Abstract
Background:
Internationally, there is a growing interest in the potential benefits of psilocybin-assisted therapy to treat existential distress at the end of life. However, the social acceptability of this therapy is not yet well known.
Aim:
This study assesses the social acceptability of the medical use of psilocybin to treat existential distress at the end of life.
Design:
An online survey was conducted in Canada between November 23 and December 4, 2022. The questionnaire included items pertaining to perceptions, attitudes and concerns towards psilocybin-assisted therapy to treat existential distress at the end of life.
Participants:
The sample (n = 2800) was stratified by province, age and sex. Participants were adults from four provinces of Canada: Québec, Ontario, Alberta and British Columbia.
Results:
Overall, 79.3% considered psilocybin-assisted therapy a reasonable medical choice for a patient suffering from existential distress at the end of life, 84.8% agreed that the public health system should cover the costs of the intervention and 63.3% would welcome the legalisation of psilocybin for medical purposes. Previous psilocybin use (p < 0.0001, for all dependent variables), exposure to palliative care (p < 0.05, for all dependent variables) and a progressive political orientation (p < 0.05, for all dependent variables) were associated with more favourable attitudes towards psilocybin-assisted therapy at the end of life.
Conclusion:
The social acceptability of psilocybin-assisted therapy for existential distress at the end of life is rather high in Canada. These findings may contribute to efforts to mobilise resources and improve access to this emerging therapy in palliative and end of life care settings.
Keywords: Psilocybin, psychedelic, palliative care, end-of-life, psychological distress, survey, public opinion
What is already known on this topic?
There is a growing interest in psilocybin-assisted therapy worldwide, particularly to treat existential distress at the end of life.
What this study adds?
In this study, we show that the social acceptability of psilocybin-assisted therapy to treat existential distress at the end of life is high in Canada and identify factors associated with favourable attitudes of the population towards it.
How this study might affect research, practice or policy
Our findings may help mobilise resources to address barriers and challenges for implementing psilocybin-assisted therapy within palliative medicine and society. This could also have implications for policies regarding medical assistance in dying.
Introduction
Over the last two decades, a growing number of clinical studies have shown the potential benefits of psychedelic substances in treating various, and often complex, mental conditions.1–4 Psilocybin, notably, was associated with a rapid and lasting reduction of the symptoms of existential distress in patients nearing the end of life.5–7
While these substances remain illegal in most jurisdictions worldwide, some have begun accepting their medical use. In January 2022, the Canadian government amended its Special Access Program to enable healthcare professionals to request a substance like MDMA or psilocybin to treat a patient with a serious or life-threatening condition, positioning the country at the forefront of such developments. The access is granted on a case-by-case basis if conventional therapies have been ineffective, are unsuitable for the patient or are unavailable. 8
The increasing demand for psilocybin-assisted therapy raises many questions regarding access, safety, intervention protocol and training of healthcare professionals.9,10 However, its social acceptability, which has significant bearing on public policy and decision-making, is not yet well known. The objective of this study is to assess the social acceptability of psilocybin-assisted therapy in order to treat existential distress at the end of life and identify factors underlying perceptions, attitudes and concerns of the general population.
Methods
Study design and participants
A population-based, cross-sectional survey was conducted online. Participants were adults from four provinces in Canada: Alberta, British Columbia, Ontario and Québec. These provinces were selected as they account for > 86% of the Canadian population. Moreover, they are further along in having an established infrastructure for accessing psilocybin. The sample size was set a priori to 2800 and stratified by province, age and sex. The study was approved by the Research Ethics Committee of the CHU de Québec – Université Laval (2023-6501).
Measures
The questionnaire was developed, revised and translated by an interdisciplinary team of researchers, and included 39 multiple-choice items and 2 open-ended questions. Definitions (e.g. Special Access Program, palliative care, existential distress and psilocybin-assisted therapy) were provided at the beginning of each section to ensure understanding among participants. Because of the adaptive nature of the questionnaire, not all respondents answered all items.
Dependent variables
The first item of the questionnaire measured the awareness of the Special Access Program established by Health Canada, the main governmental health agency: ‘Have you heard about the Special Access Program?’. Other questions examined different facets of social acceptability towards psilocybin-assisted therapy: ‘To what extent would you agree that healthcare professionals should be allowed to administer psilocybin without going through Health Canada?’; ‘Do you think psilocybin is a reasonable choice for a palliative care patient suffering from existential distress?’; ‘In your opinion, should the public health system cover the costs of psilocybin-assisted therapy?’; ‘To what extent do you support the legalisation of psilocybin for medical purposes?’. Respondents were also asked to express their level of endorsement for three hypothetical scenarios on involving the use of psilocybin to address their own existential distress at the end of life: (1) within an assisted psychotherapy with a certified healthcare professional; (2) within a guided experience with a facilitator who is not a certified therapist; and (3) in a non-regulated context.
Independent variables
Factors potentially associated with perceptions and attitudes towards psilocybin-assisted therapy included socio-demographic variables (province, age, sex, education level, household income and ethnicity), previous use of psilocybin (either in a therapeutic context, in a recreational context, in microdoses or any other way), exposure to palliative care (either as a palliative care patient, as a caregiver or volunteer, through someone close or in some other way) and political orientation assessed on a five-point Likert scale from ‘Conservative’ (right-wing) to ‘Progressive’ (left-wing).
Data collection
A pre-test (n = 30) was conducted on November 17, 2022, to assess the questionnaire’s length, clarity and completeness. No significant modification was necessary. The questionnaire was then administered online between November 23 and December 4, 2022, by Léger (https://leger360.com/), a Canadian-owned survey and analysis firm with a panel of individuals having consented to being contacted for research. This was a closed survey. A link to the questionnaire, including an information and consent sheet (see Supplemental Material), both available in French and English, was emailed to potential participants. Participation was voluntary and anonymous.
Data analysis
We used descriptive statistics to present the characteristics of the sampled population and to report proportions of participants for each dependent variable. To identify factors associated with the respondents’ attitudes, adjusted prevalence ratios and 95% confidence intervals were estimated using a generalised linear model with a log link and a Poisson working model. Models were mutually adjusted for all socio-demographic factors. Robust variances were obtained with sandwich estimators to account for the larger variance of Poisson variables compared with binomial variables. Since the point estimates and p-values were not materially different between weighted and unweighted analyses, only results of unweighted analyses are reported here. All analyses were conducted using SAS, version 9.4 (SAS Institute). Two-sided p-value <0.05 was considered statistically significant.
Results
A total of 16,252 panel members were sent the invitation to participate in the survey, of whom 11,481 did not follow the link provided, 696 could not open the questionnaire because their stratum quota had been reached and 757 were ineligible. Of the 3318 eligible who accessed the survey web page, 170 (5%) refused to answer the questionnaire, and 348 (11%) did not complete it, resulting in a total of 2800 (84%) completed questionnaires that were analysed. Characteristics of the population surveyed are shown in Table 1. Because of the stratified sampling frame, sex and age groups were proportionally represented in the final sample. Overall, 19% of the surveyed population had previously used psilocybin-containing mushrooms, ranging from 15% in Québec to 26% in British Columbia.
Table 1.
Multivariate regression model showing relationships between dependent variables and respondents’ characteristics.
| Characteristics | n (%) | Have you heard about the Special Access Program? | To what extent would you agree that healthcare professionals should be allowed to administer psilocybin without going through Health Canada? | Do you think psilocybin is a reasonable medical choice for a palliative care patient suffering from existential distress? | Currently, psilocybin-assisted therapy can be expensive. In your opinion, should the public health system cover the costs? | To what extent do you support the legalisation of psilocybin for medical purposes? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes vs. (No; I don’t know) | Strongly agree; Agree vs. (Neither disagree nor agree; Disagree; Strongly Disagree) | Yes, since it’s a personal choice; Yes, but only if other treatments have proven ineffective; Yes, for another reason vs. (No; Not sure) | Yes, fully; Yes, partially vs. (No) | Very favourable; Favourable vs. (Neither unfavourable nor favourable; Unfavourable; Very unfavourable) | ||||||||||||
| % | aPR (95% CI) | p | % | aPR (95% CI) | p | % | aPR (95% CI) | p | % | aPR (95% CI) | p | % | aPR (95% CI) | p | ||
| Province | ||||||||||||||||
| Québec | 1000 (35.7) | 14.2 | 1.00 | – | 46.8 | 1.00 | – | 80.2 | 1.00 | – | 85.8 | 1.00 | – | 60.7 | 1.00 | – |
| Ontario | 600 (21.4) | 17.6 | 1.23 (0.96–1.58) | 0.1004 | 40.7 | 0.87 (0.76–0.99) | 0.0324 | 80.9 | 1.01 (0.96–1.07) | 0.7927 | 85.2 | 0.99 (0.95–1.04) | 0.7934 | 64.1 | 1.06 (0.97–1.15) | 0.2080 |
| Alberta | 600 (21.4) | 24.2 | 1.70 (1.36–2.13) | 0.0000 | 42.3 | 0.90 (0.80–1.02) | 0.1053 | 78.2 | 0.98 (0.92–1.03) | 0.3992 | 85.8 | 1.00 (0.95–1.05) | 0.9912 | 67.0 | 1.10 (1.02–1.20) | 0.0155 |
| British Columbia | 600 (21.4) | 24.3 | 1.71 (1.37–2.14) | 0.0000 | 45.8 | 0.98 (0.87–1.10) | 0.6960 | 77.5 | 0.97 (0.91–1.02) | 0.2399 | 82,0 | 0.96 (0.91–1.00) | 0.0653 | 63.2 | 1.04 (0.96–1.13) | 0.3361 |
| Age (years) | ||||||||||||||||
| 18–34 | 716 (25.6) | 17.9 | 1.00 | – | 40.6 | 1.00 | – | 77.7 | 1.00 | – | 85.2 | 1.00 | – | 59.3 | 1.00 | – |
| 35–54 | 1050 (37.5) | 19.0 | 1.06 (0.86–1.30) | 0.5982 | 43.8 | 1.08 (0.95–1.22) | 0.2325 | 77.1 | 0.99 (0.94–1.05) | 0.7737 | 83.2 | 0.98 (0.93–1.02) | 0.2828 | 60.3 | 1.02 (0.93–1.10) | 0.7050 |
| ⩾55 | 1034 (36.9) | 19.0 | 1.06 (0.85–1.33) | 0.6023 | 47.3 | 1.17 (1.03–1.32) | 0.0181 | 82.8 | 1.07 (1.01–1.13) | 0.0252 | 86.3 | 1.01 (0.97–1.06) | 0.5880 | 69.3 | 1.17 (1.08–1.27) | 0.0003 |
| Sex | ||||||||||||||||
| Female | 1398 (50.0) | 18.2 | 1.00 | – | 41.2 | 1.00 | – | 81.2 | 1.00 | – | 86.2 | 1.00 | – | 63.7 | 1.00 | – |
| Male | 1398 (50.0) | 19.2 | 1.05 (0.89–1.24) | 0.5533 | 47,0 | 1.14 (1.04–1.25) | 0.0061 | 77.7 | 0.96 (0.92–1.00) | 0.0369 | 83.7 | 0.97 (0.94–1.01) | 0.1042 | 62.9 | 0.99 (0.93–1.05) | 0.7084 |
| Education | ||||||||||||||||
| No university degree | 1584 (56.9) | 17.3 | 1.00 | – | 44,0 | 1.00 | – | 79.4 | 1.00 | – | 87.1 | 1.00 | – | 63.3 | 1.00 | – |
| University degree(s) | 1200 (43.1) | 20.6 | 1.19 (1.01–1.40) | 0.0386 | 44.5 | 1.01 (0.91–1.11) | 0.8223 | 79.2 | 1.00 (0.96–1.04) | 0.9306 | 82.3 | 0.94 (0.91–0.98) | 0.0022 | 63.3 | 1.00 (0.94–1.06) | 0.9982 |
| Income | ||||||||||||||||
| Less than $50,000 | 751 (29.7) | 17.1 | 1.00 | – | 44.8 | 1.00 | – | 77.5 | 1.00 | – | 85.4 | 1.00 | – | 61.6 | 1.00 | – |
| $50,000–$99,999 | 895 (35.4) | 18.3 | 1.07 (0.87–1.33) | 0.5235 | 42.4 | 0.95 (0.85–1.06) | 0.3363 | 79.1 | 1.02 (0.97–1.07) | 0.4600 | 83.3 | 0.98 (0.93–1.02) | 0.2624 | 62.2 | 1.01 (0.94–1.09) | 0.7979 |
| $100,000 or more | 881 (34.9) | 20.5 | 1.20 (0.97–1.48) | 0.0962 | 45.7 | 1.02 (0.91–1.14) | 0.7233 | 81,0 | 1.04 (0.99–1.10) | 0.1138 | 86,0 | 1.01 (0.96–1.05) | 0.7542 | 65.7 | 1.07 (0.99–1.15) | 0.1039 |
| Ethnicity | ||||||||||||||||
| White | 2257 (82.0) | 19.5 | 1.00 | – | 46.2 | 1.00 | – | 80,0 | 1.00 | – | 85.4 | 1.00 | – | 65.4 | 1.00 | – |
| Indigenous | 60 (2.2) | 17.3 | 0.89 (0.54–1.47) | 0.6369 | 52.4 | 1.13 (0.89–1.45) | 0.3140 | 76.6 | 0.96 (0.83–1.10) | 0.5402 | 89.6 | 1.05 (0.96–1.14) | 0.2734 | 67.4 | 1.03 (0.87–1.22) | 0.727 |
| Other | 437 (15.9) | 15.4 | 0.79 (0.61–1.02) | 0.0692 | 34.4 | 0.74 (0.63–0.88) | 0.0004 | 76,0 | 0.95 (0.89–1.02) | 0.1337 | 81.2 | 0.95 (0.90–1.01) | 0.0880 | 52.8 | 0.81 (0.72–0.90) | 0.0001 |
| Political affiliation | ||||||||||||||||
| Conservative | 493 (20.3) | 17.1 | 1.00 | – | 42.9 | 1.00 | – | 76.1 | 1.00 | – | 78.4 | 1.00 | – | 58.5 | 1.00 | – |
| Neutral | 866 (35.7) | 15.7 | 0.92 (0.72–1.17) | 0.4852 | 39.9 | 0.93 (0.82–1.06) | 0.2836 | 74.2 | 0.98 (0.91–1.04) | 0.4674 | 81.9 | 1.04 (0.99–1.11) | 0.1458 | 55.5 | 0.95 (0.86–1.04) | 0.2854 |
| Progressive | 1070 (44.0) | 22.4 | 1.31 (1.06–1.62) | 0.0136 | 48.6 | 1.13 (0.89–1.45) | 0.0460 | 85.1 | 1.12 (1.06–1.19) | 0.0002 | 90.3 | 1.15 (1.09–1.21) | 0.0000 | 72.6 | 1.24 (1.14–1.35) | 0.0000 |
| Has used psilocybin | ||||||||||||||||
| No/no answer | 2270 (81.1) | 16.4 | 1.00 | – | 40.2 | 1.00 | – | 76.1 | 1.00 | – | 83.3 | 1.00 | – | 59.4 | 1.00 | – |
| Yes | 530 (18.9) | 31.9 | 1.95 (1.65–2.29) | 0.0000 | 64.9 | 1.62 (1.48–1.77) | 0.0000 | 93.6 | 1.23 (1.19–1.28) | 0.0000 | 91.4 | 1.10 (1.06–1.14) | 0.0000 | 80.9 | 1.36 (1.28–1.44) | 0.0000 |
| Has been exposed to palliative care | ||||||||||||||||
| No | 1535 (55.2) | 15.9 | 1.00 | – | 42.4 | 1.00 | – | 75.9 | 1.00 | – | 82.5 | 1.00 | – | 61.2 | 1.00 | – |
| Yes | 1265 (44.8) | 22.6 | 1.42 (1.21–1.67) | 0.0000 | 46.5 | 1.10 (1.01–1.20) | 0.0375 | 83.5 | 1.10 (1.06–1.15) | 0.0000 | 87.7 | 1.06 (1.03–1.10) | 0.0003 | 65.8 | 1.08 (1.01–1.14) | 0.0170 |
| Global: 18.7% | Global: 44.2% | Global: 79.3% | Global: 84.8% | Global: 63.3% | ||||||||||||
aPR: adjusted prevalence ratio; 95% CI: 95% confidence interval.
Globally, only 18.7% of respondents were aware of the Special Access Program enabling healthcare professionals to request psilocybin to treat a patient. Awareness varied significantly across provinces. University graduates, progressives, ever users of psilocybin, as well as those exposed to palliative care, were also more likely to have heard of the programme.
In terms of acceptability, 79.3% of Canadians considered psilocybin-assisted therapy a reasonable medical choice for a patient suffering from existential distress at the end of life, 44.2% agreed that healthcare professionals should be allowed to administer psilocybin without going through Health Canada, 84.8% agreed that the public health system should cover the costs of the intervention, either totally or partially and 63.3% would welcome the legalisation of psilocybin for medical purposes. Previous psilocybin use (p < 0.0001), exposure to palliative care (p < 0.05) and a progressive political orientation (p < 0.05) were positively associated with all indicators of acceptability, as observed for all dependent variables reported (see Table 1). Still, a majority of those who consider themselves politically conservative would support the legalisation of psilocybin for medical purposes. Respondents from ethnic minority groups tended to have less favourable attitudes compared to those who identified as white.
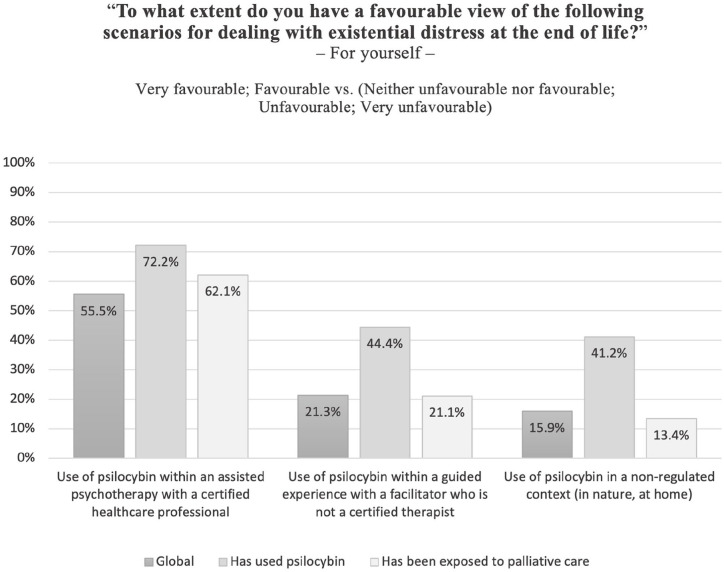
As Figure 1 illustrates, 55.5% of respondents had a favourable view of psilocybin to treat existential distress at the end of life if used within the regulated context of psychotherapy, in sharp contrast with less regulated (21.3%) and non-regulated (15.9%) contexts.
Figure 1.
Percentage of respondents having a favourable view of psilocybin to treat existential distress at the end of life depending on the context of use.
Discussion
Main findings
In this peer-reviewed academic study on the social acceptability of psilocybin-assisted therapy at the end of life, we found that most Canadians have favourable attitudes about this emerging intervention. Those results are comparable to what has been observed in smaller population-based surveys conducted in the country and elsewhere.11–14 More than three out of four respondents considered psilocybin-assisted therapy a reasonable medical option to treat existential distress at the end of life, with the vast majority agreeing that the costs should be covered, at least partially, by the universal healthcare system.
Survey results did not vary significantly across provinces. Previous experience with psilocybin, exposure to palliative care and political orientation were the main factors associated with the respondents’ attitudes, followed by ethnicity and age.
What this study adds?
Our findings are largely consistent with those of population-based surveys previously conducted in Canada,11,12 England 13 and Australia. 14 These surveys, mostly commissioned by interest groups or organisations, have not been published in scientific journals. Additionally, our study is novel as it focusses on treating existential distress at the end of life, an intricate condition for which treatment options are still limited and often ineffective. 15
In light of the meteoric rise in the interest in psychedelic-based therapies, Canada’s experience with granting therapeutic psilocybin access could have far-reaching impacts, both locally and globally. Consequently, more research is required to address the many facets of implementing psilocybin-assisted therapy within medico-legal and ethical frameworks. As acceptability is high in this study, it may help stakeholders mobilise resources to address barriers and challenges. 16 Since decision-makers and politicians are influenced by public opinion, results may also have a spillover effect on other contexts. 16 The results obtained from this survey are timely in the wake of the growing worldwide interest towards psilocybin-assisted therapy. The implications of using this type of intervention to alleviate the existential suffering of patients nearing the end of life are manifest, as evidenced by a recommendation made in February 2023 by the Canadian Special Joint Committee on Medical Assistance in Dying, calling for an improvement of access to this therapy, as part of palliative care supports. 17
Strengths and limitations of the study
Our sample was large, and we obtained a high response rate. Measures were taken to follow the reporting guidelines set forth by the CHERRIES checklist. 18 However, the present study shares the general limitations of web-based surveys, notably potential volunteer bias. 19
Despite these limitations, our findings may contribute to efforts to mobilise resources and improve access to this emerging therapy in palliative and end of life care settings.
Supplemental Material
Supplemental material, sj-pdf-1-pmj-10.1177_02692163231222430 for Social acceptability of psilocybin-assisted therapy for existential distress at the end of life: A population-based survey by Louis Plourde, Sue-Ling Chang, Houman Farzin, Pierre Gagnon, Johanne Hébert, Robert Foxman, Pierre Deschamps, François Provost, Marianne Masse-Grenier, Jean-François Stephan, Katherine Cheung, Yann Joly, Jean-Sébastien Fallu, Michel Dorval and for the P3A Study Group in Palliative Medicine
Supplemental material, sj-pdf-2-pmj-10.1177_02692163231222430 for Social acceptability of psilocybin-assisted therapy for existential distress at the end of life: A population-based survey by Louis Plourde, Sue-Ling Chang, Houman Farzin, Pierre Gagnon, Johanne Hébert, Robert Foxman, Pierre Deschamps, François Provost, Marianne Masse-Grenier, Jean-François Stephan, Katherine Cheung, Yann Joly, Jean-Sébastien Fallu, Michel Dorval and for the P3A Study Group in Palliative Medicine
Acknowledgments
We thank Éric Demers for his assistance with statistical analyses. Other members of the P3A Study Group include François Arès, Marion Barrault-Couchouron, Ariane Bélanger, Léonie Doyon, Florence Moureaux, Olivia Nguyen and Diane Tapp.
Footnotes
Data availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Houman Farzin and Jean-François Stephan are trainers for the non-profit organisation TheraPsil. Houman Farzin was the Montréal site physician for MAPPUSX, a Phase 3 clinical trial of MDMA-AT for PTSD sponsored by MAPS, and is an early investor in Beckley Psytech.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a grant from the Fonds de Recherche du Québec (AUDACE programme). Louis Plourde is recipient of a scholarship from the Fonds d’enseignement et de recherche (Faculty of Pharmacy, Université Laval). The grant agency had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Informed consent statement: Informed consent was obtained from all participants when they opened the survey link. Data were collected anonymously.
Institutional review board statement: The study was approved by the Institutional Review Board of CHU de Québec-Université Laval (2023-6501).
ORCID iD: Michel Dorval  https://orcid.org/0000-0002-3207-8211
https://orcid.org/0000-0002-3207-8211
Supplemental material: Supplemental material for this article is available online.
References
- 1. Belouin SJ, Henningfield JE. Psychedelics: where we are now, why we got here, what we must do. Neuropharmacology 2018; 142: 7–19. [DOI] [PubMed] [Google Scholar]
- 2. Curran HV, Nutt D, de Wit H. Psychedelics and related drugs: therapeutic possibilities, mechanisms and regulation. J. Psychopharmacol 2018; 235(2): 373–375. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Romeu A, Richards WA. Current perspectives on psychedelic therapy: use of serotonergic hallucinogens in clinical interventions. Int Rev Psychiatry 2018; 30(4): 291–316. [DOI] [PubMed] [Google Scholar]
- 4. Nichols DE. Psychedelics. Pharmacol Rev 2016; 68(2): 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol 2016; 30(12): 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 2016; 30(12): 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agrawal M, Emanuel E, Richards B, et al. Assessment of psilocybin therapy for patients with cancer and major depression disorder. JAMA Oncol 2023; 9(6): 864–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Government of Canada. Regulations amending certain regulations relating to restricted drugs (special access program). Canada Gazette Part 2 2022; 156(1). [Google Scholar]
- 9. Baig F. Health-care professionals fight decision to reject access to psilocybin for training. Toronto Star, https://www.thestar.com/news/canada/2023/03/28/health-care-professionals-fight-decision-to-reject-access-to-psilocybin-for-training.html (2023; accessed 29 May 2023).
- 10. Beaussant Y, Tulsky J, Guérin B, et al. Mapping an agenda for psychedelic-assisted therapy research in patients with serious illness. J Palliat Med 2021; 24(11): 1657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nanos-Canadian Psychedelic Association. Strong majority of Canadians would support or somewhat support a government that legalized mushroom-based psilocybin-assisted psychotherapy to improve the quality of life for terminally ill patients. https://nanos.co/wp-content/uploads/2021/08/2021-1913-Cdn-Psychedelic-Assoc.-June-Populated-report-with-tabs.pdf (2021, accessed 29 May 2023).
- 12. Therapsil. Public Opinion on Psilocybin Regulations in Canada 2022. https://therapsil.ca/wp-content/uploads/2023/01/Public-Perception-Poll-3.pdf (2023, accessed 29 May 2023).
- 13. PsiloNautica, Drug Science. Public attitudes topsilocy bin-assisted therapy. https://www.drugscience.org.uk/wpcontent/uploads/2021/06/PsiloNautica-Report-Embargolifts-04_06_21-at-00_01.pdf (2021, accessed 20 November 2023).
- 14. Kunstler B, Hatty M, Genat A, et al. Attitudes and beliefs about the medically supervised use of psychedelic medications for the treatment of mental health conditions. https://www.behaviourworksaustralia.org/projects/attitudes-towards-the-use-of-psychedelic-drugs-e-g-psilocybin-to-treat-mental-health-conditions (2021, accessed 20 November 2023).
- 15. Boston P, Bruce A, Schreiber R. Existential suffering in the palliative care setting: an integrated literature review. J Pain Symptom Manage 2011; 41(3): 604–618. [DOI] [PubMed] [Google Scholar]
- 16. Howse E, Cullerton K, Grunseit A, et al. Measuring public opinion and acceptability of prevention policies: an integrative review and narrative synthesis of methods. Health Res Policy Sys 2022; 20(1): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Special Joint Committee on Medical Assistance in Dying. Medical Assistance in Dying in Canada: Choices for Canadians. https://parl.ca/DocumentViewer/en/44-1/AMAD/report-2/ (2023, accessed 29 May 2023).
- 18. Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004; 6(3):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wright KB. Researching internet-based populations: advantages and disadvantages of online survey research, online questionnaire authoring software packages, and web survey services. J Comput Mediat Commun 2005; 10(3). https://www.drugscience.org.uk/wp-content/uploads/2021/06/PsiloNautica-Report-Embargo-lifts-04_06_21-at-00_01.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pmj-10.1177_02692163231222430 for Social acceptability of psilocybin-assisted therapy for existential distress at the end of life: A population-based survey by Louis Plourde, Sue-Ling Chang, Houman Farzin, Pierre Gagnon, Johanne Hébert, Robert Foxman, Pierre Deschamps, François Provost, Marianne Masse-Grenier, Jean-François Stephan, Katherine Cheung, Yann Joly, Jean-Sébastien Fallu, Michel Dorval and for the P3A Study Group in Palliative Medicine
Supplemental material, sj-pdf-2-pmj-10.1177_02692163231222430 for Social acceptability of psilocybin-assisted therapy for existential distress at the end of life: A population-based survey by Louis Plourde, Sue-Ling Chang, Houman Farzin, Pierre Gagnon, Johanne Hébert, Robert Foxman, Pierre Deschamps, François Provost, Marianne Masse-Grenier, Jean-François Stephan, Katherine Cheung, Yann Joly, Jean-Sébastien Fallu, Michel Dorval and for the P3A Study Group in Palliative Medicine