documented for macrophages infected with Shigella flexneri or Yersinia enterocolitica. IpaB, an invasin encoded by the Shigella virulence plasmid, is required for the induction of apoptosis in Shigella-infected macrophages (21). Similarly, the intracellular secretion of YopP by Yersinia enterocolitica is necessary to elicit apoptosis (11).
It is important to note that only the parental strain (RN6390) induced both the morphological changes and DNA laddering characteristic of apoptosis. Infection with RN6911 (agr) or ALC135 (agr sar) did not result in morphological changes or DNA laddering in MAC-T cells. Although infection with ALC136 (sar) did not induce apoptotic DNA laddering, this mutant induced dramatic morphological changes compared to the other mutant strains. Compared to cells infected with RN6390, cells infected with ALC136 appeared larger and with less densely mottled cell membranes, which are characteristics more commonly observed in cells undergoing necrosis (18). These morphological features were not attributed to apoptotic changes but were typical of cells exposed to cytolytic agents. While ALC136 is deficient in many secreted products, some exoproteins are still produced at various levels. For example, this strain produces decreased but detectable levels of β-toxin and increased levels of lipase (3). These, or possibly other exoproteins might account for the abnormal epithelial cell morphology induced by ALC136.
A model for the function of Agr-mediated quorum sensing in staphylococcal disease has recently been proposed (17). In that model, extracellular S. aureus cells differentially express cell wall-associated factors and exoproteins as a function of the extracellular concentrations of the AgrD octapeptide which mediates expression of the agr operon. Low levels of octapeptide lead to the expression of cell wall-associated factors, while high levels trigger a shift to the expression of exoproteins. S. aureus cells that have recently entered a host express cell wall-associated proteins, since the initial octapeptide concentration is low. These bacteria resist host defense mechanisms (potentially due to the production of surface factors such as protein A) while being primed to bind tissue surfaces (due to the production of matrix binding MSCRAMM surface proteins). Once a focus of infection had been established, the bacteria multiply and form a characteristic barrier (abscess) to provide additional protection against host defenses. As the number and concentration of bacteria increase within the abscess, the agrD-encoded octapeptide reaches a threshold concentration that promotes Agr and Sar expression and a shift to the expression of tissue-damaging exoproteins with escape from the abscess barrier. This then leads to a corresponding shift to a more progressive and invasive infection.
Based on our results, we propose a modification of this model to accommodate staphylococci in an intracellular niche. According to our model (Fig. 6), upon entry into the host or previously sterile body site, extracellular S. aureus cells are likely to express cell surface-associated adherence factors (such as fibronectin- or collagen-binding proteins) due to the dilution of AgrD octapeptide in the surrounding fluids. The bacteria are in a physical state that is optimal for binding to cell surfaces and subsequent internalization. Upon internalization, the bacteria become surrounded immediately by an endosomal membrane (2, 5). Within the confined space of the endosome, accumulation of octapeptide occurs rapidly and triggers expression of Agr and its up-regulated exoproteins. Some of these exoproteins (particularly the hemolysins) could play a role in escape from the endosome. We have shown that shortly after internalization, S. aureus escapes from the endosome via a process that results in lysis of the endosomal membrane (2). Outside the endosome, the octapeptide is immediately diluted again, resulting in an Agr-mediated shift away from the expression of exoproteins and toward a physiological state that is more adapted to promote survival and growth within the cytoplasm.
FIG. 6.
Model of Agr regulation during invasion. In an extracellular environment, levels of agrD-encoded octapeptide (triangles) are low and S. aureus (solid circles) expresses cell surface-associated adherence factors. Once internalized, a rapid accumulation of octapeptide within the endosome causes a shift to the expression of exoproteins. Some exoproteins could effect escape from the endosome, resulting in the dilution of the octapeptide and a shift away from expression of exoproteins. Once S. aureus resides in the cytoplasm of the host, we envision three possible outcomes: (i) the induction of apoptosis, (ii) the formation of small colony variants (hatched circles), or (iii) lysis of the host cell.
Once the bacteria have gained access to the cytoplasm, we envision three possible outcomes that would have dramatically different effects on pathogenesis (Fig. 6). These outcomes are based on the different effects that were observed with the MAC-T cells when infected by the S. aureus strains used in this study and the results of Proctor et al. (16). The first pathway (pathway I) would lead to the production of an Agr-regulated factor that triggers the development of apoptosis. The resulting apoptotic bodies are then engulfed by resident macrophages. Engulfment of membrane-bound bacteria could provide a protective environment within the macrophage (analogously to the Trojan horse), resulting in the inability to produce an appropriate bactericidal response. Alternatively, metabolically inactive small colony variants (SCVs) would be formed (pathway II), causing little damage to the host cell and leading to a more chronic and persistent disease (16). Finally, if the bacteria accumulated to a high enough concentration in the cytoplasm, AgrD octapeptide levels could reach a concentration sufficient to shift expression to exoproteins. Production of cytolytic proteins (hemolysins) results in lysis of the host cell (pathway III), possibly followed by spread of bacteria to adjacent cells, generation of an abscess, or perhaps a more progressive and invasive disease. Entry into this pathway depends on the ability of S. aureus to replicate, allowing the accumulation of enough octapeptide to shift gene expression back to the exoproteins. According to our model, any inhibition of growth in the cytoplasm reduces the accumulation of octapeptide and delays lysis of the host cell. Thus, the commitment to advance toward a more progressive infection, in contrast to a more persistent and chronic infection (two conditions that are not mutually exclusive), is made within the cytoplasm and could be based on the immunological state of the host. Experiments designed to test various aspects of this model are currently in progress.
ACKNOWLEDGMENTS
We thank Ambrose Cheung for providing S. aureus RN6390, RN6911, ALC135, and ALC136.
This work was funded in part by NIH grant no. R29-AI38901 (K.W.B.), NRICGP USDA grant no. 9402399 (G.A.B.), Public Health Service grant no. AI28401 (G.A.B.), the United Dairymen of Idaho (G.A.B.), and a sabbatical fellowship from the Organization for Economic Cooperation and Development (W.R.T.).
REFERENCES
- 1.Almeida R A, Matthews K R, Cifrian E, Guidry A J, Oliver S P. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 2.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung A L, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M, Bayer A S. Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamill R J, Vann J M, Proctor R A. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect Immun. 1986;54:833–836. doi: 10.1128/iai.54.3.833-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 6.Hyunh H T, Robitaille G, Turner J D. Establishment of bovine mammary epithelial cells (MAC-T): an in vivo model for bovine lactation. Exp Cell Res. 1991;197:191–199. doi: 10.1016/0014-4827(91)90422-q. [DOI] [PubMed] [Google Scholar]
- 7.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12005–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornblum J, Kreiswirth B N, Projan S J, Ross H, Novick R P. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers, Inc.; 1990. pp. 373–402. [Google Scholar]
- 9.Lester W. Rifampin: a semisynthetic derivative of rifamycin: a prototype for the future. Annu Rev Microbiol. 1972;26:85–102. doi: 10.1146/annurev.mi.26.100172.000505. [DOI] [PubMed] [Google Scholar]
- 10.Majno G, Joris I. Apoptosis, oncosis and necrosis. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 11.Mills S D, Boland A, Sory M P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neu H C. Antimicrobial chemotherapy. In: Baron S, editor. Medical microbiology. 2nd ed. Menlo Park, Calif: Addison-Wesley Publishing Co., Inc.; 1986. pp. 173–196. [Google Scholar]
- 13.Nhieu T V, Isberg R R. Bacterial internalization mediated by beta 1 chain integrins is determined by ligand affinity and receptor density. EMBO J. 1993;12:1887–1895. doi: 10.1002/j.1460-2075.1993.tb05837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patti J M, Allen B L, McGavin M, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissue. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 15.Peitsch M C, Mannherz H G, Tschopp J. The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol. 1994;4:37–41. doi: 10.1016/0962-8924(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 16.Proctor R A, Balwit J M, Vesga O. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect Agents Dis. 1994;3:302–312. [PubMed] [Google Scholar]
- 17.Projan S J, Novick R P. The molecular basis of pathogenicity. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 55–81. [Google Scholar]
- 18.Smith C A, McCarthy N J, Williams G T. Cell recognition of apoptotic cells. In: Horton M A, editor. Blood cell biochemistry. Vol. 5. New York, N.Y: Plenum Press; 1993. pp. 393–421. [Google Scholar]
- 19.Vann J M, Proctor R A. Ingestion of Staphylococcus aureus by bovine endothelial cells results in time- and dose-dependent damage to endothelial cell monolayers. Infect Immun. 1987;55:2155–2163. doi: 10.1128/iai.55.9.2155-2163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyllie A H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature (London) 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 21.Zychlinsky A, Kenny B, Menard R, Prevost M C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]