Abstract
Bordetella avium causes an upper-respiratory-tract disease called bordetellosis in birds. Bordetellosis shares many of the clinical and histopathological features of disease caused in mammals by Bordetella pertussis and Bordetella bronchiseptica. In this study we determined several parameters of infection in the domestic turkey, Meleagris galapavo, and compared these in vivo findings with an in vitro measure of adherence using turkey tracheal rings. In the in vivo experiments, we determined the effects of age, group size, infection duration, and interindividual spread of B. avium. Also, the effect of host genetic background on susceptibility was tested in the five major commercial turkey lines by infecting each with the parental B. avium strain and three B. avium insertion mutants. The mutant strains lacked either motility, the ability to agglutinate guinea pig erythrocytes, or the ability to produce dermonecrotic toxin. The susceptibilities of 1-day-old and 1-week-old turkeys to B. avium were the same, and challenge group size (5, 8, or 10 birds) had no effect upon the 50% infectious dose. Two weeks between inoculation and tracheal culture was optimal, since an avirulent mutant (unable to produce dermonecrotic toxin) persisted for a shorter time. Communicability of the B. avium parental strain between confined birds was modest, but a nonmotile mutant was less able to spread between birds. There were no host-associated differences in susceptibility to the parental strain and the three B. avium mutant strains just mentioned: in all turkey lines tested, the dermonecrotic toxin- and hemagglutination-negative mutants were avirulent whereas the nonmotile mutants showed no loss of virulence. Interestingly, the ability of a strain to cause disease in vivo correlated completely with its ability to adhere to ciliated tracheal cells in vitro.
All of the principal Bordetella species, B. pertussis, B. parapertussis, B. bronchiseptica, and B. avium, can cause upper respiratory disease. In many instances, the pathogenesis involves the interaction of the bacteria with ciliated tracheal epithelial cells (2, 22, 30), resulting first in ciliastasis and eventually in death of the ciliated cells (26, 31, 36). Further, all species can cause diseases with similar tracheal lesions and outward signs and symptoms that involve ocular-nasal discharge and persistent severe coughing (5, 28, 32). However, each Bordetella species causes the hallmark signs of disease only in particular hosts. In the case of B. pertussis, children are susceptible and the disease is called whooping cough (reviewed in reference 40). B. parapertussis causes a milder form of whooping cough in humans and a chronic pneumonia in lambs (6). B. bronchiseptica infects the upper respiratory tract of a number of domestic, companion, and laboratory animals and can cause a variety of upper respiratory diseases in these hosts (e.g., kennel cough in dogs [41]), most of which are complicated when seen naturally (reviewed in reference 16). With B. avium, birds are the susceptible host and the disease produced is called avian bordetellosis or turkey coryza, the latter name reflecting the most economically important animal commonly afflicted (reviewed in reference 32).
B. parapertussis, B. bronchiseptica, and B. avium all have characters associated with virulence in the type species, B. pertussis (reviewed in reference 39). Of those characters, B. avium has the smallest subset: dermonecrotic toxin (DNT), tracheal cytotoxin, hemagglutination, and fimbriae (reviewed in reference 32). Also, B. avium is the furthest removed from the other Bordetella species by systematic measurements (8). These observations might lead one to suspect that B. avium is fundamentally different from the other Bordetella species in its method of disease production. However, it may be that generation of the basic tracheal lesion and production of the most pronounced clinical features of the disease in the natural host depend upon characteristics (e.g., tracheal cytotoxin [7]) common to all Bordetella species. This possibility remains open in part due to the lack of a practical experimental animal that gets the clinically pronounced symptoms and the histological features of bordetellosis caused by B. pertussis and B. bronchiseptica under well-controlled experimental conditions (16, 39).
Turkeys (Meleagris galapavo) are plentiful and readily show pronounced signs of bordetellosis under experimental conditions (27). Whereas birds are indeed evolutionarily distant from humans and other mammals, the insights gained from a systematic study of avian bordetellosis may be useful in understanding the pathogenesis of all Bordetella infections. Furthermore, avian bordetellosis is of significant economic concern to producers of turkeys worldwide (32). Turkeys grow faster and use feed more efficiently than chickens (10). Consequently, worldwide agriculture has an interest in their development as a food source.
As a first step in analyzing B. avium virulence, we have systematically examined several variables in the experimental infection. These include a statistical analysis of the effects of turkey age at infection, the time required for tracheal colonization, the effect of group size on 50% infectious dose (ID50) measurements, and the communicability of B. avium within groups. In addition, since turkeys are not inbred animals (but can be divided into distinct strains or varieties, referred to here as lines to avoid confusion with bacterial strains), we examined the five major commercial lines for possible host-associated differences in susceptibility to the parental strain and three mutant strains of B. avium. Finally, we developed and employed an in vitro turkey tracheal ring assay that examined the adhesion of the B. avium parental and mutant strains.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. avium strains used in this study are all derivatives of strain 197N, a spontaneous nalidixic acid-resistant mutant of strain 197 (14). Strain 197 was chosen from a laboratory collection of three B. avium strains that were tested for virulence. One strain, GOBL271 (15) was of reduced virulence compared to the other two. Strain 197 was the better described of the two remaining (14) and was chosen for our studies. All bacterial strains, plasmids, and one generalized transducing phage are described in Table 1. B. avium and Escherichia coli (when used for matings) were grown on brain heart infusion (BHI) medium (Difco) at 37°C. Broth cultures were shaken vigorously. E. coli was routinely maintained on medium composed of L broth or L agar (Difco). Lactose MacConkey agar (composed of 1% lactose and MacConkey agar base [Difco]) was used to enrich and isolate B. avium from infected turkey tracheas. B. avium minimal medium was prepared as described previously (21) except that dextrose was omitted. Stainer-Scholte agar (18) was prepared with modifications for B. avium (SSM agar [14]) and contained 10 mM MgSO4 when used for mating. Bordet-Gengou agar was prepared as directed (Difco) with 15% sheep blood added.
TABLE 1.
Bacterial strains, bacteriophage, and plasmids used
| Strain, plasmid, or bacteriophage | Description | Source or reference |
|---|---|---|
| B. avium strains | ||
| 197a | Wild type | 14 |
| 197N | Parental strain; Dnt+ Hag+ Mot+ Nalr Kans | This study |
| P206 | Parental except for mini-Tn5phoA creating hag206; Hag− Kanr | This study |
| G146 | Parental except for mini-Tn5lacZ2 creating mot146; Mot− Kanr | This study |
| WBA16 | Parental except for neoR insertion from pKEW16-7 in dnt1, a locus required for DNT production; Dnt− Kanr | This study |
| E. coli strains | ||
| CC118(λpir) | Host for pUT/mini-Tn5 plasmids | 9 |
| MM294 | Host for pRK2013, a mobilizing plasmid for pUT/mini-Tn5 plasmids | 4 |
| Plasmids | ||
| pUT/mini-Tn5phoA | Contains a mini-Tn5 transposon encoding a promotorless phoA gene, used for making translational fusions with secreted proteins | 9 |
| pUT/mini-Tn5lacZ2 | Contains a mini-Tn5 transposon encoding a promotorless lacZ gene, used for making translational fusions | 9 |
| pRK2013 | RP4 mobilization plasmid containing a ColE1 replicon | 13 |
| pKEW16-7b | pSS1129 containing a NotI fragment of the B. pertussis dnt gene interrupted by a neoR gene insertion from Tn903 | 37 |
| pSS1129 | Mobilizable cloning vector, nonreplicating in B. avium | 34 |
| Bacteriophage | ||
| Ba1 | B. avium transducing phage | This study |
Our isolate of 197N was found to lack a plasmid conferring tetracycline resistance. Consequently, our 197N strain, in contrast to the referenced parental strain 197, is tetracycline sensitive.
Source of animals and housing conditions.
The various lines of turkeys used in this study were obtained from three sources depending upon the type of experiment to be performed. (i) Turkey poults used for comparing line susceptibility were obtained as breeding stock from a commercial supplier, and the resulting poults were laid and hatched at the North Carolina State University Poultry Science facility. Birds for these experiments were obtained at hatching from this facility. (ii) For studies involving tracheal ring assays, fertilized eggs of the BUT-A line were obtained from the British United Turkeys of America breeding location in Lewisburg, W.Va. These embryonated eggs were incubated in a Kuhl (Flemington, N.J.) model AT-600-110 incubator for 26 days before sacrifice and use. (iii) Birds not specifically used in host susceptibility tests were obtained from Tarheel Turkey Hatchers in Raeford, N.C., and from the British United Turkeys of America breeding location in Lewisburg, W.Va., at 1 day after hatching. Hatched birds were kept at the AAALAC-accredited facility at North Carolina State University and housed in stainless steel brooders (a maximum of 10 birds were kept in a single 61- by 91- by 28-cm brooder) for the 3 weeks (average) needed to complete an experiment.
Standard infection protocol.
The standard protocol for infecting turkeys was established after a number of parameters had been examined. Some of these parameters are described in Results. Features common to all experiments are mentioned here. Just prior to an experiment, approximately 5% of the population was removed (three to five birds) and tracheal cultures and serum samples were obtained. Serum samples were evaluated for antibody to B. avium in a slide agglutination test performed by Rollins Animal Diagnostic Laboratory, Raleigh, N.C. In all experiments, the tested birds were uniformly negative in both cultural and immunological tests. Birds were infected by B. avium with an inoculum obtained from overnight, 37°C, BHI plate-grown cultures that had been resuspended in phosphate-buffered saline (PBS) to give three concentrations necessary for an ID50 determination. Parent and mutant microorganisms were always compared in a given experiment in order to detect any variation in the general health of received birds. Each experiment involved an analysis of the fraction of birds infected (infection rate) in three groups of 10 birds per group, each group given a 10-fold increasing dose of B. avium. Each experiment also contained a group of control (PBS sham-inoculated) birds of the same size as an inoculated group (10 birds).
The inoculum was always administered in the right eye and right nostril (0.1 ml in each). Colonization was assessed, unless otherwise noted, at 2 weeks postinoculation by exposing the tracheal opening and inserting a swab approximately halfway down the bird’s trachea. Swabs were expressed onto lactose MacConkey agar petri plates and examined after 48 h for B. avium. Common pharyngeal contaminants grow poorly on MacConkey agar, and the asaccharolytic B. avium has a distinctive colonial appearance that makes presumptive identification straightforward (19). Single colony isolates, obtained on MacConkey agar, were patched onto selective medium with sterile toothpicks to confirm the identity of the infecting strain (both parental and mutant strains were nalidixic acid resistant, and each mutant was kanamycin resistant). Phenotypic characterization (e.g., hemagglutination and motility), analysis of any revertants, and calculation of the ID50 followed. A bird was termed colonized if B. avium exhibiting the inoculated phenotype was recovered. The proportion of the birds infected within each group was used to quantitate the infection rate. The method of Reed and Meunch (29) was used to calculate the ID50.
Genetic methods.
B. avium hemagglutination (Hag−) and motility (Mot−) mutants were generated by using pUT/mini-Tn5lacZ2 and -Tn5phoA plasmids constructed by DeLorenzo et al. (9). A triparental mating was performed by mixing overnight broth cultures of (i) E. coli MM294 containing mating plasmid pRK2013, (ii) E. coli CC118 (λpir) containing the pUT/mini-Tn5 plasmid, and (iii) the parental strain B. avium 197N in a 1:1:10 ratio. The mating mixture was dropped onto the surface of dry SSM agar plates (without selective antibiotics). Mating was allowed to proceed for 4 to 8 h at 35°C, after which the mixture was removed with a cotton swab and plated on selective L agar (containing, per ml, 30 μg of nalidixic acid, 150 μg of kanamycin, and 40 μg of 5-bromo-6-chloro-3-indolyl-β-d-galactoside or 5-bromo-6-chloro-3-indolyl phosphate) and incubated for 2 days at 35°C. Blue B. avium exconjugants were patched onto selective medium plates and tested for hemagglutination and motility phenotypes. Prospective Hag− and Mot− mutants were colony purified, the phenotypes were rechecked, and mutant strains were stored in 50% glycerol–50% L broth at −80°C. The DNT mutant (Dnt−) was isolated by insertion mutagenesis, except that in this case a cloned B. pertussis dnt gene was interrupted in vitro following cloning onto a mobilizable suicide plasmid (see Table 1 and Results).
Generalized transduction of B. avium was accomplished by using a newly discovered transducing phage, Ba1 (unpublished data). Approximately 108 PFU were used to infect 0.2 ml of a 37°C overnight BHI broth culture of B. avium (approximately 1010 CFU). After 15 min of incubation for adsorption at room temperature, the mixture was diluted to 1.0 ml in BHI broth and the bacteria were isolated by centrifugation. The cell pellet was resuspended in 1.0 ml of BHI and incubated for 1 h at 37°C with shaking. After incubation, cells were pelleted, resuspended in 0.1 ml of 0.15 N NaCl, and plated on BHI medium containing 40 mg of kanamycin per ml.
DNT measurements.
Cell-free lysates of bacteria were obtained by first growing B. avium from glycerol stock cultures on SSM agar plates at 35°C for 24 to 36 h. Bacterial cell suspensions (approximately 108 bacteria/ml) were prepared in sterile saline and sonicated (Branson Sonifier model 350; 1-cm tip) on ice at 50% power for 2 min (or until the optical density at 600 nm of the suspension was reduced by at least 50%). The sonicate was then subjected to centrifugation for 1 h at 100,000 × g at 5°C, and the supernatant was sterilized by passage through a 0.2-μm filter. DNT activity of bacterial preparations was determined in 7- to 8-day-old albino outbred Swiss Webster mice. Sonicated cell suspensions (0.05 ml/mouse) were injected intradermally on one side of the back. The mice were marked to distinguish different treatments and kept with the mother. They were regularly observed for 2 days to note the time of lesion appearance. Dark purple to black lesions at the inoculation site were considered DNT positive.
Motility and flagellin assay.
B. avium strains were tested for motility by touching an isolated colony with a sterile toothpick and stabbing it into SSM containing 0.4% agar. Plates were observed for expanding zones of bacterial growth, indicating motility, after 24 h at 35°C. The presence of flagella on nonmotile mutants was checked by transmission electron microscopy (TEM) and by colony immunoblotting using monoclonal antibody to E. coli flagellin as described by Feng et al. (11).
Hemagglutination assay.
Suspensions of 1010 bacteria/ml were prepared from strains grown on L agar overnight at 35°C. These were isolated by centrifugation and resuspended to give 1011 cells/ml in normal saline (0.15 N NaCl), and 0.1 ml of each suspension was placed in the first well of a round- or pointed-bottom 96-well plate (Costar). Normal saline (50 μl/well) was added to each subsequent well in the row, and serial twofold bacterial dilutions were made. Guinea pig erythrocytes (Cocalico, Reamstown, Pa.) that were packed at 500 × g for 5 min were used to prepare a 1% suspension in normal saline. The erythrocytes (50 μl) were then added to each well containing bacteria. After mixing, the plate was covered and incubated at 4°C for 4 to 12 h and observed. The lowest dilution in which no button was visible was recorded as the hemagglutination titer for that sample.
Tracheal attachment assay.
Bacterial strains were grown overnight on Bordet-Gengou agar containing nalidixic acid (30 μg/ml) and 15% sheep blood at 35°C. The bacteria were harvested in magnesium-free Earle’s balanced salt solution (EBSS) (Sigma Chemical Co., St. Louis, Mo.) containing 1.0 mM CaCl2 and diluted to approximately 2 × 107 bacteria/ml, and 0.5 ml of suspension was placed in each well of a 24-well plate (Costar). Twenty-six-day-old turkey embryos were decapitated, the tracheae were removed, and transverse 2-mm-long rings were cut. Three tracheal rings were placed into each well containing EBSS with or without bacteria and incubated at 42°C for 3 h with constant rocking. Microscopic observation revealed beating cilia for 4 to 6 h postculturing. Also, scanning electron microscopy (SEM) revealed an intact ciliated layer for the same time period. Upon completion of incubation, the rings were washed with rocking three times with 1.0 ml of EBSS for 2 min each at 42°C. The rings were removed, placed individually into separate tubes containing 1.0 ml of PBS with 1% Triton X-100, incubated at 4°C for 1 to 2 h, and then mixed for 1 min on a vortex mixer to distribute the bacteria. Triton X-100, at this concentration, had no effect on bacterial viability. Dilutions were plated out onto lactose MacConkey agar and incubated for 2 days at 35°C. Resulting colonies were counted, and the numbers of CFU/tracheal ring were calculated. Tracheal rings to be used for SEM were incubated with approximately 109 bacteria for 3 h. Washed rings were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.6, overnight and prepared for electron microscopy.
Recombinant DNA techniques.
Conditions for restriction endonuclease digestion, chromosomal and plasmid DNA isolation, and agarose gel electrophoresis have been previously described (3). Southern blots (33) of the Hag−, Mot−, and Dnt− strains were prepared from EcoRI-digested B. avium chromosomal DNA and a probe derived from the kanamycin resistance gene of Tn903 (bp 581 to 1501; GenBank accession no. X06404). Probes were prepared by random priming of the BamHI-XhoI fragment with biotinylated nucleotides by using a kit from Life Technologies (Gaithersburg, Md.) and detected by chemiluminescence (Tropix, Inc., Bedford, Mass.), as directed by the manufacturer.
Electron microscopy.
TEM was performed with overnight cultures grown on agar at 31°C. A drop of water containing some of the overnight growth was placed on Formvar-coated grids, the grids were rinsed, and the cells were stained with 2% phosphotungstic acid. Negatively stained preparations were examined on a Philips 910 TEM. For SEM, the glutaraldehyde-fixed tracheal ring samples were dehydrated through an ethanol-acetone series, critically point dried with liquid CO2, mounted on stubs, and sputter coated with gold-palladium (60:40), and images were acquired by the PGTIMIX image analysis system and a Topcon ABT-35 SEM at 20 kV.
Histological methods.
Tracheae were obtained at necropsy from birds at 2 weeks postinfection. Longitudinal sections were fixed in 10% buffered formalin, dehydrated, and embedded in paraffin. Sections (4 μm) were stained with hematoxylin-eosin. All microscopic histological material was viewed by an avian pathologist. Samples were coded to assure objectivity.
Statistical methods.
The statistical package developed by Microsoft, EXCEL 4.0, was used to calculate parameters (e.g., standard deviations [SD] and averages) and to determine statistical significance. Specific statistical tests (e.g., analysis of variance [ANOVA], t test) are noted, where applicable, in the text.
RESULTS
Identification and characterization of nonmotile mutants of B. avium.
Screening of several hundred Tn5lacZ2 and Tn5phoA insertion mutants of strain 197N identified four isolates that showed no motility. The motility-negative mutant (Mot−) chosen for this study (strain G146) had a Tn5lacZ2 translational fusion, did not have flagella when examined by TEM, and did not react to a monoclonal antibody (11) directed against a conserved flagellin epitope from E. coli (12). In contrast, the parental strain was positive for both features. Cotransduction experiments using Ba1 and Southern blotting with a probe made to the neoR gene both indicated that a single insertion was responsible for the mutant phenotype (data not shown). We designated the locus interrupted by this insertion mot146. No revertants to the wild type were detected under in vivo selective pressure.
Identification and characterization of hemagglutination mutants of B. avium.
Over 20 independent insertion mutants of strain 197N deficient in hemagglutination were identified. The hemagglutination-negative mutant (Hag−) chosen for this study (strain P206) had a Tn5phoA translational fusion and required at least 30 times more bacteria than the parent to show hemagglutinating activity. Cotransduction experiments using Ba1 and Southern blotting with a probe made to the neoR gene both indicated that a single insertion was responsible for the mutant phenotype (data not shown). We designated the locus interrupted by this insertion hag206. In contrast to the stable Mot− mutant, the Hag character was more problematic due to strong in vivo selection. All Hag− mutants inoculated were recovered as Kanr Hag+ pseudorevertants. That is, isolates still had the original insertion but also a second (undefined) lesion that restored the hemagglutination-positive phenotype (data not shown).
Identification and characterization of a DNT-negative B. avium mutant.
A DNT-negative mutant of strain 197N (strain WBA16) was isolated following a recombination event between the chromosome of strain 197N and the suicide plasmid pKEW16-7 (37). Plasmid pKEW16-7 carries the B. pertussis DNT gene insertionally inactivated by a neoR gene from Tn903 (Table 1). Strain WBA16 was negative for DNT production (Dnt−) when assayed in the infant mouse model (see Materials and Methods). Southern blot hybridization using a 500-bp neoR gene fragment as a probe and the antibiotic resistance phenotype of WBA16 were consistent with a single, double-crossover insertion event (37). Further, in vivo selection (ostensibly for DNT production) revealed a 100% correlation between restoration of the DNT-positive phenotype and loss of the neoR insertion. (Coreversion data was gathered from 25 Kans clonal isolates of WBA16, each independently isolated from 25 turkeys.) We have designated the locus interrupted by the neoR insertion dnt1. In spite of the above, we have been unable to formally conclude that the dnt1 locus is actually the structural gene for DNT. In part, this is because of the low homology between the B. pertussis dnt gene and its B. avium counterpart.
Defining parameters for differentiating virulent and avirulent B. avium in vivo.
The in vivo infection protocol (Materials and Methods) was based upon observations with the parental strain 197N and one of its derivatives, strain WBA16, the Dnt− mutant. In pilot experiments, these two strains represented the extremes of virulence and avirulence, respectively. Several parameters were examined: (i) the age of the birds at infection, (ii) the time required to colonize, (iii) the number of birds per group used in ID50 determinations, and (iv) communicability of B. avium within groups.
(i) The age of the birds at infection (1- and 7-day-old poults were tested) did not influence virulence. The parental strain produced statistically the same ID50 in both age groups, and the Dnt− mutant was avirulent (i.e., at least 100-fold less virulent) in both groups (Table 2). Our standard protocol adopted 1-week-old birds routinely because 1-day-old birds occasionally died for unknown reasons. Infecting birds older than 1 week was impractical because the birds became difficult to house properly by the end of the experiments.
TABLE 2.
Susceptibilities of birdsa at different ages to the parental strain (197N) and an avirulent (Dnt−) mutant (WBA16)
| B. avium strainb | Avgc ID50 (SD) for birds of age:
|
|
|---|---|---|
| 1 day | 7 days | |
| Parent | 7.5 × 106 (6.1 × 106) | 6.1 × 106 (5.7 × 106) |
| Dnt− | >109 (NCd) | >109 (NC) |
Turkeys were all of the BUT-A line.
A description of the strains can be found in Table 1. The inoculation protocol is given in the text.
For 1- and 7-day-old birds that were inoculated with the parental strain, average ID50 values expressed as CFU were calculated from seven experiments. For the Dnt− strain, three experiments with 1-day-old birds produced only one avirulent isolate in one experiment, which was recovered from a group of birds inoculated with 1.5 × 109 CFU. For 7-day-old birds, in two experiments using the mutant strain only two isolates (one from one bird in each experiment) were recovered from birds inoculated with approximately 2 × 109 CFU.
NC, not possible to calculate due to low numbers of birds inoculated.
(ii) The ability of B. avium to effect sustained colonization was tested by studies in which birds were sampled at various times after inoculation for the presence of the infecting strain. Two weeks was chosen for the duration of the standard experiment because this was the point at which the parental strain was present but the Dnt− mutant had been cleared (Table 3). Earlier times produced more equivocal results.
TABLE 3.
Proportion of birds colonized by the parental strain (197N) compared to an avirulent Dnt− mutant strain (WBA16) as a function of time
| Days postinfectiona | % (SD) of birds colonizedb
|
|
|---|---|---|
| Parent | Dnt− | |
| 0 | 0 (0) | 0 (0) |
| 3 | 100 (NCc) | 20 (NC) |
| 7 | 100 (0) | 25 (35) |
| 14 | 100 (0) | 0 (0) |
Zero refers to preinoculated birds.
Five birds were removed on each designated day and swabbed for B. avium, as described in the text. All birds received an average of 2 × 1010 CFU of B. avium on day zero and were kept in groups of 10 birds per brooder. Birds used were of the BUT-A line.
NC, not possible to calculate because only one experiment examined the 3-day time point. Other values are averages of two separate experiments.
(iii) The number of birds per group (i.e., the number given the same dose of B. avium and then housed together) could have a number of influences upon the assessment of virulence in several ways. For example, bird density would be expected to influence the spreading of bacteria between birds. We found that group size (measured from as few as 5 to as many as 10 birds per group) did not affect the calculation of ID50 significantly (Table 4). However, both the ID50 values and the SD of mean ID50 values decreased as the size of the group became larger (Table 4). Both trends should be expected: a greater bird density would tend to make any communicable disease easier to maintain, and larger bird numbers should produce a smaller SD.
TABLE 4.
Effects of the number of birds per group on ID50
| No. of birds/groupa | No. of exptsb | Avg ID50 (SD)c |
|---|---|---|
| 5 | 3 | 1.3 × 107 (1.4 × 107) |
| 8 | 2 | 8.2 × 106 (9.8 × 106) |
| 10 | 4 | 4.7 × 106 (4.1 × 106) |
Birds were kept in standard 61- by 91- by 28-cm stainless steel brooders at group numbers shown and were infected as described in the standard protocol for determining ID50 values. Birds utilized were of the Nicholas line.
Number of separate experiments performed at each group size.
Single-factor ANOVA revealed that there were no significant differences in the means for the different groups (P > 0.05).
(iv) The communicability of B. avium within the groups was compared by using the parental strain and the nonmotile (Mot−) mutant. The Mot− mutant was used in this case (rather than the Dnt− mutant) because Dnt− mutants were cleared much too rapidly for communicability comparisons. In these experiments, an index case (a single bird) was inoculated and added to a group of nine uninfected birds. The Mot− mutant was significantly less able to spread within groups than the parental strain (Table 5), indicating a possible role for motility in communicability. However, the spread of the parent was not extensive: An average of two birds were infected for each infected bird (Table 5). Further, this result was witnessed only when the index case was given a very large dose (approximately 108 CFU) and spreading was assayed after a short time interval (1 week postinoculation rather than the normal 2-week experiment). If lower doses or longer incubation times were used, the index case tended to lose its infection rather than pass it to other birds (data not shown).
TABLE 5.
Relative communicabilitiesa of the parental B. avium strain and a nonmotile (Mot−) mutant
| Strain | Total no. of birds | Avg no. of birds infected from an index bird (SD)b |
|---|---|---|
| Parent | 50 | 2.4 (0.39) |
| Mot− | 30 | 1.0 (0.33) |
Results of independent measurements with 10 birds per group with a single index case inoculated with approximately 108 CFU, per the standard protocol described in the text.
Results analyzed by Student’s t test. The parent was found to be significantly different than the Mot− mutant in an unpaired, two-tailed test (P < 0.05).
Host susceptibility to B. avium.
Turkeys are not inbred animals. However, in the United States and Great Britain, there are five major commercial turkey lines whose individual characteristics are maintained by interbreeding within the line’s restricted gene pool. These five lines constitute the majority of turkeys sold worldwide. Since each line’s gene pool is different, uniform susceptibility to infectious agents, across lines, cannot be assumed. To test the relative resistance or susceptibility of the five major commercial lines, ID50 values for the parental strain and three mutant derivatives of strain 197N were tested. Statistical analysis (ANOVA) of the results (Table 6) indicated that there were no host-associated differences in susceptibility to any of the B. avium strains tested. This is most apparent in comparing ID50 values when lines were infected with the parental strain and the Mot− mutant (Table 6). Whereas there was a wide fluctuation in the degree of resistance to our avirulent Dnt− strain, all lines were at least 100-fold more resistant to the Dnt− mutant (Table 6). This fluctuation between lines in susceptibility to the Dnt− mutant likely stems from the extremely low infection rate of Dnt− mutants, necessitating extrapolation (rather than interpolation) of ID50 calculations (Table 6, footnote c). All lines were resistant to the Hag− mutants in the sense that isolates recovered from infected birds never retained Hag− character (all were Kanr Hag+ pseudorevertants). We infer from the selection of the Hag+ character in birds that hemagglutination is important for colonization—such selection does not take place during in vitro growth. Taking all of the host susceptibility data together, we conclude that both the Dnt− and Hag− mutants were extremely avirulent, such that ID50 values were difficult or impossible (respectively) to calculate, and that lack of motility had no effect on virulence.
TABLE 6.
Effects of turkey line on the ID50 of parental and selected mutant B. avium strainsa
| B. avium strainb | Avg (SD) ID50 in linec:
|
||||
|---|---|---|---|---|---|
| Hybrid | Orlopp | BUT-A | BUT-B | Nicholas | |
| Parent | 7.9 × 106 (7.4 × 106) | 5.2 × 106 (3.4 × 106) | 6.1 × 106 (5.7 × 106) | 4.7 × 106 (4.1 × 106) | 2.1 × 106 (2.0 × 106) |
| Mot− | 9.5 × 106 (7.7 × 106) | 1.2 × 106 (ND) | 4.1 × 106 (4.7 × 106) | 1.1 × 106 (5.8 × 106) | 7.9 × 106 (6.2 × 106) |
| Dnt− | 1.8 × 1010 (ND) | 1.6 × 109 (ND) | 1.5 × 1013 (ND) | 2.2 × 1013 (ND) | 6.0 × 1010 (ND) |
| Hag− | >108 (ND) | >108 (ND) | >108 (ND) | >108 (ND) | >108 (ND) |
Birds of the listed lines were inoculated at 1 week old by the standard procedure described in the text. ID50 was assessed after 2 weeks by tracheal culturing and phenotypic characterization of the isolated B. avium organisms, again as described in the text. A bird was termed colonized when B. avium of the inoculated phenotype was recovered from a tracheal swab.
DNT-negative (Dnt−), nonmotile (Mot−), and hemagglutination-negative (Hag−) mutants were tested. Hag− mutants were not recovered from any bird in any experiment, although Kanr Hag+ pseudorevertants were readily isolated (see text for additional details).
BUT-A, British United Turkeys of America; BUT-B, British United Turkeys of Britain. ANOVA for groups in which an SD was calculated revealed no differences between lines for infectivity of the parental strain (P > 0.05) and the nonmotile (Mot−) mutant (P > 0.05). ID50 values for the Dnt− mutant were estimated by extrapolation from a very few birds infected at very high doses in a single experiment. Consequently, the significance of the ID50 value differences (e.g., between lines) could not be calculated. ND, not determined.
In vivo tracheal pathology.
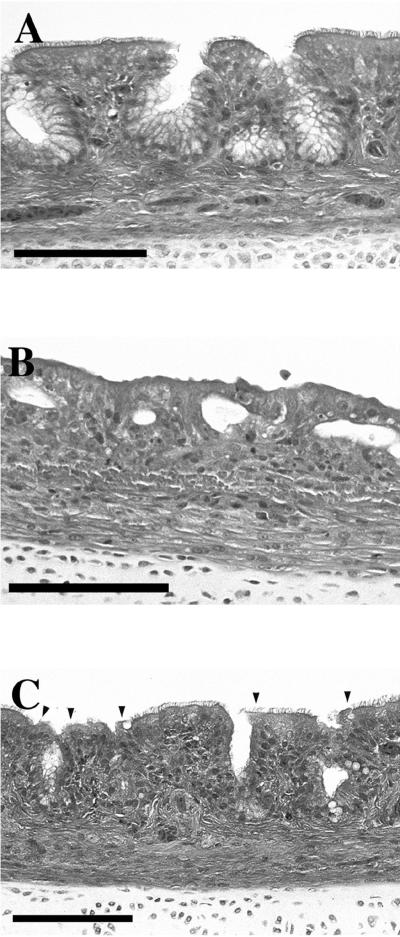
Turkeys naturally infected with B. avium exhibit severe microscopic histopathological signs of bordetellosis (32). Such signs were apparent in our experimental system, in which 10 tracheae were compared from groups of 3-week-old birds (10 birds per group) that were either sham infected or infected with approximately 2 × 1010 CFU of parental strain or the Dnt− mutant at 1 week of age. Birds were examined at 2 weeks postinfection (examples are shown in Fig. 1). Tracheae from the sham-inoculated birds had a well-defined ciliated border with a pseudostratified columnar layer with defined goblet cells and mucous secreting cells (Fig. 1A). Tracheae from birds infected with the parental strain showed a dramatic loss of ciliated epithelial cells and other changes associated with severe tracheitis (Fig. 1B). The Dnt− mutant produced much milder tracheal pathology (Fig. 1C). With a pathological scoring system, with +5 being the most severe and 0 being normal, the sham-inoculated group received an average score of +0.1, the Dnt− mutant +1.8, and the parental strain-infected birds +4. In this particular experiment, all of the Dnt− mutant-infected birds were colonized by Kans Dnt+ revertants by the time of evaluation. Consequently, the individual scores of Dnt− mutant-infected individuals were likely dictated by the time at which revertants arose in each bird rather than by any pathology caused specifically by the Dnt− mutant. This interpretation is supported indirectly by the wide variation in the scores observed in this group relative to the sham- and parental strain-inoculated groups (data not shown). Hag− and Mot− strains were not scored in this histological assay because of the high numbers of Hag+ pseudorevertants that colonized the tracheae and the negligible loss in virulence of the Mot− mutant (Table 6).
FIG. 1.
Hematoxylin-eosin-stained 4-μm sections of tracheae from 3-week-old turkeys sham inoculated or exposed 2 weeks previously to parent or Dnt− mutants of B. avium, as described in the text. (A) Sham-inoculated control with normal trachea. (B) Parental strain-infected turkey. Trachea shows marked disruption of mucosal architecture; absence of ciliated epithelium and goblet cells; epithelium composed of immature cuboidal cells; glands depleted of mucus; and hyperemia, mild fibrosis, and mixed inflammatory cell infiltrate of lamina propria and submucosa. (C) Dnt− mutant-infected turkey. Trachea shows normal mucosal architecture; normal ciliated epithelium, except for small focal areas of deciliation (arrowheads); partial depletion of mucus from glands; and increased mononuclear cells in lamina propria. Changes are intermediate between sham-inoculated and wild-type-exposed turkeys. Bars, 100 μm.
In vitro tracheal cell adherence assay.
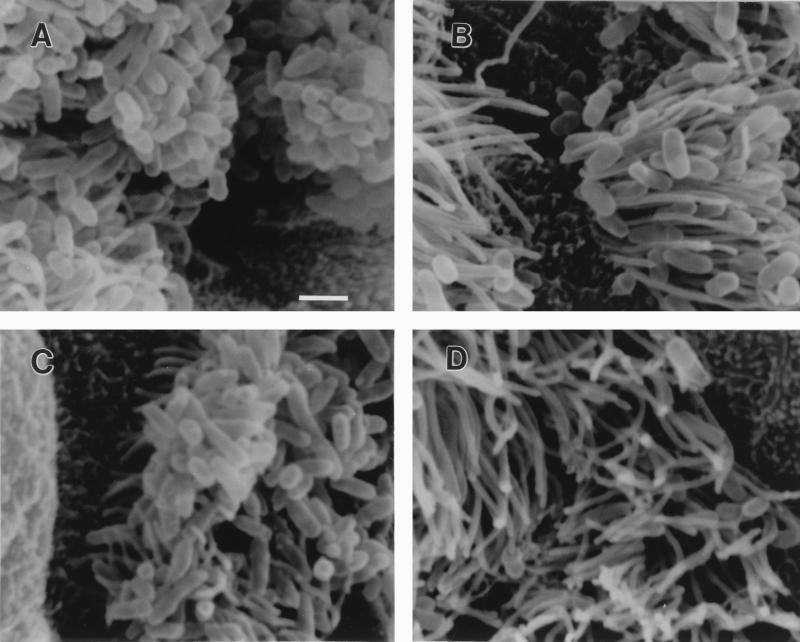
SEM revealed numerous B. avium 197N (parental strain) cells adhering to tracheal cilia but not to nonciliated cells (Fig. 2A). The Mot− strain also showed dense numbers of bacteria attached to cilia (Fig. 2B). The Dnt− mutant (Fig. 2C) showed fewer bacteria, and the Hag− mutant (Fig. 2D) showed much lower levels of attachment. In order to quantitate the relative ciliated cell binding efficiency of B. avium, an in vitro assay was developed. The tracheal ring adherence assay (see Materials and Methods) indicated that Dnt− and Hag− mutants were statistically less able to adhere to tracheal rings than were the parental strain and the nonmotile mutant (Table 7). The difference in the adherence ability between the parent and the Mot− mutant was insignificant. The difference was most dramatic with the Hag− mutant (Table 7). These results were remarkably similar to those obtained in vivo.
FIG. 2.
SEM of tracheae incubated with B. avium. Tracheal rings were incubated with approximately 109 bacteria/ml for 3 h under the same conditions as used for the tracheal attachment assay. (A) Parental strain 197N. (B) Mot− mutant, strain G146. (C) Dnt− mutant, strain WBA16. (D) Hag− mutant, strain P206. All images are shown at the same magnification. Bar, 1 μm.
TABLE 7.
Tracheal ring adherence assaya
| Strain | No. of expts | Avg CFU/ ring (104) | SD (104) | Significantly different than parentb |
|---|---|---|---|---|
| Parent | 22 | 280 | 180 | NA |
| Mot− | 8 | 170 | 150 | No |
| Dnt− | 12 | 52 | 54 | Yes |
| Hag− | 6 | 13 | 9 | Yes |
The protocol used for attachment, incubation, washing, and subsequent quantitation of adherent bacteria is described in the text.
Student’s t test was applied in an unpaired test for overlapping means between the parental and mutant strains. The test failed if P was >0.05. NA, not applicable.
DISCUSSION
The studies described herein examined some of the basic parameters for experimentally infecting turkey poults with B. avium and established host susceptibility to bordetellosis across five turkey lines. Additionally, we developed a tracheal ring adherence assay using embryonic turkeys that provided an organ, species, and line-matched in vitro corollary of colonization and tracheal pathogenesis in vivo. Interestingly, we found that examinations of several B. avium insertion mutants both in vitro and in vivo produced strikingly similar conclusions about the relative colonizing ability of each mutant.
A systematic study of the factors influencing the infection rate in experimental B. avium infections was carried out in order to firmly establish the effects of several variables that could influence the results of experimental infections. To establish the initial infection parameters (i.e., age, duration, and number of birds) and to assess the histopathological consequences of infection, we employed the parental strain 197N and an avirulent insertion mutant that did not produce DNT. Whereas the Dnt− mutant was constructed by site-directed mutagenesis, we have been unable to conclude that the insertion is in the structural gene for DNT. Nevertheless, its use here as an avirulent control strain proved to be quite valuable.
One of the more interesting results we obtained in testing parameters that could influence experimental infections was that B. avium did not spread extensively within groups. This finding is somewhat paradoxical for a respiratory pathogen. Nevertheless, in order to see any spreading among birds, we had to give the index case a very large inoculum and to assay for spreading at 1 week after inoculation rather than the normal 2 weeks. We found that at 2 weeks, rather than getting increased spreading, the index case was often cleared of infection. In spite of the modest spreading of the parental strain, we found significantly less spreading with a nonmotile mutant. This finding probably reflects the role of motility in the environment rather than in the bird, since B. avium is nonmotile and flagella are not detectable in vitro at the internal temperature (42°C) of turkeys (12).
One of the more practical and useful findings that emerged from this study was that the five major lines of turkeys showed no differences in susceptibility to experimental infection. This uniformity applied not only to the parental B. avium strain but to three mutant strains tested. The Hag−, Mot−, and Dnt− mutants provided a source of strain variation that we used here as a tool to try to detect and better understand any variation in host susceptibility to B. avium. The B. avium mutants created and employed in this study, while not fully characterized at the molecular level, did present distinctly different phenotypes to the hosts. With regard to virulence, the phenotypes ranged from no loss (Mot− mutants) to complete loss (Dnt− mutants). The only significant technical problem encountered in the use of the mutants was with the stability of the Hag− mutant, which produced Hag+ pseudorevertants under in vivo selective pressure. The basis for this pseudoreversion is not understood, but it was a property of all 20 Hag− mutants isolated and tested. In any case, all host lines reacted uniformly to the Hag mutant used in this study (i.e., pseudorevertants were isolated from all turkeys). Curiously, earlier work by Moore et al. (25), while indicating the importance of Hag in turkey virulence (and the lack of importance of motility), did not encounter instability of the Hag− character. The uniform response of all turkey lines tested suggested that practical measures for controlling the disease in one line of turkeys (e.g., development of a live vaccine strain) may be universally applicable.
Along with an in vivo model, an in vitro corollary of infection is very helpful in understanding the pathogenesis of disease at the molecular level. To our knowledge, there are no avian ciliated tracheal epithelial cell lines available. However, tracheal rings in culture have been used for a number of years (17). We utilized embryonic tracheal rings in our studies (rather than rings from live poults) because embryonic rings were easily obtained aseptically and gave uniform, reproducible results. In addition to providing an organ that is matched to the colonization site of our in vivo experiments, the rings can additionally be line matched (as they were in our experiments) so that in vitro and in vivo experiments are more closely comparable. Whereas this matching is probably not as significant a factor as originally thought, it still seems prudent to have the in vitro and in vivo experiments be as closely matched as possible. In our studies, we found that the strains that were best able to adhere to tracheal cells in culture were best able to colonize turkey poults. Whereas this finding may seem reasonable or even expected, it is remarkable from the standpoint of the different time frames involved (3 h in vitro, 2 weeks in vivo) and the number of factors coming into play in the in vivo situation (e.g., the onset of the immune response at 1 to 2 weeks postinoculation [35]).
The combination of in vivo and in vitro tools for examining avian bordetellosis and the uniform susceptibility of all commercial turkeys should facilitate development of a well-defined and practical method of preventing disease and enable a more rigorous investigation of its pathogenesis at the host and cell biological level. Also, our in vitro and in vivo studies may form a basis and provide a rationale for a more sophisticated bacterial genetic analysis of B. avium on a level comparable to those B. pertussis and B. bronchiseptica (1, 20, 23, 24, 38).
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (R15 AI/OD37773-01A1 and AI-23695), the USDA (950 934), Drew University, and the State of North Carolina.
Also, the generous donations, cooperation, and support given by British United Turkeys of America and Tarheel Turkey Hatchers were invaluable and much appreciated.
REFERENCES
- 1.Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 2.Arp L H, Cheville N F. Tracheal lesions in young turkeys infected with Bordetella avium. Am J Vet Res. 1984;45:2196–2201. [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 4.Backman K, Ptashne M, Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci USA. 1976;73:4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bemis D A, Greisen H A, Appel M J G. Pathogenesis of canine bordetellosis. J Infect Dis. 1977;135:753–762. doi: 10.1093/infdis/135.5.753. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Alley M R, Manktelow B W. Experimental induction of pneumonia in mice with Bordetella parapertussis isolated from sheep. J Comp Pathol. 1989;100:77–89. doi: 10.1016/0021-9975(89)90092-3. [DOI] [PubMed] [Google Scholar]
- 7.Cookson B T, Cho H-L, Herwaldt L A, Goldman W E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect Immun. 1989;57:2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLey J, Seger P, Kersters K, Mannheim W, Lievens A. Intra- and intergeneric similarities of the Bordetella ribosomal ribonucleic acid cistrons:proposal for a new family, Alcaligenaceae. Int J Syst Bacteriol. 1986;36:405–414. [Google Scholar]
- 9.DeLorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ensminger M E. Poultry science. Danville, Ill: The Interstate Press; 1980. p. 149. [Google Scholar]
- 11.Feng P, Sugasaware R J, Schantz A. Identification of a common enterobacterial flagellin epitope with a monoclonal antibody. J Gen Microbiol. 1990;136:337–342. doi: 10.1099/00221287-136-2-337. [DOI] [PubMed] [Google Scholar]
- 12.Fernando K, Cooper B, Temple L M, Miyamoto D, Orndorff P. Abstracts of the 96th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1996. Motility is not essential for virulence of Bordetella avium in turkeys:in vivo and in vitro testing, abstr. B-485; p. 239. [Google Scholar]
- 13.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentry-Weeks C R, Cookson B T, Goldman W E, Rimler R B, Porter S B, Curtiss R., III Dermonecrotic toxin and tracheal cytotoxin, putative virulence factors of Bordetella avium. Infect Immun. 1988;56:1698–1707. doi: 10.1128/iai.56.7.1698-1707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry-Weeks C R, Provence D L, Keith J M, Curtiss R., III Isolation and characterization of Bordetella avium phase variants. Infect Immun. 1991;59:4026–4033. doi: 10.1128/iai.59.11.4026-4033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodnow R A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–794. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray J G, Roberts J F, Dillman R C, Simmons D G. Cytotoxic activity of pathogenic Alcaligenes faecalis in turkey tracheal organ cultures. Am J Vet Res. 1981;42:2184–2186. [PubMed] [Google Scholar]
- 18.Hewlett E L, Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis:purification and characterization. J Bacteriol. 1976;127:890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackwood M W, Hilt D A, Dunn P A. Observations on colonial phenotypic variation in Bordetella avium. Avian Dis. 1991;35:496–594. [PubMed] [Google Scholar]
- 20.Kimura A, Mountzouros K T, Relman D D, Falkow S, Cowell J L. Bordetella pertussis filamentous hemagglutinin:evaluation as a protective antigen and colonization factor in a mouse respiratory model. Infect Immun. 1990;58:7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyh R D, Griffith R W, Arp L H. Transposon mutagenesis in Bordetella avium. Am J Vet Res. 1988;49:687–692. [PubMed] [Google Scholar]
- 22.Mallory F B, Horner A A. Pertussis:the histological lesion in the respiratory tract. J Med Res. 1913;27:115–123. [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez de Tejada G, Miller J F, Cotter P A. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol Microbiol. 1996;22:895–908. doi: 10.1046/j.1365-2958.1996.01538.x. [DOI] [PubMed] [Google Scholar]
- 24.Mooi F R. Genes for the filamentous hemagglutinin and fimbriae of Bordetella pertussis:colocation, coregulation, and cooperation. In: Miller V L, Kaper J B, Portnoy D A, Isberg R R, editors. Molecular genetics of bacterial pathogenesis. Washington, D.C: American Society for Microbiology; 1994. pp. 145–155. [Google Scholar]
- 25.Moore K M, Jackwood M W, Brown T P, Dreesen D W. Bordetella avium hemagglutination and motility mutants:isolation, characterization and pathogenicity. Avian Dis. 1994;38:50–58. [PubMed] [Google Scholar]
- 26.Muse K E, Findley D, Allen L, Collier A M. In vitro model of Bordetella pertussis infection:pathogenic and microbicidal interaction. In: Manclark C R, Hill J C, editors. International symposium of pertussis. U.S. Department of Health, Education, and Welfare publication no. 79-1830. U.S. Washington, D.C: Government Printing Office; 1979. pp. 41–50. [Google Scholar]
- 27.Panigrahy B, Grumbles L C, Terry R J, Millar D L, Hall C F. Bacterial coryza in turkeys in Texas. Poultry Sci. 1981;60:107–113. doi: 10.3382/ps.0600107. [DOI] [PubMed] [Google Scholar]
- 28.Pitman M. Genus Bordetella. In: Buchanan R E, Gibbons N E, editors. Bergey’s manual of determinative bacteriology. 8th ed. Baltimore, Md: The Williams and Wilkins Co.; 1974. pp. 282–283. [Google Scholar]
- 29.Reed L J, Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:293–299. [Google Scholar]
- 30.Rhea L J. The comparative pathology of the tracheal and bronchial lesions produced in man by B. pertussis (whooping cough) and those produced in dogs by B. bronchiseptica (canine distemper) J Med Res. 1915;32:471–474. [PMC free article] [PubMed] [Google Scholar]
- 31.Sekiya K, Futaesaku Y, Nakase Y. Electron microscopic observations of tracheal epithelia of mice infected with Bordetella bronchiseptica. Microbiol Immunol. 1988;32:461–472. doi: 10.1111/j.1348-0421.1988.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 32.Skeeles J K, Arp L H. Bordetellosis (turkey coryza) In: Calnek B W, Barnes H J, Beard C W, McDougal L R, Saif Y M, editors. Diseases of poultry. Ames: Iowa State University Press; 1997. pp. 275–288. [Google Scholar]
- 33.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–512. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 34.Stibitz S, Black W, Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50:133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- 35.Suresh P, Arp L H, Huffman E L. Mucosal and systemic humoral immune response to Bordetella avium in experimentally infected turkeys. Avian Dis. 1994;38:225–230. [PubMed] [Google Scholar]
- 36.Van Alstine W G, Arp L H. Histologic evaluation of lung and bronchus-associated lymphoid tissue in young turkeys infected with Bordetella avium. Am J Vet Res. 1988;49:835–839. [PubMed] [Google Scholar]
- 37.Walker K E. A genetic analysis of the dermonecrotic toxin of Bordetella. Ph.D. dissertation. Richmond, Va: Virginia Commonwealth University; 1993. [Google Scholar]
- 38.Walker K E, Weiss A A. Characterization of the dermonecrotic toxin in members of the genus Bordetella. Infect Immun. 1994;62:3817–3828. doi: 10.1128/iai.62.9.3817-3828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss A A. The genus Bordetella. In: Balows A, Trüper H G, Dworkin M, Harner W, Schleifer K-H, editors. The prokaryotes. 2nd ed. I. New York, N.Y: Springer-Verlag; 1991. pp. 2530–2543. [Google Scholar]
- 40.Weiss A A, Hewlett E. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- 41.Wright N G, Thompson H, Taylor D, Cornwell H J C. Bordetella bronchiseptica, a re-assessment of its role in canine respiratory disease. Vet Rec. 1973;93:486–487. doi: 10.1136/vr.93.18.486. [DOI] [PubMed] [Google Scholar]