Abstract
Invasion of endothelial tissues may be crucial in a Listeria monocytogenes infection leading to meningitis and/or encephalitis. Internalization of L. monocytogenes into endothelial cells has been previously demonstrated by using human umbilical vein endothelial cells as a model system. However, during the crossing of the blood-brain barrier, L. monocytogenes most likely encounters brain microvascular endothelial cells which are strikingly different from macrovascular or umbilical vein endothelial cells. In the present study human brain microvascular endothelial cells (HBMEC) were used to study the interaction of L. monocytogenes with endothelial cells, which closely resemble native microvascular endothelial cells of the brain. We show that L. monocytogenes invades HBMEC in an InlB-dependent and wortmannin-insensitive manner. Once within the HBMEC, L. monocytogenes replicates efficiently over a period of at least 18 h, moves intracellularly by inducing actin tail formation, and spreads from cell to cell. Using a green fluorescent protein-expressing L. monocytogenes strain, we present direct evidence that HBMEC are highly resistant to damage by intracellularly growing L. monocytogenes. Infection of HBMEC with L. monocytogenes results in foci of heavily infected, but largely undamaged endothelial cells. Heterologous plaque assays with L. monocytogenes-infected P388D1 macrophages as vectors demonstrate efficient spreading of L. monocytogenes into HBMEC, fibroblasts, hepatocytes, and epithelial cells, and this phenomenon is independent of the inlC gene product.
Listeria monocytogenes is a gram-positive facultative intracellular bacterium that causes severe diseases in both animals and humans. Primarily immunocompromised individuals, such as pregnant women, neonates, and elderly people, become infected and undergo bacteremia, sepsis, abortion, meningitis, and encephalitis (39). The normal route of entry of L. monocytogenes into the host is the gut via listeria-contaminated food, as shown by several recent outbreaks of listeriosis that could be traced to contaminated food such as cole slaw or Mexican-style cheese (15). During the course of infection, L. monocytogenes encounters different cell types, including epithelial cells, fibroblasts, hepatocytes, macrophages, and endothelial cells, which are all, at least in vitro, readily infected. Once inside the host cell, the bacteria rapidly lyse the phagosomal membrane in order to escape into the host cell cytoplasm, where intensive intracellular multiplication and intracellular movement occur. Direct transfer from one cell to another allows the bacteria to enter neighboring cells without an extracellular phase (reviewed in reference 26). A number of listerial virulence determinants involved in the intracellular life cycle of L. monocytogenes have been characterized. A family of internalins was discovered in L. monocytogenes (10, 14, 16). InlA and InlB have been shown to be necessary for triggering uptake by several cell types (5, 11, 16, 29). A sulfhydryl-activated pore-forming cytolysin called listeriolysin is required, along with two phospholipases, for lysis of the phagosome. ActA, a listerial cell wall protein, promotes F-actin-driven intracellular movement. The expression of most of these virulence factors is controlled in a complex manner by the positive regulatory factor PrfA (reviewed in reference 26).
As noted above, an increasing number of mouse and human cell types, including primary and permanent human umbilical vein endothelial cells, are infected by L. monocytogenes in vitro (13, 18, 33, 42, 43). However, umbilical vein endothelial cells are probably not in vivo targets of L. monocytogenes in contrast to endothelial cells from brain microvessels. The blood-brain barrier, which is responsible for maintaining the biochemical homeostasis within the central nervous system (2), is constituted of either the cerebral capillary endothelium, which is a barrier between blood and the brain parenchyma, or of the choroid plexus epithelium, which is a barrier between blood and the cerebrospinal fluid (49). The cerebral capillary endothelium is a single layer of specialized human brain microvascular endothelial cells (HBMEC) and is characterized by the possession of tight junctions (37). The tight junctions, together with the lack of pinocytosis of these cells, form a barrier even for small molecules, such as dyes and antibiotics, at the blood-brain barrier boundary. The choroid plexus is an epithelium within the cerebral vesicles that produces cerebrospinal fluid. It is a polarized epithelium exhibiting tight junctions which are lacking from the underlying endothelium of the blood vessels (28, 37). Upon intracerebral injection into mice, L. monocytogenes had a striking affinity for the epithelial cells of the choroid plexus (38).
The high incidence of meningitis and encephalitis associated with human L. monocytogenes infections implies that this microorganism is able to breach the blood-brain barrier. At the level of the cerebral capillaries, passage of bacteria through the endothelial barrier can in principle be accomplished in four different ways: (i) destruction of the barrier by killing the endothelial cells; (ii) breaking the cell-to-cell contacts and passage between the cells; (iii) direct or indirect invasion of the endothelial cells and release of the bacteria on the basolateral side; or (iv) migration of the infected monocytes through the endothelial barrier.
Direct and indirect infection of human umbilical vein endothelial cells (HUVEC) by L. monocytogenes was described recently (13, 18, 33), but the interaction of L. monocytogenes with HBMEC has not been investigated so far. In the present study we used HBMEC immortalized by simian virus 40 transformation and maintained in tissue culture monolayers. With these cells we were able to demonstrate the invasion of L. monocytogenes into HBMEC and its intracellular growth and movement. Furthermore, we show that the listerial surface protein InlB is required for efficient HBMEC infection by L. monocytogenes, infection which occurs in a cytochalasin D- and nocodazol-sensitive but wortmannin-insensitive and hence PI-3 kinase-independent process. Finally, we present evidence that L. monocytogenes is able to spread in vitro from infected macrophages into HBMEC as well as into fibroblasts, epithelial cells, and hepatocytes.
MATERIALS AND METHODS
Bacteria.
The L. monocytogenes EGD wild-type strain, the isogenic deletion mutants, and the other Listeria strains used in this study are described in Table 1. The bacteria were cultured aerobically in brain heart infusion broth (Difco) at 37°C until they reached the mid-log phase of growth. They were then washed twice with phosphate-buffered saline (PBS) and stored in aliquots in PBS with 20% glycerol (vol/vol) at −80°C until being used for the infection experiments.
TABLE 1.
Listeria strains used in this study
| Strain | Genotype | Characteristic(s) | Source or reference |
|---|---|---|---|
| L. monocytogenes | |||
| Sv 1/2a EGD | Wild type | Local strain collection | |
| WL-105 | ΔplcA | In-frame deletion in plcA | 20 |
| WL-103 | ΔplcB | In-frame deletion in plcB | 20 |
| WL-106 | ΔplcA ΔplcB | In-frame deletions in plcA and plcB | 20 |
| ΔinlC | ΔinlC | In-frame deletion in inlC | 14 |
| A76 | ΔinlA | In-frame deletion in inlA | 18 |
| WL-111 | ΔinlB | In-frame deletion in inlB | 18 |
| WL-112 | ΔinlAB | In-frame deletion of inlAB | 18 |
| A49 | ΔactA | In-frame deletion in actA | 20 |
| A42 | ΔprfA | In-frame deletion in prfA | 3 |
| LM-GFP | PactA::gfp | Expresses GFP under the control of the actA promoter | 8 |
| PKP-1 | Δ(plcA hly mpl actA plcB) | In-frame deletion and insertion of Kanr cassette | 14 |
| NCTC 7973 | Hyperhemolytic isolate | Local strain collection | |
| 10403S | Wild type | 34 | |
| DP-L2161 | Δhly | In-frame deletion in hly | 23 |
| L. innocua Sv6a | Wild type | Local strain collection |
HBMEC culture.
HBMEC were isolated from a brain biopsy of an adult female with epilepsy and cultured by previously described methods (46). The cells were positive for factor VIII-Rag, carbonic anhydrase IV, and Ulex europaeus agglutinin I. They took up fluorescently labeled acetylated low-density lipoprotein and expressed gamma glutamyl transpeptidase, thus demonstrating their brain endothelial cell properties (45). HBMEC were subsequently immortalized by transfection with simian virus 40 large T antigen and maintained their morphologic and functional characteristics for at least 30 passages. The cells are polarized and exhibit a transendothelial electric resistance of 200 Ω/cm2 (36, 47). HBMEC were cultured without the addition of antibiotics in RPMI 1640 medium (Gibco), supplemented with fetal calf serum (10%) (Gibco), NuSerum IV (10%) (Becton Dickinson, Bedford, Mass.), modified Eagle’s medium nonessential amino acids (1%) and vitamins (1%), heparin (5 U/ml), sodium pyruvate (1 mM), l-glutamine (2 mM), and endothelial cell growth supplement (30 μg/ml) (all from Sigma) (i.e., complete HBMEC medium). Then 75-ml tissue culture flasks were treated with gelatin to support the growth of the HBMEC monolayers by spreading 3 ml of a 0.2% gelatin solution in the flask to completely coat the surface. The remaining liquid was removed, and the bottom of the flask was flamed briefly; the flasks were then dried for at least 6 h. Cultures were incubated at 37°C in a humid atmosphere of 5% CO2, and the monolayers were split in a ratio of 1:4 twice a week with trypsin-EDTA according to standard procedures. Monolayers of passages 8 to 16 were used for all experiments described.
Cellular invasion and intracellular growth assays.
At 48 h prior to infection, HBMEC were split, and the cells were seeded into gelatin-treated 24-well tissue culture plates at a density of 105 cells per well. Immediately prior to the assay, each well was found to contain approximately 4 × 105 cells. The bacteria taken from the freezer were diluted in RPMI 1640 medium, and 500 μl of the suspension was added to each monolayer to reach the desired multiplicity of infection (MOI). The cultures were incubated for 1.5 h to allow the bacteria to invade the cells. The monolayers were then washed three times with PBS, 1 ml of complete HBMEC medium containing 100 μg of gentamicin (Gibco) per ml was added to each well to kill the extracellular bacteria, and the plates were further incubated for 1.5 h at 37°C. After the cells were washed three times with PBS, they were lysed by the addition of ice-cold distilled water, incubated for 10 min on ice, and sonicated for 1 s with a Branson sonifier. Serial dilutions of the lysate in 150 mM NaCl were plated on brain heart infusion agar. For intracellular growth assays, the gentamicin-containing culture medium was removed at 1.5 h postinfection, and fresh complete medium was added that contained only 10 μg of gentamicin per ml. The cultures were further incubated until being lysed at the time points indicated. All cellular invasion and growth assays were performed in triplicate and repeated three times.
Invasion inhibition studies.
Invasion assays were performed as described above, except that the media contained the indicated concentrations of the various inhibitors throughout the duration of the experiment and during a 45-min preincubation period before the bacteria were added. The inhibitors were supplied either by Sigma (cytochalasin D, nocodazole, and wortmannin) or by Calbiochem (wortmannin) and were dissolved in dimethylsulfoxide. Data were expressed as the percent invasion relative to the result of a control assay performed concurrently without the addition of an inhibitor.
Heterologous plaque assay.
The so-called heterologous plaque assay is a variant of the classical plaque assay (24, 27) and was developed to test the ability of L. monocytogenes to spread from one cell type (macrophage) into other cells (fibroblasts, epithelial cells, hepatocytes, and endothelial cells). The different cell types (with the exception of the HBMEC, which were cultured as described above) were all cultured in RPMI 1640 medium supplemented with fetal calf serum (FCS) (10%) and l-glutamine (2 mM) (all from Gibco), seeded into 6-well plates at a density of 2 × 105 cells per well, and incubated overnight. P388D1 macrophages were cultured in RPMI 1640 medium with FCS (10%) and l-glutamine (2 mM) and seeded at 5 × 106 per 90-mm-diameter plate (which were not treated for tissue culture). Upon overnight incubation, the macrophages were infected with 2.5 × 108 bacteria (MOI = 50) for 1 h at 37°C, then washed three times with PBS, and further incubated for 1.5 h in medium containing 100 μg of gentamicin per ml to kill the extracellular bacteria. After removal of the medium, 8 ml of cold PBS without Ca2+ and Mg2+ was added, and the plates were incubated on ice for 10 min, resulting in detachment of the infected macrophages from the plastic surface. The macrophages were collected, washed once with PBS and once with RPMI 1640 containing 10 μg of gentamicin per ml, resuspended in 5 ml of RPMI 1640 with 10 μg of gentamicin per ml, and counted. The suspension was adjusted to a density of 104 macrophages per ml, 200 μl of the suspension was then added to each well with the target cells in the 6-well plates, and the plates were incubated for 2.5 h at 37°C, allowing the macrophages to settle onto the monolayer. After removal of the medium, an agarose overlay (0.5% agarose in RPMI 1640 medium with 20% FCS, 2 mM l-glutamine, and 5 μg of gentamicin per ml) was added to each well. After a 24-h incubation at 37°C, a second overlay, consisting of 0.01% neutral red, 15 μg of gentamicin per ml, and 0.5% agarose in RPMI 1640 medium, was added to better visualize the plaques that appeared after an additional 24 h of incubation at 37°C.
Fluorescence microscopy.
HBMEC monolayers grown on glass coverslips were infected with L. monocytogenes EGD as described above. At 20 h postinfection, the cells were fixed with acetone, stained with tetramethyl rhodamine isocyanate (TRITC)-phalloidin as previously described (27, 31), and analyzed microscopically. Pictures were taken with a confocal microscope (Leica TCS NT). Green fluorescent protein (GFP)-expressing L. monocytogenes inside HBMEC were visualized by using a fluorescence-equipped inverted microscope (Leica DMIRP) and photographed with a 400 ASA negative film as previously described (8).
Statistical analysis.
The data in Fig. 1 are presented as means and standard deviations (error bars) of a representative experiment run in triplicate. The significance of the effect of mutations or the effect of inhibitors on L. monocytogenes invasion of HBMEC was analyzed with a two-tailed, unpaired Student’s t test.
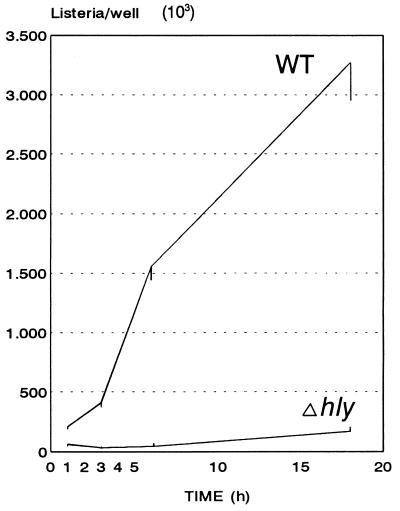
FIG. 1.
Intracellular growth of wild-type and nonhemolytic strains of L. monocytogenes in HBMEC. HBMEC were infected with L. monocytogenes 10403S (WT) and isogenic L. monocytogenes DP-L2161 (Δhly) (MOI = 5) as described in Materials and Methods, and the intracellular growth was monitored over a period of 18 h.
RESULTS
L. monocytogenes invades and replicates inside HBMEC.
As a meningitis- and encephalitis-causing bacterium, L. monocytogenes should be able to cross the blood-brain barrier and would be expected to interact with HBMEC. Up to now, however, only HUVEC, which differ in many respects from brain microvascular endothelial cells (35, 45), have been used as targets to study the interaction of L. monocytogenes with endothelial cells. We have used HBMEC in classical gentamicin-protection assays and have shown that L. monocytogenes is indeed able to enter and efficiently grow inside microvascular endothelial cells. Figure 1 shows the growth curve of L. monocytogenes in HBMEC for an 18-h period in which continuous intracellular replication occurred in the presence of 10 μg of gentamicin per ml, resulting in a 15-fold increase in the number of live intracellular bacteria between 1 and 18 h postinfection. An L. monocytogenes mutant harboring an in-frame deletion in the hly gene encoding LLO invaded the HBMEC only a little bit less than did the wild-type strain (Fig. 1). However, the number of viable intracellular bacteria of this mutant was nearly constant over the first 6 h postinfection, and only some limited intracellular growth occurred at later time points (Fig. 1).
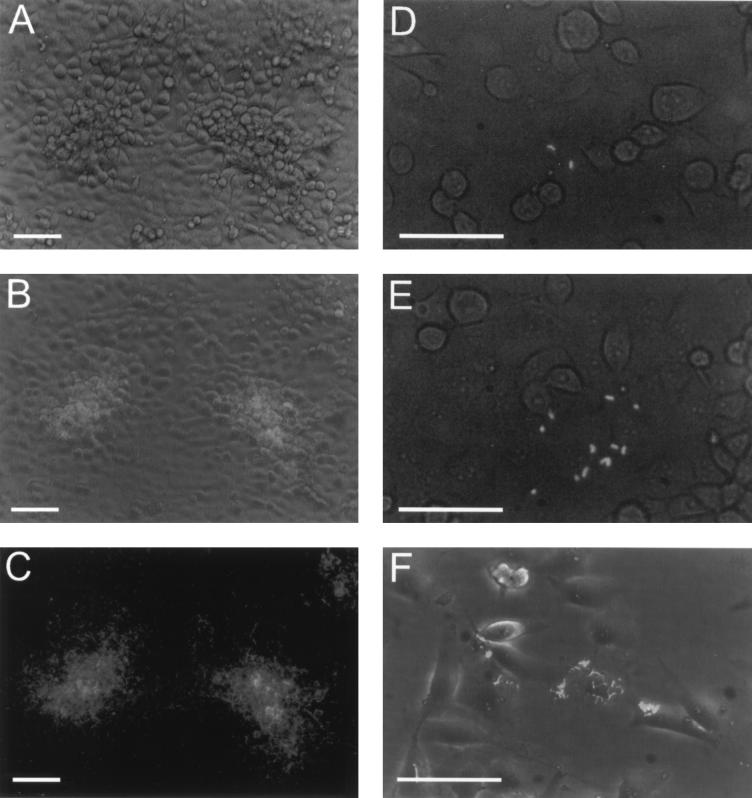
In order to directly follow the intracellular growth of L. monocytogenes in HBMEC, we used a GFP-expressing L. monocytogenes strain that was constructed in a way so that GFP is expressed preferentially by intracytoplasmic bacteria. This was achieved by cloning the gfp gene under the control of the actA promoter, which is transcribed only when the bacteria are localized in the cytoplasm of the host cell (8). By phase contrast and fluorescence microscopy, we monitored the intracellular growth and spread of L. monocytogenes in intact HBMEC monolayers (Fig. 2). Using low infection ratios, we could identify single invasion events in the monolayer and observe the growth of L. monocytogenes directly as shown in Fig. 2A to E over a period of more than 20 h. It was evident that a dramatic increase in the number of fluorescent and hence intracellular bacteria occurred and that they spread efficiently into neighboring cells. By comparing the phase-contrast picture with the fluorescent picture (Fig. 2A and C) and with a combined image of phase contrast and fluorescence (Fig. 2B), the remarkable stability of the HBMEC monolayer also became evident. Even at 21 h postinfection, at heavily infected foci the HBMEC monolayer and the individual cells appeared to be largely undamaged. From the microscopic observations it seemed that the cells that did not perfectly fit into the monolayer were preferentially infected. These cells were rounded up and settled on the monolayer. Semiconfluent monolayers of HBMEC were also readily infected by L. monocytogenes as shown with the GFP-expressing strain and as documented in Fig. 2F.
FIG. 2.
Invasion and intracellular growth of a GFP-expressing L. monocytogenes strain in HBMEC. HBMEC cultured in 75-ml flasks were infected at a low multiplicity (MOI = 5), and the monolayers were observed at different time points. (A to E) Confluent monolayers infected with GFP-expressing L. monocytogenes. The pictures shown were taken at 2.5 h (D), 4.5 h (E), and 21 h (A, B, and C) postinfection. (B, D, and E) Images from combined phase-contrast and fluorescence microscopy. Phase-contrast (A), fluorescence (C), and combined (B) images of the same region of the monolayer. (F) Infection of a subconfluent HBMEC monolayer at 17 h postinfection (combined image). Bars, 50 μm.
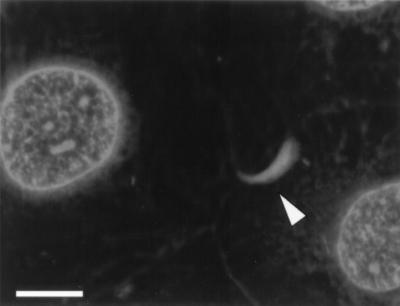
In parallel with the onset of intracellular multiplication, L. monocytogenes became associated with host cell filamentous actin inside HBMEC. This was demonstrated by TRITC-phalloidin staining of actin filaments in L. monocytogenes-infected HBMEC monolayers at 20 h postinfection as shown in Fig. 3. The filamentous actin was either found all around the bacteria or organized in actin tails of various lengths, the typical signature of ongoing intracellular movement. However, in contrast to the infection of Caco-2 cells, significant numbers of bacteria associated with actin tails were only detected upon prolonged incubation of the infected cells (10 to 20 h postinfection), suggesting that the process of actin polymerization and intracellular movement is less efficient in HBMEC than in Caco-2 cells.
FIG. 3.
Intracellular actin tail formation by L. monocytogenes inside HBMEC. The HBMEC were infected with L. monocytogenes EGD as described in the text. At 20 h postinfection the cells were fixed and treated with TRITC-phalloidin to stain the filamentous actin. A typical actin tail is marked by an arrowhead. Bar, 10 μm.
InlB- and PrfA-dependent invasion of L. monocytogenes in HBMEC.
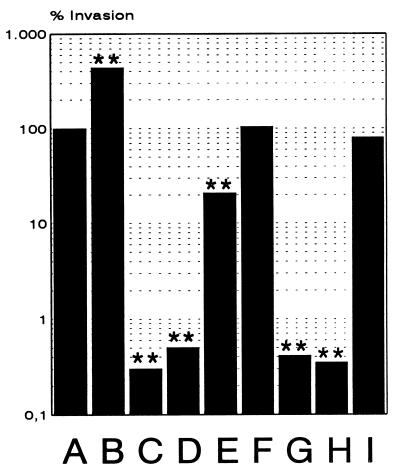
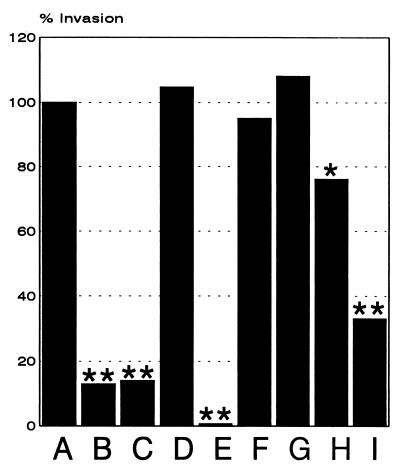
Having established the ability of L. monocytogenes to invade and to grow inside HBMEC, we next analyzed the listerial factors required for efficient entry into HBMEC. For this purpose we infected the endothelial cells with L. monocytogenes and different mutants with in-frame delections in some of the known L. monocytogenes virulence genes and then measured the number of intracellular bacteria after a 1.5-h gentamicin treatment of the cells. As shown in Fig. 4, there was a dramatic (250-fold) decrease in the invasive capacity of the ΔinlB and ΔprfA mutants, which lacked the surface protein InlB and the transcriptional regulator PrfA, respectively, compared to the otherwise isogenic L. monocytogenes EGD strain. These mutants, as well as the ΔinlAB double mutant, behaved essentially like the nonpathogenic L. innocua strain. In contrast to the dramatic effect of the deletion in the inlB gene, a deletion in the inlA gene, as well as a deletion in inlC, had no marked effect on L. monocytogenes invasion of HBMEC. A deletion in actA resulted in a slight but reproducible reduction in invasiveness, pointing to a minor role of the surface protein ActA in triggering L. monocytogenes uptake by HBMEC. The highly hemolytic strain L. monocytogenes NCTC 7973 showed a fourfold-higher invasiveness than the L. monocytogenes EGD strain.
FIG. 4.
Invasion of HBMEC by different Listeria strains and different in-frame deletion mutants of L. monocytogenes. HBMEC were infected (MOI = 20), and the percentages of the following intracellular bacteria were calculated at 1.5 h postinfection as described in the text: L. monocytogenes EGD (A), L. monocytogenes NCTC 7973 (B), L. innocua (C), L. monocytogenes ΔprfA (D), L. monocytogenes ΔactA (E), L. monocytogenes ΔinlA (F), L. monocytogenes ΔinlB (G), L. monocytogenes ΔinlAB (H), and L. monocytogenes ΔinlC (I). ∗, P < 0.01 versus strain EGD; ∗∗, P < 0.001 versus strain EGD.
From our data it is obvious that the listerial surface protein InlB, which was already shown to be important for hepatocyte invasion and invasion of some epithelial cell types such as Vero cells, is also critical for invasion of HBMEC. InlB-mediated uptake of L. monocytogenes has been shown to be associated with stimulation of the host cell PI-3 kinase in Vero cells, a kinase which can be inhibited specifically by the drug wortmannin (5, 22). We therefore tested whether wortmannin also inhibits L. monocytogenes invasion of HBMEC. As shown in Fig. 5, however, wortmannin had no effect on L. monocytogenes invasion of HBMEC at concentrations at which the invasion of Vero cells could be completely blocked (22). Only at very high concentrations of wortmannin (50 to 100 nM) did we observe a decrease in the invasion efficiency. As expected, invasion of L. monocytogenes into HBMEC was highly sensitive to the microfilament-disrupting drug cytochalasin D but also showed some degree of sensitivity to nocodazole treatment, which disrupts microtubules. The bacterial viability was not affected by treatment with the inhibitors (data not shown).
FIG. 5.
Cytochalasin D and nocodazole but not wortmannin inhibit invasion of HBMEC by L. monocytogenes. HBMEC were infected with L. monocytogenes EGD in the presence or absence of different inhibitors. The inhibitors cytochalasin D, nocodazole, and wortmannin were dissolved in dimethyl sulfoxide. The monolayers were pretreated with the inhibitors for 45 min, and the infection was performed as described in the text. Lanes: A, without inhibitor; B and C, nocodazole (10 and 20 μM); D and E, cytochalasin D (0.1 and 1 μM); F to I, wortmannin (5, 10, 50, and 100 nM). ∗, P < 0.01 versus no inhibitor; ∗∗, P < 0.001 versus no inhibitor.
L. monocytogenes spreads from infected P388D1 macrophages into HBMEC independently from the small internalin InlC.
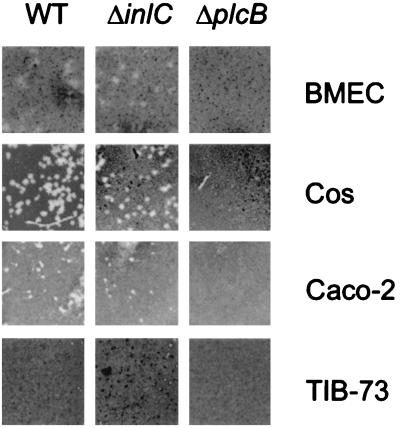
Infection of endothelial cells during the course of a listerial infection may occur either directly or by the spreading of the bacteria from infected monocytes or macrophages to the endothelial cells. Such an indirect mechanism of endothelial-cell infection was already demonstrated for HUVEC that became infected through the cocultivation with L. monocytogenes-infected U937 macrophages (13). Using the heterologous plaque assay, we showed that L. monocytogenes is able to spread from mouse P388D1 macrophages into HBMEC (Fig. 6 and Table 2). Such heterologous spreading was additionally shown to occur from infected P388D1 macrophages into epithelial (Caco-2), hepatocyte (TIB-73), and fibroblast-like (Cos-1) target cells. In order to test whether the small internalin InlC (14) is involved in the heterologous spreading event as speculated earlier (14), we tested a ΔinlC in-frame deletion mutant for its spreading ability in this assay. As seen in Fig. 6 and Table 2, the ΔinlC mutant strain behaved like the isogenic wild-type strain. Only the ΔplcB mutant and the ΔplcA ΔplcB double mutant were negative in their plaque-forming activity, as expected from published data on their behavior in standard plaque assays (6, 44, 50).
FIG. 6.
Results of heterologous plaque assays with L. monocytogenes EGD, L. monocytogenes ΔinlC, and L. monocytogenes ΔplcB demonstrating the PC-PLC-dependent but InlC-independent spread of L. monocytogenes from P388D1 macrophages to different mammalian cell types, including fibroblasts, epithelial cells, hepatocytes, and HBMEC.
TABLE 2.
Summary of the results of the heterologous plaque assays
| Target cell line | Plaque formationa with L. monocytogenes strain:
|
||||
|---|---|---|---|---|---|
| WL-105 (ΔplcA) | WL-103 (ΔplcB) | WL-106 (ΔplcA ΔplcB) | ΔinlC (ΔinlC) | EGD (WT) | |
| HBMEC (human brain, endothelial) | +* | − | − | +* | +* |
| Cos-1 (green monkey kidney, fibroblast) | + | − | − | + | + |
| Caco-2 (human colon, epithelial) | + | − | − | + | + |
| TIB 73 (mouse liver, hepatocyte) | + | − | − | + | + |
+, Plaques formed on monolayer of target cells; −, no plaques formed on monolayer of target cells; *, plaques were diffuse due to a lack of complete cell death. WT, wild type.
DISCUSSION
The final outcome of human infections with L. monocytogenes is in most cases meningitis or meningoencephalitis, resulting in a high mortality of the infected individuals (39). Both types of disease require that the bacteria breach the blood-brain barrier, but up to now it was not known whether the bacteria pass through the endothelial cells forming the brain microvessels or whether they penetrate the epithelial cells of the choroid plexus, thus gaining access to the cerebrospinal fluid. Epithelial cells from the porcine choroid plexus have been successfully cultured in vitro (17) but, to our knowledge, human epithelial-cell cultures derived from the choroid plexus are not available and in vitro analyses of the interaction of these cells with bacteria causing meningitis have not been performed. However, microvascular endothelial cells derived from human brain surgery and microvascular endothelial cell-derived cell lines (32) are now available that allow the analysis of bacterium-brain microvascular endothelial cell interactions. The simian virus 40-transformed human microvascular endothelial cells used in this study show all of the relevant markers of primary human microvascular endothelial cells (45, 47) and represent a much better model system for studying L. monocytogenes-endothelial cell interaction than the previously used human umbilical vein endothelial cells (13, 18, 33, 42, 43).
Using serum-free conditions, we show here that L. monocytogenes is able to invade HBMEC, replicate, move intracellularly, and spread into neighboring cells, thus going through the complete intracellular life cycle described for L. monocytogenes for other mammalian cell systems (26, 41, 48). The listerial invasion frequency for HBMEC (ca. 5% of the inoculum) was lower than that for HUVEC (18) or epithelial cells (29) but, once inside, the bacteria replicated and the number of intracellular bacteria increased 15-fold over a period of 18 h postinfection; this finding is in accordance with earlier findings for L. monocytogenes infections of other mammalian cell types. An isogenic nonhemolytic mutant of L. monocytogenes 10403S showed a somewhat reduced invasiveness. To our surprise, however, even the nonhemolytic L. monocytogenes strain showed some intracellular multiplication between 6 and 24 h postinfection. This intracellular multiplication might be either due to a listeriolysin-independent escape of the bacteria into the cytoplasm, which has been reported to occur in L. monocytogenes-infected human Henle 407 epithelial cells (34), or due to some intraphagosomal replication, as demonstrated for phosphatidylinositol-specific phospholipase C (PI-PLC)-expressing L. innocua strains in J774 macrophages (40). Using a GFP-expressing L. monocytogenes strain (8), we microscopically monitored invasion and intracellular growth and found that both confluent and semiconfluent HBMEC monolayers became infected. Since the gfp gene was put under the transcriptional control of the actA promoter, which is highly active, whereas the bacteria reside inside the host cell’s cytoplasm (8), GFP-labeled bacteria became visible approximately 2 h postinfection. Therefore, GFP expression under the control of the actA promoter also allows the use of GFP expression as a marker of intracytoplasmic localization. Looking carefully at the monolayers, we often found that cells that became infected by L. monocytogenes were not fully integrated into the monolayer but were located on top of other HBMEC. This location probably gives the bacteria access to those areas of the cell that allow efficient adhesion and invasion but which otherwise are protected by neighboring cells in an intact monolayer. This observation is also in line with the notion that subconfluent monolayers are more readily infected compared to confluent ones (data not shown). Surprisingly, even heavily infected HBMEC monolayers at late time points postinfection still appear undamaged, suggesting that HBMEC are highly resistant to lysis by intracellular L. monocytogenes.
As was evident from the pictures of the heavily infected foci originating from single infected cells, intracellular movement and cell-to-cell spread occur in L. monocytogenes-infected HBMEC. However, actin tails were only rarely found in L. monocytogenes-infected cells at 6 h postinfection. At 20 h postinfection, the intracellular bacteria were all covered with F actin and became visible upon TRITC-phalloidin staining, but actin tails were again rarely found and in most cases they were relatively shorter than the actin tails documented from L. monocytogenes-infected Caco-2 and PtK2 epithelial cells (7, 27, 31) and J774 macrophages (48). This suggests that the process of G actin recruitment or actin tail formation is slower in HBMEC than in other cell types such as PtK2 cells or Caco-2 cells, which were mostly used to analyze listerial intracellular movement. Whether these shorter actin tails are due to differences in listerial ActA expression or to differences in the concentration of cellular G actin (or other cellular molecules necessary for this process) is not known at present.
Internalin (InlA) was thought to be the major invasion factor of L. monocytogenes (16), but it became clear that this protein is of dominant importance only in the invasion of Caco-2 epithelial cells. In contrast, InlB was shown to be the critical protein for the invasion of numerous other cell lines, such as HeLa, HEp-2, Henle 407, L929, and Vero cells and hepatocytes (5, 11, 29). The findings presented here add HBMEC to this growing list of cell types for which efficient invasion of L. monocytogenes depends only on InlB. Our data are also in perfect agreement with the recent report of InlB-dependent HUVEC invasion (33). At present there is no way to predict which of the two proteins is the invasion factor for a given cell line. This might change as soon as the cellular receptor for InlB is known. Whether the cellular receptor for InlA, E-cadherin (30), is expressed on HBMEC is, to our knowledge, not yet known and it is also not known whether InlA binds to the vascular homologue of E-cadherin, VE-cadherin. From the sequence similarity of InlA and InlB on one side and E-cadherin and VE-cadherin on the other side, one might speculate that InlB, which is absolutely required for HBMEC invasion, might be the ligand of VE-cadherin and thereby triggers L. monocytogenes invasion. However, it is also not known whether VE-cadherin is expressed by the endothelial cells used here. The data presented here also demonstrate a strong dependence of the invasive capacity of L. monocytogenes for HBMEC on the presence of the transcriptional regulator PrfA, since a ΔprfA mutant is reduced in its invasiveness to the same extent as the ΔinlB mutant. This PrfA dependence can be based on two different possibilities. First, under the experimental conditions used here, inlB expression could be PrfA dependent, resulting in the prfA mutant’s lack of InlB protein on the listerial surface and hence a lack of invasion. The regulation of inlB expression is not yet fully understood, but it is known that the inlB gene is transcribed from multiple PrfA-dependent and PrfA-independent promoters that are located either in front of the inlA gene (resulting in bicistronic transcripts) or in the intergenic region between the inlA and the inlB genes (4, 12, 29). The second possibility to explain the PrfA dependence of HBMEC invasion is that InlB might confer invasiveness to L. monocytogenes only together with an additional, as yet unknown protein, the expression of which is strictly PrfA dependent. We recently reported on an InlA- and InlB-independent invasion of HUVEC by L. monocytogenes (18), but in contrast to the experiments described here, the invasion of HUVEC was analyzed in the presence of 20% pooled human serum, which might lead to an enhanced “nonspecific” uptake of the bacteria as described by others for HUVEC invasion in the presence of FCS (13).
A deletion in the gene inlC encoding the small secreted internalin InlC, for which no function is known, did not result in a significant alteration of the invasive capacity. This result is similar to the results of invasion assays performed with the ΔinlC mutant and other cell types such as Caco-2 (14). On the other hand, a deletion in the actA gene, the product of which was shown to participate in mediating invasion into different cell types (1), results in a weak but reproducible reduction of the invasive capacity. ActA probably binds to host cell heparan sulfate proteoglycan receptors, which are found mainly on the basolateral sides of epithelial and endothelial cells and may contribute to the invasion of HBMEC.
The recent reports (5, 22) of an InlB-triggered activation of the PI-3 kinase being necessary for invasion of Vero cells prompted us to evaluate the role of PI-3 kinase in HBMEC invasion. However, our experiments in the presence of the PI-3 kinase inhibitor wortmannin did not reveal any inhibitory effect of this drug on L. monocytogenes invasion of HBMEC at concentrations sufficient to completely inhibit Vero cell invasion. This surprising result shows that the link between InlB-mediated invasion and PI-3 kinase activation shown in Vero cells is obviously not a general phenomenon, as already mentioned in a recent study by Ireton and Cossart (21). Possibly, InlB binds to different receptors on different cell types that might be connected to different signaling pathways, some of which may not involve activation of PI-3 kinase. Cytochalasin D sensitivity of the invasion process was found as expected, but it was also found that the microtubule-depolymerizing drug nocodazole weakly, but reproducibly, inhibited invasion, adding another example to the growing number of cases in which, in addition to the well-known actin-based invasion mechanism, microtubules play a role in the uptake process of L. monocytogenes (19, 25).
The actin-based intracellular movement and cell-to-cell spread of L. monocytogenes were studied in detail in recent years by using cell culture and in vitro systems. However, besides the actin nucleator protein ActA (9, 24), only the plcB gene product, a broad-spectrum phospholipase called PC-PLC, was shown to be involved in cell-to-cell spread by being necessary for the lysis of the double-membrane vacuole (44, 50). The spread from cell to cell was mostly analyzed by evaluating plaque formation efficiency in monolayers of L2 or 3T3 fibroblasts (24, 27). Heterologous spreading of L. monocytogenes was demonstrated only once from human U937 monocyte-like cells into HUVEC by Giemsa staining of the infected HUVEC monolayer (13). In the present study we describe a new method of heterologous plaque assays in which the transfer of the bacteria from one cell type to another can be monitored. This assay, which involves the use of preinfected macrophages and monolayers of epithelial, fibroblast, hepatocyte, or endothelial cells, allowed us to demonstrate that (i) L. monocytogenes spreads efficiently from macrophages into all these cell types, (ii) the phospholipase PC-PLC is necessary for spreading from one cell type to another, and (iii) the PI-PLC and the small internalin InlC are not necessary for efficient heterologous spreading. The small internalin InlC is secreted into the cell culture supernatant in vitro and is efficiently synthesized in the late stage of a macrophage infection when L. monocytogenes starts spreading (14). It was therefore suggested that this protein could upon secretion interact with the host cell membrane during the spreading process (14). Our data, however, clearly argue against such a role for InlC. The plaques formed upon heterologous spreading in the different cell types varied slightly in size and shape showing that the spreading efficiency of L. monocytogenes differs with the cell types used. In contrast to all of the other cells, the plaques formed on the HBMEC monolayers are hardly visible since only some of the infected cells died, resulting in very faint plaques. Taken together with the results obtained with the GFP-expressing bacteria, this finding shows the high resistance of HBMEC to killing by intracellular L. monocytogenes. Whether this resistance to bacterial killing is also observed in the in vivo situation of infected experimental animals will be analyzed in the future.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft through SFB 165-B4 and SFB 479-B1, by the European Union through the BIOMED 2 Project “Listeria Eurolab,” grant CT950659, and by United States Public Health Service Grant Ro1-US 26310.
We also thank J. Daniels and A. Demuth for critically reading the manuscript.
REFERENCES
- 1.Alvarez-Dominguez C, Vazquez-Boland J-A, Carrasco-Marin E, Lopez-Mato P, Leyva-Cobian F. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun. 1997;65:78–88. doi: 10.1128/iai.65.1.78-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betz A L, Goldstein G W. Specialized properties and solute transport in brain capillaries. Annu Rev Physiol. 1986;48:241–250. doi: 10.1146/annurev.ph.48.030186.001325. [DOI] [PubMed] [Google Scholar]
- 3.Böckmann R, Dickneite C, Middendorf B, Goebel W, Sokolovic Z. Specific binding of the Listeria monocytogenes transcriptional regulator PrfA to target sequences requires additional factor(s) and is influenced by iron. Mol Microbiol. 1996;22:643–653. doi: 10.1046/j.1365-2958.1996.d01-1722.x. [DOI] [PubMed] [Google Scholar]
- 4.Bohne J, Kestler H, Uebele C, Sokolovic Z, Goebel W. Differential regulation of the virulence genes of Listeria monocytogenes by the transcriptional activator PrfA. Mol Microbiol. 1996;20:1189–1198. doi: 10.1111/j.1365-2958.1996.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 5.Braun L, Ohayon H, Cossart P. The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol Microbiol. 1998;27:1077–1087. doi: 10.1046/j.1365-2958.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 6.Camilli A, Tilney L G, Portnoy D A. Dual roles of PlcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabiri G A, Sanger J M, Portnoy D A, Southwick F S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci USA. 1990;87:6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Stimm A, Catic A, Kaufmann S H E, Hess J, Szalay A A, Goebel W. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 9.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wächter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domann E, Zechel S, Lingnau A, Hain T, Darji A, Nichterlein T, Wehland J, Chakraborty T. Identification and characterization of a novel PrfA-regulated gene in Listeria monocytogenes whose product, IrpA, is highly homologous to internalin proteins, which contain leucine-rich repeats. Infect Immun. 1997;65:101–109. doi: 10.1128/iai.65.1.101-109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of InlB, a surface protein of the internalin multigen family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 12.Dramsi S, Kocks C, Forestier C, Cossart P. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and the pleiotropic activator prfA. Mol Microbiol. 1993;9:931–941. doi: 10.1111/j.1365-2958.1993.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 13.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelbrecht F, Chun S-K, Ochs C, Hess J, Lottspeich F, Goebel W, Sokolovic Z. A new PrfA-regulated gene of Listeria monocytogenes encoding a small, secreted protein which belongs to the family of internalins. Mol Microbiol. 1996;21:823–837. doi: 10.1046/j.1365-2958.1996.541414.x. [DOI] [PubMed] [Google Scholar]
- 15.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of Listeria monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 17.Gath U, Hakvoort A, Wegener J, Decker S, Galla H J. Porcine choroid plexus cells in culture: expression of polarized phenotype, maintenance of barrier properties and apical secretion of CSF-components. Eur J Cell Biol. 1997;74:68–78. [PubMed] [Google Scholar]
- 18.Greiffenberg L, Sokolovic Z, Schnittler H-J, Spory A, Böckmann R, Goebel W, Kuhn M. Listeria monocytogenes-infected human umbilical vein endothelial cells: internalin-independent invasion, intracellular growth, movement, and host cell responses. FEMS Microbiol Lett. 1997;157:163–170. doi: 10.1111/j.1574-6968.1997.tb12768.x. [DOI] [PubMed] [Google Scholar]
- 19.Guzman C, Rhode M, Chakraborty T, Domann E, Hudel M, Wehland J, Timmis K. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect Immun. 1995;63:3665–3673. doi: 10.1128/iai.63.9.3665-3673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauf N, Goebel W, Fiedler F, Sokolovic Z, Kuhn M. Listeria monocytogenes infection of P388D1 macrophages results in a biphasic NF-κB (RelA/p50) activation induced by lipoteichoic acid and bacterial phospholipases and mediated by IκBα and IκBβ degradation. Proc Natl Acad Sci USA. 1997;94:9394–9399. doi: 10.1073/pnas.94.17.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ireton K, Cossart P. Host-pathogen interactions during entry and actin-based movement of Listeria monocytogenes. Annu Rev Genet. 1997;31:113–138. doi: 10.1146/annurev.genet.31.1.113. [DOI] [PubMed] [Google Scholar]
- 22.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 23.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. Listeria monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn M. The microtubule depolymerizing drugs nocodazole and colchicine inhibit the uptake of Listeria monocytogenes by P388D1 macrophages. FEMS Microbiol Lett. 1998;160:87–90. doi: 10.1111/j.1574-6968.1998.tb12895.x. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn M, Goebel W. Molecular studies on the virulence of Listeria monocytogenes. Genet Eng. 1995;17:31–51. [PubMed] [Google Scholar]
- 27.Kuhn M, Prévost M-C, Mounier J, Sansonetti P J. A nonvirulent mutant of Listeria monocytogenes does not move intracellularly but still induces polymerization of actin. Infect Immun. 1990;58:3477–3486. doi: 10.1128/iai.58.11.3477-3486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine S. Choroid plexus: target for systemic disease and pathway to the brain. Lab Invest. 1987;56:231–233. [PubMed] [Google Scholar]
- 29.Lingnau A, Domann E, Hudel M, Bock M, Nichterlein T, Wehland J, Chakraborty T. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect Immun. 1995;63:3896–3903. doi: 10.1128/iai.63.10.3896-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of Listeria monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 31.Mounier J, Ryter A, Coquis-Rondon M, Sansonetti P J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990;58:1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parida S K, Domann E, Rohde M, Müller S, Darji A, Hain T, Wehland J, Chakraborty T. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol Microbiol. 1998;28:81–93. doi: 10.1046/j.1365-2958.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 34.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prasadarao N V, Wass C A, Kim K S. Identification and characterization of S fimbria-binding sialoglycoproteins on brain microvascular endothelial cells. Infect Immun. 1997;65:2852–2860. doi: 10.1128/iai.65.7.2852-2860.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlossbauer B. The blood brain barrier: morphology, molecules, and neurothelin. Bioessays. 1993;15:341–346. doi: 10.1002/bies.950150508. [DOI] [PubMed] [Google Scholar]
- 38.Schlüter D, Chahoud S, Lassmann H, Schumann A, Hof H, Deckert-Schlüter M. Intracerebral targets and immunomodulation of murine Listeria monocytogenes meningoencephalitis. J Neuropathol Exp Neurol. 1996;55:14–24. doi: 10.1097/00005072-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Schuchat A, Swaminathan B, Broome C V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwan W R, Demuth A, Kuhn M, Goebel W. Phosphatidylinositol-specific phospholipase C from Listeria monocytogenes contributes to intracellular survival and growth of Listeria innocua. Infect Immun. 1994;62:4795–4803. doi: 10.1128/iai.62.11.4795-4803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld A D, Mengaud J, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr Top Microbiol Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 42.Sibelius U, Chakraborty T, Krögel B, Wolf J, Rose F, Schmidt R, Wehland J, Seeger W, Grimminger F. The listerial exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J Immunol. 1996;157:4055–4060. [PubMed] [Google Scholar]
- 43.Sibelius U, Rose F, Chakraborty T, Darji A, Wehland J, Weiss S, Seeger W, Grimminger F. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect Immun. 1996;64:674–676. doi: 10.1128/iai.64.2.674-676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stins M F, Gilles F, Kim K S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 46.Stins M F, Prasadarao N V, Ibric L, Wass C A, Luckett P, Kim K S. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am J Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 47.Stins M F, Prasadarao N V, Zhou J, Arditi M, Kim K S. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev Biol Anim. 1997;33:243–247. doi: 10.1007/s11626-997-0042-1. [DOI] [PubMed] [Google Scholar]
- 48.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuomanen E. Entry of pathogens into the central nervous system. FEMS Microbiol Rev. 1996;18:289–299. doi: 10.1111/j.1574-6976.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez-Boland J-A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon in Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]