Abstract
BACKGROUND:
Hypoglycemia in neonates is common and contributes to 4.0–5.8% of neonatal intensive care unit (NICU) admissions. In utero nicotine exposure is underexplored as a potential contributor to neonatal hypoglycemia. Rat models have shown that in utero nicotine exposure can be associated with a reduction in pancreatic beta cell mass, leading to glucose dysregulation. The primary aim of this work is to study the risk of developing hypoglycemia after birth in a population of in utero nicotine-exposed neonates.
METHODS:
We conducted a retrospective matched cohort study that augmented an existing dataset of neonates admitted to a level IV NICU with household-based in utero nicotine exposure (N = 335). Neonates in the control group parents denied household smoking (N = 325), were born within a 6-month timeframe, and were within a birthweight of 50 grams of a nicotine-exposed neonate. Data reviewed included gestational age, growth parameters, maternal history of diabetes, and glucose levels within the first three hours of life per unit protocol.
RESULTS:
660 neonates were included in the analysis. In utero nicotine exposure demonstrated a 94.3% posterior probability (PP) for greater hypoglycemia risk (RR = 1.185, 95% CrI = [0.953, 1.445]). A 94.6% PP was demonstrated when neonates who were small for gestational age, intrauterine growth-restricted, and born to diabetic mothers were excluded (n = 482; RR = 1.271, 95% CrI = [0.946, 1.669]).
CONCLUSION:
Nicotine exposure in utero was found to be a potential risk factor for developing hypoglycemia after birth. Mechanisms of action should be explored, and additional research on in utero nicotine exposure risks should follow.
Keywords: Late preterm neonate, maternal substance use, neonatal hypoglycemia, neonatology, nicotine exposure
1. Introduction
Hypoglycemia is one of the most common neonatal diagnoses [1, 2], affecting 5–10% of healthy term newborns, and contributes to neonatal intensive care unit (NICU) admissions [1, 3]. In utero nicotine exposure is underexplored as a potential contributor to neonatal hypoglycemia. Rat models have shown that in utero exposure to nicotine and continued exposure through lactation is associated with a reduction in beta cell mass in the pancreas, leading to problems with glucose homeostasis [4]. As many as 7% of women in the United States smoke throughout pregnancy, [5, 6] and approximately 33% of non-smoking women worldwide are exposed to environmental tobacco smoke [7], often in their homes [8], raising the importance of identifying whether in utero nicotine exposure contributes to neonatal hypoglycemia. However, the potential association of nicotine exposure in utero and hypoglycemia shortly after birth remains underexplored in human neonates.
Recognition of hypoglycemia is of utmost importance due to its potential adverse effects on neonates. Neonates at high risk for hypoglycemia include those born to diabetic mothers, those who are small for gestational age (SGA), those with intrauterine growth restriction (IUGR), and those born preterm (<37 weeks gestation). Other risk factors include perinatal stress, metabolic disorders, and disorders of growth hormone and cortisol [1, 9]. While some hypoglycemic neonates can be treated with oral feedings alone, others will require admission to the NICU and more extensive interventions. In addition, admission to the NICU is expensive and separates neonates from their mothers, potentially interrupting bonding and breastfeeding initiatives. Severe and persistent hypoglycemia is shown to cause poor neurodevelopmental outcomes and neurologic damage visualized on magnetic resonance imaging (MRI)[1, 10, 11], making its recognition and treatment critical.
Nicotine exposure in utero is associated with numerous adverse health outcomes for neonates [12-14]. Nicotine easily crosses the placenta [13, 14] and can affect oxygen delivery which negatively affects the growth of the fetus and increases the risk of intrauterine death [12, 13]. Nicotine exposure in utero can lead to other poor outcomes such as IUGR, preterm births [12-14], postnatal hypertonicity, lethargy, irritability, and tremors [13].
The primary aim of this cohort study was to explore the risk of developing hypoglycemia within the first three hours after birth in a population of in utero nicotine-exposed neonates admitted to the NICU. Using a retrospective matched cohort design, we hypothesized that nicotine-exposed neonates would have a greater incidence of early hypoglycemia after birth compared to non-nicotine exposed neonates.
2. Methods
2.1. Participants
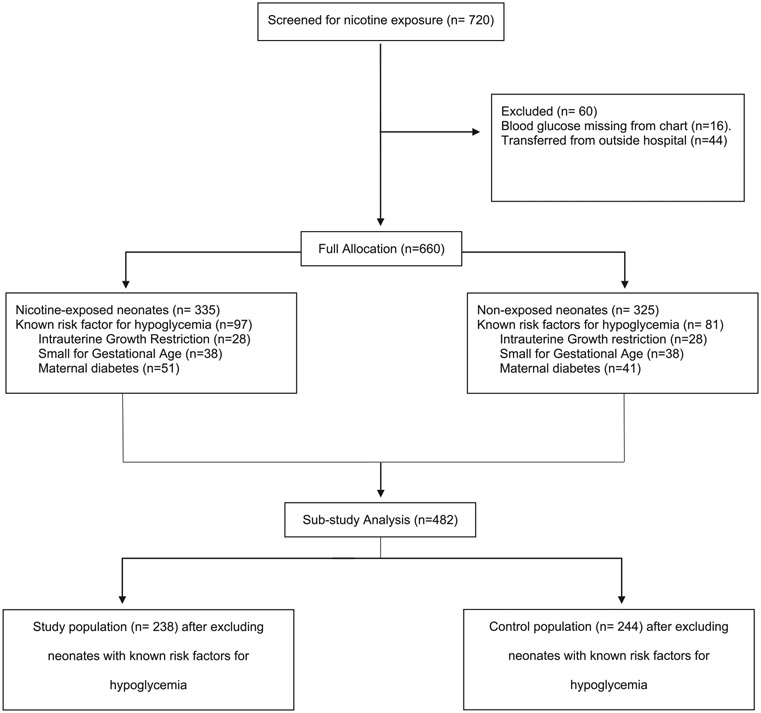
All screened participants (n = 720 neonates) were admitted to a large (>1400 admission/year) metropolitan level-4 NICU between August 2012 to January 2018. During recruitment for a randomized controlled trial (RCT) on reduction of tobacco smoke exposure (the Baby’s Breath II; clinicaltrials.gov: NCT01726062), research assistants screened infants’ parents at the NICU bedside for household tobacco smoke exposure (TSE; i.e., one or more household members smokes). Parents were screened using a multi-part, well-validated screening method [8, 15-17] to reduce underreporting and misclassification. Parents and neonates were eligible for the RCT if they screened positive for TSE (n = 360). In this secondary data analysis, these neonates comprised the nicotine-exposed cohort group. The control group infants (n = 360) screened negative for household TSE [15], were birthweight matched within 50 grams of a nicotine-exposed neonate, and were born within a 6-month timeframe to control for standard-of-care changes over time. Five control neonates were birthweight matched within 50 grams of a nicotine-exposed neonate but fell outside the 6-month timeframe. This study was approved by the institutional and hospital IRB (HSC-MS-19-0323).
2.2. Measures and procedures
We collected data from the electronic health record of 720 neonates. The data reviewed included: gestational age, anthropometric measurements at birth (length, head circumference, and weight), prenatally diagnosed congenital anomalies, race, sex, and maternal history of diabetes. The primary outcome measure was the presence or absence of hypoglycemia by point-of-care blood glucose levels in the first three hours of life per unit admission protocol. For the purposes of this study, hypoglycemia was defined as blood glucose level < 45 mg/dl per physician agreed upon NICU unit guidelines. Point-of-care blood tests were used at the bedside as part of their admission bundle. Neonates (n = 60) were excluded if blood glucose levels were not charted, could not to be obtained due to patient transfer from outside hospital, or if information was not available in the electronic health record due to previous paper charting. Of the 720 neonates screened, 660 were included in the final data analysis (see Fig. 1).
Fig. 1.
Neonate Screening and Enrolment Disposition by Household-based Nicotine Exposure and Risks Factors for Hypoglycemia.
2.3. Data analytic strategy
Generalized linear modeling (GLM) was used to fit hypoglycemia (absent vs. present) as a function of nicotine exposure in two samples: (1) the entire analyzable sample (n = 660) (regardless of known risk factors for hypoglycemia, see Fig. 1) and (2) a subsample of participants (n = 482) that demonstrated decreased confounding risk factors for hypoglycemia (i.e., SGA, IUGR, or presence of any maternal diabetes i.e. Type 1, 2, or gestational diabetes). In the present context of a dichotomous outcome, GLM provides an equivalent approach to logistic regression. Model coefficients were used to estimate relative risks (RR). Bayesian statistical inference provided the posterior probability (PP) that predictors yielded effects on model outcomes [18, 19]. Weakly informative priors (b ~Normal [μ = 0, σ2 = 100]) were used to maximize the influence of the data on posterior probabilities.
3. Results
The complete data set was comprised of 660 neonates (See Fig. 1), 335 were nicotine-exposed and 325 were non-nicotine exposed. The sample population was 52.5% female (n = 345) with 54% (n = 354) from a racial/ethnic minority. The mean infant age in the full sample was 33.7 weeks (SD ± 4.46 weeks) and average weight was 2253 grams (SD ± 920.83 grams). The subsample had overall similar baseline characteristics, except for racial/ethnic differences (See Tables 1 and 2 for full and subsample characteristics). Mothers who smoked were combined with mothers who lived with individuals who smoked as infants were exposed to nicotine in all these homes. Attempts to separate these groups (maternal smoking vs. maternal exposure to cigarette smoke) resulted in models with low precision, likely due to low sample sizes.
Table 1.
Full sample baseline characteristics
| Characteristic | Overall (n = 660) |
Non-Nicotine exposed (n = 325) |
Nicotine- exposed (n = 335) |
p value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Hypoglycemia | 0.135 | |||
| Absent | 423 (64%) | 218 (67%) | 205 (61%) | |
| Present | 237 (36%) | 107 (33%) | 130 (39%) | |
| Sex | 0.829 | |||
| Female | 345 (52%) | 168 (52%) | 177 (53%) | |
| Male | 315 (48%) | 157 (48%) | 158 (47%) | |
| Race/Ethnicity | <0.001 | |||
| African American | 217 (33%) | 71 (22%) | 146 (44%) | |
| Asian American | 15 (2.3%) | 11 (3.4%) | 4 (1.2%) | |
| Caucasian | 101 (15%) | 72 (22%) | 29 (8.7%) | |
| Hispanic | 121 (18%) | 75 (23%) | 46 (14%) | |
| Middle Eastern | 1 (0.2%) | 0 (0%) | 1 (0.3%) | |
| Missing | 205 (31%) | 96 (30%) | 109 (33%) | |
| Intrauterine Growth Restriction (IUGR) | 55 (8.3%) | 27 (8.3%) | 28 (8.4%) | >0.999 |
| Small for Gestational Age (SGA) | 74 (11%) | 36 (11%) | 38 (11%) | >0.999 |
| Maternal History of Diabetes | 91 (14%) | 40 (12%) | 51 (15%) | 0.330 |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Gestational Age at Birth (Weeks) | 33.71 (4.46) | 33.67 (4.45) | 33.74 (4.48) | 0.770 |
| Gestational Age at Birth (Days) | 2.61 (2.03) | 2.62 (2.06) | 2.60 (2.00) | 0.962 |
| Weight (grams) | 2,253.94 (920.83) | 2,241.09 (913.72) | 2,266.41 (928.87) | 0.765 |
| Length (cm) | 44.62 (22.70) | 45.64 (31.60) | 43.63 (6.80) | 0.536 |
| Head circumference (cm) | 36.74 (76.17) | 39.86 (93.71) | 33.69 (53.80) | 0.627 |
Mean ± standard deviation (SD). p-Values calculated according to baseline characteristic variable type: chi-square test (categorical) or Mann-Whitney U (continuous).
Table 2.
Subsample baseline characteristics
| Characteristic | Overall (n = 482) |
Non-Nicotine exposed (n = 244) |
Nicotine- exposed (n = 238) |
p-value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Hypoglycemia | 0.139 | |||
| Absent | 342 (71%) | 181 (74%) | 161 (68%) | |
| Present | 140 (29%) | 63 (26%) | 77 (32%) | |
| Sex | 0.844 | |||
| Female | 254 (53%) | 127 (52%) | 127 (53%) | |
| Male | 228 (47%) | 117 (48%) | 111 (47%) | |
| Race/Ethnicity | <0.001 | |||
| African American | 153 (32%) | 49 (20%) | 104 (44%) | |
| Asian American | 9 (1.9%) | 6 (2.5%) | 3 (1.3%) | |
| Caucasian | 74 (15%) | 55 (23%) | 19 (8.0%) | |
| Hispanic | 82 (17%) | 54 (22%) | 28 (12%) | |
| Missing | 164 (34%) | 80 (33%) | 84 (35%) | |
| Intrauterine Growth Restriction (IUGR) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Small for Gestational Age (SGA) | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Maternal History of Diabetes | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Gestational Age at Birth (Weeks) | 33.49 (4.67) | 33.61 (4.65) | 33.37 (4.70) | 0.530 |
| Gestational Age at Birth (Days) | 2.69 (2.06) | 2.73 (2.07) | 2.65 (2.05) | 0.664 |
| Weight (grams) | 2,250.27 (920.51) | 2,265.98 (905.79) | 2,234.17 (936.99) | 0.698 |
| Length (cm) | 44.96 (26.30) | 46.41 (36.31) | 43.46 (6.93) | 0.250 |
| Head circumference (cm) | 36.85 (77.37) | 39.00 (88.50) | 34.63 (63.97) | 0.166 |
Mean ± standard deviation (SD). Subsample analysis excluded three independent hypoglycemia risk factors (IUGR, SGA, and neonates born to diabetic mothers).
3.1. Primary analysis
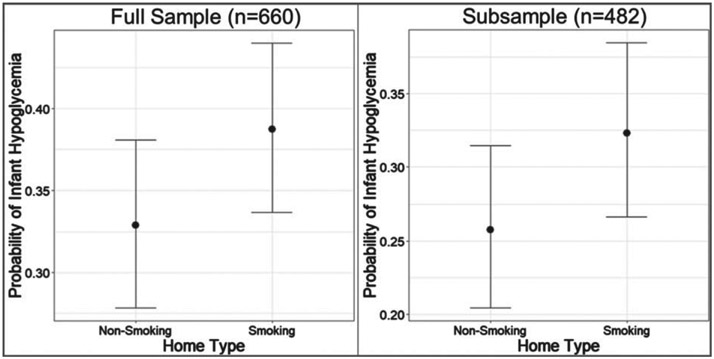
Table 3 provides the absolute number of neonates by nicotine exposure cases and hypoglycemia outcome for total sample and the subsample. In the whole sample, (n = 660), 50.8% of the neonates were nicotine-exposed. Hypoglycemia was noted in 38.8% of the nicotine-exposed neonates in comparison to only 32.9% in the non-nicotine exposed neonates (see Fig. 2). In utero nicotine exposure demonstrated a 95.0% PP for greater hypoglycemia risk (RR = 1.183, 95% CrI = [0.97, 1.458]).
Table 3.
Presence versus absence of hypoglycemia by nicotine exposure
| Full sample | Hypoglycemia present |
Hypoglycemia absent |
|---|---|---|
| Exposed | 130 | 205 |
| Non-Exposed | 107 | 218 |
| Subsample* | Hypoglycemia present | Hypoglycemia absent |
| Exposed | 77 | 161 |
| Non-Exposed | 63 | 181 |
Subsample analysis excluded three independent hypoglycemia risk factors (Intrauterine growth restriction, small for gestational age, and neonates born to diabetic mothers).
Fig. 2.
Hypoglycemia by Household Smoking Status in Full Sample & Subsample*. *Subsample analysis excluded three independent hypoglycemia risk factors (Intrauterine growth restriction, small for gestational age, and neonates born to diabetic mothers).
3.2. Sensitivity analysis of neonates with no known risks for hypoglycemia [n = 482]
A subsample of 482 neonates (238 nicotine-exposed and 244 non-nicotine exposed controls) was subsequently analyzed. Infants with increased risk factors for hypoglycemia and conditions that could be caused by nicotine exposure alone were excluded. Specifically, the subsample excluded neonates born to diabetic mothers (Type 1, 2 or gestational diabetes) and those who were SGA (less than the 10th percentile for weight) or documented as IUGR defined as “a rate of fetal growth that is less than normal for the growth potential of a specific infant” [20]. In this subsample, 49.4% of the neonates were nicotine-exposed. Hypoglycemia was noted in 32.4% of the exposed neonates in comparison to 25.8% of the non-nicotine exposed neonates (see Table 3 for absolute numbers). A 94 % PP was demonstrated when SGA, IUGR, and neonates born to diabetic mothers were excluded (RR = 1.254, 95% CrI = [0.944, 1.689]).
4. Discussion
This study found an association between in utero nicotine exposure and neonatal hypoglycemia in the first three hours of postnatal life. Even after accounting for well-known high-risk factors of neonatal hypoglycemia, 32% of nicotine-exposed neonates admitted to the NICU were identified as hypoglycemic compared to only 25.8% of NICU neonates who were not exposed to nicotine during gestation. Our study is the first investigation of in utero nicotine exposure on human neonates and the potential impact on postnatal glycemic regulation.
One proposed mechanism is that in utero nicotine exposure may lead to disruption or hypoplasia of pancreatic beta cells leading to low fetal insulin production which is essential for normal glucose homeostasis. Poor insulin production in utero disrupts normal lipid and glycogen stores leading to SGA and IUGR infants. Future research should explore these mechanisms to identify possible associations and risk factors for neonatal hypoglycemia.
Our findings are clinically significant as they heighten awareness of nicotine exposure as a potential risk factor for neonatal hypoglycemia. This may help guide prenatal counseling discussions about the risks of tobacco exposure and smoking cessation. In addition, our findings should alert pediatricians and neonatologists to screen for these additional risk factors postnatally.
This study was not without limitations due to its retrospective nature and reliance on self-reported household smoking status, which may have contributed to participation misclassification and underreporting of household tobacco smoke exposure. However, this risk was minimized by using a multi-part, well-validated, and highly sensitive method to identify tobacco smoke exposure[8, 15-17]. In addition, our mean age was 33.7 weeks gestation, which alone can be a risk factor for hypoglycemia. Moreover, we did not collect or review data on infants who were large for gestational age (LGA), another known risk factor for hypoglycemia. The study attempted to control for conditions that could be caused by nicotine exposure alone and that were also risk factors for hypoglycemia (i.e., neonates who were SGA or IUGR). Our proof-of-concept focus on tobacco smoke exposure was our primary objective; however, it is possible that other unmeasured variables are associated with an increased risk for hypoglycemia. Another limitation was our unit’s use of point-of-care glucose to determine hypoglycemia as there are known differences between point-of-care tests and standard laboratory testing. Repeat standard labs were not routinely done to confirm hypoglycemia and future work will improve our methods by modeling risk over time for repeated hypoglycemic events.
Furthermore, using a threshold of 45 mg/dl in the first three hours of life versus a lower range of 25–40 mg/dl in the first four hours of life and 35–45 mg/dl from 4–24 hours of life per AAP guidelines [2, 9] may have over-estimated the number of neonates that had transitional hypoglycemia. There has been much debate regarding the numerical value for hypoglycemia depending on context and hour of life ranging from 25–45 mg/dl in the first 24 hours of life [1, 2, 9]. Our cut off was used based on the unit guidelines for hypoglycemia screening.
Due to a relatively low sample size (n = 60) and low model precision, we were unable to run separate models on the nicotine-exposed mothers to differentiate between mothers who smoked versus mothers who were living with individuals who smoked. We chose to combine these groups since both infant groups were exposed to nicotine. Follow-up work will determine if infants whose mothers smoke have elevated risks compared to mothers who abstain from smoking but live with individuals who smoke.
Lastly, all our neonates included in this study (nicotine-exposed and non-nicotine exposed) were already admitted to the NICU, placing them in a higher risk category for hypoglycemia and limiting generalizability beyond NICU-admitted infants. It remains unclear what effect would have been observed if healthy term infants were assessed. Screening healthy term infants with nicotine exposure for hypoglycemia remains an area that should be studied in the future.
5. Conclusion
Based on our findings, neonates exposed to nicotine in utero had a greater risk of developing hypoglycemia after birth. Further mechanisms of action should be explored regarding nicotine exposure and its effect on neonatal glucose homeostasis. Physicians should be aware of these potential effects and obtain a thorough history of household tobacco smoke exposure during pregnancy, as it may help guide management after delivery and prenatal counseling.
Acknowledgments
The parent study for which the data base originated from was supported in part by the National Heart, Lung, and Blood Institute [R01 HL107404, PI = A.L. Stotts] and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development [R03 HD088847; PI: T.F. Northrup] at the US National Institutes of Health and Department of Health and Human Services.
Special thanks to the University of Texas at Houston, McGovern Medical School and Children’s Memorial Hermann Hospital.
Funding
This retrospective cohort study was partially supported by funding for two parent studies. The parent study for which the data base originated from was supported in part by the National Heart, Lung, and Blood Institute [R01 HL107404, PI = A.L. Stotts] and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development [R03 HD088847; PI: T.F. Northrup] at the US National Institutes of Health and Department of Health and Human Services.
Footnotes
Statement ethics
Written formal consent for this study was not sought due to study being a retrospective chart review. Study approval by institutional review board at University of Texas Health Science Center at Houston #HSC-MS-19-0323.
Conflict of interest
The authors have no conflicts of interest to disclose.
Data availability statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants. The data are available from one of the corresponding authors [ALS, Angela.L.Stotts@uth.tmc.edu; TFN, Thomas.F.Northrup@uth.tmc.edu] upon reasonable request.
References
- [1].Thompson-Branch A, Havranek T. Neonatal Hypoglycemia. Pediatr Rev. 2017;38(4):147–57. [DOI] [PubMed] [Google Scholar]
- [2].Adamkin DH. Neonatal hypoglycemia. Semin Fetal Neonatal Med. 2017;22(1):36–41. [DOI] [PubMed] [Google Scholar]
- [3].Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. 2012;161(5):787–91. [DOI] [PubMed] [Google Scholar]
- [4].Bruin JE, Kellenberger LD, Gerstein HC, Morrison KM, Holloway AC. Fetal and neonatal nicotine exposure and postnatal glucose homeostasis: Identifying critical windows of exposure. J Endocrinol. 2007; [DOI] [PubMed] [Google Scholar]
- [5].Drake P, Driscoll AK MT. Cigarette smoking during pregnancy: United States, 2016 [Internet]. NCHS Data Brief, no 305. 2016. Available from: https://www.cdc.gov/nchs/products/databriefs/db305.htm [PubMed] [Google Scholar]
- [6].Kondracki AJ. Prevalence and patterns of cigarette smoking before and during early and late pregnancy according to maternal characteristics: the first national data based on the 2003 birth certificate revision, United States, 2016. Reprod Health. 2019;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Second-Hand Tobacco Smoke Exposure. U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau. Women’s Health USA 2011. Rockville, Maryland: U.S. Department of Health and Human Services, 2011. 2011. [Google Scholar]
- [8].Stotts AL, Northrup TF, Green C, Suchting R, Hovell MF, Khan A, et al. Reducing Tobacco Smoke Exposure in High-Risk Infants: A Randomized, Controlled Trial. J Pediatr. 2020;218:35–41.e1. [DOI] [PubMed] [Google Scholar]
- [9].Adamkin DH, Papile LA, Baley JE, Bhutani VK, Carlo WA, Kumar P, et al. Clinical report - Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. 2011;127(3):575–9. [DOI] [PubMed] [Google Scholar]
- [10].Mao J, Chen LY, Fu JH, Li J, Duan Y, Xue XD. [Clinical evaluation of neonatal hypoglycemic brain injury demonstrated by serial MRIs]. Zhongguo Dang Dai Er Ke Za Zhi Chin J Contemp Pediatr. 2008;10(2):115–120. [PubMed] [Google Scholar]
- [11].Su J, Wang L. Research advances in neonatal hypoglycemic brain injury. Transl Pediatr. 2012;1(2):108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5(3):231–41. [DOI] [PubMed] [Google Scholar]
- [13].Bailey NA, Diaz-Barbosa M. Effect of maternal substance abuse on the fetus, neonate, and child. Pediatr Rev. 2018;39(11):550–9. [DOI] [PubMed] [Google Scholar]
- [14].Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci Off J Soc Toxicol. 2010;116(2):364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stotts AL, Green C, Northrup TF, Dodrill CL, Evans P, Tyson J, et al. Feasibility and efficacy of an intervention to reduce secondhand smoke exposure among infants discharged from a neonatal intensive care unit. J Perinatol. 2013;33(10):811–6. [DOI] [PubMed] [Google Scholar]
- [16].Northrup TF, Stotts AL, Suchting R, Khan AM, Green C, Klawans MR, et al. Thirdhand Smoke Contamination and Infant Nicotine Exposure in a Neonatal Intensive Care Unit: An Observational Study. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2021;23(2):373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Northrup TF, Suchting R, Klawans MR, Khan AM, Villarreal YR, Green C, et al. Proactive delivery of nicotine replacement therapy to families of hospitalized infants in a NICU: A randomized controlled pilot trial. J Neonatal Nurs JNN. 2020;26(4):201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis, third edition. Bayesian Data Analysis, Third Edition. 2013. [Google Scholar]
- [19].McElreath R Statistical rethinking: A bayesian course with examples in R and stan. Statistical Rethinking: A Bayesian Course with Examples in R and Stan. 2018. [Google Scholar]
- [20].Hay WW, Thureen PJ, Anderson MS. Intrauterine Growth Restriction. NeoReviews. 2001;2(6):e129–38. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants. The data are available from one of the corresponding authors [ALS, Angela.L.Stotts@uth.tmc.edu; TFN, Thomas.F.Northrup@uth.tmc.edu] upon reasonable request.