Abstract
Purpose:
To describe the impact of fractionation scheme and tumor location on toxicities in stereotactic body radiation therapy (SBRT) for ≥5-cm non-small cell lung cancer (NSCLC), as part of a multi-institutional analysis.
Methods:
Patients with primary ≥5-cm N0 M0 NSCLC who underwent ≤5-fraction SBRT were examined across multiple high-volume SBRT centers. Collected data included clinical/treatment parameters; toxicities were prospectively assessed at each institution according to the Common Terminology Criteria for Adverse Events. Patients treated daily were compared with those treated every other day (QOD)/other nondaily regimens. Stratification between central and peripheral tumors was also performed.
Results:
Ninety-two patients from 12 institutions were evaluated (2004–2016), with median follow-up of 12 months. In total there were 23 (25%) and 6 (7%) grade ≥2 and grade ≥3 toxicities, respectively. Grades 2 and 3 pulmonary toxicities occurred in 9% and 4%, respectively; 1 patient treated daily experienced grade 5 radiation pneumonitis. Of the entire cohort, 46 patients underwent daily SBRT, and 46 received QOD (n = 40)/other nondaily (n = 6) regimens. Clinical/treatment parameters were similar between groups; the QOD/other group was more likely to receive 3-/4-fraction schemas. Patients treated QOD/other experienced significantly fewer grade ≥2 toxicities as compared with daily treatment (7% vs 43%, P<.001). Patients treated daily also had higher rates of grade ≥2 pulmonary toxicities (P = .014). Patients with peripheral tumors (n = 66) were more likely to receive 3-/4-fraction regimens than those with central tumors (n = 26). No significant differences in grade ≥2 toxicities were identified according to tumor location (P >.05).
Conclusions:
From this multi-institutional study, toxicity of SBRT for ≥5-cm lesions is acceptable, and daily treatment was associated with a higher rate of toxicities.
Summary
There are toxicity concerns from irradiating large (≥5-cm) non-small cell lung cancers with stereotactic body radiation therapy (≤5 fractions). We describe the impact of fractionation scheme and tumor location on toxicities as part of a multi-institutional analysis. Although there was no association with tumor location (central vs peripheral), receipt of daily radiation therapy (as opposed to every other day/other regimens) was associated with a higher rate of toxicity.
Introduction
Stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiation therapy, affords few high-grade treatment morbidities and high local control (1–6). Patients with non-small cell lung cancer (NSCLC) lesions ≥5 cm without evidence of nodal/distant metastases are uncommon (7), but the incidence of such cases is expected to rise with the institution of low-dose computed tomography screening (8–11). However, concern exists regarding toxicities when delivering high ablative radiation therapy doses to large tumor volumes. In the largest report prior to these data, the grade ≥3 toxicity rate was 7.5%, and the overall rate of grade ≥2 pulmonary toxicities was 12.5% (12).
We previously performed a multi-institutional analysis of 92 patients from 12 academic centers examining outcomes (13). Herein, as part of a secondary analysis, we evaluate factors predicting for treatment-related toxicities as a function of 2 major parameters: fractionation schemes (daily vs every other day [QOD]/other nondaily treatment) and tumor location (central vs peripheral).
Methods and Materials
Details of the primary multi-institutional analysis are described elsewhere. Patients had nonmetastatic ≥5-cm primary lung NSCLC and underwent ≤5-fraction SBRT (13). Despite the same patient population (largely owing to the lack of data in this cohort), the original investigation herein is beyond the scope of a general analysis on outcomes and patterns of failure. For the purposes of this analysis, central tumors were defined as those within 2 cm of the proximal bronchial tree or immediately adjacent to mediastinal/pericardial pleura (2). Toxicities were prospectively assigned by the treating physician per Common Terminology Criteria for Adverse Events criteria and retrospectively reviewed.
Statistical analysis (all tests 2-sided) included the Wilcoxon rank-sum and Fisher exact tests to compare population means and proportions between groups, respectively. Owing to the overall uncommon occurrence of toxicities in these small cohorts, multivariate analysis was judged statistically inappropriate by a professional biostatistician. Because toxicity is a time-dependent variable with competing risks (eg, death), actuarial (not crude) rates were utilized, with the endpoint of time to grade ≥2 toxicities, with deaths censored therein (12).
Results
Fractionation schemes
The 92 patients were first separated by those receiving daily (n = 46) versus QOD/other nondaily (n = 46) treatment. In the latter group, 40 of 46 patients (87%) were treated QOD. The remaining 6 patients were treated every third day (n = 1), 4 to 5 fractions given in 12 to 21 days (n = 3), or other substantially different paradigms (n = 2). Clinical/treatment variables were balanced between groups (Table 1). The group treated daily was nearly twice as likely to undergo 5-fraction SBRT, whereas the QOD/other group more often underwent 3- or 4-fraction regimens (P = .003).
Table 1.
Clinical and treatment characteristics of groups based on fractionation
| Parameter | Daily (n = 46) | QOD/other (n = 46) | P |
|---|---|---|---|
| Median age at diagnosis (y) (range) | 73 (50–94) | 72 (51–95) | .810 |
| Ethnicity | .406 | ||
| Caucasian | 35 (76) | 44 (96) | |
| African American | 3 (7) | 1 (2) | |
| Other | 1 (2) | 1 (2) | |
| Unknown | 7 (15) | 0 (0) | |
| Sex | .999 | ||
| Male | 31 (67) | 32 (68) | |
| Female | 15 (33) | 14 (32) | |
| Median smoking history (pack-years) (range) | 65 (0–168) | 50 (0–160) | .153 |
| History of prior malignancy* | .139 | ||
| None | 35 (76) | 29 (63) | |
| NSCLC (early-stage) | 9 (20) | 5 (11) | |
| Head and neck | 1 (2) | 3 (7) | |
| Gastrointestinal | 0 (0) | 4 (9) | |
| Breast | 1 (2) | 2 (4) | |
| Bladder | 0 (0) | 2 (4) | |
| Skin | 1 (2) | 1 (2) | |
| Prostate | 0 (0) | 2 (4) | |
| Other | 0 (0) | 6 (13) | |
| Prior thoracic irradiation | .999 | ||
| Yes | 3 (7) | 2 (4) | |
| No | 43 (93) | 44 (95) | |
| Indication for SBRT | .412 | ||
| Medically inoperable | 42 (91) | 44 (92) | |
| Refused surgery | 4 (9) | 2 (4) | |
| Lobe of lung | .727 | ||
| Right upper | 11 (24) | 16 (35) | |
| Left lower | 13 (28) | 10 (22) | |
| Right lower | 10 (22) | 8 (17) | |
| Left upper | 10 (22) | 8 (17) | |
| Right middle | 2 (4) | 4 (9) | |
| Location | .817 | ||
| Peripheral | 34 (74) | 32 (72) | |
| Central | 12 (26) | 14 (28) | |
| Lesion size | .689 | ||
| Median (cm) (range) | 5.4 (5.0–7.5) | 5.5 (5.0–7.5) | |
| Histology | .399 | ||
| Squamous cell carcinoma | 19 (41) | 26 (57) | |
| Adenocarcinoma | 13 (28) | 15 (33) | |
| NSCLC, not otherwise specified | 6 (13) | 2 (4) | |
| Large cell carcinoma | 1 (2) | 1 (1) | |
| Mixed adenosquamous | 1 (2) | 0 (0) | |
| No biopsy | 6 (13) | 2 (4) | |
| AJCC clinical T stage | .841 | ||
| T2a | 5 (11) | 5 (11) | |
| T2b | 40 (87) | 39 (86) | |
| T3 | 1 (2) | 2 (4) | |
| Median SUVmax on pre-SBRT PET (range) | 11.5 (2.0–29.6) | 9.4 (3.0–20.3) | .078 |
| Mediastinal nodal sampling | .065 | ||
| Performed | 21 (46) | 11 (24) | |
| Not performed | 25 (54) | 34 (74) | |
| Unknown | 0 (0) | 1 (2) | |
| ECOG performance status at diagnosis | .815 | ||
| 0 | 6 (13) | 4 (9) | |
| 1 | 25 (54) | 24 (52) | |
| 2 | 13 (28) | 15 (33) | |
| 3 | 2 (4) | 2 (4) | |
| Unknown | 0 (0) | 1 (2) | |
| SBRT dose and fractionation | .003† | ||
| 50 Gy in 5 fractions | 28 (61) | 15 (33) | |
| 48 Gy in 4 fractions | 4 (9) | 17 (37) | |
| 54 Gy in 3 fractions | 3 (7) | 8 (17) | |
| 60 Gy in 5 fractions | 5 (11) | 2 (4) | |
| 55 Gy in 5 fractions | 2 (4) | 1 (2) | |
| Other | 4 (9) | 3 (7) | |
| Total SBRT dose | .066 | ||
| Median (Gy) (range) | 50 (40–60) | 50 (36–60)‡ | |
| Biologically effective dose§ | .211 | ||
| Median (Gy) (range) | 100 (72–151.2) | 105.6 (72–151.2) | |
| SBRT technique | .149 | ||
| Fixed-beam 3D | 13 (28) | 21 (46) | |
| Fixed-beam IMRT | 11 (24) | 14 (30) | |
| Dynamic arcs | 3 (7) | 0 (0) | |
| VMAT | 14 (30) | 11 (24) | |
| Unknown | 5 (11) | 0 (0) | |
| Image guidance | .145 | ||
| Kilovoltage (cone-beam) CT | 37 (80) | 30 (65) | |
| Orthogonal X rays | 9 (20) | 9 (20) | |
| Megavoltage CT | 0 (0) | 7 (8) | |
| Receipt of chemotherapy | .612 | ||
| Yes | 1 (2) | 3 (7) | |
| No | 45 (98) | 43 (93) | |
| Primary tumor responseǁ | .407 | ||
| Complete response | 1 (2) | 2 (4) | |
| Partial response | 25 (54) | 25 (54) | |
| Stable | 16 (35) | 10 (22) | |
| Progression | 1 (2) | 1 (2) | |
| Unknown | 3 (7) | 8 (17) |
Abbreviations: AJCC = American Joint Committee on Cancer; CT = computed tomography; ECOG = Eastern Cooperative Oncology Group; IMRT = intensity modulated radiation therapy; NSCLC = non-small cell lung cancer; PET = positron emission tomography; QOD = every other day; SBRT = stereotactic body radiation therapy; SUV = standard uptake value; VMAT = volumetric modulated arc therapy.
Values are number (percentage) unless otherwise noted.
Values do not add up to 100% because of patients with synchronous/metachronous neoplasms.
Statistically significant P value.
36 Gy was delivered to a patient of a planned 48 Gy; the patient did not complete treatment.
Assuming α/β ratio of 10.
As per Response Evaluation Criteria in Solid Tumors.
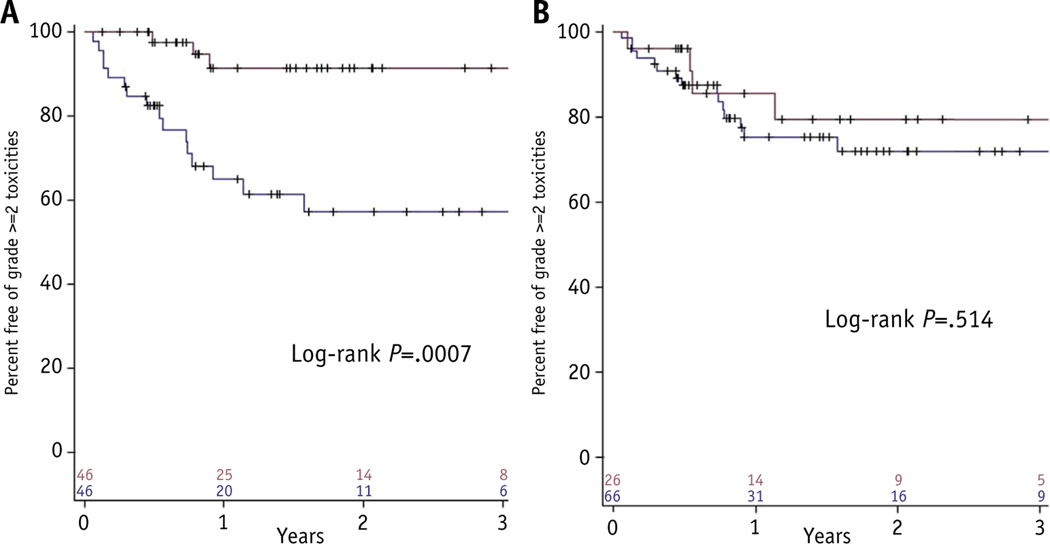
Table 2 displays toxicities in both groups. Patients treated QOD/other experienced 3 (7%) grade ≥2 toxicities, as compared with 20 (43%) in the daily treatment group (P<.001). When specifically examining grade ≥2 pulmonary adverse events, rates were similarly lower in the QOD/other cohort (4% vs 24%, P = .014). Toxicities of any grade were also fewer in the QOD/other group (P<.001). Kaplan-Meier analysis of freedom from grade ≥2 toxicities (Fig. 1A) shows differences in favor of the QOD/other group (P<.001).
Table 2.
Toxicity profiles of groups based on fractionation
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Daily treatment (n = 46) | |||||
| Pulmonary | 4 | 7 | 3 | 0 | 1 |
| RP | 1 | 4 | 3 | 0 | 1 |
| Cough/SOB | 3 | 2 | 0 | 0 | 0 |
| Pleural effusion | 0 | 1 | 0 | 0 | 0 |
| CW pain | 1 | 7 | 0 | 0 | 0 |
| Dermatitis | 3 | 1 | 0 | 0 | 0 |
| Rib fracture | 2 | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 1 | 0 | 0 | 0 |
| Anorexia | 1 | 0 | 0 | 0 | 0 |
| Total | 11 | 16 | 3 | 0 | 1 |
| QOD/other treatment (n = 46) | |||||
| Pulmonary | 5 | 1 | 1 | 0 | 0 |
| RP | 3 | 1 | 1 | 0 | 0 |
| Cough/SOB | 2 | 0 | 0 | 0 | 0 |
| Pleural effusion | 0 | 0 | 0 | 0 | 0 |
| CW pain | 1 | 0 | 0 | 0 | 0 |
| Dermatitis | 0 | 0 | 1 | 0 | 0 |
| Rib fracture | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 2 | 0 | 0 | 0 | 0 |
| Anorexia | 0 | 0 | 0 | 0 | 0 |
| Total | 8 | 1 | 2 | 0 | 0 |
Abbreviations: CW = chest wall; QOD = every other day; RP = radiation pneumonitis; SOB = shortness of breath.
Numbers indicate instances of a given toxicity.
Fig. 1.
Kaplan-Meier plot of freedom from grade ≥2 toxicities as stratified for treatment timing (A) and tumor location (B).
There were no differences in outcomes or failure patterns between groups. Median overall survival was 17 months (range, 4–123 months) in the daily group and 18 months (range, 2–69 months) in the QOD/other cohort (P = .91).
Location
Toxicity was next assessed for central (n = 26) as compared with peripheral tumors (n = 66). Table 3 demonstrates balanced clinical/treatment parameters, although 3- and 4-fraction regimens were more frequently administered to peripheral lesions (P = .048).
Table 3.
Clinical and treatment characteristics of groups based on location
| Parameter | Central (n = 26) | Peripheral (n = 66) | P |
|---|---|---|---|
| Median age at diagnosis (y) (range) | 71 (50–95) | 74 (51–93) | .246 |
| Ethnicity | .423 | ||
| Caucasian | 20 (77) | 59 (89) | |
| African American | 2 (8) | 2 (3) | |
| Other | 1 (4) | 1 (2) | |
| Unknown | 3 (12) | 4 (6) | |
| Sex | .213 | ||
| Male | 15 (58) | 48 (73) | |
| Female | 11 (42) | 18 (27) | |
| Median smoking history (pack-years) (range) | 60 (20–160) | 56.5 (0–168) | .453 |
| History of prior malignancy* | .973 | ||
| None | 19 (73) | 45 (68) | |
| NSCLC (early-stage) | 4 (15) | 10 (15) | |
| Head and neck | 1 (4) | 3 (5) | |
| Gastrointestinal | 1 (4) | 3 (5) | |
| Breast | 1 (4) | 2 (3) | |
| Bladder | 1 (4) | 1 (2) | |
| Skin | 0 (0) | 2 (3) | |
| Prostate | 1 (4) | 1 (2) | |
| Other | 1 (4) | 5 (8) | |
| Prior thoracic irradiation | .999 | ||
| Yes | 1 (4) | 4 (6) | |
| No | 25 (96) | 62 (94) | |
| Indication for SBRT | .665 | ||
| Medically inoperable | 23 (88) | 62 (94) | |
| Refused surgery | 2 (8) | 4 (6) | |
| Unknown | 1 (4) | 0 (0) | |
| Lobe of lung | .263 | ||
| Right upper | 9 (35) | 18 (27) | |
| Left lower | 9 (35) | 14 (21) | |
| Right lower | 5 (19) | 13 (20) | |
| Left upper | 3 (12) | 15 (23) | |
| Right middle | 0 (0) | 6 (9) | |
| Lesion size | .103 | ||
| Median (cm) (range) | 5.4 (5.0–6.7) | 5.5 (5.0–7.5) | |
| Histology | .426 | ||
| Squamous cell carcinoma | 12 (46) | 33 (50) | |
| Adenocarcinoma | 9 (35) | 19 (29) | |
| NSCLC, not otherwise specified | 2 (8) | 6 (9) | |
| Large cell carcinoma | 1 (4) | 1 (2) | |
| Mixed adenosquamous | 1 (4) | 0 (0) | |
| No biopsy | 1 (4) | 7 (11) | |
| AJCC clinical T stage | .163 | ||
| T2a | 5 (19) | 5 (8) | |
| T2b | 21 (81) | 58 (87) | |
| T3 | 0 (0) | 3 (5) | |
| Median SUVmax on pre-SBRT PET (range) | 10.4 (4.0–29.6) | 10.5 (2.0–27.3) | .749 |
| Mediastinal nodal sampling | .634 | ||
| Performed | 8 (31) | 24 (36) | |
| Not performed | 18 (69) | 41 (62) | |
| Unknown | 0 (0) | 1 (2) | |
| ECOG performance status at diagnosis | .234 | ||
| 0 | 5 (19) | 5 (8) | |
| 1 | 11 (42) | 38 (58) | |
| 2 | 7 (27) | 21 (32) | |
| 3 | 2 (8) | 2 (3) | |
| Unknown | 1 (4) | 0 (0) | |
| SBRT dose and fractionation | .048† | ||
| 50 Gy in 5 fractions | 17 (65) | 26 (39) | |
| 48 Gy in 4 fractions | 3 (12) | 18 (27) | |
| 54 Gy in 3 fractions | 1 (4) | 10 (15) | |
| 60 Gy in 5 fractions | 1 (4) | 6 (9) | |
| 55 Gy in 5 fractions | 2 (8) | 1 (2) | |
| Other | 2 (8) | 5 (8) | |
| Total SBRT dose | .897 | ||
| Median (Gy) (range) | 50 (40–60) | 50 (36–60)‡ | |
| Biologically effective dose§ | .190 | ||
| Median (Gy) (range) | 100 (80–151.2) | 105.6 (72–151.2) | |
| SBRT technique | .760 | ||
| Fixed-beam 3D | 8 (31) | 26 (39) | |
| Fixed-beam IMRT | 9 (35) | 16 (24) | |
| Dynamic arcs | 1 (4) | 2 (3) | |
| VMAT | 8 (31) | 17 (26) | |
| Unknown | 0 (0) | 5 (8) | |
| Image guidance | .471 | ||
| Kilovoltage (cone-beam) CT | 21 (81) | 46 (69) | |
| Orthogonal X rays | 3 (12) | 15 (23) | |
| Megavoltage CT | 2 (8) | 5 (8) | |
| SBRT schedule | .817 | ||
| Daily | 12 (46) | 34 (52) | |
| Every other day/other | 14 (54) | 32 (48) | |
| Receipt of chemotherapy | .316 | ||
| Yes | 2 (8) | 2 (3) | |
| No | 24 (92) | 64 (96) | |
| Primary tumor responseǁ | .871 | ||
| Complete response | 1 (4) | 2 (3) | |
| Partial response | 13 (50) | 37 (56) | |
| Stable | 8 (31) | 18 (27) | |
| Progression | 1 (4) | 1 (2) | |
| Unknown | 3 (12) | 8 (12) |
Abbreviations as in Table 1.
Values are number (percentage) unless otherwise noted.
Values do not add up to 100% owing to patients with synchronous/metachronous neoplasms.
Statistically significant P value.
36 Gy was delivered to a patient of a planned 48 Gy; the patient did not complete treatment.
Assuming α/β ratio of 10.
As per Response Evaluation Criteria in Solid Tumors.
Table 4 illustrates toxicities; there were no differences in grade ≥2 toxicities (P = .285) and grade ≥2 pulmonary adverse events (P = .334). When examining any toxicities, there were fewer in the central cohort (P = .003), with all cases of dermatitis (n = 5), fatigue (n = 3), rib fracture (n = 2), and anorexia (n = 1) observed in the peripheral location cohort. Kaplan-Meier analysis of freedom from grade ≥2 toxicities (Fig. 1B) revealed no significant differences (P = .51).
Table 4.
Toxicity profiles of groups based on location
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Peripheral (n = 66) | |||||
| Pulmonary | 7 | 7 | 3 | 0 | 1 |
| RP | 3 | 4 | 3 | 0 | 1 |
| Cough/SOB | 4 | 2 | 0 | 0 | 0 |
| Pleural effusion | 0 | 1 | 0 | 0 | 0 |
| CW pain | 1 | 5 | 0 | 0 | 0 |
| Dermatitis | 3 | 1 | 1 | 0 | 0 |
| Rib fracture | 2 | 0 | 0 | 0 | 0 |
| Fatigue | 2 | 1 | 0 | 0 | 0 |
| Anorexia | 1 | 0 | 0 | 0 | 0 |
| Total | 16 | 14 | 4 | 0 | 1 |
| Central (n = 26) | |||||
| Pulmonary | 2 | 1 | 1 | 0 | 0 |
| RP | 1 | 1 | 1 | 0 | 0 |
| Cough/SOB | 1 | 0 | 0 | 0 | 0 |
| Pleural effusion | 0 | 0 | 0 | 0 | 0 |
| CW pain | 1 | 2 | 0 | 0 | 0 |
| Dermatitis | 0 | 0 | 0 | 0 | 0 |
| Rib fracture | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | 0 | 0 |
| Anorexia | 0 | 0 | 0 | 0 | 0 |
| Total | 3 | 3 | 1 | 0 | 0 |
Abbreviations as in Table 2.
Numbers indicate instances of a given toxicity.
There were no differences in outcomes or failure patterns. The median overall survival was 18 months (range, 2–58 months) and 17 months (range, 4–123 months) in central and peripheral lesions, respectively (P = .91).
Discussion
Although administering high fractional doses to large treatment volumes may lead to toxicity concerns given the existing limited available data, we demonstrate that SBRT to appropriately selected tumors ≥5 cm can generally be delivered safely; there may be measures to reduce toxicities, such as spacing of treatments to every other day. Doing so may allow interfractional normal tissue repair, consistent with radiobiological principles (14). Emerging data even suggest improvements in local control when spacing treatments, potentially from reoxygenation effects (15). In fact, QOD SBRT treatments are often performed in recurrent head/neck cancers to minimize the risk of carotid blowout and other complications (16). Every other day spacing in prostate cancer SBRT also produces fewer gastrointestinal/genitourinary toxicities (17). Herein, despite more patients in the QOD/other group receiving 3- or 4-fraction treatments, we observed fewer toxicities in this cohort—including just 1 case each of grade 2 and 3 radiation pneumonitis in 46 patients. These data are consistent with the Cleveland Clinic series, which reported an 8% rate of grade ≥3 toxicities (12).
We did not find a difference in toxicity rates between central and peripheral cohorts. Several factors may explain this. First, the vast majority of the central cohort received 5 fractions (lower doses per fraction). Next, although unproven, more stringent treatment planning constraints may have been used for centrally located tumors, potentially consistent with other data showing similar results as ours (12). Although there were many planning techniques utilized, the sample sizes were too small to stratify according to technique to assess effects; however, dosimetry is likely a more robust parameter than planning technique alone. Last, central lesions generally abut the chest wall less than peripheral lesions, leading potentially to reduced risks of chest wall, rib, and skin toxicities.
This study is not without limitations. In any retrospective study, there is little standardization over receipt of particular treatment regimens, such as specific doses/fractionation or SBRT, and a comparison with hypofractionated regimens of >5 fractions is not possible. Limitations of any multicenter analysis include heterogeneity in workup (eg, pathologic nodal staging) and treatment (eg, target volume margins, breathing control, planning constraints). Next, availability of pulmonary function tests and dosimetry was also limited. Similarly, differences in choice of daily image-guidance modality were seen between centers, which may be clinically significant, especially for larger tumors (18). Additionally, biases in recording/reporting toxicities are always a concern, especially grade 1 events such as pneumonitis. We also combined acute and chronic toxicities, similar to other publications (12). Furthermore, despite our inclusion of 13 patients with prior thoracic irradiation or lobectomy/pneumonectomy, just 1 suffered grade ≥2 toxicity (chest wall pain). Next, multivariate analysis to firmly establish a correlation between treatment regimens and toxicities was determined by a professional biostatistician to be statistically unfeasible, owing to the overall low incidences of toxicities, consistent with other work (17). As such, we cannot rule out a correlation between factors such as tumor location, fractionation regimens, and timing of fractions. Nevertheless, groups were well-balanced between many parameters. Next, 6 patients in the QOD/other group received “other” treatment regimens, limiting generalizability/applicability. Lastly, the definition of “large” NSCLC lesions has been set at 5 cm in this and other studies, but this refers to just the greatest tumor dimension; dimensions of other axes are also important, because toxicities likely correlate with treated volumes and not just the greatest dimension.
Conclusions
According to this multi-institutional experience, the largest to date, SBRT is an appropriate treatment option for appropriately selected ≥5-cm node-negative NSCLC. However, to reduce potential toxicities, spacing out treatment to at least QOD may be advantageous.
Footnotes
Conflict of interest: none.
References
- 1.National Comprehensive Cancer Network. Non-small cell lung cancer. Version 1. Available at: www.nccn.org/professionals/physician_gls/PDF/nsclc.pdf. Accessed March 24, 2016.
- 2.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303: 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simone CB 2nd, Wildt B, Haas AR, et al. Stereotactic body radiation therapy for lung cancer. Chest 2013;143:1784–1790. [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol 2015;16:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma V Stereotactic radiotherapy versus surgery for early-stage operable lung cancer: More questions than answers. J Natl Compr Canc Netw 2015;13:1293–1295. [DOI] [PubMed] [Google Scholar]
- 6.Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: Surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma V, McMillan MT, Grover S, Simone CB 2nd. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;97:146–154. [DOI] [PubMed] [Google Scholar]
- 8.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma V Lung cancer: Implementing lung-cancer screeningd oncological ‘grey areas’. Nat Rev Clin Oncol 2015;12:256–257. [DOI] [PubMed] [Google Scholar]
- 10.Verma V, Zhen W. Treatment costs of early-stage lung cancers detected by low-dose computed tomography screening. Int J Radiat Oncol Biol Phys 2015;93:207–208. [DOI] [PubMed] [Google Scholar]
- 11.Verma V, Beriwal S. Medicare approves coverage for lung cancer screening: The case for symptomatic screening. JAMA Oncol 2015;1: 1027–1028. [DOI] [PubMed] [Google Scholar]
- 12.Woody NM, Stephans KL, Marwaha G, et al. Stereotactic body radiation therapy for non-small cell lung cancer tumors greater than 5 cm: Safety and efficacy. Int J Radiat Oncol Biol Phys 2015;92:325–331. [DOI] [PubMed] [Google Scholar]
- 13.Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer. 2016. Oct 14. 10.1002/cncr.30375. [DOI] [PMC free article] [PubMed]
- 14.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 R’s involved? Int J Radiat Oncol Biol Phys 2014;88:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alite F, Stang K, Balasubramanian N, et al. Local control dependence on consecutive vs. nonconsecutive fractionation in lung stereotactic body radiation therapy. Radiother Oncol 2016;121:9–14. [DOI] [PubMed] [Google Scholar]
- 16.Yazici G, Sanli TY, Cengiz M, et al. A simple strategy to decrease fatal carotid blowout syndrome after stereotactic body reirradiation for recurrent head and neck cancers. Radiat Oncol 2013;8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877–882. [DOI] [PubMed] [Google Scholar]
- 18.Corradetti MN, Mitra N, Bonner Millar LP, et al. A moving target: Image guidance for stereotactic body radiation therapy for early-stage non-small cell lung cancer. Pract Radiat Oncol 2013;3:307–315. [DOI] [PubMed] [Google Scholar]