Abstract
INTRODUCTION
Emotionally driven cognitive complaints represent a major diagnostic challenge for clinicians and indicate the importance of objective confirmation of the accuracy of depressive patients’ descriptions of their cognitive symptoms.
METHODS
We compared cognitive status and structural and functional brain connectivity changes in the pulvinar and hippocampus between patients with total depression and healthy controls. The depressive group was also classified as “amnestic” or “nonamnestic,” based on the members’ subjective reports concerning their forgetfulness. We then sought to determine whether these patients would differ in terms of objective neuroimaging and cognitive findings.
RESULTS
The right pulvinar exhibited altered connectivity in individuals with depression with objective cognitive impairment, a finding which was not apparent in depressive patients with subjective cognitive impairment.
DISCUSSION
The pulvinar may play a role in depression‐related cognitive impairments. Connectivity network changes may differ between objective and subjective cognitive impairment in depression and may play a role in the increased risk of dementia in patients with depression.
Keywords: amnestic depression, cognition, depression, functional magnetic resonance imaging, pulvinar
1. INTRODUCTION
Major depressive disorder (MDD) is characterized by a range of cognitive deficits in executive functioning, learning, and memory. 1 , 2 , 3 These are observed in a significant majority of individuals diagnosed with clinical depression. 4 These cognitive deficit impairments in individuals diagnosed with MDD have a considerable adverse impact on overall well‐being and hinders such individuals’ ability to perform daily tasks in an efficient manner. 5 , 6 , 7
Consistent with current neurobiological models of depression, 4 , 5 , 6 , 7 , 8 several studies have suggested that cognitive deficits in depression result from maladaptive bottom‐up processes between hierarchically higher cortical regions and lower subcortical brain regions. This in turn hinders the ability of higher‐level cognitive regions to adequately modulate activity in the lower regions. 8
As summarized above, depressive cognitive impairment is associated with significant neuroimaging changes in depressive patients. For instance, a recent study reported greater activity in the dorsolateral and ventrolateral prefrontal cortex and anterior cingulate gyrus in patients with depression. 9 The findings of subsequent studies were also consistent with these results, showing a significant association between impaired cognitive performance and decreased cortical region functionality in depressive patients. 9 , 10 A good example of this is a recent study showing that increased cortical activity associated with a heightened effort to retain an intact performance decreases continuously during the progression of depressive disease. 11 Due to the importance of the hippocampus and thalamus in depressive pathophysiology, previous studies have only explored structural and general functional alterations in these two subcortical regions, rather than specifically investigating their subregion connectivity with other critical cortico‐subcortical regions, a procedure made possible by advanced neuroimaging analysis methods. 12 , 13 Although a number of previous studies have investigated brain imaging markers of depressive cognitive impairment, to the best of our knowledge, none has focused on structural and functional differences between subjective and objective cognitive impairment in depressive patients. Furthermore, previous cognition studies of depressive patients have primarily focused on alterations in the hippocampus, amygdala, and cingulate gyrus, without considering functional alterations in other critical subcortical structures, such as the thalamus, and especially the pulvinar. 14 Such alterations may indicate a critical dynamic phase associated with subtle pathophysiological changes occurring before the manifestation of structural changes. 15 , 16 , 17 From that perspective, functional connectivity analysis is a valuable method for examining voxel‐wise functional magnetic resonance imaging (MRI) signals and that identifies functionally related brain areas and distributed networks. This therefore justifies an investigation of the differences between amnestic and other depressed individuals. The limited data available in this area prompted us to examine potential differences between amnestic and other depressed individuals using structural and functional (connectivity) MRI.
The pulvinar nucleus is the largest nucleus of the thalamus, representing approximately 30% of its overall volume. 18 The pulvinar is categorized as a higher‐order nucleus due to its substantial participation in receiving the majority of cortical inputs and in the transmission of crucial outputs to the cortex. Due to its robust and reciprocal connectivity with numerous cortical regions, such as the frontal, parietal, temporal, occipital, and cingulate cortices, the pulvinar performs a significant function in a wide range of complex cognitive, affective, and sensorimotor processes. 19 , 20 , 21 , 22
Within the context of this cortico‐subcortical circuiting, the pulvinar is also significant in defining the objective and/or subjective nature of cognitive symptoms through its critical role in imbalanced emotional states by altering selective attention toward unpleasant stimuli. 23 , 24 A good example is the role of the anterior pulvinar in maintaining a biased cognitive schema to aversive stimuli in depression. 23
In addition to constituting an essential component of depression, memory dysfunction in depression may also be associated with Alzheimer's disease. 25 , 26 , 27 , 28 This may be attributable to depressive symptoms and subsequent dementia possibly representing different manifestations of the same underlying pathophysiological process. The important role of serotonergic degeneration and amyloid beta accumulation in the pathogenesis of late‐life depression represents a good example of this. 29 Conversely, depressive symptoms may also be an important indicator of early stages of dementia, associated with the concomitant degeneration of serotonergic and dopaminergic neurons before the manifestation of cognitive degeneration. 30 , 31 However, despite all such evidence, the role of the pulvinar in the development of cognitive disorder in depressive disorder, as previously determined for the hippocampus, is still unclear. 32 , 33 , 34 Additionally, in contrast to the well‐known role of the pulvinar in depression and antidepressant therapy, 35 to the best of our knowledge, no research to date has implicated the pulvinar in the development of dementia in patients with MDD. From the clinical perspective, several studies of cognition in depression have shown that many depressed patients have subjective cognitive complaints that cannot be confirmed by means of objective cognitive tests. 9 , 36 Current clinical interviews represent subjective assessments of cognitive performance that can be impacted by affective states and emotions, rendering these less reliable and consistent than objective testing. 37 , 38 In other words, these so‐called “emotionally driven” cognitive complaints can pose a considerable difficulty for clinicians in this field, and emphasize the importance of objective confirmation of whether depressive patients accurately define their cognitive symptoms. 39
We sought to determine whether the subjective interpretation of cognitive status with and without real cognitive impairment may be associated with special connectivity features and the severity of depression. Cognitive status and functional brain connectivity changes were therefore compared between patients with total depression and healthy controls. In the second step, the members of the depressive group were classified as “amnestic” or “nonamnestic” based on their subjective forgetfulness in order to investigate whether these groups’ objective neuroimaging and neuropsychometric findings would differ. Based on previous studies indicating the role of the pulvinar and hippocampus in degenerative cognitive disorders, 40 we explored the structural and functional features of both regions in depressive disorder with and without objective cognitive impairment.
The Turkish‐language version of the Alzheimer's Disease Assessment Scale–Cognitive Subscore (ADAS‐Cog) has been shown to exhibit a high degree of validity and reliability. 41 It also has a proven ability to distinguish MCI from MDD 41 and to detect associations between subjective cognitive decline and depressive symptoms in the development of potential dementia . 42 , 43 ADAS‐Cog tests were therefore applied to discriminate between MDD and Alzheimer's disease in the present study.
2. METHODS
2.1. Participants
For this cross‐sectional study, 31 depression outpatients (aged 24–63 years; 15 amnestic and 16 nonamnestic) and 31 healthy control patients (aged 18–64 years) were recruited by their clinicians from Alanya Alaaddin Keykubat University Hospital. Patients met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐V) for MDD. Diagnoses were confirmed by experienced psychiatrists using the Hamilton Depression Rating Scale (HDRS). The HDRS was used to determine the mean depression scores. Exclusion criteria for all patients were as follows: (a) a score of less than 23 on Mini‐Mental State Examination (MMSE) 44 ; (b) patients with a history of previous head trauma or stroke, or current or previous substance abuse/dependence; (c) clinical evidence of other major psychiatric or neurological disorders; (d) prescribed antidementia medications. For the cognitive assessment of the participants, MMSE, and ADAS‐Cog were applied. The protocol of this study was approved by the Ethics Committee of Istanbul Medipol University (the number of ethical‐ report: 10840098‐604.01.01‐E.19402), and all patients provided written informed consent after a complete examination of the study.
RESEARCH IN CONTEXT
Systematic review: Depression is a chronic disease that may affect person's life, social interactions and work life. Also, it may cause some cognitive changes that can be detected by licensed clinicians. The pulvinar which is a part of hippocampus may play a role in these cognitive changes in depression patients. We aimed to study the role of pulvinar on depressive patients with cognitive impairment
Interpretation: We collected major depressive disorder (MDD) patients and performed neurocognitive tests and functional MR imaging. We compared cognitive status and structural and functional brain connectivity changes in the pulvinar and hippocampus between patients with total depression and healthy controls.
Future directions: Our study suggests that MDD may cause cognitive deficits and pulvinar may play a role in depression‐related cognitive impairments.
2.2. MRI data acquisition
Structural and resting‐state functional magnetic resonance imaging (fMRI) data were conducted using a Signa Explorer MR device (General Electric Company, USA) at Alanya Alaaddin Keykubat University, Turkey. Each individual's T1‐weighted structural scans consisted of 190 slices (TR/TE: 8.1/3.7), FOV 256 × 256 × 190 mm (FHxAPxRL), and a voxel size of 1 × 1 × 1 mm. Eyes‐open resting state fMRI scan recording were collected using an echo‐planar imaging sequence (EPI). The scanning process lasted approximately 12 min, and 300 volumes were recorded with the following parameters: TR 2230 ms, TE 30 ms, FOV 240 × 240 × 140 mm (RL × AP × FH), voxel size 3 × 3 × 4 mm, flip angle 770, and slice number 35. Before the scanning, all participants were instructed to keep their eyes closed, relax and move as little as possible, think nothing, and not fall asleep during the scanning.
2.3. Image preprocessing
Resting‐state preprocessing and seed‐based analyses were carried out using tools from FMRIB Soft‐ ware Library, FSL 6.0. 45 Data preprocessing procedures consists of (1) discarding the first five volumes of each scan in order to stabilize the signal; (2) brain extraction by using fsl_anat script; (3) calculating and applying linear and nonlinear registration in transforming between the resting‐state, anatomical and MNI spaces; (4) slice timing correction; (5) motion correction using rigid‐body transformations (three rotations and three translations) in motion correction tool (MCFLIRT) 46 ; (6) spatial smoothing applying a Gaussian kernel of FWHM 6 mm; and (7) performing band‐pass filter 0.01–0.1 Hz range.
2.4. Extraction of hippocampal and pulvinar masks
A seed‐based functional connectivity was conducted to identify voxels temporally correlated with the mean time series of bilateral hippocampi and pulvinar nuclei. Anatomical images were reoriented using Statistical Parametric Mapping (SPM12, Wellcome Trust Centre for Neuroimaging, London https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Each subject's anatomical image was manually set to the anterior commissure and aligned each brain along the anterior commissure – posterior commissure line passing through the centers of each commissure. 47 The MRI data was segmented with Freesurfer image analysis suite (version 7.1.1) (http://surfer.nmr.mgh.harvard.edu/), on 3D T1‐weighted images after optimized nonlocal means denoising. 47 The recon‐all in parallel pipeline was implemented for segmentations and volumetric measurements.
The output data of the recon‐all process were analyzed to extract masks for the left and right hippocampi. Furthermore, we segmented the thalamus with the thalamic nuclei tool in Freesurfer after recon‐all pipeline and extracted the masks of pulvinar nuclei one in each hemisphere of the brain from that anatomical segmentation. Output segmentations were inspected visually. The following steps involved in framework of creating anatomical mask for hippocampi and pulvinar nuclei with Freesurfer: (1) converting the segmentations from Freesurfer space to native anatomical space, (2) extraction of the anatomical binary mask for our ROI's for each subjects individually via mri_binarize function in Freesurfer, (3) converting the masks from the .mgz file type to .nii(.gz) to use them in FSL using mri_convert function in Freesurfer, and (4) converting masks of hippocampi and pulvinar nuclei from anatomical space to functional space in FSL using an affine transformation. Afterward, T1 weighted anatomical images and masks were analyzed to make average masks for hippocampi and pulvinar nuclei. The masks were normalized in SPM12 with deformation field images which created after the SPM12‐based segmentation of T1 weighted images. The “imcalc” tool in SPM is used for performing image calculations on per subjects’ masks to create an average of hippocampi and pulvinar nuclei masks for functional connectivity analysis. Thresholding of the masks was performed using fslmaths which is a command‐line tool in FSL (FMRIB Software Library) at a value of 0.8 (i.e., 80% probability) to generate binary masks. Consequently, the masks of hippocampi and pulvinar nuclei one in each hemisphere were created for seed based functional connectivity analyses.
2.5. Functional connectivity analysis
To evaluate pulvinar nuclei and hippocampal functional connectivity using the outputs from these masks segmentation, that is, the masks per subject, a seed‐based functional connectivity analysis was conducted. The analysis consists in extracting the arithmetic mean BOLD time course across all voxels within the masks, followed by a voxel‐wise bivariate Pearson's correlation with each other voxel in the brain. 30 , 48 Independent component analysis was performed with the FSL MELODIC ICA tool to regressed out The following nuisance predictors in the analysis: cerebrospinal fluid (CSF), white matter (WM), global signal, motion outlier volume masks, and six motion parameters obtained from the motion correction step. The artifact free data were registered to the MNI152 standard space using FSL apply warp tool. Two experimental matrixes were set up with FSL's GLM tool including designs for comparing to groups. In the first experimental design both amnestic depression and nonamnestic depression patients was accepted as a one group called depressive group. The depressive group (both amnestic and nonamnestic) and control individuals were compared in seed‐based functional connectivity analysis to depict of general effect of depression on functional connectivity. In the second experimental design consists of 3 group as amnestic depression‐ nonamnestic depression and control groups in order to whether effect of to be amnesia in addition to depression on functional connectivity. We favor using these experimental designs to address our research questions: (1) Does depression result in changes to functional connectivity in the hippocampus and pulvinar? (2) Does co‐occurring amnesia and depression exacerbate the impairment of hippocampal and pulvinar connectivity? (3) Can the depression or amnesia status of an individual be identified by analyzing possible alterations in functional connectivity in the hippocampus and pulvinar? FSL dual regression tool was used to identify of subject‐specific connectivity patterns and to compare connectivity between groups. The distribution of the data was calculated by the permutation method (5000 times). The masks are defined in dual regression analysis and final stage of the analysis was runed with bilateral pulvinar and hippocampi masks, and group‐level differences were compared for each mask. all those voxels whose signal time series were significantly correlated with the seed region (p < 0.05) were identified by calculating the subject‐specific contributions to the group level ICA. The z score statistical maps for the masks for the all groups separately (am, dep, con, am + dep) was obtained using one‐sample t test (cluster level p < 0.05, corrected for familywise error [FWE]). Between group comparison was used a two‐sample t test (unpaired cluster level p < 0.05, corrected for FEW) (Figure 1).
FIGURE 1.
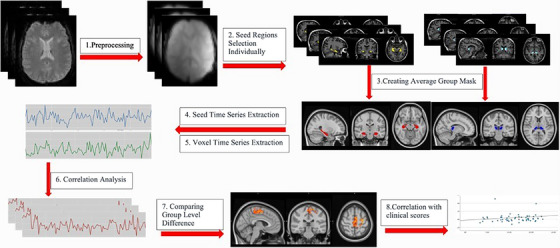
The flowchart of resting state fMRI analysis steps.
2.6. Statistical analysis
Simple descriptive statistics and cognitive scores were carried out using Jamovi (version 2.3.19.0). Shapiro–Wilk test was used to check the normality of the variables. Continuous variables are presented as mean ± standard deviation (mean ± SD) and categorical variables as frequency (n) and percentage (%). The one‐way analysis of variance (ANOVA) was used to analyze the between groups of ADAS scores and educational years while Kruskal–Wallis was used to analyze age differences between groups based on according to the normal distribution of the variables. Two‐sided p‐value ≤ 0.05 was interpreted as statistically significant. Correlation linear relationships between ADAS‐Cog scores and regional functional connectivity were assessed through correlation matrices while for each variable a Spearman's r value indicated the strength and direction of the relationship between those two variables based on according to the normal distribution of the connectivity values.
3. RESULTS
3.1. Demographic factors
Subject demographic and clinical data, including age, education, HDRS, and ADAS scores, are presented in Table 1. All three groups had similar demographic characteristics in terms of age (p > 0.05), educational level, and gender (p > 0.05), except for the amnestic depression and control groups. Based on the normal distribution of the variables, we applied either parametric [ADAS (p = 0.908); education (p = 0.113)] or nonparametric [age (p = 0.023)] tests for the dependent and independent variables (Table 2).
TABLE 1.
Demographics and ADAS scores of three groups (amnestic depression, depression, and control).
| Descriptives | Group | N | Missing | Mean | Median | SD | Minimum | Maximum | P1 | P2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Education year | C | 31 | 0 | 13.8065 | 14 | 3.763 | 5 | 24 | ||
| AD | 15 | 0 | 9.1333 | 12 | 4.138 | 5 | 16 | 0.002 a | ||
| D | 16 | 0 | 11.25 | 12 | 4.583 | 5 | 16 | |||
| Age | C | 31 | 0 | 36.2581 | 33 | 12.399 | 18 | 64 | ||
| AD | 15 | 0 | 46.5333 | 49 | 12.563 | 28 | 63 | 0.043 a | ||
| D | 16 | 0 | 37.5 | 34 | 11.009 | 24 | 55 | |||
| HDRS | C | 31 | 0 | 2.9032 | 3 | 2.427 | 0 | 9 | 0.001 a | |
| AD | 15 | 0 | 12.0667 | 11 | 5.812 | 4 | 24 | 0.001 b | ||
| D | 16 | 0 | 10.5625 | 10 | 4.83 | 1 | 22 | |||
| ADAS‐Cog | C | 31 | 0 | 5.6394 | 5.66 | 2.044 | 1.99 | 10.66 | 0.001 a | |
| AD | 15 | 0 | 8.4187 | 8.33 | 2.267 | 3.66 | 12.33 | 0.031 b | ||
| D | 16 | 0 | 7.4731 | 7.825 | 2.722 | 3.33 | 11.33 |
Note: P1 Kruskal–Wallis, P2 One‐way ANOVA, Tukey post hoc.
Abbreviations: AD, amnestic depression; C, control; D, depression.
AD‐C.
D‐C.
TABLE 2.
Three groups after adjusting for different ages and educational years (amnestic depression, depression, and control).
| ANCOVA—ADAS‐Cog | |||||
|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | p | |
| Age | 10.3 | 1 | 10.26 | 2.47 | 0.121 |
| Education year | 38.3 | 1 | 38.32 | 9.23 | 0.004 |
| Type (AD‐C) | 22.5 | 2 | 11.26 | 2.71 | 0.075 |
| Residuals | 236.5 | 57 | 4.15 | ||
| Post Hoc Comparisons—Type (AD‐C) | ||||||
|---|---|---|---|---|---|---|
| Comparison | ||||||
| Type | Type | Mean difference | SE | df | t | ptukey |
| AD | C | −1.41 | 0.72 | 57.0 | −1.96 | 0.132 |
Note: Comparisons are based on estimated marginal means.
Abbreviations: AD, amnestic depression; C, control.
Finally, our partial correlation analysis adjusted for age and education revealed a significant correlation between the right pulvinar (RPulv) and ADAS scores (r = −0.31*, p = 0.014) in the entire group (Table 3a), and the depression group (r = −0.37*, p = 0.05) (Table 3b) which was not evident in the control group (p = 0.581) (supTable 3c).
TABLE 3a.
Partial correlation analysis of whole group (amnestic depression (AD), depression (D) and Control (C).
| Partial correlation | ||||
|---|---|---|---|---|
| adas cog | RPulvCon>Dep | LPulvDep>Con | ||
| adas cog | Spearman's rho | — | ||
| p‐value | — | |||
| RPulvCon>Dep | Spearman's rho | −0.32 * | — | |
| p‐value | 0.014 | — | ||
| LPulvDep>Con | Spearman's rho | 0.04 | 0.11 | — |
| p‐value | 0.776 | 0.409 | — | |
Note: controlling for ‘age’ and ‘education year’.
p < .05.
**p < .01.
***p < .001.
TABLE 3b.
Correlation of ADAS Cog levels and voxel‐based left and right pulvinar connectivity values in nonamnestic and amnestic depression.
| Partial correlation | ||||
|---|---|---|---|---|
| adas cog | RPulvCon > Dep | hdrs | ||
| adas cog | Spearman's rho | — | ||
| p‐value | — | |||
| LPulvDep > Con | Spearman's rho | −0.31 | ||
| p‐value | 0.102 | |||
| RPulvCon > Dep | Spearman's rho | −0.37 * | — | |
| p‐value | 0.050 | — | ||
| hdrs | Spearman's rho | 0.38 * | −0.37 * | — |
| p‐value | 0.042 | 0.050 | — | |
Note: ADAS‐Cog scores were sinigicantly correlated with RpulvCon > Dep group. Controlling for “age” and “education year”.
Abbreviations: Con, control; Dep, depression; Left Pulv Dep, left pulvinar depression; RPulvCon, right pulvinar control.
p < 0.05.
**p < 0.01.
***p < 0.001.
3.2. Structural volume
There were no significant differences in three group (amnestic depression vs. nonamnestic depression vs. control) comparisons in hippocampal and pulvinar volumes (p > 0.05, Kruskal–Walis).
3.3. Functional connectivity
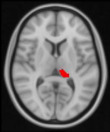
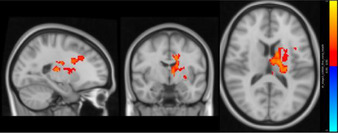
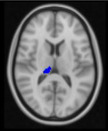
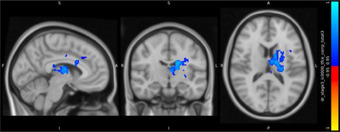
Between‐group differences of functional connectivity of seeds are showed in Table 4. In determining bilateral hippocampus and pulvinar nuclei as seed ROIs, unexpectedly, no significant group differences in functional connectivity were observed for hippocampus. Interestingly, adverse alterations were observed in right and left pulvinar between patient and control groups. Surprisingly, between‐3 group difference revealed that there is no significant functional connectivity alteration in pulvinar between amnestic depressive group and nonamnestic depression group. However, compared with healthy control group, nonamnestic depression group had increased functional connectivity between left pulvinar and, left superior frontal gyrus and somatosensory regions in left hemisphere such as precentral, postcentral gyri and supplementary motor area (p = 0.01). In contrast, we detected decreased functional connectivity of right pulvinar with putamen, caudate, pallidum, thalamus, and paracingulate gyrus in left hemisphere in nonamnestic depression group compared to control (p = 0.01).
TABLE 4.
Differences in resting state functional connectivity between nonamnestic depression and control groups which shows that pulvinar is significantly different between groups (p < 0.05).
| Size (voxels) | MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|---|
| Contrasts | Seeds | Regions | X | Y | Z | p‐Value | ||
| Non‐amnestic depression group > Control |
Left pulvinar
|
|
Left supplementary cortex Left precentral Left postcentral Left superior frontal gyrus |
1091 | 62 | 51 | 61 | 0.01* |
| Control>non‐amnestic depression group |
Right pulvinar
|
|
Left putamen Left caudate Left pallidum Left paracingulate Left thalamus |
1838 | 52 | 57 | 47 | 0.01* |
4. DISCUSSION
No difference in terms of cognitive deficits was observed between the amnestic (AD) and nonamnestic (D) patients or between the amnestic patients (AD) and healthy controls (Tables 1 and 2). However, a significant difference was found between the nonamnestic (D) patients presenting with objective cognitive impairment and the healthy controls (Table 1). This was also suggested by our fMRI findings showing significant alterations between the nonamnestic depressive patients and controls, a phenomenon that was not observed between the amnestic and control groups. Our partial correlation analysis also confirmed this finding, showing a significant correlation between right pulvinar activity and ADAS scores that was not observed in the amnestic and control groups. Our comparative and correlative data for behavioral and fMRI parameters in the amnestic depression and nonamnestic depression groups showed that neither the hippocampal subfield analysis nor the pulvinar‐based analysis revealed any significant differences between the amnestic, nonamnestic, and control groups.
No significant differences were observed between the amnestic and nonamnestic depression groups in terms of objective cognitive scores and connectivity changes. This led us to exclude the possibility of real cognitive impairment as well as cognitive anosognosia in both groups. Our finding of overestimated cognitive symptoms in the amnestic depression group seems to be particularly valuable for clinicians who are especially challenged by profound subjective neuropsychological complaints in the absence of objective cognitive deficits. This is an intriguing finding, and one that has been validated by several task‐based fMRI and neurotransmitter‐based projection investigations of emotional memory disturbances in depression. 23 , 49 However, although we identified significant differences in fMRI and cognitive scores between the nonamnestic depressive and control groups, this was not replicated between our amnestic depression and control groups, in which no significant differences in cognitive performances were determined.
The idea summarized above is also suggested by our finding of a strong link between cognitive scores and pulvinar functional activity in the nonamnestic depression patients, which was not evident in the amnestic group. This is in good agreement with the subjective nature of cognitive complaints without objective neuroimaging or clinical evidence in this specific (amnestic) depression patient population. More generally, increased depression severity and decreased cognitive scores associated with relevant functional connectivity changes in the right and left pulvinar regions in the entire depression group fits well with previous data showing separate roles of the right and left pulvinars in MDD and the treatment thereof. 50 , 51 Our finding of altered pulvinar connectivity in this mixed (both amnestic and nonamnestic) group is particularly noteworthy since several studies of depression have highlighted the role of the pulvinar in the pathophysiology of depression and also in the response to antidepressant therapy and remission. 35 , 52 Xiong et al. identified decreased gray matter volume in the anterior pulvinar as a trait‐marker of depression, 14 a phenomenon which was subsequently reversed with antidepressant therapy. 35 , 50 This was also suggested by novel connectivity data showing that decreased functional connectivity between the pulvinar and the parietal cortex and precuneus may be associated with memory impairments after electroconvulsive therapy in depressive patients . 53 Additionally, right pulvinar volume seems to increase with appropriate antidepressant medication in MDD. 35 , 50 That finding confirmed previous data reported by Tadayonnejad et al. showing increased connectivity between the right pulvinar and the right precuneus and decreased connectivity between the right pulvinar and the left thalamus and right putamen in depression, a phenomenon that is modulable after antidepressant therapy. 54
The absence of statistical significance in comparisons between the amnestic and control groups warrants further discussion. Recent neuropsychological evidence has suggested the involvement of disrupted pulvinar activation in biased selective attention toward negative stimuli among individuals with depression. This may represent a reasonable explanation for the negative bias toward their cognitive complaints in our depressed population with subjective cognitive impairment, thus causing them to overestimate their cognitive symptoms. McTeague et al.’s meta‐analysis involving 298 studies also supported the idea of pulvinar involvement in MDD. 23 Although not entirely relevant, these findings also accord with attentional symptoms of schizophrenia, exhibiting decreased functional connectivity between the left pulvinar and anterior cingulate cortex. 55
To summarize, as attested by self‐reported cognitive symptoms, our data appear to highlight an overestimation of subjective cognitive symptoms among amnestic depressive patients compared to nonamnestic individuals. The activity of the pulvinar, with its multifunctional role, may be specifically associated with objective cognitive impairment, thus representing a potential discriminating factor between subjective and objective cognitive complaints.
In order to avoid any potential confusion, we would emphasize that our findings do not mean that subjective cognitive symptoms in depressed individuals can be ignored. Indeed, they indicate that cognitive symptoms of any kind should be taken seriously, since their association with depressive symptomatology may be linked to some unusual brain regions that exhibit multimodal properties under healthy and disease conditions such as dementia. The pulvinar, for instance, differs from other well‐known cognitive regions such as the hippocampus. However, recent research, including investigation by ourselves, 40 , 56 suggests that this unique region with its diverse cortical connections and gatekeeper function also plays a critical role in cognitive impairment in neurodegenerative diseases. 51 In terms of cognition, these results, together with our current findings, are especially valuable in the light of growing evidence of an increased risk of dementia in MDD, irrespective of whether any subjective evidence is available for those cognitive symptoms. 26 , 57
4.1. Study limitations
It is important to note that the present study has several limitations, starting with a possible recruitment bias based on antidepressant therapy. Although we found no difference in terms of type or duration of antidepressant therapy and HDRS severity between individuals with amnestic depression and depressive patients, more anxious patients, who were particularly interested in the study, may have been in the amnestic depression group. Second, our depressive group was not drug‐naïve, which may, over the long term, have had an effect on cognitive factors and brain connectivity changes, although all drug groups and treatment durations were homogenously distributed. Third, there is no validated method for calculating self‐reported cognitive awareness in depression, which makes a statistical comparison of self‐reported scores and functional connectivity impossible. Despite these limitations, however, our findings go beyond merely replicating previously reported general results concerning altered network activity in depression. Our findings extend our current understanding of cognition by linking subjective cognitive awareness with specially altered network activity and the severity of symptoms in patients with MDD.
5. CONCLUSIONS
In conclusion, our findings, together with those from other studies, suggest that the pulvinar plays a unique role in orchestrating structural and functional changes in the pathology of depression and also in the treatment response. 35 , 54 Our findings not only confirm previously reported results concerning objective cognitive impairment in MDD, but also highlight the potential role of the pulvinar in discriminating between subjective and objective cognitive complaints in patients with MDD. They also emphasize that diagnostic approaches should be strengthened by means of practicable dynamic neuroimaging modalities, an approach which may help to overcome the current difficulties in the diagnosis of cognitive impairment in MDD. This may also be of great importance for the future management of depressive patients.
AUTHOR CONTRIBUTIONS
Conceptualization: B.Y., S.A., and D.S. Methodology: S.A., S.C., B.A., R.K., L.I., A.B.S., C.M., and N.Y. Investigation: E.O.O., A.O., and C.M. Writing – original draft preparation: B.Y., D.S., R.K., S.A., and B.A. Writing – review and editing: B.Y., D.S., R.K., and L.I. Supervision: L.H. and H.A.V. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the Supporting information.
Supporting information
Supporting Information
Suptable 3c
ACKNOWLEDGMENTS
The authors declare that no funding was received from any source for this study.
Yulug B, Ayyildiz S, Sayman D, et al. The functional role of the pulvinar in discriminating between objective and subjective cognitive impairment in major depressive disorder. Alzheimer's Dement. 2024;10:1–11. 10.1002/trc2.12450
Burak Yulug, Sevilay Ayyildiz, and Halil Aziz Velioglu contributed equally to this study and share senior authorship.
REFERENCES
- 1. Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur J Pharmacol. 2010;626(1):83‐86. [DOI] [PubMed] [Google Scholar]
- 2. Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacol. 2012;37(1):117‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaremba D, Kalthoff IS, Förster K, et al. The effects of processing speed on memory impairment in patients with major depressive disorder. Prog Neuro‐Psychoph. 2019;92:494‐500. [DOI] [PubMed] [Google Scholar]
- 4. Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3‐year prospective study. Psychol Med. 2011;41(6):1165‐1174. [DOI] [PubMed] [Google Scholar]
- 5. Knight MJ, Air T, Baune BT. The role of cognitive impairment in psychosocial functioning in remitted depression. J Affect Disorders. 2018;235:129‐134. [DOI] [PubMed] [Google Scholar]
- 6. Knight MJ, Lyrtzis E, Baune BT. The association of cognitive deficits with mental and physical Quality of Life in Major Depressive Disorder. Compr Psychiat. 2020;97:152147. [DOI] [PubMed] [Google Scholar]
- 7. Koenig AM, Bhalla RK, Butters MA. Cognitive functioning and late‐life depression. J Int Neuropsych Soc. 2014;20(5):461‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467‐477. [DOI] [PubMed] [Google Scholar]
- 9. Furtado CP, Maller JJ, Fitzgerald PB. A magnetic resonance imaging study of the entorhinal cortex in treatment‐resistant depression. Psychiat Res‐Neuroim. 2008;163(2):133‐142. [DOI] [PubMed] [Google Scholar]
- 10. Schlösser RGM, Wagner G, Koch K, Dahnke R, Reichenbach JR, Sauer H. Fronto‐cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage. 2008;43(3):645‐655. [DOI] [PubMed] [Google Scholar]
- 11. Harvey PO, Fossati P, Pochon JB, et al. Cognitive control and brain resources in major depression: an fMRI study using the ‐back task. Neuroimage. 2005;26(3):860‐869. [DOI] [PubMed] [Google Scholar]
- 12. Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using, ultra‐high resolution MRI: application to adaptive segmentation of MRI. Neuroimage. 2015;115:117‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iglesias JE, Insausti R, Lerma‐Usabiaga G, et al. A probabilistic atlas of the human thalamic nuclei combining MRI and histology. Neuroimage. 2018;183:314‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiong G, Dong DF, Cheng C, et al. Potential structural trait markers of depression in the form of alterations in the structures of subcortical nuclei and structural covariance network properties. Neuroimage‐Clin. 2021;32:102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Velioglu HA, Ayyildiz B, Ayyildiz S, et al. A structural and resting‐state functional connectivity investigation of the pulvinar in elderly individuals and Alzheimer's disease patients. Alzheimers Dement. 2023;19(7):2774‐2789. [DOI] [PubMed] [Google Scholar]
- 16. Yulug B, Ayyıldız B, Ayyıldız S, et al. Infection with COVID‐19 is no longer a public emergency: but what about degenerative dementia? J Med Virol. 2023;95(9):e29072. [DOI] [PubMed] [Google Scholar]
- 17. Berron D, van Westen D, Ossenkoppele R, Strandberg O, Hansson O. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer's disease. Brain. 2020;143:1233‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karunakaran KD, Yuan R, He J, et al. Resting‐state functional connectivity of the thalamus in complete spinal cord injury. Neurorehab Neural Re. 2020;34(2):122‐133. [DOI] [PubMed] [Google Scholar]
- 19. Arend I, Henik A, Okon‐Singer H. Dissociating emotion and attention functions in the pulvinar nucleus of the thalamus. Neuropsychology. 2015;29(2):191‐196. [DOI] [PubMed] [Google Scholar]
- 20. Guedj C, Vuilleumier P. Functional connectivity fingerprints of the human pulvinar: decoding its role in cognition. Neuroimage. 2020;221:117162. [DOI] [PubMed] [Google Scholar]
- 21. Koizumi A, Zhan MY, Ban H, et al. Threat anticipation in the pulvinar and superficial layers of the primary visual cortex (V1). Evidence from layer‐specific ultra‐high‐field 7T fMRI. Eneuro. 2019;6(6):ENEURO.0429‐19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Romanski L, Giguere M, Bates J, Goldman‐Rakic P. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1997;379(3):313‐332. [PubMed] [Google Scholar]
- 23. McTeague LM, Rosenberg BM, Lopez JW, et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiat. 2020;177(5):411‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bell PT, Shine JM. Subcortical contributions to large‐scale network communication. Neurosci Biobehav R. 2016;71:313‐322. [DOI] [PubMed] [Google Scholar]
- 25. Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann Ny Acad Sci. 2015;1345:36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease—systematic review, meta‐analysis, and metaregression analysis. Arch Gen Psychiat. 2006;63(5):530‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson L, Adams S. Cognitive deficits in patients with depression. Jnp‐J Nurse Pract. 2018;14(6):437 ‐+. [Google Scholar]
- 28. Semkovska M, Quinlivan L, O'Grady T, et al. Cognitive function following a major depressive episode: a systematic review and meta‐analysis. Lancet Psychiat. 2019;6(10):851‐861. [DOI] [PubMed] [Google Scholar]
- 29. Smith GS, Kuwabara H, Nandi A, et al. Molecular imaging of beta‐amyloid deposition in late‐life depression. Neurobiol Aging. 2021;101:85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700‐711. [DOI] [PubMed] [Google Scholar]
- 31. Proitsi P, Lupton MK, Reeves SJ, et al. Association of serotonin and dopamine gene pathways with behavioral subphenotypes in dementia. Neurobiol Aging. 2012;33(4):791‐803. [DOI] [PubMed] [Google Scholar]
- 32. Rayner G, Jackson G, Wilson S. Cognition‐related brain networks underpin the symptoms of unipolar depression: evidence from a systematic review. Neurosci Biobehav R. 2016;61:53‐65. [DOI] [PubMed] [Google Scholar]
- 33. Steffens DC, Payne ME, Greenberg DL, et al. Hippocampal volume and incident dementia in geriatric depression. Am J Geriat Psychiat. 2002;10(1):62‐71. [PubMed] [Google Scholar]
- 34. Zackova L, Jani M, Brazdil M, Nikolova YS, Mareckova K. Cognitive impairment and depression: meta‐analysis of structural magnetic resonance imaging studies. Neuroimage‐Clin. 2021;32:102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kraus C, Klöbl M, Tik M, et al. The pulvinar nucleus and antidepressant treatment: dynamic modeling of antidepressant response and remission with ultra‐high field functional MRI (vol. 24, p. 746, 2018). Mol Psychiatr. 2019;24(5):746‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perini G, Ramusino MC, Sinforiani E, Bernini S, Petrachi R, Costa A. Cognitive impairment in depression: recent advances and novel treatments. Neuropsych Dis Treat. 2019;15:1249‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knight MJ, Fourrier C, Lyrtzis E, et al. Cognitive deficits in the THINC‐Integrated Tool (THINC‐it) are associated with psychosocial dysfunction in patients with major depressive disorder. J Clin Psychiat. 2019;80(1):18m12472. [DOI] [PubMed] [Google Scholar]
- 38. McIntyre RS, Subramaniapillai M, Park C, et al. The THINC‐it tool for cognitive assessment and measurement in major depressive disorder: sensitivity to change. Front Psychiatry. 2020;11:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ragguett RM, Cha DS, Kakar R, Rosenblat JD, Lee Y, McIntyre RS. Assessing and measuring cognitive function in major depressive disorder. Evid‐Based Ment Heal. 2016;19(4):106‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Velioglu HA, Ayyildiz B, Ayyildiz S, et al. A structural and resting‐state functional connectivity investigation of the pulvinar in elderly individuals and Alzheimer's disease patients. Alzheimers Dement. 2023;19(7):2774‐2789. [DOI] [PubMed] [Google Scholar]
- 41. DUMAN B, TÖ KIZIL, BARAN Z, Kirici S, Turan E. A comparison between subjective memory complaints and objective memory deficits in elderly patients with depression or mild cognitive impairment. Turk Psikiyatr Derg. 2016;27(1):1‐7. [PubMed] [Google Scholar]
- 42. Choi J, Lee S, Motter JN, et al. Models of depressive pseudoamnestic disorder. Alzh Dement‐Trci. 2022;8(1):e12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lanza C, Sejunaite K, Steindel C, Scholz I, Riepe MW. Cognitive profiles in persons with depressive disorder and Alzheimer's disease. Brain Commun. 2020;2(2):fcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Folstein MF. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 45. Smith SH, Reth M. Perspectives on the nature of BCR‐mediated survival signals. Mol Cell. 2004;14(6):696‐697. [DOI] [PubMed] [Google Scholar]
- 46. Thompson PM, Stein JL, Medland SE, et al. The ENIGMA Consortium: large‐scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8(2):153‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coupé P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3‐D magnetic resonance images. Ieee T Med Imaging. 2008;27(4):425‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo‐planar mri. Magnet Reson Med. 1995;34(4):537‐541. [DOI] [PubMed] [Google Scholar]
- 49. Thomas EJ, Elliott R. Brain imaging correlates of cognitive impairment in depression. Front Hum Neurosci. 2009;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bhome R, Zarkali A, Thomas G, Iglesias J, Cole J, Weil R. Thalamic white matter macrostructure and subnuclei volumes in Parkinson's disease depression. Movement Disord. 2022;37:S366‐S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fredericks CA, Brown JA, Deng J, et al. Intrinsic connectivity networks in posterior cortical atrophy: a role for the pulvinar? Neuroimage‐Clin. 2019;21:101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bennabi D, Haffen E, Van Waes V. Vortioxetine for cognitive enhancement in major depression: from animal models to clinical research. Front Psychiatry. 2019;10:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wei Q, Bai TJ, Brown EC, et al. Thalamocortical connectivity in electroconvulsive therapy for major depressive disorder. J Affect Disorders. 2020;264:163‐171. [DOI] [PubMed] [Google Scholar]
- 54. Tadayonnejad R, Ajilore O, Mickey BJ, et al. Pharmacological modulation of pulvinar resting‐state regional oscillations and network dynamics in major depression. Psychiat Res‐Neuroim. 2016;252:10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Penner J, Osuch EA, Schaefer B, et al. Higher order thalamic nuclei resting network connectivity in early schizophrenia and major depressive disorder. Psychiat Res‐Neuroim. 2018;272:7‐16. [DOI] [PubMed] [Google Scholar]
- 56. Yulug B, Altay O, Li XY, et al. Combined metabolic activators improve cognitive functions in Alzheimer's disease patients: a randomised, double‐blinded, placebo‐controlled phase‐II trial. Transl Neurodegener. 2023;12(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pan ZH, Park C, Brietzke E, et al. Cognitive impairment in major depressive disorder. Cns Spectrums. 2019;24(1):22‐29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Suptable 3c