Abstract
Background
The prevalence of obesity is a growing global public health concern. Certain dietary amino acids have been shown to have a potential therapeutic role in improving metabolic syndrome parameters and body composition in individuals with obesity. However, some amino acids have been linked to an increased risk of cardiometabolic disorders. This cross-sectional study aims to investigate the association between dietary amino acid patterns and cardiometabolic risk factors in individuals with obesity.
Methods
This cross-sectional study included 335 participants with obesity (57.9% males and 41.5% females) from Tabriz and Tehran, Iran. The participants were between the ages of 20–50, with a body mass index (BMI) of 30 kg/m2 or higher, and free from certain medical conditions. The study examined participants’ general characteristics, conducted anthropometric assessments, dietary assessments, and biochemical assessments. The study also used principal component analysis to identify amino acid intake patterns and determined the association between these patterns and cardiometabolic risk factors in individuals with obesity.
Results
Upon adjusting for potential confounders, the study found that individuals in the third tertiles of pattern 1 and 2 were more likely to have lower LDL levels (OR = 0.99 and 95% CI (0.98–0.99)) for both. Additionally, a significant decrease in total cholesterol was observed in the third tertiles of pattern 2 in model II (OR = 0.99, 95% CI (0.98–0.99)). These findings suggest a potential cardioprotective effect of these amino acid patterns in managing cardiometabolic risk factors in individuals with obesity.
Conclusions
This study found that two identified amino acid patterns were associated with lower serum LDL and total cholesterol levels, while a third pattern was associated with higher serum triglycerides. The specific amino acids contributing to these patterns highlight the importance of targeted dietary interventions in managing cardiometabolic risk factors in individuals with obesity.
Keywords: Amino acids, Dietary protein, Factor analysis, Cardi-metabolic factors, Obesity, Amino acid pattern, Cardio-metabolic risk factors, Diet
Introduction
The escalating global prevalence of obesity, characterized by a body mass index (BMI) of 30 kg/m2 or higher, has become a major public health concern worldwide [1–3]. The World Health Organization (WHO) reports that the number of individuals affected by obesity has nearly tripled since 1975 [4]. In Iran, Tabrizi et al. found that approximately 25% of adults are obese [5]. Obesity is strongly associated with various cardio-metabolic disorders, including high blood pressure, elevated blood glucose, insulin resistance, high cholesterol, coronary heart disease, stroke, and certain types of cancers [6, 7]. Considering the high prevalence of obesity and its associated health consequences, achieving a healthy weight is of utmost importance. The pathophysiology of obesity is complex and multifactorial, involving both unmodifiable factors such as genetics, as well as modifiable factors such as sedentary lifestyle and diet [8, 9].
Emerging research suggests that specific dietary amino acids may have a potential therapeutic role in improving metabolic syndrome parameters and body composition in individuals with obesity [10]. Epidemiological studies have also explored the relationship between dietary amino acid patterns and various chronic diseases, including hypertension, type 2 diabetes, and metabolic biomarkers [11–13]. Certain dietary amino acids have been linked to an increased risk of hypertension and vascular dysfunction [14–16]. For example, amino acids like tyrosine and tryptophan have been shown to induce a vasodilator response in specific regions of the brain or blood vessel walls [15, 16]. Teymoori et al. discovered that a high intake of branched-chain amino acids (BCAA), alcoholic amino acids, and proline may increase the risk of developing hypertension [13]. Conversely, plant-derived L-arginine has been associated with a lower risk of cardiovascular disease (CVD) events due to its ability to produce nitric oxide [17]. A randomized controlled trial conducted by Mone et al. to evaluated the effect of L-arginine on cardiac rehabilitation revealing that L-arginine enhances the response to cardiac rehabilitation regardless of factors such as age, gender, baseline functional capacity, and comorbid conditions [18]. Additionally, a case-control study suggested that a high consumption of BCAA, aromatic, and sulfuric amino acids may increase the likelihood of developing non-alcoholic fatty liver disease (NAFLD) [19]. NAFLD is associated with dyslipidemia and CVD [20]. Yu et al. also reported that higher intakes of BCAAs can elevate serum levels of specific lipids and increase the risk of dyslipidemia [21]. Furthermore, some studies have demonstrated novel roles for specific amino acids in the atherogenicity of macrophages through the modulation of triglyceride metabolism [22].
Despite the growing body of evidence regarding the relationship between dietary amino acids and chronic diseases, there is a lack of cross-sectional studies investigating the association between dietary amino acid patterns and the likelihood of cardiometabolic risk factors such as dyslipidemia, hyperglycemia, and hypertension in individuals with obesity. Therefore, the objective of this cross-sectional study is to determine whether dietary amino acid patterns can contribute to the alteration of cardiometabolic risk factors among individuals with obesity.
Methods and materials
Participants
This cross-sectional study included 335 individuals with obesity (57.9% males and 41.5% females) who were selected from two previous studies [23, 24] conducted in Tabriz and Tehran, Iran. Public announcements were made to invite eligible participants. When determining the sample size, we considered the relationship between dietary quality indices and obesity as an important dependent variable. We used G-power software with specific parameters, including a correlation coefficient (r) of 0.25, a significance level (α) of 0.05, and a desired statistical power of 80%. Based on these factors, the minimum sample size was estimated to be 150 individuals. To conduct a sex-stratified analysis and considering an 11% drop-out rate, the final sample size for the study was increased to 335 participants [25]. The study enrolled subjects between the age of 20–50 and a BMI of 30 kg/m2 and above. Pregnant, lactating, and menopausal women were excluded from the study. Other exclusion criteria included recent bariatric surgery, CVD, diabetes mellitus, hepatic and renal diseases, cancer, malabsorptive disorders, and the use of weight-altering drugs or supplements. Ethical considerations were taken into account, and written informed consent was obtained from all participants prior to their involvement in the study. The study concept was approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran.
General characteristics and anthropometric assessments
A face-to-face interview was conducted with each participant to gather sociodemographic information including sex, age, smoking status, education attainment, marital status, occupation, medical histories, and family size using a demographic questionnaire. The socio-economic status (SES) score was determined by considering Individual factors such as education level (highest level of educational attainment), family size, occupational status, and home ownership. The physical activity level of the participants was assessed using a concise form of the International Physical Activity Questionnaire (IPAQ) [26]. Body composition evaluations were performed using the bioelectrical impedance analysis (BIA) method (Tanita, BC-418 MA, Tokyo, Japan). Measurements of body fat percentage, fat mass (FM), fat free mass (FFM) and predicted muscle mass were obtained. The participants’ weights and heights were measured using a Seca scale (Seca co., Hamburg, Germany) and a wall-mounted stadiometer, respectively, and the results were rounded to the nearest 0.1 kg and 0.5 cm, respectively. Each subject’s waist circumference (WC) and hip circumference (HC) were measured to the nearest 0.1 cm using a fixed tension tape. WC was measured at the midpoint between the lower costal margin and the iliac crest, and HC was measured at the widest part of the hip. BMI and waist-to-hip ratio (WHR) were then calculated. Blood pressure was assessed twice in the same arm using a standard mercury sphygmomanometer, with a minimum rest period of 15 min between measurements. The average of the two readings was used for analysis. The participants appetite state was assessed using the visual analogue scale (VAS) [27] in the fasting state in the morning. The VAS questionnaire asked about hunger, satiety, fullness, and future food intake as well as cravings for sweet, salty, and fatty foods. The appetite was determined by the distance between the left side of the line and the mark.
Dietary assessments
We used a validated semi-quantitative Food Frequency Questionnaire (FFQ) consisting of 168-item, adapted for Iranian population to assess dietary intakes of the subjects during the prior year [28]. The FFQ included a comprehensive list of food items that are frequently consumed in specified portion sizes in Iran. Participants were instructed to report the frequency and amount of each food item consumed, indicating whether they consumed them on a daily, weekly, monthly, or yearly basis during the past year. Household measures were then used to convert reported frequency of consumed foods and portion sizes for each food items into grams [29]. Due to the incompleteness of the Iranian Food Composition Table (FCT), the US Department of Agriculture (USDA) was utilized to analyze the energy and nutrient content of the reported foods. The intake of 18 individual amino acids from each food item was derived from the USDA. Amino acid intake was calculated as the frequency of consumption of each food item multiplied by the amino acid content of the food.
Biochemical assessment
Following a 12-h fasting period, a morning venous blood sample of 10 ml was obtained from each participant and centrifuged at 4500 rpm for 10 min at 4°C to separate serum and plasma. The samples were then stored at -80°C until measurement. Commercial kits (Pars Azmoon, Tehran, Iran) were used to assess serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and fasting blood sugar (FBS). Furthermore, low-density lipoprotein cholesterol (LDL-C) level was evaluated by the Friedewald equation [30]. Serum insulin concentration was measured using enzyme-linked immunosorbent assay (ELISA) kits (Bioassay Technology Laboratory, Shanghai Korean Biotech, Shanghai City, China).
Statistical analysis
Data analysis was carried out by SPSS version 27 (Statistical Package for Social Analysis, version 27, SPSS Inc., Chicago, IL, USA) with a significance level set at < 0.05. The principal component analysis (PCA) was used to determine amino acid intake patterns based on dietary intakes of amino acids (g/day). The ProMax rotation (an oblique rotation) was utilized due to the high correlation between the amino acids. Three patterns for amino acid intake were derived based on eigenvalues > 0.3, the scree plot, and the interpretability of the factors. Amino acid with an absolute component loading ≥ 0.40 were selected to describe each pattern. However, all amino acids contributed to the pattern score calculation. The Kaiser-Mayer-Olkin statistic, which serves as a measure of sampling adequacy, was 0.914, indicating good proportionality of factor analysis. We used Bartlett’s test of sphericity to assess the appropriateness of the correlation matrix for factor analysis and the P value was found to be < 0.001. Participants’ factor scores were calculated by multiplying the amino acid intake by their respective factor loadings in each amino acid pattern. All subjects were classified into tertiles based on the amino acid patterns. The normality distribution of the data was assessed through the Kolmogorov–Smirnov test. The participants’ baseline characteristics were presented as either median (interquartile range) and mean (standard deviation) for quantities variables, while frequency (%) were used for categorical variables. Chi-square test was used to compare the differences in categorical variables across different tertiles of amino acid patterns. For continuous variables, both the one-way analysis of variance (ANOVA) and the Kruskal-Wallis test were employed for parametric and non-parametric variables, respectively. Multivariate multinomial logistic regression with adjusted models (Model I: crude, Model II: adjusted for age and sex, Model III: adjusted for age, BMI, sex, physical activity, SES and energy intake) was used to obtain the odds ratio (OR) and 95% confidence interval (CI) for cardiometabolic risks across tertiles of amino acid patterns.
Results
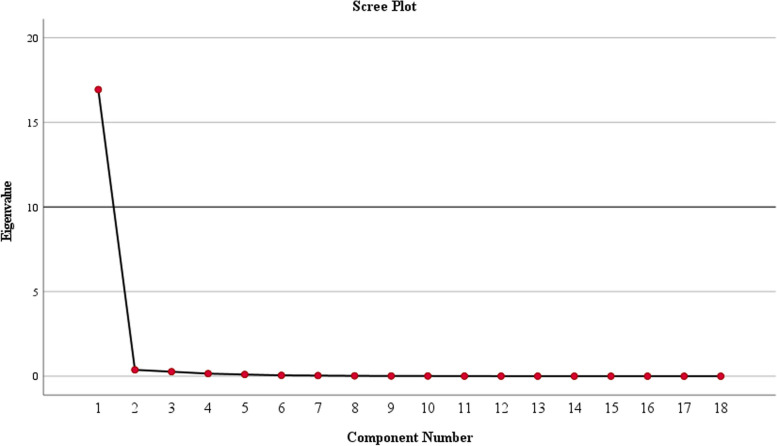
Three prominent amino acid patterns were identified using PCA, collectively accounting for 97.6% of the total variance in amino acid intake. Table 1 presents the factor loadings and the variances of each pattern and Fig. 1 depicts the scree plot exhibiting the eigenvalues. Pattern 1 exhibited higher loads of arginine, glycine, tryptophan, aspartic acids, cysteine and alanine; pattern 2 was characterized by higher loads of proline, phenylalanine, valine, serine, glutamic acid, tyrosine and leucine, whereas pattern 3 displayed higher loads of histidine, lysine, methionine, isoleucine and threonine.
Table 1.
Component loadings for dietary amino acid patterns
| Amino acid patterns | |||
|---|---|---|---|
| Pattern 1 | Pattern 2 | Pattern 3 | |
| Arginine | 0.899 | - | - |
| Glycine | 0.814 | - | - |
| Tryptophan | 0.651 | - | - |
| Aspartic acid | 0.571 | - | - |
| Cysteine | 0.524 | - | - |
| Alanine | 0.455 | - | - |
| Proline | - | 0.891 | - |
| Phenylalanine | - | 0.699 | - |
| Valine | - | 0.675 | - |
| Serine | - | 0.656 | - |
| Glutamine | - | 0.520 | - |
| Tyrosine | - | 0.501 | - |
| Leucine | - | 0.495 | - |
| Histidine | - | - | 0.763 |
| Lysine | - | - | 0.694 |
| Methionine | - | - | 0.657 |
| Isoleucine | - | - | 0.433 |
| Threonine | - | - | 0.409 |
| Variance | 94.092 | 2.092 | 1.478 |
Factor loadings of food patterns measured by factor analysis. Absolute value > 0.4
Fig. 1.
Scree plot representing the eigenvalues of dietary pattern analysis among study participants
Table 2 provides an overview of the demographic, anthropometric, and biochemical characteristics of the study participants across the tertiles of dietary amino acid patterns. Participants in upper tertiles of pattern 1 showed higher WC and WHR (P = 0.01 and P = 0.04, respectively). Moreover, individuals in the upper tertiles of pattern 2 were more likely to be male (P = 0.03) and had higher WC (P = 0.03) and FFM (P = 0.007). Individuals in the higher tertiles of pattern 3 exhibited higher serum TG levels (P = 0.01) compared to those in the lower tertiles. Additionally, participants in the upper tertiles of all three amino acid patterns had significantly higher energy intake and appetite (P < 0.001 for energy intake and P = 0.02 for appetite across all patterns).
Table 2.
General demographic characteristics of study participants by tertiles of amino acid patterns
| Variable | Pattern 1 | Pattern 2 | Pattern 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 n = 113 |
T2 n = 112 |
T3 n = 110 |
P Value | T1 n = 112 |
T2 n = 110 |
T3 n = 113 |
P Value | T1 n = 109 |
T2 n = 114 |
T3 n = 112 |
P Value | |
|
Age (y) Median (IQR) |
40.00 (11.00) | 36.00 (10.75) | 39.00 (12.75) | 0.152 | 40.00 (11.00) | 37.00 (11.00) | 38.00 (13.00) | 0.783 | 39.00 (10.75) | 38.00 (11.00) | 37.50 (12.75) | 0.692 |
|
Sex (Male) n (%) |
57 (50.4%) | 69 (61.6%) | 68 (60.2%) | 0.140 | 56 (80.0%) | 66 (58.4%) | 72 (63.7%) | 0.038 | 56 (50.0%) | 70 (61.4%) | 68 (60.7%) | 0.105 |
| Education (diploma ≤) n (%) | 54 (93.1%) | 59 (95.2%) | 61 (89.5%) | 0.880 | 56 (91.8%) | 61 (92.3%) | 57 (93.4%) | 0.421 | 69 (94.5%) | 48 (87.3%) | 56 (94.9%) | 0.169 |
| Marital status (Single) n (%) | 14 (12.4%) | 17 (15.2%) | 17 (15.0%) | 0.384 | 16 (14.0%) | 13 (11.5%) | 19 (14.2%) | 0.743 | 14 (12.5%) | 13 (11.4%) | 18 (16.1%) | 0.382 |
| Family size (> 4) n (%) | 9 (15.5%) | 7 (11.3%) | 11 (16.2%) | 0.545 | 9 (14.8%) | 8 (12.2%) | 10 (16.1%) | 0.967 | 11 (15.1%) | 7 (12.4%) | 9 (15.1%) | 0.981 |
| BMI (kg/m2) Median (IQR) | 33.34 (5.75) | 34.12 (5.03) | 34.58 (5.18) | 0.055 | 33.19 (5.59) | 34.19 (5.36) | 34.37 (4.67) | 0.315 | 33.75 (5.36) | 35.04 (5.81) | 33.57 (3.68) | 0.626 |
|
WC (cm) Mean (SD) |
104.83 (8.77) | 106.59 (10.40) | 108.68 (9.46) | 0.011 | 104.84 (8.66) | 107.19 (9.95) | 108.06 (10.10) | 0.035 | 106.05 (9.83) | 106.60 (9.78) | 107.46 (9.40) | 0.545 |
|
WHR Mean (SD) |
0.92 (0.08) | 0.92 (0.07) | 0.94 (0.06) | 0.043 | 0.92 (0.07) | 0.93 (0.08) | 0.94 (0.06) | 0.190 | 0.92 (0.08) | 0.93 (0.07) | 0.93 (0.07) | 0.383 |
|
FM (%) Median (IQR) |
34.90 (10.45) | 32.40 (12.43) | 32.85 (14.05) | 0.942 | 33.80 (10.70) | 32.30 (12.80) | 32.90 (14.30) | 0.966 | 34.00 (11.28) | 35.20 (16.00) | 30.40 (12.90) | 0.469 |
|
FFM (%) Median (IQR) |
55.00 (22.10) | 64.05 (22.75) | 65.30 (24.52) | 0.065 | 52.90 (20.80) | 63.10 (21.90) | 66.50 (23.90) | 0.007 | 58.15 (22.80) | 65.20 (21.60) | 64.40 (24.63) | 0.313 |
|
SES Score Mean (SD) |
9.51 (2.69) | 10.24 (2.46) | 10.08 (2.37) | 0.252 | 9.47 (2.74) | 10.15 (2.44) | 10.24 (2.29) | 0.179 | 9.50 (2.68) | 10.16 (2.65) | 10.33 (2.05) | 0.130 |
|
Energy (Kcal) Median (IQR) |
2078.28 (597.18) | 2960.31 (877.05) | 3477.74 (1003.59) | < 0.001 | 2121.42 (786.98) | 2945.59 (870.75) | 3485.90 (1319.24) | < 0.001 | 2293.44 (643.34) | 3084.18 (862.00) | 3516.12 (1229.23) | < 0.001 |
|
Appetite Median (IQR) |
31.07 (10.50) | 35.00 (8.75) | 33.55 (12.75) | 0.022 | 32.00 (10.00) | 34.00 (13.00) | 35.00 (12.00) | 0.032 | 31.82 (9.22) | 33.00 (10.00) | 36.00 (13.75) | 0.027 |
|
LDL (mg/dl) Median (IQR) |
120.60 (36.00) | 122.20 (34.95) | 111.40 (47.35) | 0.112 | 120.60 (31.60) | 122.00 (45.20) | 110.00 (41.40) | 0.154 | 121.00 (32.15) | 120.20 (49.30) | 111.40 (40.75) | 0.411 |
|
HDL (mg/dl) Median (IQR) |
45.00 (14.00) | 43.50 (10.50) | 45.00 (13.00) | 0.445 | 45.00 (16.00) | 42.00 (10.00) | 45.00 (13.00) | 0.243 | 45.00 (13.00) | 42.00 (16.00) | 45.00 (13.55) | 0.515 |
|
TG (mg/dl) Median (IQR) |
105.00 (50.00) | 103.50 (62.25) | 118.50 (82.25) | 0.503 | 98.00 (48.0) | 120.00 (64.00) | 111.00 (77.00) | 0.453 | 100.00 (45.75) | 111.00 (80.50) | 118.00 (72.00) | 0.011 |
|
TC (mg/dl) Mean (SD) |
196.80 (39.81) | 190.66 (36.30) | 187.83 (33.66) | 0.292 | 195.35 (38.42) | 193.22 (39.15) | 186.78 (32.12) | 0.241 | 192.97 (36.81) | 192.36 (39.82) | 189.96 (33.62) | 0.977 |
|
FBS (mg/dl) Median (IQR) |
90.00 (14.00) | 91.00 (11.75) | 92.00 (20.25) | 0.380 | 90.00 (13.00) | 91.00 (15.00) | 92.00 (15.00) | 0.936 | 88.00 (13.00) | 93.00 (16.00) | 90.50 (13.25) | 0.682 |
|
Insulin (mIU/l) Median (IQR) |
12.60 (11.00) | 13.10 (15.33) | 13.90 (16.50) | 0.714 | 13.00 (10.00) | 13.80 (16.40) | 13.20 (15.20) | 0.695 | 12.40 (11.80) | 13.40 (13.75) | 13.25 (17.75) | 0.665 |
|
SBP (mmHg) Median (IQR) |
110.00 (19.00) | 110.00 (15.00) | 117.50 (23.75) | 0.872 | 110.00 (23.00) | 110.00 (15.00) | 115.00 (18.00) | 0.954 | 121.40 (15.63) | 110.00 (20.00) | 111.00 (10.00) | 0.161 |
|
DBP (mmHg) Median (IQR) |
80.00 (11.50) | 70.00 (10.00) | 75.00 (16.00) | 0.270 | 75.00 (10.00) | 75.00 (15.00) | 70.00 (10.00) | 0.831 | 81.66 (10.96) | 75.00 (15.00) | 70.00 (10.00) | 0.773 |
Continuous values are shown as median (interquartile range) or mean (standard deviation). Categorical values are shown as number (%); Kruskal-Wallis test was used for estimate p-value of non-parametric variables. One-way ANOVA test was used for estimate p-value of parametric variables
BMI Body mass index, WC Waist Circumference, WHR Waist-to-hip ratio, FM Fat Mass, FFM Fat Free Mass, SES Socio-economic status, BMR Basal Metabolic Rate, LDL-C Low Density Lipoprotein Cholesterol, HDL-C High Density Lipoprotein Cholesterol, TG Triglyceride, TC Total Cholesterol, SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure
Table 3 displays the dietary energy and nutrient intakes of individuals across different tertiles of dietary amino acid patterns. Those in the upper tertiles had significantly higher intake of energy, protein, total cholesterol (Cho), saturated fat (SF), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA) and fiber (P < 0.01). In pattern 1 and pattern 3, subjects in the higher tertiles had lower intakes of carbohydrates (P < 0.01).
Table 3.
Dietary energy and nutrient intake of participants according to amino acid pattern
| Variable | Pattern 1 | Pattern 2 | Pattern 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | P Value* | T1 | T2 | T3 | P Value * | T1 | T2 | T3 | P Value * | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Energy(kcal/d) | 2163.83 (550.84) | 3069.71 (837.16) | 3817.16 (1117.36) | < 0.001 | 2183.45 (585.17) | 2990.93 (792.39) | 3860.81 (1118.74) | < 0.001 | 2293.44 (643.34) | 3043.76 (952.19) | 3706.77 (1147.89) | < 0.001 |
| CHO (% of energy) | 61.28 (7.52) | 59.76 (7.78) | 59.03 (6.72) | < 0.001 | 60.69 (7.71) | 60.17 (7.72) | 59.22 (6.70) | 0.325 | 61.58 (7.90) | 60.17 (7.66) | 58.33 (6.21) | 0.004 |
| Pro (% of energy) | 12.94 (2.06) | 13.05 (2.08) | 14.11 (2.21) | < 0.001 | 12.94 (2.30) | 13.14 (1.99) | 14.03 (2.10) | < 0.001 | 12.42 (1.81) | 13.21 (1.96) | 14.47 (2.25) | < 0.001 |
| Fat (%of energy) | 28.70 (6.72) | 30.33 (7.36) | 29.92 (6.20) | 0.175 | 29.20 (7.26) | 29.99 (7.10) | 29.74 (5.99) | 0.678 | 28.98 (7.44) | 29.93 (6.94) | 30.02 (5.92) | 0.450 |
| Cholesterol (g) | 213.83 (239.14) | 289.69 (133.80) | 388.32 (171.94) | < 0.001 | 212.92 (234.44) | 293.66 (153.39) | 383.89 (166.19) | < 0.001 | 209.92 (241.59) | 288.67 (142.57) | 392.72 (159.51) | < 0.001 |
| SF (g) | 20.00 (7.31) | 30.56 (15.74) | 37.44 (14.95) | < 0.001 | 19.92 (7.33) | 28.60 (13.78) | 39.30 (15.64) | < 0.001 | 20.16 (7.23) | 29.24 (14.02) | 38.50 (16.16) | < 0.001 |
| MUFA (g) | 22.50 (7.59) | 35.29 (17.52) | 42.06 (16.82) | < 0.001 | 24.12 (11.51) | 34.10 (16.29) | 41.44 (17.16) | < 0.001 | 24.37 (10.72) | 34.63 (18.08) | 40.72 (16.27) | < 0.001 |
| PUFA (g) | 15.26 (6.12) | 24.08 (14.18) | 28.59 (14.15) | < 0.001 | 16.84 (9.94) | 22.63 (11.23) | 28.34 (15.46) | < 0.001 | 17.60 (9.48) | 23.30 (14.18) | 26.95 (13.94) | < 0.001 |
| Fiber(g) | 55.71 (36.22) | 69.81 (35.46) | 88.31 (51.35) | < 0.001 | 54.67 (31.07) | 70.35 (36.17) | 91.24 (54.14) | < 0.001 | 62.50 (36.41) | 69.60 (47.04) | 86.23 (46.56) | 0.007 |
All data are mean (± SD)
CHO Carbohydrate, Pro Protein, SF Saturated Fat, MUFA Monounsaturated Fatty Acid, PUFA Polyunsaturated Fatty Acid
P* values derived from unadjusted ANOVA
Table 4 presents the ORs and 95% CIs for participants’ anthropometric variables based on the tertiles of dietary amino acid patterns. Participants in the highest tertiles of pattern 1 exhibited a higher BMI in the crude model (OR = 1.06, CI = 1.00–1.12, P = 0.03). increased adherence to pattern 1 and pattern 2 was associated with higher WC in the crude model (OR = 1.04, CI = 1.01–1.07, P = 0.003 and OR = 1.03, CI = 1.00–1.05, P = 0.01, respectively). However, after adjusting for potential confounders, the significance of the association between BMI and WC and amino acid patterns was no longer observed. The third tertile of pattern 2 was consistently associated with higher FFM across all three models, in comparison to the first tertiles (OR = 1.04, CI = 1.01–1.08, P = 0.002 for the crud model, OR = 1.08, CI = 1.01–1.16, P = 0.01 for model II and OR = 1.10, CI = 1.00–1.20, P = 0.03, for model III).
Table 4.
Association between anthropometric variables and amino acid patterns among participants
| Variable | Tertiles of Amino Acid Pattern 1 | Tertiles of Amino Acid Pattern 2 | Tertiles of Amino Acid Pattern 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |||||||
| OR (CI) | P-value | OR (CI) | P-value | OR (CI) | P-value | OR (CI) | P-value | OR (CI) | P-value | OR (CI) | P-value | ||||
| BMI (kg/m2) | |||||||||||||||
| Model I | 1 REF | 1.021 (0.966–1.080) | 0.456 | 1.062 (1.006–1.123) | 0.031 | 1 REF | 1.043 (0.987–1.102) | 0.137 | 1.039 (0.983–1.098) | 0.176 | 1 REF | 0.990 (0.938–1.045) | 0.714 | 1.004 (0.951–1.059) | 0.891 |
| Model II | 1.032 (0.973–1.094) | 0.297 | 1.067 (0.999–1.122) | 0.069 | 1.056 (0.997–1.118) | 0.065 | 1.059 (0.999–1.118) | 0.054 | 1.003 (0.949–1.061) | 0.905 | 1.016 (0.960–1.074) | 0.589 | |||
| Model III | 1.014 (0.944–1.089) | 0.699 | 1.063 (0.982–1.151) | 0.129 | 1.049 (0.979–1.124) | 0.176 | 1.044 (0.964–1.131) | 0.287 | 0.978 (0.918–1.041) | 0.481 | 0.979 (0.914–1.049) | 0.555 | |||
| WC (cm) | |||||||||||||||
| Model I | 1 REF | 1.020 (0.992–1.050) | 0.159 | 1.043 (1.014–1.073) | 0.003 | 1 REF | 1.027 (0.998–1.056) | 0.064 | 1.036 (1.008–1.056) | 0.013 | 1 REF | 1.006 (0.979–1.034) | 0.667 | 0.988 (1.043–1.015) | 0.274 |
| Model II | 1.016 (0.986–1.046) | 0.309 | 1.030 (0.998–1.059) | 0.114 | 1.023 (0.994–1.050) | 0.122 | 1.031 (1.001–1.062) | 0.079 | 0.998 (0.970–1.027) | 0.901 | 0.680 (0.390–1.185) | 0.511 | |||
| Model III | 1.007 (0.971–1.046) | 0.696 | 1.033 (0.991–1.076) | 0.123 | 1.020 (0.983–1.057) | 0.296 | 1.022 (0.981–1.065) | 0.298 | 0.985 (0.953–1.017) | 0.348 | 0.990 (0.956–1.026) | 0.591 | |||
| WHR (cm) | |||||||||||||||
| Model I | 1 REF | 1.017 (0.986–1.049) | 0.282 | 1.016 (0.985–1.048) | 0.309 | 1 REF | 0.996 (0.699–1.027) | 0.804 | 1.016 (0.986–1.047) | 0.304 | 1 REF | 0.984 (0.955–1.015) | 0.310 | 0.992 (0.963–1.022) | 0.619 |
| Model II | 1.025 (0.992–1.059) | 0.137 | 1.024 (0.991–1.058) | 0.150 | 1.002 (0.970–1.035) | 0.926 | 1.026 (0.994–1.060) | 0.108 | 0.990 (0.959–1.021) | 0.517 | 0.999 (0.968–1.030) | 0.939 | |||
| Model III | 1.025 (0.981–1.070) | 0.273 | 1.023 (0.985–1.063) | 0.263 | 1.004 (0.967–1.042) | 0.832 | 1.032 (0.988–1.078) | 0.157 | 0.984 (0.951–1.019) | 0.372 | 0.989 (0.953–1.027) | 0.560 | |||
| FM (%) | |||||||||||||||
| Model I | 1 REF | 1.012 (0.973–1.053) | 0.556 | 1.006 (0.967–1.046) | 0.779 | 1 REF | 1.008 (0.970–1.048) | 0.683 | 1.011 (0.972–1.051) | 0.584 | 1 REF | 1.014 (0.977–1.053) | 0.469 | 0.993 (0.956–1.033) | 0.735 |
| Model II | 1.039 (0.988–1.094) | 0.138 | 1.034 (0.983–1.088) | 0.192 | 1.035 (0.983–1.089) | 0.192 | 1.052 (1.000–1.107) | 0.052 | 1.028 (0.982–1.076) | 0.243 | 1.009 (0.962–1.058) | 0.714 | |||
| Model III | 1.032 (0.971–1.097) | 0.311 | 1.030 (0.963–1.103) | 0.390 | 1.037 (0.977–1.101) | 0.228 | 1.067 (0.997–1.142) | 0.062 | 1.019 (0.969–1.071) | 0.464 | 0.999 (0.944–1.057) | 0.976 | |||
| FFM (%) | |||||||||||||||
| Model I | 1 REF | 1.019 (0.989–1.050) | 0.207 | 1.025 (0.995–1.056) | 0.081 | 1 REF | 1.031 (1.001–1.062) | 0.044 | 1.048 (1.017–1.080) | 0.002 | 1 REF | 1.016 (0.987–1.046) | 0.276 | 1.016 (0.988–1.045) | 0.253 |
| Model II | 1.038 (0.972–1.109) | 0.264 | 1.063 (0.996–1.134) | 0.066 | 1.079 (1.009–1.153) | 0.025 | 1.088 (1.017–1.164) | 0.014 | 1.048 (0.957–1.053) | 0.150 | 1.008 (0.948–1.072) | 0.804 | |||
| Model III | 1.036 (0.958–1.120) | 0.373 | 1.085 (0.993–1.185) | 0.070 | 1.091 (1.012–1.177) | 0.022 | 1.102 (1.000–1.206) | 0.038 | 1.039 (0.970–1.113) | 0.273 | 1.005 (0.934–1.081) | 0.893 | |||
The multivariate multinomial logistic regression was used for estimation of ORs and 95% CI. Model I: crude, Model II: adjusted for age and sex, Model III: adjusted for age, sex and energy intake
BMI Body mass index, WC Waist Circumference, WHR Waist-to-hip ratio, FM Fat Mass, FFM Fat Free Mass, OR Odds ratio, CI Confidence interval
The ORs and 95% CIs for biochemical variables of the subjects displayed in Table 5. Individuals in the third tertiles of pattern 1 and pattern 2, were more likely to have lower level of LDL (OR = 0.99, CI = 0.98–0.99, P = 0.02 for both pattern in the crude model, and OR = 0.99, CI = 0.98–0.99, P = 0.01 for both pattern in model I) compared to those in the first tertiles, both in the crude and age- and sex-adjusted models. In model II, pattern 2 showed a significant decrease in TC level in the third tertiles (OR = 0.99, CI = 0.98–0.99, P = 0.04). Furthermore, the results indicated a positive association between the third amino acid pattern and increased TG levels, even after controlling for potential confounders such as age, sex, BMI, PA and energy intake (OR = 1.004, CI = 1.000–1.007, P = 0.03).
Table 5.
Association between biochemical variables and amino acid patterns among participants
| Variable | Tertiles of Amino Acid Pattern 1 | Tertiles of Amino Acid Pattern 2 | Tertiles of Amino Acid Pattern 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |||||||
| OR (CI) | P-value | OR (CI) | P-value | OR (CI) | P-value | OR (CI) | P-value | OR (CI) | P-value | OR (CI) | P-value | ||||
| LDL (mg/dl) | |||||||||||||||
| Model I | 1 REF | 0.997 (0.989–1.005) | 0.442 | 0.990 (0.982–0.999) | 0.023 | 1 REF | 0.995 (0.987–1.003) | 0.243 | 0.990 (0.982–0.999) | 0.024 | 1 REF | 1.001 (0.992–1.009) | 0.890 | 0.995 (0.987–1.003) | 0.233 |
| Model II | 0.997 (0.989–1.006) | 0.517 | 0.990 (0.982–0.998) | 0.019 | 0.995 (0.987–1.003) | 0.232 | 0.990 (0.981–0.998) | 0.019 | 1.000 (0.992–1.008) | 0.963 | 0.995 (0.989–1.003) | 0.218 | |||
| Model III | 1.003 (0.989–1.017) | 0.654 | 0.989 (0.972–1.006) | 0.202 | 1.001 (0.988–1.015) | 0.827 | 0.988 (0.971–1.005) | 0.176 | 0.999 (0.986–1.012) | 0.876 | 0.991 (0.977–1.006) | 0.224 | |||
| HDL (mg/dl) | |||||||||||||||
| Model I | 1 REF | 0.983 (0.956–1.010) | 0.213 | 0.986 (0.960–1.014) | 0.329 | 1 REF | 0.981 (0.954–1.008) | 0.166 | 0.988 (0.961–1.015) | 0.379 | 1 REF | 0.997 (0.970–1.024) | 0.803 | 0.985 (0.958–1.013) | 0.287 |
| Model II | 0.989 (0.961–1.018) | 0.457 | 0.992 (0.964–1.021) | 0.576 | 0.985 (0.957–1.014) | 0.302 | 0.996 (0.968–1.025) | 0.792 | 1.005 (0.976–1.034) | 0.757 | 0.991 (0.962–1.020) | 0.543 | |||
| Model III | 0.980 (0.929–1.033) | 0.451 | 0.997 (0.940–1.058) | 0.919 | 0.975 (0.928–1.024) | 0.313 | 1.004 (0.947–1.065) | 0.890 | 0.987 (0.941–1.035) | 0.589 | 0.996 (0.946–1.048) | 0.867 | |||
| TG (mg/dl) | |||||||||||||||
| Model I | 1 REF | 1.000 (0.997–0.003) | 0.992 | 1.000 (0.997–1.003) | 0.941 | 1 REF | 1.002 (0.999–1.005) | 0.271 | 1.001 (0.998–1.004) | 0.420 | 1 REF | 1.004 (1.000–1.007) | 0.027 | 1.004 (1.001–1.007) | 0.021 |
| Model II | 1.000 (0.997–1.003) | 0.847 | 0.999 (0.997–1.002) | 0.989 | 1.001 (0.998–1.004) | 0.397 | 1.001 (0.998–1.004) | 0.712 | 1.004 (1.001–1.008) | 0.046 | 1.004 (1.000–1.007) | 0.040 | |||
| Model III | 0.998 (0.989–1.006) | 0.581 | 1.001 (0.992–1.010) | 0.852 | 1.006 (0.998–1.014) | 0.126 | 1.003 (0.994–1.013) | 0.505 | 1.006 (0.999–1.014) | 0.097 | 1.004 (1.000–1.007) | 0.032 | |||
| TC (mg/dl) | |||||||||||||||
| Model I | 1 REF | 0.995 (0.988–1.003) | 0.212 | 0.993 (0.986–1.000) | 0.068 | 1 REF | 0.998 (0.991–1.006) | 0.664 | 0.994 (0.986–1.001) | 0.081 | 1 REF | 1.000 (0.992–1.007) | 0.901 | 0.998 (0.991–1.005) | 0.542 |
| Model II | 0.996 (0.989–1.003) | 0.266 | 0.993 (0.986–1.000) | 0.057 | 0.998 (0.991–1.005) | 0.630 | 0.992 (0.985–0.999) | 0.048 | 0.999 (0.992–1.006) | 0.783 | 0.998 (0.990–1.005) | 0.515 | |||
| Model III | 1.000 (0.987–1.013) | 0.981 | 0.991 (0.976–1.007) | 0.274 | 1.003 (0.991–1.015) | 0.654 | 0.992 (0.977–1.008) | 0.321 | 1.002 (0.990–1.014) | 0.797 | 0.995 (0.982–1.008) | 0.476 | |||
| FBS (mg/dl) | |||||||||||||||
| Model I | 1 REF | 0.990 (0.972–1.009) | 0.310 | 1.012 (0.997–1.028) | 0.128 | 1 REF | 1.009 (0.994–1.024) | 0.262 | 1.006 (0.990–1.022) | 0.467 | 1 REF | 1.011 (0.994–1.028) | 0.218 | 1.013 (0.997–1.030) | 0.117 |
| Model II | 0.992 (0.973–1.011) | 0.400 | 1.011 (0.996–1.027) | 0.155 | 1.009 (0.993–1.025) | 0.284 | 1.005 (0.989–1.021) | 0.542 | 1.010 (0.992–1.028) | 0.269 | 1.014 (0.996–1.031) | 0.126 | |||
| Model III | 1.000 (0.964–1.037) | 0.979 | 1.016 (0.979–1.054) | 0.410 | 1.029 (0.994–1.067) | 0.109 | 1.023 (0.986–1.062) | 0.232 | 1.030 (0.997–1.063) | 0.077 | 1.028 (0.994–1.062) | 0.104 | |||
| Insulin (mIU/l) | |||||||||||||||
| Model I | 1 REF | 0.984 (0.955–1.014) | 0.984 | 1.008 (0.986–1.030) | 0.493 | 1 REF | 1.007 (0.982–1.033) | 0.592 | 1.010 (0.985–1.036) | 0.431 | 1 REF | 1.002 (0.976–1.030) | 0.865 | 1.014 (0.989–1.039) | 0.273 |
| Model II | 0.985 (0.956–1.015) | 0.337 | 1.007 (0.984–1.031) | 0.543 | 1.009 (0.981–1.037) | 0.536 | 1.011 (0.984–1.039) | 0.421 | 1.003 (0.976–1.032) | 0.816 | 1.014 (0.988–1.041) | 0.282 | |||
| Model III | 1.022 (0.971–1.075) | 0.405 | 1.033 (0.975–1.095) | 0.268 | 1.021 (0.974–1.070) | 0.395 | 1.012 (0.953–1.075) | 0.693 | 0.995 (0.948–1.044) | 0.839 | 1.041 (0.992–1.094) | 0.104 | |||
| SBP (mmHg) | |||||||||||||||
| Model I | 1 REF | 1.003 (0.987–1.019) | 0.726 | 0.993 (0.977–1.009) | 0.399 | 1 REF | 1.000 (0.984–1.016) | 0.977 | 0.995 (0.979–1.011) | 0.541 | 1 REF | 1.015 (0.998–1.032) | 0.080 | 1.000 (0.984–1.016) | 0.984 |
| Model II | 1.005 (0.987–1.023) | 0.603 | 0.989 (0.972–1.007) | 0.229 | 0.997 (0.980–1.015) | 0.776 | 0.990 (0.973–1.007) | 0.252 | 1.012 (0.994–1.030) | 0.197 | 0.998 (0.981–1.015) | 0.828 | |||
| Model III | 1.023 (0.986–1.061) | 0.220 | 1.017 (0.976–1.059) | 0.421 | 0.998 (0.967–1.031) | 0.919 | 0.988 (0.949–1.029) | 0.557 | 1.007 (0.976–1.039) | 0.653 | 0.999 (0.964–1.035) | 0.956 | |||
| DBP (mmHg) | |||||||||||||||
| Model I | 1 REF | 0.987 (0.964–1.009) | 0.244 | 0.986 (0.963–1.008) | 0.211 | 1 REF | 0.999 (0.977–1.022) | 0.953 | 0.989 (0.967–1.012) | 0.341 | 1 REF | 1.007 (0.984–1.030) | 0.549 | 0.992 (0.970–1.015) | 0.494 |
| Model II | 0.988 (0.964–1.012) | 0.327 | 0.981 (0.958–1.005) | 0.128 | 0.998 (0.974–1.022) | 0.842 | 0.984 (0.961–1.008) | 0.189 | 1.002 (0.979–1.027) | 0.841 | 0.990 (0.967–1.014) | 0.400 | |||
| Model III | 0.995 (0.952–1.040) | 0.814 | 1.005 (0.957–1.054) | 0.853 | 1.018 (0.978–1.060) | 0.375 | 0.992 (0.945–1.042) | 0.756 | 0.988 (0.951–1.027) | 0.555 | 0.977 (0.935–1.020) | 0.282 | |||
The multivariate multinomial logistic regression was used for estimation of ORs and 95% CI. Model I: crude, Model II: adjusted for age and sex, Model III: adjusted for age, sex, BMI and energy intake
LDL-C Low Density Lipoprotein Cholesterol, HDL-C High Density Lipoprotein Cholesterol, TG Triglyceride, TC Total Cholesterol, FBS Fasting Blood Sugar, SBP Systolic Blood Pressure, DBP Diastolic Blood Pressure, OR Odds ratio, CI Confidence interval
Discussion
The study revealed that factors 1 and 2, which had a major contribution of amino acids such as arginine, glycine, tryptophan, aspartic acid for factor 1, and proline, phenylalanine, valine, serine, and glutamic acid for factor 2, exhibited a negative correlation with serum LDL and total cholesterol levels. Conversely, factor 3, which consisted of histidine, lysine, methionine, and isoleucine, displayed an association with elevated serum triglycerides.
Among the amino acids contributing to a more favorable lipid profile (Factors 1 and 2), arginine -the highest loading component in Factor 1 - has undergone extensive investigation. Available evidence suggests that arginine, as a substrate for nitric oxide (NO) synthesis, fosters lipid oxidation by increasing the phosphorylation of hormone-sensitive lipase [31]. The L-arginine-nitric oxide pathway, through which cell signal protein can be activated, has shown to regulate carbohydrate and lipid metabolism [32]. Furthermore, the functional relevance of L- arginine in T2DM has been shown to improve insulin sensitivity, glucose disposal and reduce glucose intolerance [33, 34]. Arginine provides the substrate to improve endothelial function and diminish the susceptibility of LDL to oxidation in patients with stable coronary artery disease, exerting its effects through multiple mechanisms, including the direct, NO-dependent, and NO-independent antioxidant capacity [35]. Additionally, in vivo supplementation of L-arginine has shown a reduction in oxidative stress by restoring superoxide dismutase and catalase activities as well as glutathione concentration [36]. Steven et al. conducted a study investigating the effects of plant extracts on blood parameters and oxidative/antioxidant status and concluded that plant-derived L-arginine improved lipid and glycemic profiles, reduced oxidant parameters, increased antioxidant tissue activity [37]. While dietary glutamate has shown an inverse association with cardiometabolic risk, the impact of glutamine on cardiometabolic risk appears to be unclear [38, 39]. However, it is important to state that our data did not detect these two amino acids. Other amino acids found in the protective factors have also been studied for their antioxidative effects on cardiometabolic factors. Glutathione (GSH), a major endogenous antioxidants synthesized in almost all tissues by sequential addition of the precursor amino acids cysteine, glutamic acid and glycine, appears to be associated with cardiometabolic risk due to its antioxidant role [38–41]. In addition to these amino acids, other studies have observed an increase in the total antioxidant capacity of plasma in connection with other antioxidant amino acids, including tryptophan and tyrosine (predominant in Factor 1 and 2, respectively) [42] which our conclusion is consistent with these findings. Rom et al. also found an anti-atherogenic role of glycine, cysteine, alanine, and leucine, which decreased macrophage triglyceride content by reducing VLDL uptake [22]. Furthermore, F Teymoori et al. demonstrated a negative association between higher intake of tryptophan and changes in serum TG, LDL-C, and TC levels which could be explained by the role of tryptophan as a serotonin precursor [43]. Serotonin, which assists in regulating glucose and lipid metabolism, has emerged as a novel therapeutic target for lipid metabolic disorders [44]. Phenylalanine and tyrosine, both precursors to the neurotransmitter dopamine, which plays a role in appetite and mood regulating, have been negatively associated with increased food intake [45]. As a non-essential amino acid, proline possesses antioxidant and anti-inflammatory effects in animal models, which may contribute to its beneficial effects on lipid metabolism and cardiovascular health [46]. High intake of sulfur-containing AAs, including methionine and cysteine, may contribute to cardiometabolic risk factors such as higher TCs and TGs by increasing oxidative stress and insulin resistance [43, 47]. Our results are in line with these findings for methionine, while cysteine in our study appears to have a cardiometabolic protective role which can be explained through modulation of redox status. Methionine, as a precursor of homocysteine, may play an increasing role in cardiovascular risk [48]. Nevertheless, evidence for the effect of methionine in interaction with other amino acids and the protein source is limited [49]. In an animal model, methionine supplementation result in hypercholesterolemia [50]. Moreover, a positive relationship between homocysteine and liver triglyceride levels has been demonstrated [51]. A potential cardioprotective role has been demonstrated for the intake of 7 amino acids (arginine, cysteine, glutamic acid, glycine, histidine, leucine, and tyrosine) [14], which is consistent with our results except for histidine. Leucine and valine, on the other hand, play a role in regulating lipid metabolism and insulin sensitivity by activating the mammalian target of rapamycin (mTOR) signaling pathway [46, 52].
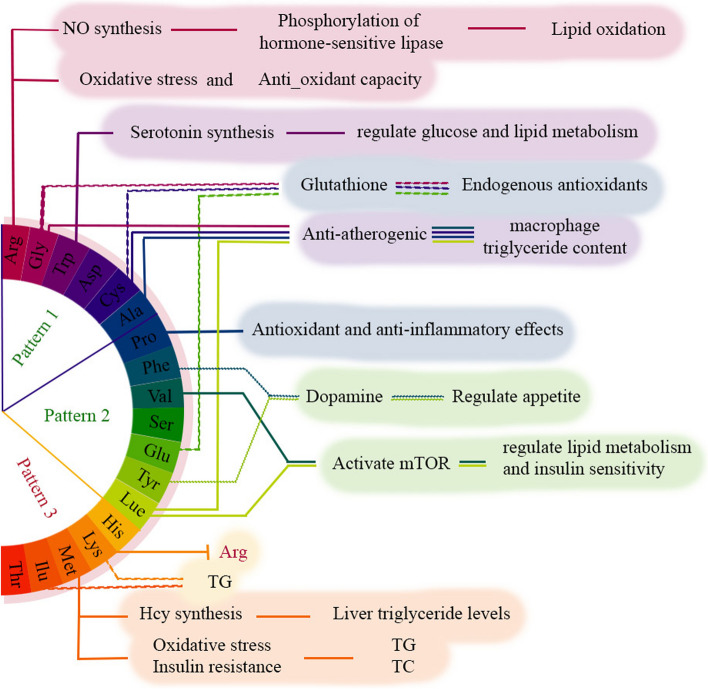
In contrast to our results, animal studies have shown that histidine supplementation reduce liver TGs and TCs content [53]. Additionally, studies by Javidan et al. reported a positive correlation between dietary lysine and isoleucine and serum TG levels, contradicting the results of Bel-Serrat et al. [54, 55]. Unfavorable effects of lysine on hypertriglyceridemia and hypercholesterolemia have also been reported in animal studies [56, 57]. It appears that the specific composition of accompanying amino acid influences the role of dietary amino acids in the body. In animal proteins, lysine competes with the intracellular transport of arginine due to a higher lysine-to-arginine ratio, thereby altering the physiological effects of arginine in the body [58, 59]. A randomized controlled crossover trial has shown that a low lysine-to-arginine ratio diet reduces postprandial TG levels [60]. Therefore, there may be substances associated with food proteins that strongly affect the function of amino acids. Figure 2 illustrates some of the mechanistic pathways depicting the involvement of amino acids in cardiometabolic factors.
Fig. 2.
Mechanistic pathways of amino acids role in cardio-metabolic factors. Arg contributes in cardio metabolic health by increasing lipid oxidation through No synthesis as well as its anti-oxidant role. Serotonin which derived from Trp has a therapeutic role in regulating glucose and fat metabolism. Gly, Cys and Glu are involved in the synthesis of glutathione - an endogenous antioxidant - which has a cardioprotective role. Amino acids such as Gly, Cys, Ala and Lue had anti-atherogenic role through reducing VLDL uptake and macrophage triglyceride content. Phe and Tyr regulate appetite by dopamine synthesis. Val and Lue contribute in regulating lipid metabolism and insulin sensitivity by activating mTOR. Met—a sulfur-containing amino acid—increases oxidative stress and insulin resistance, as well as liver triglyceride levels. It has been shown a positive association between Lys and Ilu and serum TG level. Abbreviates: Arg, arginine; Gly, glycine; Trp, tryptophan; Asp, aspartate; Cys, cysteine; Ala, alanine; Pro, Proline; Phe, phenylalanine; Val, valine Ser, serine; Glu, glutamate; Tyr, tyrosine; Lue, Lucine; His, histidine; Lys, lysine; Met, methionine; Ilu, isoleucine; Thr, threonine; NO, nitric oxide; TG, triglyceride; Hcy, homocysteine; TC, total cholesterol. Pattern 1: Arg, Gly, Trp, Asp, Cys and Ala, Pattern 2: Pro, Phe, Val, Ser, Glu, Tyr and Lue, pattern 3: His, Lys, Met, Ilu and Thr
To the best of our knowledge, this is the first study to examine the relationship between dietary amino acid patterns and cardiometabolic risk among Iranian adults. All phases of recruitment and data collection were conducted by a trained nutritionist, increasing the accuracy of the evaluation. Additionally, to achieve an independent relationship between dietary amino acid patterns and cardiometabolic factors, numerous potential confounders were considered in the analysis. The use of factor analysis to identify amino acid patterns not only better describes the dietary amino acid composition in our population but also takes into account any inter-correlation between amino acids and their cumulative effects. However, our study does have some potential limitations; First, the cross-sectional design complicates causal inference and longitudinal studies are required to better understand the cause-effect relationship. Second, the study had a small number of participants. Third, despite adjusting for the confounders, residual confounding factors may still exist. Forth, the FFQ used in this study was not originally developed for assessing amino acid intake. FFQs have the advantage of assessing participants’ usual dietary intake over a long period of time, but the risk of recall and data reporting errors is greater. However, the FFQ is the most widely used and population-based questionnaire for dietary assessment, which has been successfully validated for the Iranian population [28].
Conclusion
This study found that two identified amino acid patterns, factor 1 and factor 2, were negatively associated with serum LDL and TC levels. These patterns had major contributions from amino acids such as arginine, glycine, tryptophan, aspartic acid, proline, phenylalanine, valine, serine, and glutamic acid. On the other hand, factor 3, consisting of histidine, lysine, methionine, and isoleucine, was associated with higher serum TG levels. These findings highlight the importance of specific amino acid patterns in managing cardiometabolic risk factors in individuals with obesity.
Acknowledgements
We are thankful from all of the study’s participants. We also thank research undersecretary of Tabriz University of Medical Sciences for their financial support (Grant number: 73770).
Authors’ contributions
The final paper was read and approved by all writers. Research was supervised by MAF, and she also played a key role in designing the study. Together, FA and MM helped with the statistical analysis and drafted the article.
Funding
Present study has been financially supported by a grant from Tabriz University of Medical Sciences. (Registration code: IR.TBZMED.REC.1398.460 and IR. TBZMED.REC.1396.768). The funders had no role in hypothesis generation, recruiting and designing the study. Their role was only financial supporting.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to some restrictions that applied by the ethical committee but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study protocol has been approved by the ethics committee of the Tabriz University of Medical Sciences (registration code: IR.TBZMED.REC.1398.460 and IR. TBZMED.REC.1396.768). Written informed consent was obtained from all of the participants before participation in the study. All methods in the current research were performed in accordance with the declaration of Helsinki’s guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. doi: 10.1016/j.metabol.2022.155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao X, et al. Higher dietary choline and betaine intakes are associated with better body composition in the adult population of Newfoundland, Canada. PLoS One. 2016;11(5):e0155403. doi: 10.1371/journal.pone.0155403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176–s185. [PubMed] [Google Scholar]
- 4.World Health organization. Obesity and overweight. 2023. [9 June 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 5.Tabrizi JS, et al. Prevalence of dyslipidemia in urban and rural areas of the Northwest of Iran: the sociodemographic, dietary and psychological determinants. Iran J Public Health. 2019;48(5):925. [PMC free article] [PubMed] [Google Scholar]
- 6.Poirier P, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 7.Forouzanfar MH, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hruby A, et al. Determinants and consequences of obesity. Am J Public Health. 2016;106(9):1656–1662. doi: 10.2105/AJPH.2016.303326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samodien E, et al. Diet-induced DNA methylation within the hypothalamic arcuate nucleus and dysregulated leptin and insulin signaling in the pathophysiology of obesity. Food Sci Nutr. 2019;7(10):3131–3145. doi: 10.1002/fsn3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonson M, Boirie Y, Guillet C. Protein, amino acids and obesity treatment. Rev Endocr Metab Disord. 2020;21(3):341–353. doi: 10.1007/s11154-020-09574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, et al. Association between dietary essential amino acids intake and metabolic biomarkers: influence of obesity among Chinese children and adolescents. Amino Acids. 2021;53(5):635–644. doi: 10.1007/s00726-021-02970-4. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016;45(5):1482–1492. doi: 10.1093/ije/dyw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teymoori F, et al. Dietary amino acids and incidence of hypertension: a principle component analysis approach. Sci Rep. 2017;7(1):16838. doi: 10.1038/s41598-017-17047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings A, et al. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J Nutr. 2015;145(9):2130–2138. doi: 10.3945/jn.115.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altorf-van der Kuil W, et al. Dietary amino acids and the risk of hypertension in a Dutch older population: the Rotterdam study. Am J Clin Nutr. 2013;97(2):403–410. doi: 10.3945/ajcn.112.038737. [DOI] [PubMed] [Google Scholar]
- 16.Tuttle KR, et al. Dietary amino acids and blood pressure: a cohort study of patients with cardiovascular disease. Am J Kidney Dis. 2012;59(6):803–809. doi: 10.1053/j.ajkd.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Bahadoran Z, et al. Dietary L-arginine intake and the incidence of coronary heart disease: Tehran lipid and glucose study. Nutr Metab. 2016;13(1):1–9. doi: 10.1186/s12986-016-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mone P, et al. L-Arginine enhances the effects of cardiac rehabilitation on physical performance: new insights for managing cardiovascular patients during the COVID-19 pandemic. J Pharmacol Exp Ther. 2022;381(3):197–203. doi: 10.1124/jpet.122.001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokhtari E, et al. The association between dietary amino acids and the risk of nonalcoholic fatty liver disease among Tehranian adults: a case-control study. BMC Nutr. 2022;8(1):155. doi: 10.1186/s40795-022-00656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65(8):1109–1123. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, et al. Dietary branched-chain amino acids (BCAAs) and risk of dyslipidemia in a Chinese population. Nutrients. 2022;14(9):1824. doi: 10.3390/nu14091824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rom O, et al. Atherogenicity of amino acids in the lipid-laden macrophage model system in vitro and in atherosclerotic mice: a key role for triglyceride metabolism. J Nutr Biochem. 2017;45:24–38. doi: 10.1016/j.jnutbio.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 23.AbbasalizadFarhangi M, et al. Interaction between vascular endothelial growth factor-A (rs2010963) gene polymorphisms and dietary diversity score on cardiovascular risk factors in patients with metabolic syndrome. Lifestyle Genom. 2020;13(1):1–10. doi: 10.1159/000503789. [DOI] [PubMed] [Google Scholar]
- 24.Khodarahmi M, Asghari-Jafarabadi M, AbbasalizadFarhangi M. A structural equation modeling approach for the association of a healthy eating index with metabolic syndrome and cardio-metabolic risk factors among obese individuals. PLoS One. 2019;14(7):e0219193. doi: 10.1371/journal.pone.0219193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray AE, et al. Dietary quality in a sample of adults with type 2 diabetes mellitus in Ireland; a cross-sectional case control study. Nutr J. 2013;12:1–11. doi: 10.1186/1475-2891-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washburn RA. Assessment of physical activity in older adults. Res Q Exerc Sport. 2000;71(sup2):79–87. doi: 10.1080/02701367.2000.11082790. [DOI] [PubMed] [Google Scholar]
- 27.Flint A, et al. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 28.Mirmiran P, et al. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13(5):654–662. doi: 10.1017/S1368980009991698. [DOI] [PubMed] [Google Scholar]
- 29.Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran Nashre Olume Keshavarzy. 1999;7(213):42–58. [Google Scholar]
- 30.Sampson M, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5(5):540–548. doi: 10.1001/jamacardio.2020.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jobgen WS, et al. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17(9):571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Hu S, et al. L-arginine modulates glucose and lipid metabolism in obesity and diabetes. Curr Protein Pept Sci. 2017;18(6):599–608. doi: 10.2174/1389203717666160627074017. [DOI] [PubMed] [Google Scholar]
- 33.Szlas A, Kurek JM, Krejpcio Z. The potential of L-arginine in prevention and treatment of disturbed carbohydrate and lipid metabolism—a review. Nutrients. 2022;14(5):961. doi: 10.3390/nu14050961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forzano I, et al. L-Arginine in diabetes: clinical and preclinical evidence. Cardiovasc Diabetol. 2023;22(1):89. doi: 10.1186/s12933-023-01827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin WH, et al. L-arginine improves endothelial function and reduces LDL oxidation in patients with stable coronary artery disease. Clin Nutr. 2005;24(6):988–997. doi: 10.1016/j.clnu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 36.El-Missiry MA, Othman AI, Amer MA. L-Arginine ameliorates oxidative stress in alloxan-induced experimental diabetes mellitus. J Appl Toxicol. 2004;24(2):93–97. doi: 10.1002/jat.952. [DOI] [PubMed] [Google Scholar]
- 37.Hertzler SR, et al. Plant proteins: assessing their nutritional quality and effects on health and physical function. Nutrients. 2020;12(12):3704. doi: 10.3390/nu12123704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng S, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma W, et al. Dietary glutamine, glutamate and mortality: two large prospective studies in US men and women. Int J Epidemiol. 2018;47(1):311–320. doi: 10.1093/ije/dyx234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin J, et al. l-Cysteine metabolism and its nutritional implications. Mol Nutr Food Res. 2016;60(1):134–146. doi: 10.1002/mnfr.201500031. [DOI] [PubMed] [Google Scholar]
- 41.Senthilkumar R, Sengottuvelan M, Nalini N. Protective effect of glycine supplementation on the levels of lipid peroxidation and antioxidant enzymes in the erythrocyte of rats with alcohol-induced liver injury. Cell Biochem Funct. 2004;22(2):123–128. doi: 10.1002/cbf.1062. [DOI] [PubMed] [Google Scholar]
- 42.Meucci E, Mele M. Amino acids and plasma antioxidant capacity. Amino Acids. 1997;12:373–377. doi: 10.1007/BF01373017. [DOI] [Google Scholar]
- 43.Teymoori F, et al. Are dietary amino acids prospectively predicts changes in serum lipid profile? Diabetes Metab Syndr. 2019;13(3):1837–1843. doi: 10.1016/j.dsx.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe H, Rose MT, Aso H. Role of peripheral serotonin in glucose and lipid metabolism. Curr Opin Lipidol. 2011;22(3):186–191. doi: 10.1097/MOL.0b013e3283462273. [DOI] [PubMed] [Google Scholar]
- 45.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-B. [DOI] [PubMed] [Google Scholar]
- 46.Li F, et al. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011;41:1185–1193. doi: 10.1007/s00726-011-0983-2. [DOI] [PubMed] [Google Scholar]
- 47.Dong Z, et al. Association of sulfur amino acid consumption with cardiometabolic risk factors: cross-sectional findings from NHANES III. EClinicalMedicine. 2020;19:100248. doi: 10.1016/j.eclinm.2019.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chambers JC, Obeid OA, Kooner JS. Physiological increments in plasma homocysteine induce vascular endothelial dysfunction in normal human subjects. Arterioscler Thromb Vasc Biol. 1999;19(12):2922–2927. doi: 10.1161/01.ATV.19.12.2922. [DOI] [PubMed] [Google Scholar]
- 49.Verhoef P, et al. Dietary serine and cystine attenuate the homocysteine-raising effect of dietary methionine: a randomized crossover trial in humans. Am J Clin Nutr. 2004;80(3):674–679. doi: 10.1093/ajcn/80.3.674. [DOI] [PubMed] [Google Scholar]
- 50.Hirche F, et al. Effect of dietary methionine on plasma and liver cholesterol concentrations in rats and expression of hepatic genes involved in cholesterol metabolism. Br J Nutr. 2006;95(5):879–888. doi: 10.1079/BJN20061729. [DOI] [PubMed] [Google Scholar]
- 51.Obeid R, Herrmann W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett. 2009;583(8):1215–1225. doi: 10.1016/j.febslet.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 52.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136(1):227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 53.Shanshan D, et al. Effects of histidine supplementation on global serum and urine 1H NMR-based metabolomics and serum amino acid profiles in obese women from a randomized controlled study. 2017. [DOI] [PubMed]
- 54.Javidan AN, et al. Is the pattern of dietary amino acids intake associated with serum lipid profile and blood pressure among individuals with spinal cord injury? J Spinal Cord Med. 2017;40(2):201–212. doi: 10.1080/10790268.2015.1109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bel-Serrat S, et al. The role of dietary fat on the association between dietary amino acids and serum lipid profile in European adolescents participating in the HELENA Study. Eur J Clin Nutr. 2014;68(4):464–473. doi: 10.1038/ejcn.2013.284. [DOI] [PubMed] [Google Scholar]
- 56.Leszczynski D, Kummerow F. Excess dietary lysine induces hypercholesterolemia in chickens. Experientia. 1982;38(2):266–267. doi: 10.1007/BF01945105. [DOI] [PubMed] [Google Scholar]
- 57.Giroux I, Kurowska EM, Carroll KK. Role of dietary lysine, methionine, and arginine in the regulation of hypercholesterolemia in rabbits. J Nutr Biochem. 1999;10(3):166–171. doi: 10.1016/S0955-2863(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 58.Luiking YC, Deutz NE. Biomarkers of arginine and lysine excess. J Nutr. 2007;137(6):1662S–1668S. doi: 10.1093/jn/137.6.1662S. [DOI] [PubMed] [Google Scholar]
- 59.Venho B, et al. Arginine intake, blood pressure, and the incidence of acute coronary events in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2002;76(2):359–364. doi: 10.1093/ajcn/76.2.359. [DOI] [PubMed] [Google Scholar]
- 60.Vega-López S, et al. Altering dietary lysine: arginine ratio has little effect on cardiovascular risk factors and vascular reactivity in moderately hypercholesterolemic adults. Atherosclerosis. 2010;210(2):555–562. doi: 10.1016/j.atherosclerosis.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to some restrictions that applied by the ethical committee but are available from the corresponding author on reasonable request.