Abstract
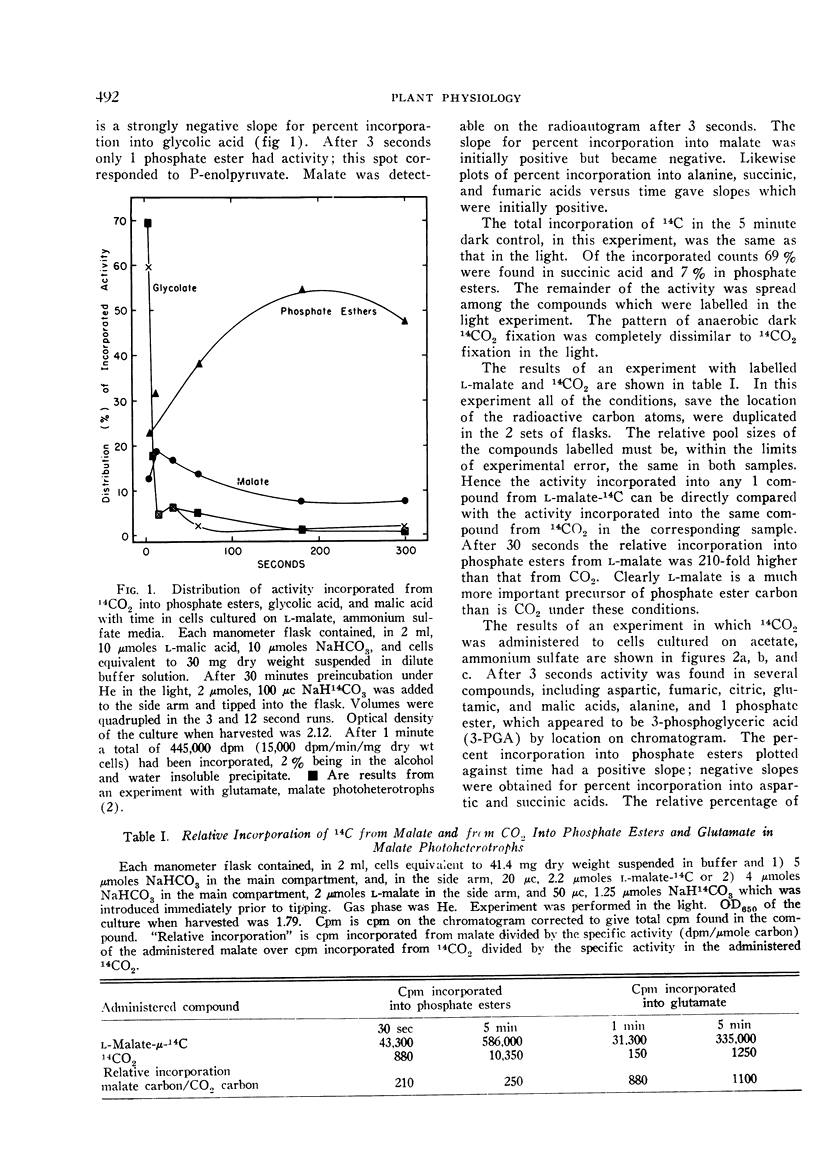
The contribution of the reductive pentose phosphate cycle to the photometabolism of carbon dioxide and to carbon metabolism in Rhodospirillum rubrum grown photoheterotrophically with l-malate as the carbon source is nil, unlike autotrophically grown R. rubrum. Glycolic acid appears to be the first stable product of CO2 fixation in R. rubrum cultured photoheterotrophically on l-malate. The results obtained in 14CO2 fixation experiments suggest that the photometabolism of CO2 through glycolate into malate is a major pathway of CO2 fixation in such cells. However, l-malate was a much more efficient precursor of phosphate esters, and of glutamic acid, than was carbon dioxide; l-malate is therefore, in this case, a far more important source of cell carbon than is carbon dioxide.
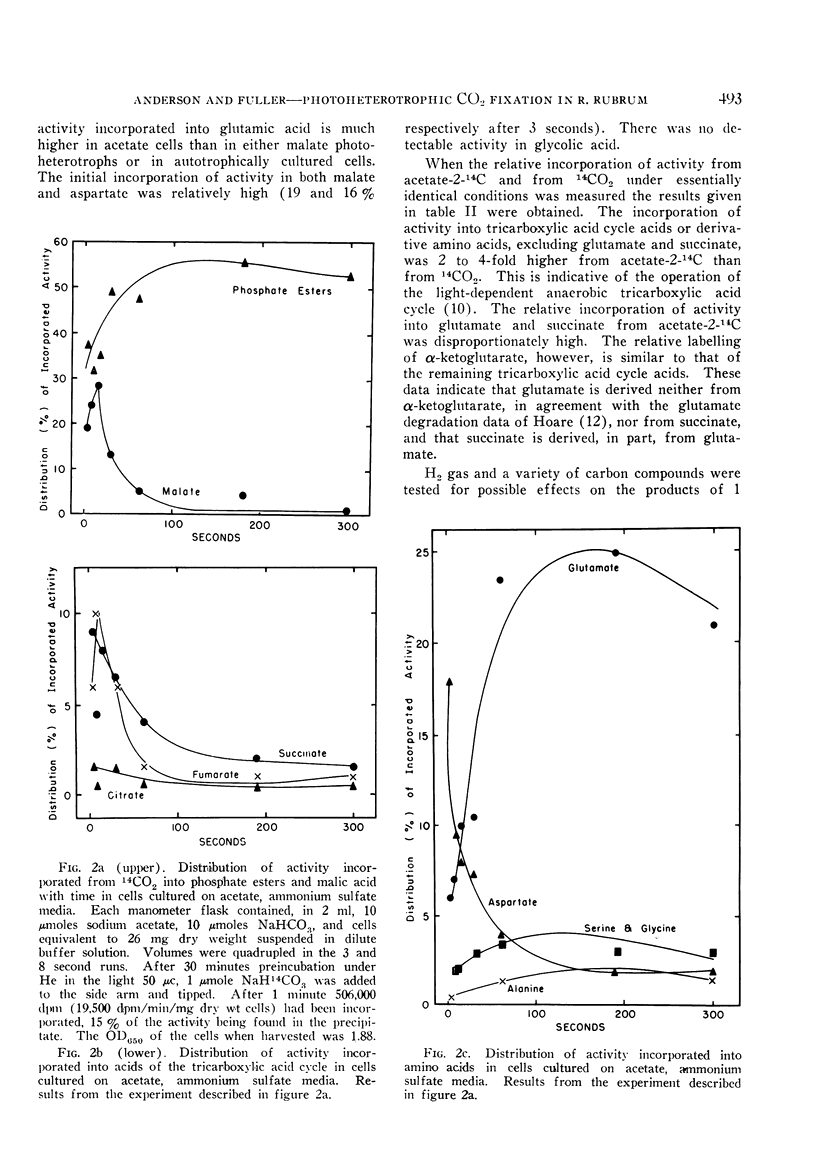
The products of the light-dependent incorporation of CO2 and of acetate were investigated in R. rubrum grown photoheterotrophically on acetate. Carboxylation reactions and the reductive pentose phosphate cycle are apparently of greater significance in the photometabolism of acetate heterotrophs than in malate heterotrophs; the photometabolism of the acetate photoheterotrophs seems to be intermediate between the photoheterotrophy of malate heterotrophs and strict autotrophy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Fuller R. C. Photosynthesis in Rhodospirillum rubrum. 3. Metabolic control of reductive pentose phosphate and tricarboxylic acid cycle enzymes. Plant Physiol. 1967 Apr;42(4):497–509. doi: 10.1104/pp.42.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxin-dependent synthesis of labelled pyruvate from labelled acetyl coenzyme A and carbon dioxide. Biochem Biophys Res Commun. 1965 Jul 12;20(2):163–168. doi: 10.1016/0006-291x(65)90340-2. [DOI] [PubMed] [Google Scholar]
- FULLER R. C., SMILLIE R. M., SISLER E. C., KORNBERG H. L. Carbon metabolism in Chromatium. J Biol Chem. 1961 Jul;236:2140–2149. [PubMed] [Google Scholar]
- ORMEROD J. G. The use of radioactive carbon dioxide in the measurement of carbon dioxide fixation in Rhodospirillum rubrum. Biochem J. 1956 Oct;64(2):373–380. doi: 10.1042/bj0640373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. E., TOLBERT N. E. Oxidation of glyoxylic acid to oxalic acid by glycolic acid oxidase. J Biol Chem. 1961 May;236:1280–1284. [PubMed] [Google Scholar]
- STERN J. R. Enzymic activation and cleavage of D- and L-malate. Biochim Biophys Acta. 1963 Feb 5;69:435–438. doi: 10.1016/0006-3002(63)91288-5. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Doudoroff M., Kunisawa R., Contopoulou R. THE ROLE OF ORGANIC SUBSTRATES IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1246–1260. doi: 10.1073/pnas.45.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]



