Abstract
Background
Rapid-deployment aortic valve replacement (RDAVR) is an alternative to conventional AVR (cAVR) for aortic stenosis. Benefits include a reduction in operative times, facilitation of minimal access surgery and superior haemodynamics compared to conventional valves. However, further evidence is required to inform guidelines, preferably in the form of propensity-matched studies that include mid-term follow-up data.
Methods
This was a single-centre, retrospective, propensity-matched cohort study comparing the Perceval and conventional Perimount Magna Ease valve for short- and mid-term clinical parameters and size-matched mid-term echocardiographic parameters (n = 102 in both groups) from 2014 to 2020. Data were extracted from a nationally managed dataset.
Results
There were no demographic differences between the matched groups. The Perceval group had shorter cross-clamp time (Perceval 62 [49–81] minutes; Perimount 79 [63–102] minutes, P < 0.001), shorter bypass time (Perceval 89 [74–114] minutes; Perimount 104 [84–137] minutes, P < 0.001), and more frequent minimally-invasive approaches (Perceval 28%; Perimount 5%, P < 0.001). Size-matched haemodynamics showed initially higher gradients in the Perceval group, but haemodynamics equalised at 12 + months. The Perceval group had a more favourable % change in the left ventricular posterior wall dimension at 2 + years (Perceval − 4.8 ± 18; Perimount 17 ± 2).
Conclusions
The Perceval facilitated shorter operations, which may benefit intermediate-high-risk, elderly patients with comorbidities requiring concomitant procedures. It also facilitated minimally invasive surgery. Size-matched haemodynamic performance was similar at mid-term follow-up, with the Perceval possibly better facilitating regression of left ventricular hypertrophy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-024-02575-4.
Keywords: Aortic valve stenosis, Aortic valve implantation, Sutureless aortic valve replacement, Minimally invasive aortic valve replacement
Background
Surgical aortic valve replacement (AVR) remains the gold standard treatment for severe aortic stenosis [1]. Within bioprosthetic AVR, rapid-deployment AVR (RDAVR) is an emerging alternative to conventional (sutured) AVR (cAVR). The Perceval (Corcym Canada Corp, Burnaby, BC, Canada) valve is effectively the only truly sutureless valve surgically implanted worldwide [2].
Previous work suggests that Perceval RDAVR reduces cross-clamp and cardiopulmonary bypass (CPB) time compared to cAVR [3], thus enhancing many aspects of recovery [4–11]. The Perceval may enable lower peak and mean pressure gradients (PG and MG) than cAVR, and higher effective orifice area (EOA) and therefore lower risk of patient-prosthesis-mismatch. However, the Perceval may increase pacemaker implantation, post-operative thrombocytopaenia, and para-valvular leakage (PVL) [3, 9, 12, 13].
There are no definitive recommendations on RDAVR (Perceval or otherwise) in the European Society of Cardiology or American College of Cardiology/American Heart Association guidelines [14, 15], and conventional AVR remains the gold standard. Although NICE Interventional Procedures Guidance validated sutureless valves as an option in 2018 [7], definitive recommendations for clinical decision-making have not been made. A paucity of mid- to long-term data and robust, matched comparisons is one reason for the lack of definitive guidelines.
This retrospective cohort study aimed to compare Perceval RDAVR with Perimount Magna Ease (Edwards Lifesciences, Irvine, CA, USA) cAVR at a single institution, by analysing intra-operative, clinical post-operative, and short- and mid-term echocardiographic post-operative outcomes in a propensity-matched cohort. Survival was also compared.
Methods
Aim
To compare Perceval RDAVR with Perimount Magna Ease cAVR at a single institution, using a propensity-matched cohort.
Study design
This was a retrospective, propensity-matched cohort study of a prospectively collected, nationally managed database, with data extracted from two hospitals in the United Kingdom. Data collection was carried out in the first half of 2022.
Inclusion and exclusion criteria
Our institution began implanting the Perceval valve in 2014, with most operations carried out by two experienced surgeons in the department. The number of aortic valve replacements with or without concomitant CABG has been constant in the years from 2014 to 2019, with around 630 cases. There were fewer cases in 2020 due to the COVID pandemic. Patient selection for the Perceval was at the surgeons’ discretion following pre-operative assessment and relied on the presence of favourable anatomy for its implantation. All patients who had undergone AVR with the sutureless Perceval or conventional Perimount Magna Ease between 2014 and 2020 were identified (some patients underwent concomitant CABG and very few underwent non-CABG concomitant procedures; further details are provided in the Results section). Redo cardiac surgery was excluded. Patients with aortic annular enlargement were also excluded, due to the longer duration of the operation and potential altered clinical outcome. Propensity matching was conducted on the resulting cohort of 136 Perceval patients and 296 Perimount patients. After matching, the decision was made to exclude pairs in which either patient had predominantly regurgitation rather than stenosis. The final matched population size was 204, with 102 patients in each group.
Data collection
An encrypted spreadsheet proforma was used to collect patient data from electronic patient records. Demographic, pre-operative, and short-term post-operative data for all patients, as well as mid-term follow-up (> 6 months post-op) echocardiograms were extracted across two hospital sites. Mid-term follow-up echocardiographic outcomes included mean gradients, peak gradients, indexed effective orifice area, maximum velocity through aortic valve, and ventricular dimensions. Follow-up echocardiograms were organised into three categories: 6–12 months, 1–2 years, and 2 + years (following surgery, the usual departmental protocol is to carry out echocardiographic follow-up on the patient between 3 and 6 months, after 1 year and in a second year). If a patient had more than one echocardiogram per category, the latest was used. Intrahospital mortality and mid-term mortality data were collected for all patients.
Statistical analysis
The groups were propensity-matched by gender, age, ejection fraction (< 30% = poor, 30–50% = moderate, > 50% = normal), concomitant CABG, and valve size (21, 23, 25, 27 mm) using the ‘nearest neighbour’ method. Details of the propensity matching work conducted by our statistician is provided in Supplement 1. Normality was assessed with the Shapiro-Wilk test. For continuous variables, comparisons were performed using unpaired student’s t-tests (parametric), Mann-Whitney U tests (non-parametric unpaired), or Wilcoxon signed-rank tests (non-parametric paired). For categorial variables, comparisons were performed using chi-squared tests if non-binary, and two-sample tests of proportion if binary. For echocardiographic comparisons, sample sizes fell with longer follow-up. Mid-term mortality was compared using a log-rank test and the construction of crude Kaplan-Meier curves for visual comparison. Statistical analysis was conducted using Stata BE 17.0 (Stata Corp., TX, USA). The following variables were also compared using sub-group analysis to separate isolated AVR and concomitant CABG patients: cross-clamp time, cardiopulmonary bypass time, and all post-operative clinical parameters at discharge. Parametric continuous variables are expressed as mean ± standard deviation. Non-parametric continuous variables are expressed as median (lower quartile to upper quartile). Binary and categorical variables are expressed as % in text and % (n) in tables.
Results
Pre-operative characteristics
In the unmatched comparison, patients who received the sutureless Perceval valve were significantly older (P < 0.001) and had a different distribution of valve sizes (P = 0.004), but the matched cohort showed no differences in clinical or haemodynamic parameters (Tables 1 and 2).
Table 1.
Unmatched pre-operative demographic, clinical, and echocardiographic parameters (without regurgitation patients)
| Perimount, n=250 | Perceval, n=132 | P-value | |
|---|---|---|---|
| Age / years | 71 (65; 76) | 74 (69; 79) | <0.001* |
| % Female (n) | 32% (80) | 55% (55) | 0.060 |
| BMI / kgm-2 | 28.6 ± 5.2 | 28.6 ± 5.1 | 0.990 |
| EuroSCORE II | 1.69 (1.1; 2.7) | 1.89 (1.3; 2.9) | 0.081 |
| Diabetes | 26% (66) | 23% (31) | 0.534 |
| Previous or current smoker | 54% (136) | 56% (74) | 0.756 |
| Previous PCI | 9.6% (24) | 6.1% (8) | 0.235 |
| Hypertension | 76% (190) | 73% (97) | 0.589 |
| ‘Good’ EF (>50%) | 85% (213) | 84% (111) | 0.508 |
| ‘Moderate’ EF (30-49%) | 12% (31) | 11% (15) | |
| Poor EF (<30%) | 2.4% (6) | 4.6% (6) | |
| Concomitant CABG / % | 42% (106) | 39% (51) | 0.477 |
| Valve size 21 mm / % | 18% (44) | 17% (22) | 0.004* |
| Valve size 23 mm / % | 46% (115) | 33% (43) | |
| Valve size 25 mm / % | 31% (78) | 36% (48) | |
| Valve size 27 mm / % | 5.2% (13) | 14% (19) |
Values are mean ± SD or median (LQ to UQ), and % (n). * P <0.05
BMI: body mass index, PCI: percutaneous coronary intervention, LVEF: left ventricular ejection fraction
Table 2.
Matched pre-operative demographic, clinical, and echocardiographic parameters
| Perimount, n=102 | Perceval, n=102 | P-value | |||
|---|---|---|---|---|---|
| Age / years | 74.0 ± 6.4 | 73.7 ± 6.7 | 0.733 | ||
| % Female | 33.3% (34) | 39.2% (40) | 0.382 | ||
| BMI / kgm-2 | 27.2 (24; 30) | 28.6 (25; 32) | 0.163 | ||
| EuroSCORE II | 1.96 (1.3; 3.3) | 1.95 (1.5; 3.0) | 0.937 | ||
| Diabetes | 26.5% (27) | 24.5% (25) | 0.748 | ||
| Previous or current smoker | 50% (51) | 55.9% (57) | 0.400 | ||
| Previous PCI | 12.7% (13) | 6.9% (7) | 0.158 | ||
| Hypertension | 78.4% (80) | 72.5% (74) | 0.329 | ||
| Perimount | n | Perceval | n | P-value | |
| LVEF | 60 (55; 65) | 100 | 59.5 (55; 65) | 102 | 0.135 |
| PG / mmHg | 72.3 (62; 89) | 96 | 76 (63; 95) | 97 | 0.736 |
| MG / mmHg | 41 (35; 52) | 94 | 45 (36; 57) | 96 | 0.393 |
| iEOA / cm2 | 0.42 (0.37; 0.52) | 75 | 0.44 (0.36; 0.51) | 84 | 0.730 |
| Vmax aortic valve / ms-1 | 4.3 (4.0; 4.7) | 88 | 4.4 (3.9; 4.9) | 94 | 0.762 |
| Mixed AS + AR / % (n) | 10.8% (11) | 102 | 12.7% (13) | 102 | 0.664 |
| LVOT / cm | 2.3 (2.2; 2.5) | 86 | 2.3 (2.1; 2.3) | 79 | 0.311 |
| LVDd / cm | 4.9 (4.2; 5.3) | 87 | 5.1 (4.7; 5.4) | 86 | 0.280 |
| LVSd / cm | 3.1 ± 0.65 | 84 | 3.1 ± 0.67 | 86 | 0.734 |
| IVSd / cm | 1.4 (1.3; 1.5) | 87 | 1.3 (1.0; 1.4) | 85 | 0.300 |
| LVPWd / cm | 1.14 ± 0.28 | 87 | 1.18 ± 0.23 | 86 | 0.292 |
Values are mean ± SD or median (LQ to UQ), and % (n)
BMI: body mass index, PCI: percutaneous coronary intervention, LVEF: left ventricular ejection fraction, PG: peak gradient, MG: mean gradient, iEOA: indexed effective orifice area, Vmax: maximum velocity across aortic valve, LVDd: left ventricular diastolic dimension, LVSd: left ventricular systolic dimension, IVSd: interventricular septal diastolic dimension, LVOT: left ventricular outflow tract dimension, LVPWd: left ventricular posterior wall dimension
Intra-operative parameters
In the matched comparison, Perceval patients had a shorter cross-clamp time (Perceval 62 [50–80] minutes; Perimount 79 [64–105] minutes, P < 0.001) and CPB time (Perceval 92 [75–116] minutes; Perimount 104 [86–147] minutes, P < 0.001) (Table 3). Minimally invasive approaches, mostly mini-sternotomy, were more common in the Perceval group (Perceval 28%; Perimount 5%, P < 0.001) (Table 3).
Table 3.
Intra-operative parameters
| Perimount, n=102 | Perceval, n=102 | P-value | |
|---|---|---|---|
| XCT time isolated AVR † / minutes | 70.0 (58; 82) | 52 (43; 63) | <0.001* |
| CPB time isolated AVR † / minutes | 91 (79; 103) | 82 (67; 96) | 0.012* |
| XCT AVR + CABG / minutes | 107 (92; 123) | 74 (62; 91) | <0.001* |
| CPB time AVR + CABG / minutes | 147 (118; 167) | 110 (80; 133) | <0.001* |
| Minimally invasive / % (n) | 5% (5) | 28% (29) | <0.001* |
| Isolated AVR † / % (n) | 58% (59) | 53% (54) | 0.481 |
| Concomitant CABG / % (n) | 42% (43) | 47% (48) | |
| Valve size 21 mm / % (n) | 15.7% (16) | 16.7% (17) | 0.802 |
| Valve size 23 mm / % (n) | 36.3% (37) | 38.2% (39) | |
| Valve size 25 mm / % (n) | 39.2% (40) | 33.3% (34) | |
| Valve size 27 mm / % (n) | 8.8% (9) | 11.8% (12) |
† Some patients received other short, non-CABG procedures in addition to AVR: Perimount: 3 x LAA clip + ablation, 1 x LAA clip, 1 x AMVL decalcification, 1 x septal myotomy; Perceval: 3 x LAA clip, 1 x AMVL decalcification, 1 x LAA clip + ablation, 1 x ablation; note that 1 patient with CABG also received ablation
Values: median (LQ to UQ) or % (n). * P <0.05
XCT: cross-clamp time, CPB: cardiopulmonary bypass, AVR: aortic valve replacement, CABG: coronary artery bypass graft, MVR: mitral valve replacement
Post-operative parameters & follow-up
Clinical parameters were compared between the matched patient groups at discharge (Table 4). Echocardiographic parameters were compared by valve size at four time points: discharge (Table 5), 6–12-month follow-up (Table 6), 1-2-year-follow-up (Table 7), and 2+-year-follow-up (Table 8).
Table 4.
Post-operative (discharge) parameters
| Perimount, n=102 | Perceval, n=102 | P-value | |
|---|---|---|---|
| Operative mortality | 2.0% (2) | 2.0% (2) | 1.000 |
| Need for hemofiltration or dialysis | 1.0% (1) | 2.9% (3) | 0.313 |
| Pacemaker implantation | 7.8% (8) | 5.9% (6) | 0.580 |
| Bleeding req. transfusion† | 2% (2) | 2.9% (3) | 0.667 |
| Transient stroke | 2.0% (2) | 0% (0) | 0.155 |
| Permanent stroke | 2.9% (3) | 0% (0) | 0.081 |
| Return to theatre for bleeding / tamponade | 5.9% (6) | 3.9% (4) | 0.517 |
Values: % (n)
† for this variable, Perimount n=100, Perceval n=102
Table 5.
Echocardiographic parameters by size, in propensity-matched population: discharge
| Size / mm | Perimount | n | Perceval | n | P-value | |
|---|---|---|---|---|---|---|
| MG | 21 | 11.5 (9; 15) | 16 | 17 (12; 20) | 17 | 0.010* |
| 23 | 11 (9; 14.0) | 36 | 15 (12; 17) | 37 | <0.001* | |
| 25 | 10 (8; 12) | 39 | 12 (10; 15) | 32 | 0.013* | |
| 27 | 10 (7; 11) | 9 | 13 (10; 16) | 12 | 0.195 | |
| PG | 21 | 21 (17; 26) | 16 | 30 (24; 37) | 17 | 0.007* |
| 23 | 21 (18; 24) | 36 | 27 (23; 33) | 37 | <0.001* | |
| 25 | 20 (16; 24) | 39 | 23 (18; 28) | 32 | 0.031* | |
| 27 | 17 (14; 24) | 9 | 25 (18; 28) | 12 | 0.211 | |
| EF % change | 21 | 4.3 (-11; 8) | 15 | -1.7 (-4.8; 6.8) | 17 | 0.948 |
| 23 | 1.6 (-13; 9) | 35 | 1.6 (-1.7; 9.1) | 37 | 0.489 | |
| 25 | 3.4 (-6.7; 11) | 39 | 1.7 (-6.8; 11) | 33 | 0.730 | |
| 27 | -3.9 (-8.8; 14) | 9 | 1.7 (-5.7; 13) | 12 | 0.589 | |
| Vmax | 21 | 2.29 (1.9; 2.5) | 15 | 2.91 (2.5; 3.2) | 15 | 0.005* |
| 23 | 2.32 (2.1; 2.5) | 34 | 2.58 (2.2; 2.9) | 37 | 0.010* | |
| 25 | 2.3 (2; 2.5) | 37 | 2.51 (2.0; 2.6) | 32 | 0.609 | |
| 27 | 2.22 (1.6; 2.4) | 8 | 2.3 (2.0; 2.7) | 12 | 0.374 | |
| iEOA | 21 | 0.81 (0.78; 0.87) | 10 | 0.76 (0.65; 0.88) | 8 | 0.203 |
| 23 | 0.83 (0.61; 1.1) | 25 | 0.80 (0.70; 1.0) | 30 | 0.874 | |
| 25 | 0.94 (0.89; 1.1) | 25 | 0.77 (0.64; 0.84) | 21 | 0.002* | |
| 27 | 0.90 (0.8; 1.2) | 7 | 1.06 (0.70; 1.3) | 8 | 0.908 | |
| % PVL (n) | 21 | 0 | 16 | 0 | 17 | - |
| 23 | 0 | 35 | 5.1% (2) | 39 | 0.174 | |
| 25 | 7.5 (3) | 40 | 0 | 34 | 0.103 | |
| 27 | 0 | 9 | 0 | 12 | - |
Values are mean ± SD or median (LQ to UQ), and % (n). * P <0.05
PG: peak gradient, MG: mean gradient, iEOA: indexed effective orifice area, Vmax: maximum velocity across aortic valve, LVDd: left ventricular diastolic dimension, LVSd: left ventricular systolic dimension, IVSd: interventricular septal diastolic dimension, LVOT: left ventricular outflow tract dimension, LVPWd: left ventricular posterior wall dimension
Table 6.
Echocardiographic parameters & ventricular dimensions by size, in matched patients: 6-12 months
| Size / mm | Perimount | n | Perceval | n | P-value | |
|---|---|---|---|---|---|---|
| MG | 21 | 7 (7; 10) | 3 | 18 (10; 25) | 4 | 0.114 |
| 23 | 10 (9; 18) | 7 | 13 (11; 14) | 7 | 0.444 | |
| 25 | 10 (8; 12) | 11 | 9 (7; 13) | 9 | 0.809 | |
| 27 | 6.5 (5; 8) | 2 | 10.8 (10; 12) | 2 | 0.333 | |
| PG | 21 | 16.3 (13; 19) | 3 | 31 (20.0; 43) | 4 | 0.229 |
| 23 | 19 (17; 33) | 7 | 26 (20.0; 28) | 7 | 0.330 | |
| 25 | 19.5 (16; 23) | 11 | 18.1 (13; 21) | 8 | 0.642 | |
| 27 | 13.5 (9; 18) | 2 | 20.5 (19; 22) | 2 | 0.333 | |
| LVEF % change | 21 | -11 (-16; -4.9) | 3 | 21 (1.7; 41) | 2 | 0.200 |
| 23 | 1.5 (-5; 10) | 7 | 4.6 (1.6; 5.5) | 5 | 0.755 | |
| 25 | 1.7 (-4.5; 10) | 11 | -1.4 (-7.5; 36) | 8 | 0.732 | |
| 27 | 16 (14; 18) | 2 | 45 (5; 86) | 2 | 1.000 | |
| Vmax | 21 | 1.99 ± 0.19 | 3 | 2.2 ± 1.1 | 4 | 0.801 |
| 23 | 2.30 ± 0.43 | 7 | 2.46 ± 0.24 | 7 | 0.393 | |
| 25 | 2.21 ± 0.30 | 11 | 2.12 ± 0.34 | 5 | 0.547 | |
| 27 | 1.81 ± 0.44 | 2 | 2.24 (2.2; 2.3) | 2 | 0.306 | |
| iEOA | 21 | 0.73 ± 0.17 | 3 | 0.5 (0.47; 0.82) | 3 | 0.400 |
| 23 | 0.78 (0.6; 0.97) | 6 | 0.74 (0.73; 0.82) | 3 | 1.000 | |
| 25 | 0.91 (0.84; 0.94) | 8 | 0.97 (0.91; 0.99) | 9 | 0.500 | |
| 27 | 1.30 (1.1; 1.5) | 2 | 1.01 | 1 | 0.667 | |
| % PVL (n) | 21 | 0 | 3 | 0 | 3 | - |
| 23 | 0 | 6 | 0 | 6 | - | |
| 25 | 0 | 11 | 0 | 6 | - | |
| 27 | 0 | 2 | 0 | 2 | - | |
| LVOT % change | 21 | -8.3 ± 5.4 | 3 | -10.0 ± 5.3 | 3 | 0.703 |
| 23 | -4.3 ± 11.5 | 6 | 0.83 ± 11 | 4 | 0.503 | |
| 25 | -4.7 ± 9 | 6 | -7.50 ± 6.8 | 6 | 0.558 | |
| 27 | -3.8 ± 5.4 | 2 | -4.5 | 1 | - | |
| LVDd % change | 21 | -6.7 (-28; 4.7) | 3 | -3.5 (-9; 4.7) | 4 | 0.629 |
| 23 | -2.1 (-5; 1.1) | 7 | -11 (-16; -9.6) | 6 | 0.051 | |
| 25 | -11 (-17; -6) | 8 | -3.1 (-9.5; 14) | 8 | 0.038* | |
| 27 | -9.6 (-9.9; -9.4) | 2 | -15.4 (-19; -12) | 2 | 0.333 | |
| LVSd % change | 21 | -2.24 ± 18 | 3 | 6.0 ± 28 | 4 | 0.679 |
| 23 | 2.55 ± 11 | 6 | 1.34 ± 21 | 6 | 0.701 | |
| 25 | -8.6 ± 6.9 | 7 | 5.3 ± 25 | 8 | 0.167 | |
| 27 | -9.35 ± 0.29 | 2 | -25 ± 11 | 2 | 0.301 | |
| IVSd % change | 21 | -19 ± 25 | 3 | -10.1 ± 8.4 | 4 | 0.515 |
| 23 | -4.3 ± 10 | 7 | -14 ± 27 | 6 | 0.450 | |
| 25 | 0.83 ± 15 | 8 | -14 ± 15 | 8 | 0.061 | |
| 27 | -9.4 ± 3 | 2 | -17 ± 16 | 2 | 0.549 | |
| LVPWd % change | 21 | -29 ± 19 | 3 | -8.8 ± 30 | 4 | 0.371 |
| 23 | -5.6 ± 14 | 7 | -9.3 ± 15 | 6 | 0.660 | |
| 25 | 4.1 ± 23 | 7 | -22.5 ± 20 | 7 | 0.040* | |
| 27 | -4.3 ± 11 | 2 | -21 ± 11 | 2 | 0.266 |
Values are mean ± SD or median (LQ to UQ), and % (n). * P <0.05
PG: peak gradient, MG: mean gradient, iEOA: indexed effective orifice area, Vmax: maximum velocity across aortic valve, LVDd: left ventricular diastolic dimension, LVSd: left ventricular systolic dimension, IVSd: interventricular septal diastolic dimension, LVOT: left ventricular outflow tract dimension, LVPWd: left ventricular posterior wall dimension
Table 7.
Echocardiographic parameters & ventricular dimensions by size, in matched patients: 1-2 years
| Size / mm | Perimount | n | Perceval | n | P-value | |
|---|---|---|---|---|---|---|
| MG | 21 | 13 | 1 | 15 (10; 17) | 7 | 0.750 |
| 23 | 10 (5; 14) | 3 | 11 (10; 14) | 12 | 0.462 | |
| 25 | 11 (9; 16) | 11 | 11 (9; 11) | 8 | 0.525 | |
| 27 | 7 | 1 | 8.3 (6; 11) | 6 | 1.000 | |
| PG | 21 | 20.6 | 1 | 28.1 (19; 31) | 7 | 1.000 |
| 23 | 17.1 (8; 26) | 3 | 22 (19; 27) | 12 | 0.750 | |
| 25 | 26 (16; 27) | 11 | 19.9 (18; 23) | 8 | 0.365 | |
| 27 | 13 | 1 | 15.6 (11; 18) | 6 | 0.298 | |
| LVEF % change | 21 | -3.2 | 1 | 8.6 (-3.3; 11) | 5 | 1.000 |
| 23 | 35 (-12; 83) | 2 | -1.6 (-3; 11) | 9 | 0.873 | |
| 25 | 1.7 (0; 9) | 11 | 0.85 (-4.6; 3.6) | 4 | 0.881 | |
| 27 | 29 | 1 | 10.3 (-5.5; 22) | 6 | 0.571 | |
| Vmax | 21 | 2.27 | 1 | 2.33 ± 0.97 | 7 | - |
| 23 | 2.01 ± 0.58 | 3 | 2.29 ± 0.48 | 12 | 0.395 | |
| 25 | 2.34 ± 0.41 | 11 | 2.04 ± 0.51 | 8 | 0.168 | |
| 27 | 1.8 | 1 | 1.83 ± 0.47 | 6 | - | |
| iEOA | 21 | 0.85 | 1 | 0.70 (0.5; 0.9) | 4 | - |
| 23 | 0.87 (0.85; 0.9) | 2 | 0.69 (0.6; 0.9) | 4 | 0.533 | |
| 25 | 0.81 (0.70; 1.0) | 11 | 0.80 (0.70; 0.9) | 2 | 0.769 | |
| 27 | 1.16 | 1 | 1.3 (1.1; 1.4) | 4 | - | |
| % PVL (n) | 21 | 0 | 1 | 0 | 7 | - |
| 23 | 0 | 3 | 0 | 12 | - | |
| 25 | 0 | 11 | 0 | 8 | - | |
| 27 | 0 | 1 | 0 | 6 | - | |
| LVOT % change | 21 | - | 0 | -6.2 ± 10 | 6 | - |
| 23 | -1.6 ± 2.7 | 3 | -7.5 ± 8.4 | 6 | 0.291 | |
| 25 | -2.5 ± 15 | 8 | -1.9 ± 10 | 2 | 0.962 | |
| 27 | -13.0 | 1 | -3.4 ± 13 | 5 | - | |
| LVDd % change | 21 | - | 0 | 0.96 ± 10 | 7 | - |
| 23 | 1.3 ± 3.4 | 3 | -7.1 ± 14 | 11 | 0.339 | |
| 25 | -4.9 ± 12 | 8 | -8.4 ± 9.3 | 5 | 0.584 | |
| 27 | -19 | 1 | -16.5 ± 18 | 6 | - | |
| LVSd % change | 21 | - | 0 | 9.0 ± 23 | 7 | - |
| 23 | 4.1 ± 8.5 | 2 | -11 ± 13 | 10 | 0.144 | |
| 25 | 3.1 ± 8 | 7 | 15.2 ± 12 | 5 | 0.064 | |
| 27 | -10.8 | 1 | -17.3 ± 16 | 6 | - | |
| IVSd % change | 21 | - | 0 | -14 ± 12 | 7 | - |
| 23 | -0.21 ± 29 | 3 | -8.3 + 15 | 11 | 0.500 | |
| 25 | -15 ± 11 | 8 | -6.8 ± 14 | 5 | 0.298 | |
| 27 | 12.4 | 1 | -7.8 ± 13 | 6 | - | |
| LVPWd % change | 21 | - | 0 | -8.7 ± 20 | 7 | - |
| 23 | -6.1 ± 18 | 3 | -7.2 ± 18 | 11 | 0.923 | |
| 25 | 5.6 ± 26 | 7 | -4.2 ± 27 | 5 | 0.464 | |
| 27 | -19.3 | 1 | 4.7 ± 26 | 6 | - |
Values are mean ± SD or median (LQ to UQ), and % (n)
PG: peak gradient, MG: mean gradient, iEOA: indexed effective orifice area, Vmax: maximum velocity across aortic valve, LVDd: left ventricular diastolic dimension, LVSd: left ventricular systolic dimension, IVSd: interventricular septal diastolic dimension, LVOT: left ventricular outflow tract dimension, LVPWd: left ventricular posterior wall dimension
Table 8.
Echocardiographic parameters by size in propensity-matched population: 2+ years
| Size / mm | Perimount | n | Perceval | n | P-value | |
|---|---|---|---|---|---|---|
| MG | 21 | - | 0 | 18 (17; 19) | 6 | - |
| 23 | 11 (9; 11) | 6 | 12.7 (11; 15) | 10 | 0.362 | |
| 25 | 11 (10; 21) | 7 | 10.3 (7.3; 12) | 10 | 0.351 | |
| 27 | 9 | 1 | 7.7 (7; 13) | 5 | 1.000 | |
| PG | 21 | - | 0 | 34.9 (30; 43) | 6 | - |
| 23 | 20 (16; 21) | 6 | 24 (20; 27) | 10 | 0.274 | |
| 25 | 20 (18 40) | 7 | 20.4 (14; 23) | 10 | 0.550 | |
| 27 | 17.3 | 1 | 14.9 (13; 23) | 5 | 1.000 | |
| EF % change | 21 | - | 0 | 3.6 (-1.6; 48) | 3 | - |
| 23 | -5.6 (-17; 3.3) | 6 | -1.5 (-19; 15) | 5 | 0.970 | |
| 25 | 6.7 (-18; 12) | 7 | 9.7 (3.3; 20) | 8 | 0.220 | |
| 27 | 26 | 1 | 17 (0.85; 41) | 4 | 1.000 | |
| Vmax | 21 | - | 1 | 2.9 ± 0.44 | 6 | - |
| 23 | 2.34 ± 0.55 | 6 | 2.44 ± 0.29 | 10 | 0.645 | |
| 25 | 2.32 ± 0.75 | 7 | 2.1 ± 0.40 | 10 | 0.425 | |
| 27 | 2.08 | 1 | 2.0 ± 0.36 | 5 | - | |
| iEOA | 21 | - | 0 | 0.69 ± 0.26 | 3 | - |
| 23 | 2.3 ± 0.55 | 6 | 2.4 ± 0.29 | 10 | 0.726 | |
| 25 | 2.3 ± 0.75 | 7 | 2.1 ± 0.40 | 10 | 0.762 | |
| 27 | 2.1 ± 0.75 | 1 | 2.0 ± 0.36 | 5 | - | |
| % PVL (n) | 21 | - | 0 | 16.7 (1) | 6 | - |
| 23 | 0 | 5 | 0 | 10 | - | |
| 25 | 0 | 6 | 10 (1) | 10 | 0.625 | |
| 27 | 0 | 1 | 0 | 5 | - | |
| LVOT % change | 21 | - | 0 | -10 (-26; 0) | 3 | - |
| 23 | 0.95 (-4.8; 5) | 6 | 0 (0; 5) | 3 | 0.976 | |
| 25 | -5 (-10; -1.8) | 5 | -8.7 (-30; 2.4) | 4 | 0.730 | |
| 27 | -7.7 | 1 | -1.3 (-3.6; 2.3) | 4 | 0.400 | |
| LVDd % change | 21 | - | 0 | -3.1 ± 12 | 6 | - |
| 23 | -4.8 ± 13 | 6 | 2.1 ± 8 | 9 | 0.237 | |
| 25 | -11 ± 7.6 | 5 | -2.6 ± 13 | 7 | 0.212 | |
| 27 | -10.5 | 1 | -15 ± 16 | 4 | - | |
| LVSd % change | 21 | - | 0 | -11.4 | 5 | - |
| 23 | -4.7 ± 19.9 | 6 | 5.6 ± 24 | 9 | 0.394 | |
| 25 | -12 ± 8 | 4 | -3.8 ± 33 | 7 | 0.378 | |
| 27 | -19.6 | 1 | -25.1 | 3 | - | |
| IVSd % change | 21 | - | 1 | -5.8 ± 19 | 6 | - |
| 23 | -8.9 ± 19 | 6 | -12 ± 12 | 9 | 0.687 | |
| 25 | -4.1 ± 22 | 5 | -11 ± 22 | 7 | 0.606 | |
| 27 | -24.6 | 1 | 0.59 ± 9.2 | 4 | - | |
| LVPWd % change | 21 | - | 0 | -8.6 ± 28 | 6 | |
| 23 | -18 ± 22 | 6 | -12.1 ± 19 | 9 | 0.613 | |
| 25 | 17 ± 2 | 4 | -4.8 ± 18 | 7 | 0.017* | |
| 27 | -15.5 | 1 | -12.0 ± 12 | 4 |
Values are mean ± SD or median (LQ to UQ), and % (n). * P <0.05
PG: peak gradient, MG: mean gradient, iEOA: indexed effective orifice area, Vmax: maximum velocity across aortic valve, LVDd: left ventricular diastolic dimension, LVSd: left ventricular systolic dimension, IVSd: interventricular septal diastolic dimension, LVOT: left ventricular outflow tract dimension, LVPWd: left ventricular posterior wall dimension
There were no clinical differences between the two groups at discharge (Table 4), including in subgroup analyses by isolated AVR and concomitant CABG.
The Perceval patients had a higher MG and PG in the 21-, 23-, and 25-mm sizes, higher Vmax in the 21- and 23-mm sizes, and a lower iEOA in the 25-mm size at discharge (Table 5). However, discharge % LVEF change, paravalvular regurgitation, and composite regurgitation were comparable (Table 5).
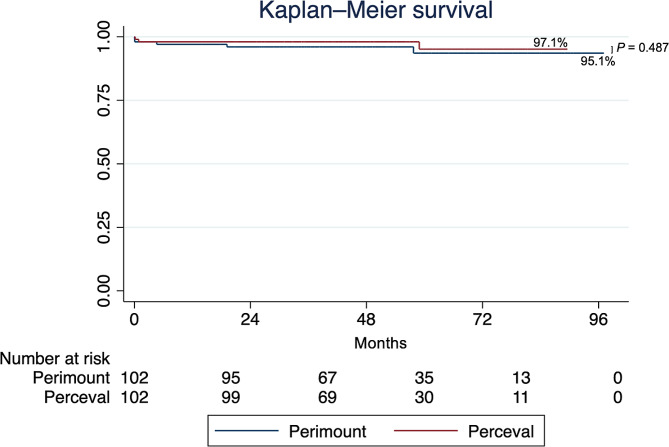
At 6–12 months (median follow-up: 266 days [Perceval], 283 days [Perimount]), the Perimount group had a more negative LVDd % change in the 25-mm size, and the LVPWd % change was more negative with in the Perceval group in the 25-mm size; there were no other differences. At 1–2 years (median follow-up: 506 days [Perceval], 595 days [Perimount]), there were no differences. At 2 + years (median follow-up: 959 days [Perceval], 1363 days [Perimount]), the only difference was a more negative LVPWd % change in the Perceval group in the 25-mm size (as seen at 6–12 months). Mid-term mortality did not vary by valve choice (Fig. 1).
Fig. 1.
Kaplan-Meier survival estimate. Log-rank P=0.487. Median follow-up: 49.3 months (Perimount), 48.4 months (Perceval). Event rates: 5 (Perimount), 3 (Perceval)
Discussion
Intra- and post-operative parameters
Cross-clamp and CPB times were shorter in the Perceval group. CPB time may independently influence post-operative morbidity and mortality, likely because CPB places the patient in a non-physiologic state where the blood withstands non-physiologic surfaces and atypical shear forces, causing systemic inflammation [16]. Although the differences in cross-clamp and CPB time were significant, one may argue that clinically this may not produce a large benefit for the cohort of Perceval patients. However, there might be a benefit for the individual, given that older patients with higher comorbidities also receive surgical aortic valve replacement. In combination with the possibility of carrying out the procedure via minimal invasive access without sewing in the valve, this may help the frail patient to get through the procedure with a cumulatively lower risk. Although this study found no clinical differences at discharge parameters, (in-line with some work such as PERSIST-AVR [8]), clinical benefits have been shown elsewhere including in a meta-analysis [6, 9–11]. We studied a matched population, thereby controlling for confounding factors and strengthening the comparison; nevertheless, it is possible that shorter operation times may translate into clinical benefits in larger populations or into benefits not measured in this study. The Perceval may be helpful for higher-risk patients benefitting from shorter operations, such as elderly patients with comorbidities, those with calcified aortas requiring minimal manipulation, and those undergoing concomitant procedures, particularly as operative mortality was comparable between groups.
MI-AVR was more frequent in the Perceval group, likely reflecting facilitation of MI-AVR by sutureless valves. This is consistent with the literature, although MI-AVR frequency varies depending on surgeon preferences [6, 9]. Converging sutureless valves with MI-AVR may further benefit recovery, as is supported by recent meta-analyses [3, 17].
The pacemaker rate was similar between groups, but the rate in both groups was relatively high compared to other studies [9]. Pacing of non-complete heart block arrhythmias may have contributed to this; moreover, our department has a low threshold for ensuring safe long-term outcome from new-onset dysrhythmias. Furthermore, among the patients receiving a permanent pacemaker, 1 Perceval patient had an ablation, and 1 Perimount patient underwent septal myotomy, increasing the pacing risk.
Echocardiographic parameters
MG, PG, and Vmax were higher for most valve sizes in the Perceval group at discharge, and iEOA was lower for the two smaller valve sizes. This seemingly contrasts with previous work, including a recent meta-analysis [3], the ongoing PERSIST-AVR trial [18], and a recent propensity-matched study [19], which all suggest comparable or superior early hemodynamic performance with the Perceval. Although higher discharge MG with the Perceval has been previously reported [4], we found that haemodynamics equalised between groups beyond 12 months, suggesting limited clinical impact. The reduced early iEOA with the Perceval was unexpected, given its lack of a sewing ring [9]. Another technical explanation for the higher gradients in the two smaller valve sizes may be the effect of a planned slight oversizing leading to impaired leaflet motion due to incompletely deployed valves. In particular, during the early days of Perceval implantation, there was a tendency to oversize with an M-size valve rather than opting for the S-size valve, in an attempt to avoid paravalvular leak. In this study, paravalvular leakage was similar between groups; this contrasts with the 2022 meta-analysis, which showed higher rates of PVL with the Perceval [3], although a different meta-analysis [20] and other studies [4, 13] found no difference.
Overall, a key finding of this study is that haemodynamic performance was comparable using propensity- and size-matched comparisons between the valves at mid-term follow-up, suggesting the Perceval reduces operative times and improves post-operative recovery without a haemodynamic cost. There was a more negative LVPWd % change in the Perceval group, possibly suggesting greater regression of left ventricular hypertrophy. Indeed, at the early stages of Perceval implantation in 2014, beneficial LV remodelling had been noticed in the first year after implantation [21], and this positive effect seems to be confirmed across all sizes after a mean follow up of 5 years reporting continuously low gradients [22]. The main cause of the beneficial remodelling of the LV is unclear. One could speculate that patients benefit from the fact that the sutureless valve has no sewing ring, providing a larger orifice area than the corresponding stented valve; another explanation could be lower long-term gradients with the Perceval; unfortunately, because the cohorts became smaller with time in the present study, potentially beneficial haemodynamics in the long-term may have gone undetected due to low power.
Limitations
This study faces the standard drawbacks of a retrospective, observational, single-centre design. Another limitation is the declining sample size with time (due to a combination of propensity matching and loss to follow-up). Although the resulting comparisons were fair and less likely to be confounded, they are likely underpowered. Echocardiograms taken / read from 2 different sites were included in this study, possibly introducing inconsistency. Functional outcomes such as symptoms or quality of life were not assessed.
Five staff surgeons perform conventional aortic valve replacement in our department. Out of the five operators, both Perceval implanters are the surgeons with the highest number of isolated AVRs performed and prefer the minimal-invasive access through upper mini-sternotomy. This may explain why, in the cohort of classic Perimount Magna Ease valves, the number of full sternotomies is higher.
Conclusions
This study supports the operative advantage of the Perceval valve in reducing operative times when compared to conventional sutured valves in a propensity-matched cohort. The Perceval could function as a ‘bridge’ between cAVR and TAVR in intermediate-high-risk, elderly patients with comorbidities or calcified aortic roots undergoing concomitant procedures, who may benefit from shorter operations. Valve haemodynamics, compared between propensity-matched and size-matched groups, were similar at mid-term follow-up, but the Perceval may better facilitate regression of left ventricular hypertrophy. More real-world follow-up data is required to ascertain whether the Perceval delivers superior iEOA and gradients in the long-term.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- AVR
Aortic valve replacement
- RDAVR
Rapid-deployment aortic valve replacement
- cAVR
Conventional aortic valve replacement
- PG
Peak gradient
- MG
Mean gradient
- iEOA
Indexed effective orifice area
- Vmax
Maximum velocity across aortic valve
- LVDd
Left ventricular diastolic dimension
- LVSd
Left ventricular systolic dimension
- IVSd
Interventricular septal diastolic dimension
- LVOT
Left ventricular outflow tract dimension
- LVPWd
Left ventricular posterior wall dimension
Author contributions
SJK, MYS, AZ, and GA conceptualized the study. SJK, MYS, and AZ collected pre-operative, intra-operative, and short-term post-operative data; SJK also collected mid-term follow-up data. AZ provided the propensity-matched patient table. SJK performed the statistical analysis, generated the figures and tables, and wrote the manuscript. MYS, AZ, IR, CQ, and GA reviewed the manuscript. CQ and GA supervised the project.
Funding
None.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The need for ethical approval was waived by the research and ethics office at Royal Brompton and Harefield Trust, given the retrospective nature of this study.
Consent for publication
Not applicable.
Competing interests
George Asimakopoulos is a proctor for Perceval.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 2.Dalén M, Sartipy U, Cederlund K, Franco-Cereceda A, Svensson A, Themudo R, et al. Hypo‐attenuated leaflet thickening and reduced leaflet motion in sutureless bioprosthetic aortic valves. J Am Heart Association. 2017;6(8):e005251. doi: 10.1161/JAHA.116.005251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmasi MY, Ramaraju S, Haq I, Mohamed RAB, Khan T, Oezalp F, et al. Rapid deployment technology versus conventional sutured bioprostheses in aortic valve replacement. J Card Surg. 2022;37(3):640–55. doi: 10.1111/jocs.16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ensminger S, Fujita B, Bauer T, Möllmann H, Beckmann A, Bekeredjian R, et al. Rapid deployment versus conventional bioprosthetic valve replacement for aortic stenosis. J Am Coll Cardiol. 2018;71(13):1417–28. doi: 10.1016/j.jacc.2018.01.065. [DOI] [PubMed] [Google Scholar]
- 5.Dedeilias P, Baikoussis NG, Prappa E, Asvestas D, Argiriou M, Charitos C. Aortic valve replacement in elderly with small aortic root and low body surface area; the Perceval S valve and its impact in effective orifice area. J Cardiothorac Surg. 2016;11(1):54. doi: 10.1186/s13019-016-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gersak B, Fischlein T, Folliguet TA, Meuris B, Teoh KHT, Moten SC, et al. Sutureless, rapid deployment valves and stented bioprosthesis in aortic valve replacement: recommendations of an International Expert Consensus Panel. Eur J Cardiothorac Surg. 2016;49(3):709–18. doi: 10.1093/ejcts/ezv369. [DOI] [PubMed] [Google Scholar]
- 7.Overview | Sutureless aortic valve replacement for aortic stenosis | Guidance | NICEhttps://www.nice.org.uk/guidance/ipg624.
- 8.Fischlein T, Folliguet T, Meuris B, Shrestha ML, Roselli EE, McGlothlin A, et al. Sutureless versus conventional bioprostheses for aortic valve replacement in severe symptomatic aortic valve stenosis. J Thorac Cardiovasc Surg. 2021;161(3):920–32. doi: 10.1016/j.jtcvs.2020.11.162. [DOI] [PubMed] [Google Scholar]
- 9.Meco M, Montisci A, Miceli A, Panisi P, Donatelli F, Cirri S, et al. Sutureless perceval aortic valve versus conventional stented bioprostheses: meta-analysis of postoperative and midterm results in isolated aortic valve replacement. J Am Heart Association. 2018;7(4):e006091. doi: 10.1161/JAHA.117.006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariani C, Murana G, Botta L, Gliozzi G, Folesani G, Santamaria V, et al. Single centre experience in 1202 biological prosthesis: a comparison between sutured, sutureless and surgical transcatheter aortic valve. Eur Heart J. 2021;42.ehab724.2259.
- 11.Muneretto C, Alfieri O, Cesana BM, Bisleri G, De Bonis M, Di Bartolomeo R, et al. A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in real-world patients with aortic stenosis and intermediate- to high-risk profile. J Thorac Cardiovasc Surg. 2015;150(6):1570–9. doi: 10.1016/j.jtcvs.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Hanedan MO, Yuruk MA, Parlar AI, Ziyrek U, Arslan AK, Sayar U, et al. Sutureless versus conventional aortic valve replacement: outcomes in 70 high-risk patients undergoing concomitant cardiac procedures. Tex Heart Inst J. 2018;45(1):11–6. doi: 10.14503/THIJ-16-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam KY, Reardon MJ, Yakubov SJ, Modine T, Fremes S, Tonino PAL, et al. Surgical sutureless and sutured aortic valve replacement in low-risk patients. Ann Thorac Surg. 2022;113(2):616–22. doi: 10.1016/j.athoracsur.2021.03.048. [DOI] [PubMed] [Google Scholar]
- 14.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2021;143(5). 10.1161/CIR.0000000000000923. [DOI] [PubMed]
- 15.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Oxford University Press (OUP); 2021.
- 16.Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(6):814–22. doi: 10.1053/j.jvca.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Salmasi MY, Papa K, Mozalbat D, Ashraf M, Zientara A, Chauhan I, et al. Converging rapid deployment prostheses with minimal access surgery: analysis of early outcomes. J Cardiothorac Surg. 2021;16(1):355. doi: 10.1186/s13019-021-01739-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischlein T, Caporali E, Asch FM, Vogt F, Pollari F, Folliguet T, et al. Hemodynamic performance of sutureless vs. conventional bioprostheses for aortic valve replacement: the 1-year core-lab results of the randomized persist-avr trial. Front Cardiovasc Med. 2022;9. 10.3389/fcvm.2022.844876. https://www.frontiersin.org/articles/. [DOI] [PMC free article] [PubMed]
- 19.Kueri S, Berger T, Puiu P, Alhamami Y, Diab N, Czerny M, et al. The hemodynamic performance of the Perceval Sutureless Aortic Valve in a propensity-matched comparison to the Carpentier–Edwards Perimount and Perimount magna ease valves for aortic valve replacement. Georg Thieme Verlag KG; 2022. [DOI] [PubMed]
- 20.Sohn SH, Jang M, Hwang HY, Kim KH. Rapid deployment or sutureless versus conventional bioprosthetic aortic valve replacement: a meta-analysis. J Thorac Cardiovasc Surg. 2018;155(6):2402–2412e5. doi: 10.1016/j.jtcvs.2018.01.084. [DOI] [PubMed] [Google Scholar]
- 21.Santarpino G, Pfeiffer S, Pollari F, Concistrè G, Vogt F, Fischlein T. Left ventricular mass regression after sutureless implantation of the Perceval S aortic valve bioprosthesis: preliminary results. Interact Cardiovasc Thorac Surg. 2014;18(1):38–42. doi: 10.1093/icvts/ivt362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldea GS, Burke CR, Fischlein T, Heimansohn DA, Haverich A, Suri RM, et al. Does valve size impact hemodynamic, left ventricular mass regression, and prosthetic valve deterioration with a sutureless aortic valve? J Thorac Cardiovasc Surg. 2023 doi: 10.1016/j.jtcvs.2023.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.