Abstract
INTRODUCTION
The prevalence of poor sleep quality and sleep apnea differs by race and ethnicity and may contribute to racial disparities in cognitive aging. We investigated whether sleep quality and sleep apnea risk were associated with cognitive function and decline and whether the associations differed by race/ethnicity.
METHODS
Participants from the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE; N = 1690; mean age: 75.7 years) study, a cohort of Asian, Black, Latino, and White participants, completed a modified Pittsburgh Sleep Quality Index assessing subjective sleep quality, latency, duration, disturbances, sleep medication use, and daytime dysfunction. Sleep apnea risk was measured by questions about snoring, tiredness, and whether apnea was observed. Executive function and verbal episodic memory were assessed at three time points over an average of 2.7 years with the Spanish and English Neuropsychological Assessment Scale. We fit linear mixed‐effect models and stratified analyses by race/ethnicity.
RESULTS
Higher sleep apnea risk was associated with faster declines in verbal episodic memory ( sleep apnea = −0.02, 95% confidence interval [CI], −0.04, −0.001) but not in executive function. Poorer sleep quality was associated with lower levels of and faster decline in executive function but not in verbal episodic memory. Race/ethnicity modified these associations: compared to estimated effects among White participants, poorer global sleep quality ( sleep*time = −0.02, 95% CI, −0.02, −0.01) was associated with larger effects on decline in executive function among Black participants. Estimated effects of some individual sleep quality components were also modified by race/ethnicity; for example, sleep medication use was associated with faster declines in executive function ( sleep*time = −0.05, 95% CI, −0.07, −0.03) and verbal episodic memory sleep*time = −0.04, 95% CI, −0.07, −0.02) among Black participants compared to White participants.
DISCUSSION
Observational evidence indicates sleep quality is a promising target for addressing racial/ethnic disparities in cognitive aging, especially among Black older adults.
Highlights
Sleep apnea risk was associated with faster declines in verbal episodic memory but not executive function among all participants.
Global sleep quality was associated with lower levels of and faster decline in executive function but not verbal episodic memory among all participants.
Black older adults were particularly susceptible to the estimated adverse cognitive impacts of global sleep quality, particularly the use of sleep medication.
Keywords: cognitive decline, cognitive function, race, sleep apnea, sleep quality
1. INTRODUCTION
Disparities in late‐life cognitive health by race and ethnicity status are well documented, with most research showing that non‐Hispanic Black and Hispanic older adults have higher risk of Alzheimer's disease and related dementias (ADRD) than non‐Hispanic White and Asian older adults. 1 , 2 , 3 Socioeconomic and health‐related inequalities resulting from interlocking historical and contemporary systems of racial discrimination, such as education, psychosocial stressors, and cardiovascular diseases drive these disparities, but proximal mechanisms for the inequities remain unexplained. 2 , 3 , 4 Evaluating factors that could be modified in old age, such as sleep quality and apnea, may offer avenues to reduce dementia disparities in the short term.
Sleep is a modifiable factor that plausibly influences late‐life cognitive functioning and disparities by race and ethnicity. Sleep problems, including poor sleep quality and obstructive sleep apnea, are especially common among older adults, with ≈ 20% reporting poor sleep quality and > 50% reporting obstructive sleep apnea. 5 , 6 , 7 Prior research has documented heterogeneity in sleep quality and apnea across different races and ethnicities. 8 For example, compared to White older adults, Black, Latino, and Asian American older adults have higher prevalence of sleep apnea syndrome. 8 , 9 , 10 , 11 Moreover, Black older adults report shorter sleep duration, poorer sleep quality, and more daytime sleepiness than White older adults. 8 , 9 Given the heterogeneity in sleep quality and apnea by racial and ethnic identity, the impact of sleep on cognitive function and decline may also differ by these factors. Nevertheless, limited research has investigated race differences in the associations of sleep quality and apnea with cognitive function.
Using a racially and ethnically diverse cohort, this study aimed to investigate the associations of sleep quality and sleep apnea risk with cognitive function and decline and to examine the heterogeneity in these associations by race and ethnicity. We hypothesized that higher risk of sleep apnea and poorer sleep quality are associated with lower levels of and greater decline in executive function and verbal episodic memory scores and that these associations are stronger among Black, Latino, and Asian older adults than among White older adults.
2. METHODS
2.1. Data
The Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) study is a prospective cohort of community‐dwelling older adults residing in the San Francisco Bay and Sacramento areas of California. 4 , 9 Individuals eligible for KHANDLE were long‐term Kaiser Permanente Northern California (KPNC) members; age ≥ 65 years on January 1, 2017; spoke English or Spanish; and had previously participated in Kaiser Permanente multiphasic health checkup (MHC) exams between 1964 and 1985. Participants were ineligible for enrollment if they had: (1) an electronic medical record diagnosis of dementia or other neurodegenerative diseases, (2) health conditions that would impede participation in study interviews in the past 12 months, or (3) a history of severe chronic obstructive pulmonary disease or congestive heart failure hospitalizations in the past 6 months at the time of their baseline interview.
The KHANDLE study enrolled 1712 older participants, but our analyses were restricted to 1690 persons with measures of executive function and verbal episodic memory at baseline. Of these participants, 293 were lost to follow‐up at wave 2, and 127 were lost to follow‐up at wave 3 (see Figure S1 in supporting information for sample selection flowchart). Over a mean follow‐up of 2.7 years (standard deviation [SD] = 0.74), participants in our analyses contributed 5070 observations. Participants had completed nearly two waves of in‐person interviews when the COVID‐19 pandemic began. The third wave was conducted via phone for participant safety and to comply with pandemic restrictions. This study was approved by the KPNC Institutional Review Board, and all participants provided informed consent.
2.2. Measures
Racial/ethnic identity was self‐reported and categorized as non‐Hispanic Asian, non‐Hispanic Black, Latino, or non‐Hispanic White. We conceptualized racial and ethnic identities as relevant due to the historical and contemporary patterning of social experiences by these dimensions of identities, including the consequences of structural racism and discrimination.
Sleep quality was assessed at baseline by a modified version of the well‐validated Pittsburgh Sleep Quality Index (PSQI), a self‐reported measure designed to assess sleep quality and disturbances over the past month. 12 The modified version of the PSQI assessed six components of sleep, including subjective sleep quality, latency, duration, disturbances, sleep medication use, and daytime dysfunction. Each domain is scored on a scale of 0 to 3, and we created a global composite score for overall sleep quality (range 0–18) by summing the scores of the six components, with a higher score indicating poorer sleep quality (see Table S1 in supporting information for detailed information of the PSQI items and the scoring of each sleep quality domain). To reduce respondent burden, KHANDLE did not assess sleep efficiency. As a result, the cut‐off for “poor sleep quality” based on all components of the original PSQI was not applicable. The PSQI has been widely used in both research and clinical settings, and it has been validated in older adult populations and across racially and ethnically diverse populations. 13 , 14 , 15 , 16
RESEARCH IN CONTEXT
Systematic review: Using PubMed, we reviewed the literature on sleep apnea, sleep quality, and cognitive functioning, as well as the literature on racial/ethnic differences in these associations. Very few studies have examined the heterogeneity in how sleep parameters relate to cognitive outcomes by race and ethnicity.
Interpretation: Sleep apnea risk was associated with faster declines in verbal episodic memory. Poorer global sleep quality was associated with lower levels of and faster decline in executive function. When compared to associations among White older adults, the associations of global sleep quality with cognitive decline were more pronounced among Black older adults.
Future directions: Future research should confirm and extend our results by incorporating objective sleep measures, quasi‐experimental approaches, and additional cognitive outcomes in diverse samples.
Sleep apnea risk was assessed at baseline by three questions derived from the STOP‐BANG (snoring/tiredness/observed apnea/pressure/body mass index/age/neck circumference/gender) measure asking the participants whether they: (1) snore loudly; (2) often felt tired, fatigued, or sleepy during daytime; or (3) had ever been observed not breathing during sleep. We excluded questions such as high blood pressure from the STOP‐BANG measure while creating the score for sleep apnea risk. As high blood pressure is strongly associated with cognitive function, including it would make it difficult to accurately evaluate the role of sleep apnea in cognitive function. All questions were with a binary response (yes/no). We summed the scores of the three items to obtain a score for sleep apnea risk (range 0–3).
Cognitive function included executive function and verbal episodic memory that were assessed at each assessment by the Spanish and English Neuropsychological Assessment Scales (SENAS). 17 The SENAS is a battery of cognitive tests that has previously undergone extensive development for valid comparisons of cognitive change across racial/ethnic and linguistically diverse groups. Executive function composite scores were obtained using component tasks of category fluency, phonemic (letter) fluency, and working memory (digit span backward, visual span backward, list sorting). Verbal episodic memory composite scores were derived from a multitrial word‐list‐learning test. The cognitive function assessments remained unchanged throughout the study, but the mode of interview administration transitioned from in person to phone before and during the pandemic. We z standardized executive function and verbal episodic memory scores at each wave to baseline mean and SD.
All models adjusted for interview mode (a time‐updated variable indicating whether the interview was conducted in person vs. over the phone), baseline age (centered at mean baseline age), sex/gender (men vs. women), and race and ethnicity (Asian, Black, Latino, and White). A second set of covariates additionally adjusted for educational attainment and household income. Educational attainment was assessed by asking participants the highest degree or last grade in school they completed and was categorized into four groups: (1) high school or lower, (2) some college but no college credential, (3) associate degree, and (4) bachelor's degree or more. Household income was self‐reported in 13 categories and converted to a continuous variable using the lowest value for each non‐missing category.
2.3. Statistical analysis
We performed separate analyses for executive function and verbal episodic memory with sleep apnea risk and the composite global PSQI sleep quality score. The correlations among the sleep components used to create the PSQI score are weak to moderate, ranging from 0.02 to 0.44 (see Figure S2 in supporting information). Therefore, we also examined the associations of individual domains of PSQI component with executive function and verbal episodic memory separately. We fit linear mixed‐effects random intercept models with current age (in years) as the time scale to estimate the mean difference in performance on cognitive test per unit difference in levels of each sleep parameter. The coefficient for sleep in these models estimated the associations of sleep with average level of cognitive function across all waves.
We then assessed the associations between sleep apnea risk and sleep quality and rate of cognitive decline by fitting linear mixed‐effects models with random intercepts. These models used time since baseline (in years) as the time scale and included the cross‐product of sleep and time as well as the cross‐products of covariates and time. Our primary interest, the cross‐product of sleep and time, can be interpreted as the difference in annual rate of cognitive decline across levels of the sleep parameter. For each sleep quality domain and cognitive outcome, we sequentially adjusted for the two sets of covariates as outlined in the previous section. All models restricted the possible range of effect estimates to correct for practice effects based on prior work in KHANDLE. 18 To facilitate the interpretation of findings, we compared associations of sleep apnea risk and sleep quality with cognitive function to the estimated age‐related differences in cognitive function.
We evaluated heterogeneity in the associations of sleep apnea risk and sleep quality with level and decline in cognitive function, by stratifying the analyses by race and ethnicity. Because exposure–outcome confounders (e.g., education and income) are likely influenced by race and ethnicity, instead of testing race/ethnic differences using interaction terms, we computed the differences by subtracting the estimates of interests (e.g., sleep x time for cognitive decline) obtained from each subsample in stratified models to estimates derived from the comparison group. In this analysis, we selected White participants as the comparison group for two reasons: (1) we are interested in understanding whether sleep is a compelling target for reducing racial disparities in cognitive aging and (2) most prior research in this area has used predominantly White samples and we would like to evaluate whether results are likely to generalize to other racial/ethnic groups. Confidence intervals for these differences were calculated through bootstrapping with 1000 iterations.
In our analytical sample, 569 (34%) individuals had either missing exposure or covariates (see Table S2 in supporting information for details about the missingness of each exposure and covariate). Compared to those who had complete information, those with missing exposure and covariates were more likely to be older, with lower income and education, report poorer sleep quality, and identify as Black or Latino. Individuals excluded due to missing data also had averaged worse executive function or verbal episodic memory (see Table S3 in supporting information). We imputed missing exposures and covariates using multivariate imputation by chained equations (MICE) implemented in the R package “mice.” We generated 35 imputed datasets and combined the point estimates and standard errors using Rubin's rule. We imputed continuous variables using predictive mean matching, binary variables using logistic regression, and categorial variables using polytomous logistic regression. We included all analytical variables in the imputation models. Additionally, the risk of attrition in our sample increased with lower baseline cognitive function, poorer sleep quality, and higher risk of obstructive sleep apnea (see Table S4 in supporting information). Therefore, we calculated the inverse probability of continuation weights (IPCW) to account for differential attrition.
In additional analyses, we examined the potential non‐linear associations of sleep duration (i.e., the actual hours of sleep participants reported [treated as a continuous score]) with levels of cognitive function by including a quadratic term for sleep duration in the linear mixed‐effects models. As sleep medication use may confound the relationship between sleep quality and cognitive function, we conducted a sensitivity analysis to treat sleep medication as a covariate instead of a component of the PSQI. All analyses were performed using R version 4.2.1.
3. RESULTS
3.1. Baseline characteristics of the full sample and by race and ethnicity
Mean age of the 1690 older adults at baseline was 76.0 years (SD = 6.77), 59.4% were women, 48.2% had a college degree or higher, and 59.2% had central adiposity (Table 1). Black older adults reported the worse sleep quality and sleep apnea compared to Asian, Latino, or White respondents. White older adults reported better sleep quality than Black, Latino, or Asian participants, but were the most likely to use sleep medication.
TABLE 1.
Characteristics of Kaiser Healthy Aging and Diverse Life Experiences participants, stratified by racial and ethnic identity (N = 1690).
| Racial and ethnic identity | |||||
|---|---|---|---|---|---|
| All participants | Asian participants | Black Participants | Latino Participants | White participants | |
| N = 1690 | N = 413 | N = 435 | N = 345 | N = 496 | |
| Age (years), mean (SD) | 76.0 (6.77) | 75.6 (6.63) | 75.4 (6.60) | 76.0 (6.43) | 76.9 (7.20) |
| Sex/gender, N (%) | |||||
| Women | 1004 (59.4) | 220 (53.3) | 294 (67.6) | 203 (58.8) | 287 (57.9) |
| Men | 686 (40.6) | 193 (46.7) | 141 (32.4) | 142 (41.2) | 209 (42.1) |
| Income in thousands of dollars, mean (SD) | 88.7 (46.7) | 103.0 (47.5) | 81.1 (45.6) | 76.0 (42.9) | 92.4 (45.9) |
| Education, N (%) | |||||
| High school or less | 353 (20.9) | 49 (11.9) | 96 (22.1) | 123 (35.7) | 85 (17.1) |
| Some college but no degree | 334 (19.8) | 54 (13.1) | 123 (28.3) | 73 (21.2) | 84 (16.9) |
| Associate degree | 181 (10.7) | 35 (8.5) | 65 (14.9) | 36 (10.4) | 44 (8.9) |
| Bachelor's degree | 814 (48.2) | 273 (66.1) | 151 (34.7) | 110 (31.9) | 280 (56.5) |
| a Sleep apnea risk (0–3), mean (SD) | 1.00 (0.89) | 1.00 (0.91) | 1.05 (0.89) | 0.99 (0.89) | 0.97 (0.87) |
| a Global sleep quality (0–18), mean (SD) | 4.69 (3.13) | 4.53 (3.05) | 5.34 (3.42) | 4.45 (3.02) | 4.43 (2.93) |
| Sleep latency (0–3) | 1.14 (1.13) | 1.14 (1.10) | 1.28 (1.21) | 1.12 (1.13) | 1.05 (1.08) |
| Sleep duration (0–3) | 0.76 (0.96) | 0.85 (0.96) | 1.06 (1.05) | 0.69 (0.95) | 0.45 (0.80) |
| Sleep disturbance (0–3) | 1.07 (0.75) | 0.98 (0.73) | 1.14 (0.80) | 1.03 (0.75) | 1.12 (0.71) |
| Sleep medication use (0–3) | 0.60 (1.09) | 0.45 (0.98) | 0.59 (1.08) | 0.53 (1.05) | 0.78 (1.19) |
| Daytime sleep dysfunction (0–3) | 0.17 (0.53) | 0.17 (0.53) | 0.22 (0.62) | 0.18 (0.57) | 0.11 (0.40) |
| Subjective sleep quality (0–3) | 0.97 (0.71) | 0.94 (0.68) | 1.07 (0.82) | 0.95 (0.70) | 0.92 (0.64) |
| Average sleep duration (hours), mean (SD) | 6.59 (1.36) | 6.43 (1.32) | 6.18 (1.39) | 6.62 (1.29) | 7.06 (1.29) |
| Any sleep medication use, N (%) | 440 (26.0) | 80 (19.4) | 112 (25.7) | 79 (22.9) | 169 (34.1) |
| b Depression (range: 2–38), mean (SD) | 9.01 (2.59) | 9.23 (2.68) | 9.10 (2.49) | 9.18 (2.98) | 8.64 (2.25) |
| c Physical activity (range 0–16), mean (SD) | 8.82 (3.20) | 8.83 (3.05) | 8.63 (3.37) | 8.73 (3.27) | 9.06 (3.10) |
| d Central adiposity, N (%) | 1001 (59.2) | 146 (35.4) | 145 (33.3) | 124 (35.9) | 211 (42.5) |
Note: Results reflect the original (unimputed) data.
Abbreviation: SD, standard deviation.
Higher sleep quality scores indicate poorer sleep quality; higher sleep apnea scores indicate greater risk of sleep apnea.
Depression was ascertained using the National Institutes of Health Toolbox Patient‐Reported Outcomes Measurement Information System (PROMIS) Depression assessment with a Ί‐score >60 indicating depression.
Physical activity included light and vigorous leisure and sport activity with a higher score indicating more physically active.
Central adiposity was defined as a waist circumference of ≥90 cm for Asian men; ≥80 cm for Asian women; ≥102 cm for Black, Latino, and White men; and ≥88 cm for Black, Latino, and White women.
3.2. Sleep apnea risk and sleep quality with level of cognitive function among all participants
Overall, after adjusting for demographic and socioeconomic variables, sleep apnea risk was not associated with levels of executive function or verbal episodic memory (Figure 1).
FIGURE 1.
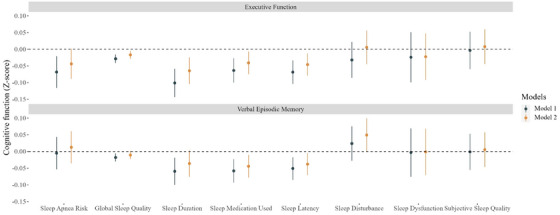
Adjusted mean difference (95% confidence interval) in standardized cognitive function test scores per 1 unit higher (worse) sleep quality and sleep apnea risk in the Kaiser Healthy Aging and Diverse Life Experiences Study (N = 1690). Notes: The executive function and verbal memory scores were z scored to the baseline sample and were evaluated in separate models. Higher scores indicate poorer sleep quality and higher risk of sleep apnea. Estimates were based on linear mixed effect models. Model 1 was adjusted for age, sex, race, and interview mode. Model 2 was additionally adjusted for socioeconomic variables including income and education. Results were based on 35 imputed datasets. Inverse probability of continuation weights were applied to account for loss to follow‐up
Each unit higher (poorer) global sleep quality was associated with 0.02 standard units lower (95% confidence interval [CI], −0.03, −0.005) executive function score. Global sleep quality was not associated with levels of verbal episodic memory. To provide context for these findings, the observed differences are comparable to the cognitive function differences between participants in our sample who were about 4 months apart in age, with each additional year corresponding to a decrease of 0.06 units in cognitive function (= −0.06 per year; 95% CI, −0.06, −0.05).
Of the individual global sleep quality domains, shorter sleep duration (= −0.07; 95% CI, −0.10, −0.02), more frequent difficulty falling sleep (sleep latency; = −0.05; 95% CI, −0.08, −0.01), and more frequent sleep medication use ( = −0.04; 95% CI, −0.08, −0.01) were associated with worse executive function. More frequent difficulty falling sleep (sleep latency; = −0.04; 95% CI, −0.07, −0.005) and more frequent sleep medication use ( = −0.04; 95% CI, −0.08, −0.01) were also associated with lower verbal episodic memory (Figure 1). Our analyses revealed an inverted U‐shaped relationship between sleep duration and levels of executive function and verbal episodic memory, with the highest cognitive scores occurring with sleep duration of ≈ 7 hours (Figure 2). Most associations remained similar after adjusting for sleep medication use except sleep latency, of which the associations with executive function became null (Table S5 in supporting information).
FIGURE 2.
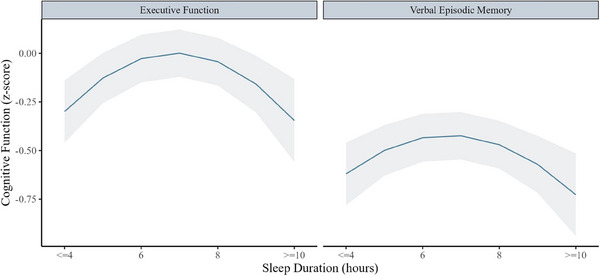
Associations between baseline sleep duration (continuous score) and average level of executive function and verbal episodic memory in the Kaiser Healthy Aging and Diverse Life Experiences Study (N = 1690). Linear mixed effects models adjusted for age, sex, race, income, and education. Results were based on 35 imputed datasets
3.3. Sleep apnea risk and sleep quality with rate of decline among all participants
Sleep apnea risk was associated with declines in verbal episodic memory but not with executive function score. Specifically, for every 1 unit higher in sleep apnea score, average annual decline in verbal episodic memory accelerated slightly (= −0.02 SD; 95% CI = −0.04, −0.001; Figure 3).
FIGURE 3.
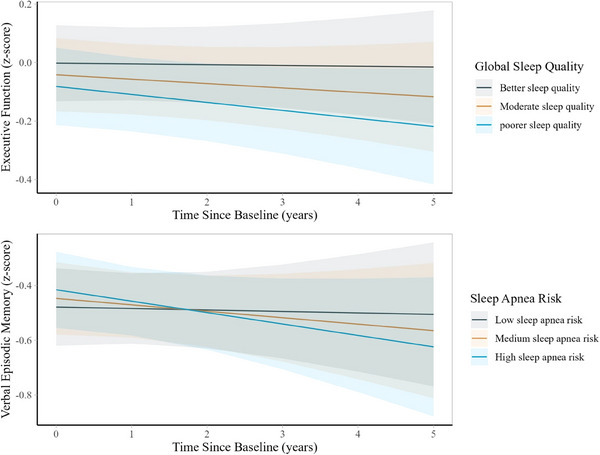
Predicted changes in executive function and verbal episodic memory scores from baseline to wave 3 by baseline sleep quality and sleep apnea risk scores in the Kaiser Healthy Aging and Diverse Life Experiences Study (N = 1690). Notes: The executive function and verbal episodic memory scores were z scored to the baseline sample. Higher sleep scores indicate greater risk of sleep apnea, while higher cognitive function scores indicate better cognition. Linear mixed effect models were adjusted for baseline age, sex, race, interview mode, income, education, interactions between time and sleep, and interactions between time and all covariates except for interview mode. All models used time since baseline as the time scale and accounted for practice effects and interview mode (phone vs. in person). All models included random intercepts and random slopes. Results were based on 35 imputed datasets. Inverse probability weights were included to account for loss to follow‐up
Global sleep quality was associated with faster declines in executive function but not declines in verbal episodic memory. Each 1 unit worse global sleep quality was associated with faster annual decline in executive function (= −0.004 SD; 95% CI, −0.01, −0.001; Figure 3). These associations remained similar after adjusting for sleep medication use (Table S5).
Regarding individual sleep quality domains, increases in sleep medication use were associated with faster annual declines in executive function (= −0.02 SD; 95% CI, −0.02, −0.01).
3.4. Heterogeneity in the associations of sleep apnea risk and sleep quality with levels of cognitive function by race and ethnicity
We observed no race and ethnicity differences in the associations of sleep apnea and global sleep quality with levels in executive function or verbal episodic memory.
When considering individual aspects of sleep quality, sleep duration was associated with lower levels of executive function scores among Black ( sleep duration = −0.09; 95% CI, −0.16, −0.03) and Latino ( sleep duration = −0.09; 95% CI, −0.18, −0.01) older adults but not among White older adults. Unexpectedly, more frequent difficulty falling sleep (sleep latency) was associated with lower levels of verbal episodic memory performance among White older adults only ( sleep latency = −0.07; 95% CI, −0.14, −0.01; Table S6 in supporting information).
3.5. Heterogeneity in the associations of sleep apnea risk and sleep quality with decline in cognitive function by race and ethnicity
The associations between sleep apnea risk and rate of decline in executive function and verbal episodic memory did not differ by race and ethnicity status. However, differences were observed for the relationship between global sleep quality and decline in executive function. Specifically, over a mean follow‐up of 2.7 (SD = 0.7) years, a 1 unit worse global sleep quality score was associated with −0.02 (95% CI, −0.03, −0.01) standard units faster annual declines in executive function among Black older adults, compared to White older adults (Figure 4). Placing the results in context, these differences were similar to the difference in rate of change between participants in our sample who were half a year apart in age (= −0.04 per year; 95% CI, −0.07, −0.01).
FIGURE 4.
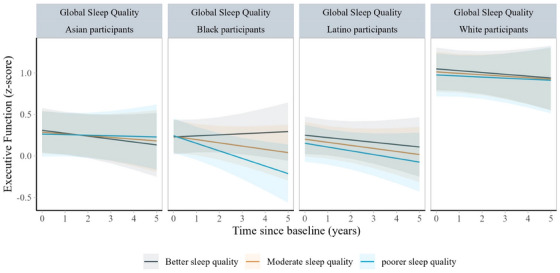
Predicted changes in executive function scores from baseline to wave 3 by baseline sleep quality score in the Kaiser Healthy Aging and Diverse Life Experiences Study (N = 1690), by race and ethnicity. Notes: Analyses were stratified by race and ethnicity. The executive function scores were z scored to the baseline sample. Linear mixed effect models were adjusted for baseline age, sex, race, interview mode, income, education, interactions between time and sleep, and interactions between time and all covariates except for interview mode. All models used time since baseline as the time scale and accounted for practice effects and interview mode (phone vs. in person). All models included random intercepts and random slopes
We also observed differences across races and ethnicities when examining specific aspects of sleep quality. These included sleep latency (differences for executive function in Black participants vs. White participants = −0.03; 95% CI, −0.06, −0.001), sleep medication use (difference for executive function in Black participants vs. White participants = −0.06; 95% CI, −0.09, −0.02; difference for verbal episodic memory in Black participants vs. White participants = −0.05; 95% CI,−0.08, −0.005), and subjective sleep quality (difference for executive function in Black participants vs. White participant = −0.07; 95% CI,−0.12, −0.02; Table S7 in supporting information). For Black older adults, more sleep medication use ( medication*time = −0.05; 95% CI, −0.07, −0.03), more frequent difficulty falling sleep ( latency*time = −0.03; 95% CI, −0.05, −0.02), and poorer subjective sleep quality ( subjective sleep quality*time = −0.05; 95% CI, −0.08, −0.02) were all associated with faster declines in executive function compared to estimated associations among White older adults. For verbal episodic memory, sleep medication use ( medication*time = −0.04; 95% CI, −0.07, −0.02) was associated with faster declines among Black older adults compared to White older adults (Figure S3 in supporting information).
4. DISCUSSION
In this prospective cohort of racially diverse older adults, sleep apnea risk was not associated with levels of executive function or verbal episodic memory but was associated with faster declines in verbal episodic memory. Poorer global sleep quality was associated with both lower levels and faster declines in executive function. Some racial/ethnic differences in these associations also emerged: poorer global sleep quality score, for example, was associated with faster declines in executive function among Black older adults compared to White older adults.
Consistent with previous studies, we showed that higher sleep apnea risk is associated with faster declines in verbal episodic memory. 19 Sleep apnea may lead to intermittent hypoxemia or alter sleep macro‐ and microarchitecture, reduce neurogenesis, and promote accumulation of amyloid plaques, resulting in declines in episodic memory. 20 Our finding that poorer global sleep quality was associated with lower levels and faster declines in executive function also aligns with much of the existing research, which suggests that poor sleep quality may negatively impact cognitive function. 21 Although the mechanisms underlying sleep quality and executive function are not yet established, poorer sleep quality may be associated with increases in inflammation and enhanced amyloid beta aggregation, which in turn affects executive function. Our findings on the inverted U‐shaped relationship between sleep duration and cognitive levels are consistent with previous evidence that both short and excessive sleep duration are harmful for cognitive health. 22 , 23 , 24 We provided further evidence that these associations are observed among racially and ethnically diverse populations.
Our findings align with recent studies conducted in the Baltimore Longitudinal Study of Aging and the Study of Women's Health Across the Nation, demonstrating that the sleep–cognition relationship is stronger among Black than White older adults. 25 Exposure to race‐specific stressors, such as interpersonal racism, may exacerbate the effects of sleep on cognitive outcomes. 26 Moreover, unequal access to sleep health education and services among marginalized racial/ethnic populations, including Black older adults, may worsen sleep problems, potentially contributing to the stronger associations between sleep and cognitive function observed in Black populations. As poor sleep quality was more prevalent for Black participants, the stronger effect of sleep quality on cognitive decline is superimposed on higher prevalence of the risk factors, making sleep potentially especially pertinent for dementia risk among Black older adults. Potential interventions aimed at increasing access to sleep‐related health‐care services and sleep hygiene education may not only help address sleep disparities but also reduce cognitive health disparities in the long term. 27
Our findings that the associations between sleep medication use and cognitive function were stronger among Black older adults are in contrast to a recent study, which showed that sleep medication use was associated with a higher risk of dementia among White compared to Black older adults. 28 These differences in findings may be attributable to variations in the populations, the prevalence of sleep medication use, or the outcomes assessed (e.g., dementia vs. cognitive decline). Given that sleep medication use has become increasingly common among older adults and the inconclusive evidence on the racial differences in its impact on cognitive outcomes, more research is needed to investigate the impact of sleep medication use in racial/ethnic groups historically underrepresented in cognitive aging research. For example, electronic health records with detailed information on sleep medication and dementia diagnosis would be especially helpful to shed light on the racial/ethnic differences revealed in our study.
Our study has some limitations, most importantly the reliance on self‐reported sleep quality and sleep apnea measures. Subjective sleep measures, such as PSQI, may not fully capture sleep symptoms. 29 Measurement error in self‐reported sleep measures could have biased our results, either toward or away from the null. Despite these drawbacks, the PSQI offers key advantages over polysomnography or actigraphy measures, especially for disparities research. It is easier to administer, less expensive, captures subjective aspects of sleep quality, and is widely used in clinical settings. Additionally, it has been validated across diverse populations. 30 , 31 When health records become available, future KHANDLE studies will be able to link physician diagnosis of sleep problems (e.g., insomnia) to sleep quality assessed by the PSQI, although underdiagnoses of sleep disorders suggest the importance of assessing self‐reported experiences. Despite the longitudinal design, we cannot rule out reverse causation, that is, poor sleep quality or obstructive sleep apnea may be markers of preclinical or prodromal brain conditions. 32 Furthermore, we did not have information on the type, dosage, and duration of sleep medication use, which would need to be investigated further to clarify any observed racial differences. For example, racial bias associated with the prescription of controlled substances may lead to different types of sleep medications used across racial and ethnic groups, resulting in differential impacts on cognitive decline. 33 We also did not have information about the use of sleep treatment such as continuous positive airway pressure machines, which could have influenced the impacts of sleep on cognitive function. Last, while we did not find major differences in the associations between sleep and cognitive function between Hispanic/Latino and Asian populations compared to White populations, our sample does not allow us to distinguish the associations between subgroups of the Hispanic/Latino or Asian populations. Our findings may obscure meaningful differences across subgroups. 11 Future research should disaggregate data for Hispanic/Latino and Asian populations to better understand the nuanced associations between sleep and cognitive function within these heterogeneous populations.
The study has several strengths, including a racially and ethnically diverse sample, examination of various sleep parameters, and repeated measures of cognitive function with minimal floor or ceiling effects. 34 Our study represents an important step toward unraveling the heterogeneity in the associations of sleep quality and sleep apnea risk with cognitive functioning. If our findings are supported by other studies, sleep quality may emerge as an important modifiable factor for improving cognitive functioning among Black older adults, potentially narrowing racial disparities in cognitive decline.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants provided informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This study is supported by the National Institute on Aging (NIA; R01AG052132). R.C. is supported in part by NIA (K00AG068431). S.A. is support by NIA (K99AG073454). Dr. Aric A. Prather reports grants from Eisai, Ltd and Big Health. Additionally, he serves as an advisor to NeuroGeneces.
Chen R, Wang J, Pederson AM, et al. Evaluation of racial and ethnic heterogeneity in the associations of sleep quality and sleep apnea risk with cognitive function and cognitive decline. Alzheimer's Dement. 2024;10:e12441. 10.1002/trc2.12441
REFERENCES
- 1. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216‐224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen R, Weuve J, Misra S, Cuevas A, Kubzansky LD, Williams DR. Racial disparities in cognitive function among middle‐aged and older adults: the roles of cumulative stress exposures across the life course. J Gerontol Ser A. 2022;77(2):357‐364. doi: 10.1093/gerona/glab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weuve J, Barnes LL, de Leon CFM, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology (Cambridge, Mass). 2018;29(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peterson RL, George KM, Gilsanz P, et al. Racial/ethnic disparities in young adulthood and midlife cardiovascular risk factors and late‐life cognitive domains: the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) study. Alzheimer Dis Assoc Disord. 2021;35(2):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaidel C, Musich S, Karl J, Kraemer S, Yeh CS. Psychosocial factors associated with sleep quality and duration among older adults with chronic pain. Popul Health Manag. 2021;24(1):101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon NP, Yao JH, Brickner LA, Lo JC. Prevalence of sleep‐related problems and risks in a community‐dwelling older adult population: a cross‐sectional survey‐based study. BMC Public Health. 2022;22(1):2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braley TJ, Dunietz GL, Chervin RD, Lisabeth LD, Skolarus LE, Burke JF. Recognition and diagnosis of obstructive sleep apnea in older americans. J Am Geriatr Soc. 2018;66(7):1296‐1302. doi: 10.1111/jgs.15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi‐Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. George KM, Peterson RL, Gilsanz P, et al. Racial/ethnic differences in sleep quality among older adults: Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) Study. Ethn Dis. 2020;30(3):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen R, Slopen N, Lee S. Perceived stress, recent stressors, and distress in relation to sleep disturbance and duration among middle‐aged and older Asian immigrants. Sleep Health. 2023;9(2):211‐217. doi: 10.1016/j.sleh.2022.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson DA, Jackson CL, Williams NJ, Alcántara C. Are sleep patterns influenced by race/ethnicity–a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep. 2019;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193‐213. [DOI] [PubMed] [Google Scholar]
- 13. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non‐clinical samples: a systematic review and meta‐analysis. Sleep Med Rev. 2016;25:52‐73. [DOI] [PubMed] [Google Scholar]
- 14. Hashmi AM, Khawaja IS, Butt Z, Umair M, Naqvi SH, Ul‐Haq J. The Pittsburgh Sleep Quality Index: validation of the Urdu translation. J Coll Physicians Surg Pak. 2014;24(2):123‐126. [PubMed] [Google Scholar]
- 15. Beaudreau SA, Spira AP, Stewart A, et al. Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Med. 2012;13(1):36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smyth CA. Evaluating sleep quality in older adults: the Pittsburgh Sleep Quality Index can be used to detect sleep disturbances or deficits. AJN Am J Nurs. 2008;108(5):42‐50. [DOI] [PubMed] [Google Scholar]
- 17. Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347. [DOI] [PubMed] [Google Scholar]
- 18. Chen R, Calmasini C, Swinnerton K, et al. Pragmatic approaches to handling practice effects in longitudinal cognitive aging research. Alzheimers Dement. 2023;19(9):4028‐4036. doi: 10.1002/alz.13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta‐analysis. Sleep. 2013;36(2):203‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gosselin N, Baril AA, Osorio RS, Kaminska M, Carrier J. Obstructive sleep apnea and the risk of cognitive decline in older adults. Am J Respir Crit Care Med. 2019;199(2):142‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramos AR, Tarraf W, Wu B, et al. Sleep and neurocognitive decline in the Hispanic Community Health Study/Study of Latinos. Alzheimers Dement. 2020;16(2):305‐315. doi: 10.1016/j.jalz.2019.08.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34(5):575‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self‐reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18(4):436‐446. [DOI] [PubMed] [Google Scholar]
- 24. Ramos AR, Tarraf W, Daviglus M, et al. Sleep duration and neurocognitive function in the Hispanic Community Health Study/Study of Latinos. Sleep. 2016;39(10):1843‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swanson LM, Hood MM, Hall MH, et al. Associations between sleep and cognitive performance in a racially/ethnically diverse cohort: the Study of Women's Health Across the Nation. Sleep. 2021;44(2):zsaa182. doi: 10.1093/sleep/zsaa182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ji L, Zhaoyang R, Jiao JL, et al. Discrimination and education quality moderate the association of sleep with cognitive function in older black adults: results from the Einstein Aging Study. J Gerontol Ser B. 2023;78(4):596‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Billings ME, Cohen RT, Baldwin CM, et al. Disparities in sleep health and potential intervention models: a focused review. Chest. 2021;159(3):1232‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leng Y, Stone KL, Yaffe K. Race differences in the association between sleep medication use and risk of dementia. J Alzheimer's Dis. 2023;91(3):1133‐1139; (Preprint): 1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cabanel N, Speier C, Müller MJ, Kundermann B. Actigraphic, but not subjective, sleep measures are associated with cognitive impairment in memory clinic patients. Psychogeriatrics. 2020;20(2):133‐139. [DOI] [PubMed] [Google Scholar]
- 30. Hita‐Contreras F, Martínez‐López E, Latorre‐Román PA, Garrido F, Santos MA, Martínez‐Amat A. Reliability and validity of the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) in patients with fibromyalgia. Rheumatol Int. 2014;34:929‐936. [DOI] [PubMed] [Google Scholar]
- 31. Lee J, Shin C, Ko YH, et al. The reliability and validity studies of the Korean version of the Perceived Stress Scale. KJPM. 2012;20(2):127‐134. [Google Scholar]
- 32. Leng Y, Ackley SF, Glymour MM, Yaffe K, Brenowitz WD. Genetic risk of Alzheimer's disease and sleep duration in non‐demented elders. Ann Neurol. 2021;89(1):177‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cook B, Creedon T, Wang Y, et al. Examining racial/ethnic differences in patterns of benzodiazepine prescription and misuse. Drug Alcohol Depend. 2018;187:29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hernandez Saucedo H, Whitmer RA, Glymour M, et al. Measuring cognitive health in ethnically diverse older adults. J Gerontol Ser B. 2022;77(2):261‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


