Abstract
Background:
Concurrent chemoradiotherapy is a standard therapy for patients with stage III non-small-cell lung cancer (NSCLC). Durvalumab is an approved treatment option following concurrent chemoradiotherapy in the absence of disease progression. The multicenter, phase III, randomized, placebo- and active-controlled, double-blind KEYLYNK-012 study evaluates whether initiation of immunotherapy with pembrolizumab concurrently with chemoradiotherapy, followed by post-chemoradiotherapy pembrolizumab with or without olaparib improves outcomes for participants with stage III NSCLC. (ClinicalTrials.gov: NCT04380636)
Methods:
Eligible participants are aged ≥18 years with previously untreated, pathologically confirmed, stages IIIA-C, squamous or nonsquamous NSCLC not suitable for surgery with curative intent. Participants will be randomized 1:1:1 to platinum-doublet chemotherapy plus radiotherapy with pembrolizumab (Groups A and B) or concurrent chemoradiotherapy alone (Group C) for 3 cycles. In the absence of disease progression, participants will receive pembrolizumab plus olaparib placebo (Group A), pembrolizumab plus olaparib (Group B), or durvalumab monotherapy (Group C). Dual primary endpoints are progression-free survival per RECIST version 1.1 by independent central review and overall survival.
Results:
Enrollment began on July 6, 2020, and is ongoing at approximately 190 sites.
Conclusion:
KEYLYNK-012 will provide important information on the efficacy and safety of pembrolizumab combined with concurrent chemoradiotherapy and subsequent pembrolizumab with or without olaparib in participants with unresectable stage III NSCLC.
Keywords: Immunotherapy, Chemotherapy, Radiation therapy, PARP inhibition, Non-small-cell lung cancer
Introduction
Stage III non-small-cell lung cancer (NSCLC) is commonly treated with concurrent chemoradiotherapy (CCRT).1 For patients without disease progression following CCRT, the phase III PACIFIC trial showed that durvalumab, an anti–PD-L1 antibody, significantly prolongs overall survival (OS) versus placebo.2 Based on these results, durvalumab was approved for the treatment of all-comer adult patients with unresectable, stage III NSCLC (in the United States) or patients with NSCLC expressing PD-L1 on ≥1% of tumor cells (in the European Union) whose disease has not progressed following platinum-based CCRT.3,4 Although locoregional NSCLC is treated with curative intent, this population has a high risk of disease progression or recurrence to metastatic sites.5 Additionally, up to 27% of patients who complete CCRT are not eligible to receive subsequent durvalumab due to disease progression or prohibitive toxicity.6 Thus, there remains an unmet clinical need for therapeutic approaches to prolong progression-free survival (PFS) and OS in patients with stage III NSCLC.
Pembrolizumab, a monoclonal anti–PD-1 antibody, is a first-line standard of care for metastatic NSCLC as monotherapy (for patients whose tumors have a PD-L1 tumor proportion score [TPS] ≥1% in the United States or TPS ≥50% in the European Union) or in combination with platinum-based chemotherapy.7,8 Pembrolizumab monotherapy is also an approved treatment in the United States for patients with stage III NSCLC with TPS ≥1% who are not candidates for surgical resection or definitive CCRT.8 Phase I and II trials of CCRT and concomitant pembrolizumab showed acceptable tolerability and promising antitumor activity in participants with unresectable stage III NSCLC.9,10
Poly(ADP-ribose) polymerase (PARP) inhibition was shown to upregulate PD-L1 expression in cancer cell lines and animal models, thus stimulating immune escape mechanisms in cancer cells.11,12 This effect potentially sensitizes tumor cells to PD-1/PD-L1 inhibitor therapy, and provides a rationale for the combination of pembrolizumab and PARP inhibition in cancer treatment. Olaparib is a potent PARP inhibitor currently approved for use in advanced ovarian, breast, pancreatic, and prostate cancer,13 and has an acceptable safety profile when combined with pembrolizumab for the treatment of advanced solid tumors.14,15
We describe the design of KEYLYNK-012, a multicenter, phase III, randomized, placebo- and active-controlled, double-blind study that investigates whether the initiation of immunotherapy, such as pembrolizumab, in combination with CCRT (ie, earlier in the treatment), followed by pembrolizumab with or without olaparib in the post-CCRT setting, improves the outcomes of participants with locally advanced, unresectable stage III NSCLC.
Participants and Methods
Study Design and Objectives
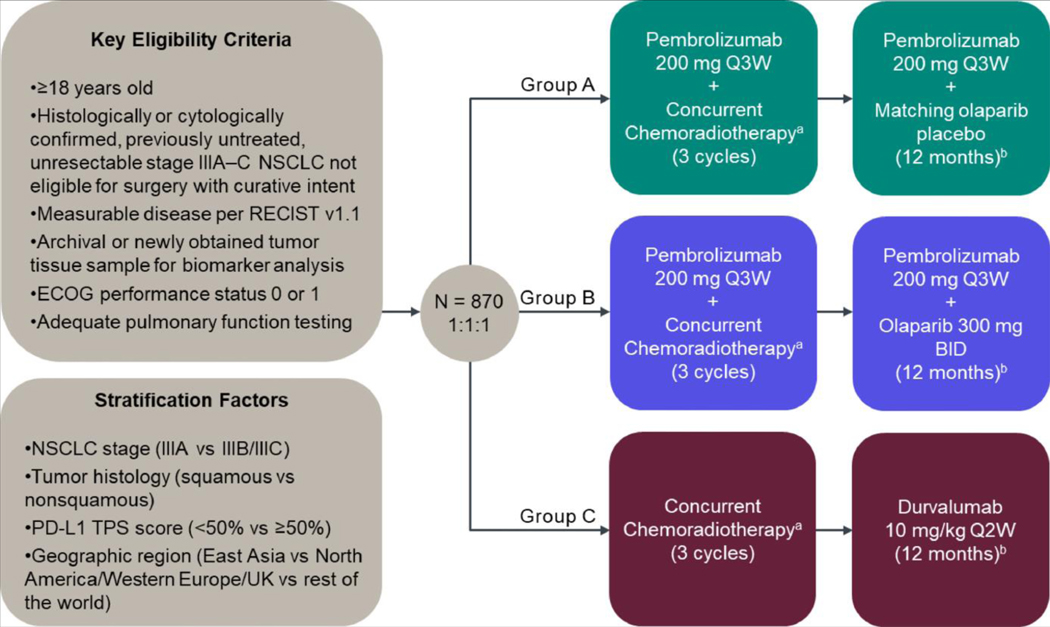
In the KEYLYNK-012 study (ClinicalTrials.gov identifier NCT04380636), participants with unresectable, locally advanced, stage III NSCLC will receive pembrolizumab in combination with CCRT followed by pembrolizumab plus olaparib placebo or olaparib (Groups A and B, respectively) or CCRT followed by durvalumab (Group C) (Figure 1). Participants will be randomly allocated in a 1:1:1 ratio across the 3 treatment groups. Participants and investigators will be blinded to treatment assignment in Groups A and B only (ie, whether the participant is receiving olaparib placebo or olaparib).
Figure 1.
KEYLYNK-012 study design. BID = twice daily; ECOG = Eastern Cooperative Oncology Group; NSCLC = non-small-cell lung cancer; Q2W = every 2 weeks; Q3W = every 3 weeks; TPS = tumor proportion score. aPlatinum doublet chemotherapy and concurrent standard thoracic radiotherapy (60 Gy in 2 Gy fractions; during cycles 2 and 3). Platinum doublet options (per investigator’s choice) include cisplatin 75 mg/m2 IV and pemetrexed 500 mg/m2 IV every 3 wk on day 1 of cycles 1–3 (for participants with nonsquamous histology only); cisplatin 50 mg/m2 IV on days 1 and 8 in cycles 1 and 2 and d 8 and 15 in cycle 3 plus etoposide 50 mg/m2 IV on days 1–5 in cycles 1 and 2 and days 8–12 in cycle 3; or carboplatin area under the curve (AUC) 6 mg/mL/min IV plus paclitaxel 200 mg/m2 IV on day 1 in cycle 1 and carboplatin AUC 2 mg/mL/min IV plus paclitaxel 45 mg/m2 IV on days 1, 8, and 15 in cycles 2 and 3. bTreatment will continue for 12 mo or until the participant or legally acceptable representative requests to discontinue study treatment, treatment assignment to Group A or B is unblinded, disease progression or new malignancy, surgery with curative intent, unacceptable toxicity, interruption of olaparib placebo or olaparib for more than 28 consecutive days, interruption of pembrolizumab or durvalumab post-radiotherapy for more than 12 wk, intercurrent illness, evidence of myelodysplastic syndrome or acute myeloid leukemia, investigator decision, or pregnancy. Participants in Groups A and B may receive a maximum of 17 cycles of pembrolizumab post-chemoradiotherapy (~1 y) and participants in Group C may receive a maximum of 26 cycles of durvalumab (~1 y).
The dual primary endpoints of the study are PFS per RECIST version 1.1 by blinded independent central review and OS. Secondary endpoints include safety/tolerability, objective response rate, duration of response, and patient-reported outcomes. Exploratory endpoints included biomarkers and PFS on the next line of therapy.
Participants
Detailed participant inclusion and exclusion criteria are shown in Table 1. Briefly, participants must be ≥18 years of age, with histologically or cytologically confirmed, previously untreated, unresectable stage IIIA-C NSCLC by the American Joint Committee on Cancer version 8 criteria16 that are not eligible for surgery with curative intent, with adequate performance status and organ function. Participants with squamous and nonsquamous histology are eligible, although those with squamous NSCLC are not eligible to receive pemetrexed-based chemotherapy.
Table 1.
Key Inclusion and Exclusion Criteria for the KEYLYNK-012 Study
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • Age ≥18 y | • Has SCLC or mixed tumor with presence of small-cell elements |
| • Histologically/cytologically confirmed, previously untreated, unresectable stage IIIA-C NSCLC not eligible for surgery with curative intent and without evidence of metastatic disease | • Known MDS/AML, or features suggestive of MDS/AML |
| • Measurable disease per RECIST version 1.1 as assessed by the local investigator | • Able to have radiation treatment plan with V20 >34% of lung volume |
| • No prior chemotherapy, targeted therapy or radiotherapy for stage III NSCLC | Prior thoracic radiotherapy, prior olaparib or other PARP inhibitor or prior anti–PD-1, PD-L1, PD-L2 or other stimulatory or co-inhibitory T-cell receptor therapy |
| • Provision of archival or newly obtained tumor tissue sample for PD-L1 assessment | • Major surgery <4 wk prior to initiation of study treatment |
| • ECOG PS of 0 or 1 | • Received colony-stimulating factors <28 d prior to initiation of study treatment |
| • Adequate pulmonary function testing, defined as defined as a FEV1 >50% of predicted normal volume and a DLCO >40% of predicted normal value | • Known additional malignancy that is progressing or required treatment within 5 y |
| • Adequate organ function | • History of/current pneumonitis/interstitial lung disease or active tuberculosis |
| • Life expectancy at least 6 months | • Active infection requiring systemic therapy |
| • Written informed consent | • Diagnosis of immunodeficiency or is receiving chronic systemic steroid therapy within 7 d |
| • Active autoimmune disease requiring systemic treatment within 2 y | |
| • Known history of HIV or hepatitis B or active hepatitis C |
Abbreviations: AML = acute myeloid leukemia; DLCO = carbon monoxide lung diffusing capacity; FEV = forced expiratory volume; HIV = human immunodeficiency virus; MDS = myelodysplastic syndrome; PARP = poly (ADP-ribose) polymerase; V20 = volume of lung receiving >20 Gy.
Treatments
Participants in Group A will receive pembrolizumab 200 mg intravenously (IV) every 3 weeks in combination with 3 cycles of platinum-doublet chemotherapy and concurrent standard thoracic radiotherapy (60 Gy in 2 Gy fractions during Cycles 2 and 3) followed by pembrolizumab plus matching olaparib placebo. Participants in Group B will receive pembrolizumab 200 mg IV every 3 weeks in combination with 3 cycles of platinum-doublet chemotherapy and concurrent standard thoracic radiotherapy followed by pembrolizumab plus olaparib 300 mg orally twice daily. Participants in Group C will receive 3 cycles of platinum-doublet chemotherapy and concurrent standard thoracic radiotherapy followed by durvalumab 10 mg/kg IV every 2 weeks. Chemotherapy regimens will be selected by the investigator from protocol-specified options (Figure 1). Interruption and discontinuation, but not dose reduction, are permitted for pembrolizumab and durvalumab. Olaparib dose can be reduced to 250 mg twice daily, and then to 200 mg twice daily if needed, with no further dose reductions permitted.
Following completion of the CCRT phase and in the absence of disease progression, participants will receive pembrolizumab plus olaparib placebo or olaparib, or durvalumab monotherapy for a maximum of 12 months or until protocol-specified discontinuation criteria are met (including request to discontinue study treatment, disease progression, unblinding of treatment assignment, or unacceptable toxicity).
Assessments
Tumor response per RECIST version 1.1 will be evaluated at the end of the CCRT (with or without pembrolizumab) phase, which is approximately 12 weeks after study treatment initiation. Subsequent imaging assessments will be performed every 9 weeks for the first 2 years, every 12 weeks in the third year, every 26 weeks until the end of the fifth year, and every 52 weeks thereafter until disease progression, the start of new anticancer therapy, withdrawal of consent or death, whichever occurs first.
Adverse event data will be collected from time of randomization through to 30 days after study treatment discontinuation. Serious adverse event data will be reported for up to 90 days after study treatment discontinuation.
Statistical Analysis
Efficacy will be evaluated in all randomized participants (the intention-to-treat population). Safety will be assessed in all participants who receive at least 1 dose of study treatment. Treatment differences in the dual primary endpoints of PFS and OS will be compared between Groups A and B versus C using a stratified log-rank test. Hazard ratios will be estimated using a stratified Cox regression model, and event rates over time will be estimated using the Kaplan-Meier method.
External Data Monitoring Committee
An independent, external data and safety monitoring committee will oversee the trial and assess efficacy at protocol-specified interim analyses. Pneumonitis events will be monitored and adjudicated by an external clinical adjudication committee.
Results
Current Status
Enrollment for this study began on July 6, 2020 and is ongoing at approximately 190 sites. The current estimated study completion date is July 6, 2026. The planned sample size is approximately 870 participants.
Discussion
The KEYLYNK-012 study will evaluate the combination of pembrolizumab with CCRT followed by pembrolizumab plus olaparib placebo or olaparib versus CCRT followed by durvalumab for the treatment of unresectable, locally advanced, stage III NSCLC. Pembrolizumab efficacy and safety have been extensively investigated and characterized in NSCLC, and pembrolizumab as monotherapy or in combination with chemotherapy is a standard of care in the advanced disease setting.7,8 Furthermore, phase I and II studies of pembrolizumab administered sequentially or concurrently with CCRT have shown promising efficacy for stage III NSCLC9,10,17 that appeared consistent with observations in the PACIFIC trial.2 These studies also indicated that pembrolizumab in combination with or following the completion of CCRT for patients with stage III NSCLC is feasible and tolerable. Therefore, it is hoped that pembrolizumab will be active and well tolerated in combination with CCRT and as a subsequent therapy with or without olaparib in the absence of disease progression in the KEYLYNK-012 trial.
Preclinical models suggest that olaparib is a suitable combination candidate for pembrolizumab.11,12 Olaparib has been established as maintenance therapy in platinum-sensitive ovarian and pancreatic cancer.13 NSCLC has loss of heterozygosity scores as high as those observed in ovarian and pancreatic cancers,18 suggesting similar homologous recombination deficiency and potential for antitumor activity of PARP inhibition in this tumor type.19 Furthermore, the combination of olaparib and pembrolizumab is being investigated in multiple advanced solid tumors, with early-phase results suggesting antitumor activity and a safety profile that is consistent with expectations based on the agents’ individual profiles.14
In conclusion, the KEYLYNK-012 study aims to identify whether the addition of pembrolizumab to CCRT and pembrolizumab with or without olaparib following CCRT potentially will improve outcomes for participants with stage III NSCLC.
Acknowledgments
This study and assistance with manuscript editing were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. We thank the participants and their families and caregivers, all primary investigators and their site personnel; Humberto Lara-Guerra (Merck & Co., Inc., Rahway, NJ, USA) for study support; and Ina Nikolaeva (Merck & Co., Inc., Rahway, NJ, USA) for medical writing assistance.
Disclosure
S.K.J. reports research funding/grants from Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc, Rahway, NJ, USA, consulting fees from MSD, Syntactx, and IMX Medical; B.C.C. reports research funding/grants from Abbvie, AstraZeneca, Bayer, Blueprint Medicines, Champions Oncology, Dizal Pharma, Dong-A ST, Eli Lilly, GI Innovation, Interpark Bio Convergence Corp, Janssen, Medpacto, MOGAM Institute, MSD, Novartis, Ono, Yuhan, royalties/licenses from Champions Oncology, consulting fees from AstraZeneca, Blueprint Medicines, BMS, Boehringer-Ingelheim, Eli Lilly, Janssen, Medpacto, MSD, Novartis, Ono, Pfizer, Roche, Takeda, Yuhan, scientific advisory role for Bridgebio Therapeutics, Cyrus Therapeutics, Guardant Health, Joseah BIO, KANAPH Therapeutic Inc, board membership for Gencurix Inc, Interpark Bio Convergence Corp, founder role for DAAN Biotherapeutics, and stock ownership Bridgebio Therapeutics, Cyrus Therapeutics, Gencurix Inc, KANAPH Therapeutic Inc, TheraCan-Vac Inc; E.B. reports research funding/grants from MSD, speaker honoraria and advisory boards from AstraZeneca, BMS, Eli Lilly, MSD, Pfizer, Roche; T.K. reports research funding/grants from Abbvie, Amgen, AstraZeneca, Blueprint, Chugai, Eli Lilly, Haihe, Merck Serono, MSD, Novartis, Pfizer, Regeneron, Takeda, speaker honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Daiichi-Sankyo, Eli Lilly, Merck Serono, MSD, Novartis, Ono, Pfizer, Roche, advisory board honoraria from Abbvie, Amgen, AstraZeneca, Beigene, Chugai, Daiichi-Sankyo, Eli Lilly, Glaxo, Merck Serono, MSD, Nippon Kayaku, Novartis, Ono, Pfizer, Taiho, Takeda, and employment (spouse) by Eli Lilly; J.F.G. reports research funding/grants from MSD, Adaptimmune, Alexo, Ariad/Takeda, Array Biopharma, AstraZeneca, Blueprint, Bristol-Myers Squibb, Genentech/Roche, Jounce, Merck Serono, Moderna, Novartis, Tesaro, consulting fees from Ariad/Takeda, Agios, Amgen, Array, AstraZeneca, Blueprint, Bristol-Myers Squibb, EMD Serono, Genentech, Gilead Sciences, GlydeBio, Incyte, Loxo/Lilly, Moderna, MSD, Novartis, Oncorus, Pfizer, and Regeneron, honoraria from Ariad/Takeda, AstraZeneca, Blueprint, Bristol-Meyers Squibb, CStone, Genentech, Gilead, GlydeBio, Loxo/Lilly, Moderna, MSD, Novartis, Oncorus, Pfizer, Regeneron, and advisory board for Amgen, BeiGene, iTeos, Pfizer, Sanofi Genzyme, Silverback and Regeneron. N.R. reports consulting fees from Amgen, AstraZeneca, BMS, Boehringer-Ingelheim, Merck & Co., Inc., MSD, Novartis, Pfizer, Roche, Sanofi, Takeda and honoraria from Amgen, AstraZeneca, BMS, Boehringer-Ingelheim, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi, and Takeda. D.M. reports consulting fees from: Abbvie, Arcus, Eli Lilly, and Gilead and was the institutional principal investigator at Abbvie, Arcus, Boehringer-Ingelheim, Bristol-Myers, Eli Lilly, Epicentrx, Incyte, Merck, NeoImmuno Tech, Novartis, Pfizer, and Surface. M.R. reports consulting fees from Amgen, AstraZeneca, BMS, Boehringer-Ingelheim, Beigene, Lilly, Merck & Co., Inc., MSD, Mirati, Novartis, Pfizer, Roche, and Sanofi and honoraria from Amgen, AstraZeneca, BMS, Boehringer-Ingelheim, Beigene, Lilly, Merck & Co., Inc., MSD, Mirati, Novartis, Pfizer, Roche, and Sanofi, and advisory board from Daiichi and Sanofi. J.B. and L.W. report research funding/grants from MSD. Y.S., S.J.K. and F.S. are employees of MSD, and may own stock options in the company.
References
- 1.National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: non-small cell lung cancer (Version 4.2021). [DOI] [PubMed]
- 2.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 3.IMFINZI (durvalumab) Product Information. EMEA/H/C/004771 - IB/0031/G.Last updated July 27, 2021.
- 4.AstraZeneca. IMFINZI (durvalumab): U.S. prescribing information. 2021.
- 5.Curran WJ Jr., Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaverdian N, Offin MD, Rimner A, et al. Utilization and factors precluding the initiation of consolidative durvalumab in unresectable stage III non-small cell lung cancer. Radiother Oncol. 2020;144:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KEYTRUDA (pembrolizumab) Product Information. EMEA/H/C/003820 - II/0090. Last updated March 15, 2021.
- 8.KEYTRUDA (pembrolizumab) for injection, for intravenous use. Whitehouse Station, NJ, USA, Merck Sharp & Dohme Corp., 2020. [Google Scholar]
- 9.Jabbour SK, Berman AT, Decker RH, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: a nonrandomized controlled trial. JAMA Oncol. 2020;6:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage iii non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 2021;7(9):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabanon RM, Muirhead G, Krastev DB, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest. 2019;129:1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23:3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AstraZeneca. Lynparza (olaparib). Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf Accessed: May 2020.
- 14.Yu EY PJ, Gravis G, Fong P, et al. Pembrolizumab (pembro) plus olaparib in patients with docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC): update of KEYNOTE-365 cohort a with a minimum of 11 months of follow-up for all patients. Ann Oncol. 2021;32:S652–S6S3. [Google Scholar]
- 15.Maio MS-F R; Yap TA; Ciuleanu T; et al. Olaparib plus pembrolizumab in patients with previously treated advanced solid tumors with homologous recombination repair mutation (HRRm) and/or homologous recombination deficiency (HRD): Initial results of the phase 2 KEYLYNK-007 study. AACR (Abstr #CT178) 2021.
- 16.Brierley JD GM, Wittekind C. TNM Classification of Malignant Tumours. 8th ed. Hoboken: John Wiley & Sons, Ltd; 2017. [Google Scholar]
- 17.Durm GA, Jabbour SK, Althouse SK, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier cancer research network LUN 14–179. Cancer. 2020;126:4353–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols CA, Gibson WJ, Brown MS, et al. Loss of heterozygosity of essential genes represents a widespread class of potential cancer vulnerabilities. Nat Commun. 2020;11:2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. [DOI] [PubMed] [Google Scholar]