Abstract
Background:
Inflammatory bowel disease (IBD) has a major economic impact on healthcare costs.
Objectives:
The aim of this study was to evaluate the current healthcare expenditure associated with IBD in a population-wide study in Catalonia.
Design:
Retrospective observational study.
Methods:
All patients with IBD included in the Catalan Health Surveillance System (CHSS) were considered eligible. The CHSS compiles data on more than 7 million individuals in 2020 (34,823 with IBD). Data on the use of healthcare resources and its economic impact were extracted applying the International Classification of Diseases, 10th revision, Clinical Modification codes (ICD-10-CM codes). Health expenditure, comorbidities, and hospitalization were calculated according to the standard costs of each service provided by the Department of Health of the Catalan government. The data on the IBD population were compared with non-IBD population adjusted for age, sex, and income level. IBD costs were recorded separately for Crohn’s disease (CD) and ulcerative colitis (UC).
Results:
Prevalence of comorbidities was higher in patients with IBD than in those without. The risk of hospitalization was twice as high in the IBD population. The overall healthcare expenditure on IBD patients amounted to 164M€. The pharmacy cost represents the 60%. The average annual per capita expenditure on IBD patients was more than 3.4-fold higher (IBD 4200€, non-IBD 1200€). Average costs of UC were 3400€ and 5700€ for CD.
Conclusion:
The risk of comorbidities was twice as high in patients with IBD and their use of healthcare resources was also higher than that of their non-IBD counterparts. Per capita healthcare expenditure was approximately 3.4 times higher in the population with IBD.
Trial registration:
The study was not previously registered.
Keywords: Catalonia, economy, inflammatory bowel disease
Plain language summary
Economic impact of inflammatory bowel disease in Catalonia
The manuscript includes data of the most recent epidemiologic data about the high economic impact of IBD in Catalonia.
Introduction
Inflammatory bowel disease (IBD) comprises two chronic intestinal disorders: Crohn’s disease (CD) and ulcerative colitis (UC). 1 IBD affects more than 5 million people worldwide and presents a variable clinical course, alternating periods of remission and flares.2,3 The reported annual hospitalization rate approaches 20%, and around 50% of patients require surgery within 10 years of diagnosis.4–7 Current therapeutic approaches to IBD are made up of relatively expensive biological treatments, although most patients have mild to moderate disease and do not receive these drugs.8,9
Recently Burisch et al. published a cost commission document where the direct and indirect costs of IBD were described. Before the introduction of biological treatments, most direct costs were associated with IBD-related hospitalization and surgery. However, since the emergence of these therapies 20 years ago direct costs of IBD management have shifted, the biological treatments being the predominant driver of direct health-care costs in high-income countries. 10
In 2020, Park et al. 11 reported the annual cost of IBD-patients in USA to be over three times higher than in matched non-IBD patients ($22,987 versus $6956). Major drivers of costs were hospitalization (40%), emergency room visits (18%), and pharmacotherapy (12%). 11 In 2003–2004, Kappelman et al. 12 reported mean annual costs of $8265 and $5066 for CD and UC, respectively.
The Catalan Health Service (CatSalut) created the Catalan Health Surveillance System (CHSS) database in 2011. This population-based health register contains data on the entire population of Catalonia, a region with more than 7,500,000 inhabitants in 2020. Previous studies by our group using this database recorded a high prevalence and incidence of IBD 13 and a fall in the rates of hospitalization in recent years; this latter finding was negatively correlated with the increase in the use of biological treatments. 14 Despite this recent data, population-based estimated costs of IBD are still sparse.
The aim of the present study was to report the expenditure on patients with IBD in 2020. Secondary objectives were to evaluate the sociodemographic and clinical characteristics and utilization of healthcare services among patients with IBD, and to compare them with the data from patients without IBD.
Methods
Study design, participants, and database
A retrospective analysis of an administrative database including all patients registered in the CHSS during 2020 was performed. IBD patients were identified, and classified according to the International Classification of Diseases, 10th revision, Clinical Modification codes (ICD-10-CM codes). The ICD-10-CM codes used are shown in Supplemental Table 1. A case was considered valid only if there was also a IBD treatment registered. The anatomical therapeutic chemical (ATC) codes used for drugs are shown in Supplemental Table 2. Patients without IBD included the entire population that did not meet the previously described criteria. Groups were matched through indirect standardization, adjusted for age, sex, and income level.
Sociodemographic and clinical data were obtained from the CHSS database which, since 2011, has recorded detailed information on the utilization of healthcare resources by the entire population of Catalonia (7,727,029 inhabitants in 2019). The concept of healthcare utilization, which has been analyzed in previous publications in other areas,15–17 covers multiple settings, including primary care, acute and intermediate care hospitals, mental health centers, outpatient clinics, and emergency services. The CHSS database contains comprehensive diagnostic information according to the ICD-9-CM and ICD-10-CM classification. This database also records information regarding pharmacological dispensing and pharmacy expenses, both outpatient pharmacy and more specific and expensive treatments from hospital pharmacy. Cost of each treatment were according CatSalut registries, and it also included invoices, from outpatient clinics, non-urgent medical transportation, outpatient rehabilitation, home oxygen therapy, dialysis, and orthopedic services. No data about private healthcare could be recorded, because private centers use different codes for patient identification; however, we expect this to be a lower percentage, because as IBD is a chronic disease and both pharmacological and non-pharmacological treatments are relatively expensive, most patients require financial support from the public system at some stage of the disease.
Variables
New cases of IBD were defined as patients residing in Catalonia who were diagnosed with IBD for the first time during 2020. Prevalent cases were defined as the total number of patients with IBD, residing in Catalonia and alive on 31 December 2020, and also in relation to the total Catalan population. Reference population for the calculation of incidence and prevalence rates was the population of Catalonia provided by the IDESCAT 18 and expressed per 100,000 inhabitants.
The sociodemographic variables considered in this study were age, sex, and income level, classified as high (annual income >100,000€), intermediate (18,000–100,000€), low (<18,000€), and very low (receiving welfare support from the government). 19 Clinical variables were diagnoses (or comorbidities), as they appear in the CHSS database according to standard clinical practice, and coded according to ICD-10-CM. The multimorbidity burden was stratified based on the Adjusted Morbidity Groups (Spanish acronym GMA), which considers the type of disease (acute or chronic), the number of systems affected, and complexity of each disease.20–22 The GMA allows the classification of the entire population into four strata based on their associated morbidity risk. These strata are (a) baseline risk (healthy stage), with a GMA score up to the 50th percentile of the total population; (b) low risk, with a GMA score between the 50th and 80th percentiles; (c) moderate risk, with a GMA score between the 80th and 95th percentiles; and (d) high risk, with a GMA score above the 95th percentile.23,24 Variables associated with the utilization of healthcare services were: the number of: visits to primary healthcare centers; outpatient visits; emergency service admissions; acute care hospital admissions; intermediate care hospital admissions; admissions to psychiatric centers, drugs according to the different chemical and therapeutic (ATC) classification groups, and medication units dispensed by IBD and non-IBD patients. Health expenditure was calculated according to the standard costs of each service provided by the Department of Health of the Catalan government for each year. 25 Expenditures were estimated for the overall IBD population and also separately for CD and UC populations.
As 2020 was the year of the COVID-19 pandemic, we performed a sensitivity analysis by studying the same variables for 2019 to determine whether the pandemic had significantly altered the distribution of expenditure.
Statistical analysis
Categorical variables were described as frequencies and percentages and quantitative variables as means and standard deviations (SD) and/or medians and interquartile ranges (Q1–Q3). Incidence and prevalence rates were expressed per 100,000 inhabitants. Categorical variables were compared using Pearson’s Chi-squared test with Yate’s continuity correction. The statistical significance threshold was set at a two-sided alpha value of 0.05.
The prevalence of comorbidities and the utilization of healthcare services and the associated expenditure were compared in the populations of patients with and without this pathology (IBD and non-IBD) through indirect standardization, adjusted for age, sex, and income level. Comorbidities and healthcare services utilization were compared using the rate ratio by median-unbiased estimation (mid-p), and healthcare services expenditure was compared using the Mann–Whitney U test. All analyses were performed using the R statistical package (version 4.2.0; R Core Team; Vienna, Austria).
Ethical issues
The research used retrospective anonymized data from the CHSS database. No personal data were used, and all the patient data were encrypted so that no personal identifications were retrievable or traceable to the original source from the database. This study was conducted in accordance with the Ethical Principles for Medical Research Involving Human Subjects of the Helsinki Declaration 26 and the local Personal Data Protection Law (LOPD 03/2018). The results are presented in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) and REporting of studies Conducted using Observational Routinely collected health Data (RECORD) guidelines for observational studies,27,28 and studies using routinely collected health data were also considered.
Results
Analysis of the CHSS database identified 34,823 patients diagnosed with IBD. The prevalence of IBD per 100,000 inhabitants in 2020 was 511.01 (204.62 for CD and 317.7 for UC). There were 2558 new diagnoses (739 CD and 1538 UC), with an incidence per 100,000 inhabitants of 37.5 (10.8 for CD and 22.6 for UC).
The prevalence of comorbidities was higher in IBD than in non-IBD patients. Relative figures for certain comorbidities were: 14.5% versus 10.4% for cancer, 13.4% versus 6.2% for arthritis, 11.6% versus 7% for chronic obstructive pulmonary disease, and 11.1% versus 7% for asthma.
According to the GMA stratification, multimorbidity in IBD patients was twice that recorded in non-IBD patients. Moderate and high multimorbidity risk strata were 33% and 16%, respectively, for IBD, 20% and 7% for non-IBD, and 15% and 5% for the general population.
Baseline characteristics for age, sex, comorbidities, and multimorbidity of our population are shown in Table 1, Supplemental Table 3, and Supplemental Figures 1–3.
Table 1.
Detailed age, sex, comorbidities, and GMA stratification of IBD, CD, and UC in Catalonia in 2020.
| Variables | IBD (n = 38,954) | CD (n = 14,192) | UC (n = 24,762) | p |
|---|---|---|---|---|
| Sex | <0.001 | |||
| Men | 19,411 (49.8%) | 6846 (48.2%) | 12,565 (50.7%) | |
| Women | 19,543 (50.2%) | 7346 (51.8%) | 12,197 (49.3%) | |
| Age | 53.8 (18.3) | 49.5 (18.4) | 56.3 (17.8) | <0.001 |
| Age group | <0.001 | |||
| <15 | 535 (1.37%) | 323 (2.28%) | 212 (0.86%) | |
| 15–44 | 11,949 (30.7%) | 5534 (39.0%) | 6415 (25.9%) | |
| 45–64 | 15,190 (39.0%) | 5291 (37.3%) | 9899 (40.0%) | |
| 65–74 | 5465 (14.0%) | 1546 (10.9%) | 3919 (15.8%) | |
| 75–84 | 3814 (9.79%) | 1024 (7.22%) | 2790 (11.3%) | |
| >84 | 2001 (5.14%) | 474 (3.34%) | 1527 (6.17%) | |
| Morbidity index | 13.7 (12.4) | 13.5 (11.7) | 13.9 (12.8) | 0.013 |
| Risk groups | <0.001 | |||
| Baseline risk | 4271 (11.0%) | 1246 (8.78%) | 3025 (12.2%) | |
| Low risk | 15,336 (39.4%) | 5730 (40.4%) | 9606 (38.8%) | |
| Moderate risk | 13,294 (34.1%) | 5192 (36.6%) | 8102 (32.7%) | |
| High risk | 6053 (15.5%) | 2024 (14.3%) | 4029 (16.3%) | |
| Comorbidities | ||||
| Neoplasia | 5651 (14.5%) | 1820 (12.8%) | 3831 (15.5%) | <0.001 |
| Arthritis | 5207 (13.4%) | 2198 (15.5%) | 3009 (12.2%) | <0.001 |
| Diabetes | 4917 (12.6%) | 1495 (10.5%) | 3422 (13.8%) | <0.001 |
| COPD | 4520 (11.6%) | 1502 (10.6%) | 3018 (12.2%) | <0.001 |
| Asthma | 4334 (11.1%) | 1715 (12.1%) | 2619 (10.6%) | <0.001 |
| Ischemic HD | 2668 (6.85%) | 802 (5.65%) | 1866 (7.54%) | <0.001 |
| Heart failure | 2534 (6.51%) | 805 (5.67%) | 1729 (6.98%) | <0.001 |
| Stroke | 2485 (6.38%) | 774 (5.45%) | 1711 (6.91%) | <0.001 |
| Complex chronic | 1832 (4.70%) | 578 (4.07%) | 1254 (5.06%) | <0.001 |
| Alcohol | 1201 (3.08%) | 487 (3.43%) | 714 (2.88%) | 0.003 |
| Dementia | 1065 (2.73%) | 267 (1.88%) | 798 (3.22%) | <0.001 |
| Major depression | 1028 (2.64%) | 368 (2.59%) | 660 (2.67%) | 0.692 |
| Obsessive compulsive | 520 (1.33%) | 220 (1.55%) | 300 (1.21%) | 0.006 |
| Psychosis | 483 (1.24%) | 176 (1.24%) | 307 (1.24%) | 1.000 |
| Cirrhosis | 436 (1.12%) | 159 (1.12%) | 277 (1.12%) | 1.000 |
CD, Crohn’s disease; COPD, Chronic obstructive pulmonar disease; GMA, Adjusted Morbidity Groups; HD, heart disease; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Economic impact
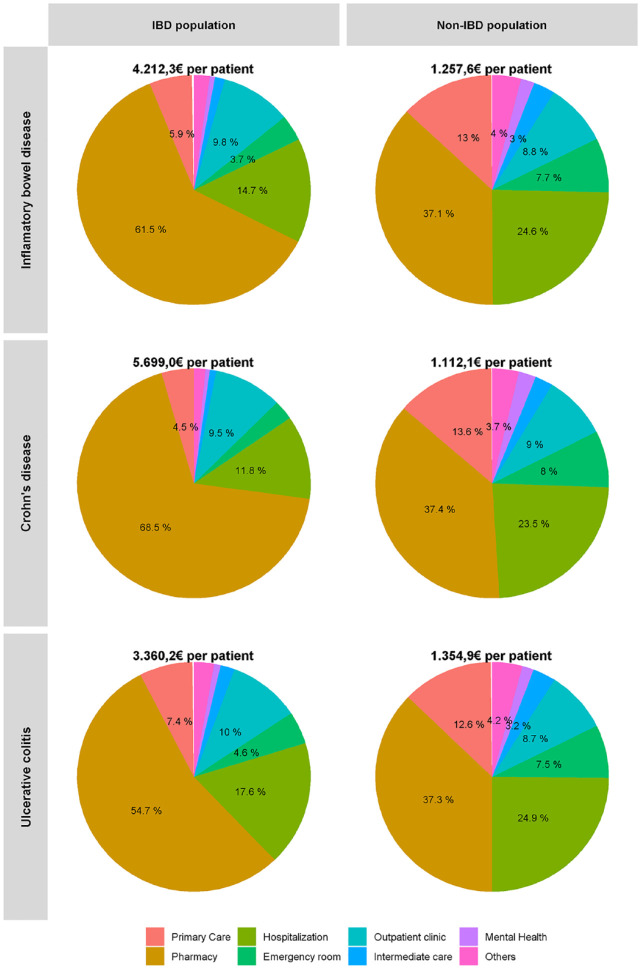
In 2020, patients with IBD had a global healthcare expenditure of 164M€, more than three times higher than the figure for the non-IBD group (49M€) (Table 2); which suggests that an expenditure of 115M€ can be attributed to IBD. The highest health cost was pharmacy (total 101M€; 55M€ CD and 46M€ UC) followed by hospitalization (total 24M€; 9M€ CD and 15M€ UC), outpatient care (total 16M€; 8M€ CD and 8M€ UC), primary health care (total 10M€; 4M€ CD and 6M€ UC), and emergency room visits (total 6M€; 2M€ CD and 4M€ UC) (Table 2).
Table 2.
Detailed healthcare expenditures for IBD, CD, UC, and non-IBD population in 2020.
| Sanitary resource | IBD population | Non-IBD population | p 1 * | CD | UC | P 2$ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual expenditure | Average per capita | % | Annual expenditure | Average per capita | % | Annual expenditure | Average per capita | % | Annual expenditure | Average per capita | % | |||
| Primary healthcare | 9,750,778 | 230.3 | 5.9 | 6,375,231 | 163.7 | 13 | <0.0001 | 3,630,832 | 255.8 | 4.5 | 6,119,947 | 247.2 | 7.2 | <0.0001 |
| Pharmacy | 100,888,316 | 2589.9 | 61.5 | 18,122,860 | 466.1 | 37.1 | <0.0001 | 55,389,041 | 3902.8 | 68.5 | 45,499,275 | 1837.5 | 54.7 | <0.0001 |
| Hospitalization | 24,157,004 | 620.1 | 14.7 | 12,030,129 | 308.8 | 24.6 | <0.0001 | 9,511,156 | 670.2 | 11.8 | 14,645,848 | 591.5 | 17.6 | <0.0001 |
| Emergency room | 6,092,252 | 156.4 | 3.7 | 3,778,156 | 97 | 7.7 | <0.0001 | 2,237,617 | 157.7 | 2.8 | 3,854,634 | 155.7 | 4.6 | <0.0001 |
| Outpatient visit | 16,014,048 | 411.1 | 9.8 | 4,314,309 | 110.8 | 8.8 | <0.0001 | 7,674,064 | 540.7 | 9.5 | 8,339,984 | 336.8 | 10 | <0.0001 |
| Socio-sanitary | 2,351,141 | 60.4 | 1.4 | 1,458,510 | 37.4 | 3.0 | <0.0001 | 696.583 | 49.1 | 0.9 | 1,654,558 | 66.8 | 2 | <0.0001 |
| Mental health | 1,208,956 | 31 | 0.7 | 96.461 | 23.3 | 1.9 | <0.0001 | 465.132 | 34.2 | 0.6 | 723.824 | 29.2 | 0.9 | <0.0001 |
| Dialysis | 2,008,945 | 51.6 | 1.2 | 1,062,773 | 27.3 | 2.2 | <0.0001 | 721.08 | 50.8 | 0.90 | 1,287,865 | 52 | 1.5 | 0.9376 |
| Sanitary transport | 1,177,870 | 30.2 | 0.7 | 606.338 | 15.6 | 1.2 | <0.0001 | 390.804 | 27.5 | 0.5 | 787.065 | 31.8 | 0.9 | 0.0003 |
| Other | 436.489 | 11.2 | 0.3 | 300.003 | 7.7 | 0.6 | <0.0001 | 144.171 | 10.2 | 0.2 | 292.318 | 11.8 | 0.4 | <0.0001 |
| Total | 164,085,799 | 4212.3 | 100 | 48,987,769 | 1257.6 | 100 | <0.0001 | 80,880,480 | 5.699 | 100 | 83,205,319 | 3360.2 | 100 | <0.0001 |
p1 compares IBD population with non-IBD population.
p2 compares CD with UC population.
CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis.
As regards cost per patient, the average annual expenditure was 4212€ for IBD and 1258€ for non-IBD; thus, a per capita expenditure of 2954€ was attributable to IBD.
Most of the expenditure corresponded to pharmacy, which accounted for 61.5% of the total, followed by hospitalization and outpatient clinic visits (14.7% and 9.8%, respectively: see Table 2 and Figure 1 for details). The annual per capita cost in 2020 for UC was 3360€ and for CD 5699€. The average per capita expenditure for pharmacy was 2590€ (CD 3903€, UC 1838€), followed at some distance by hospitalization (620€; CD 670€, UC 592€). Variables with lower costs were outpatient care, primary health care, and emergency room care.
Figure 1.
Healthcare expenditure distribution of IBD; CD and UC in 2020.
CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Use of health resources
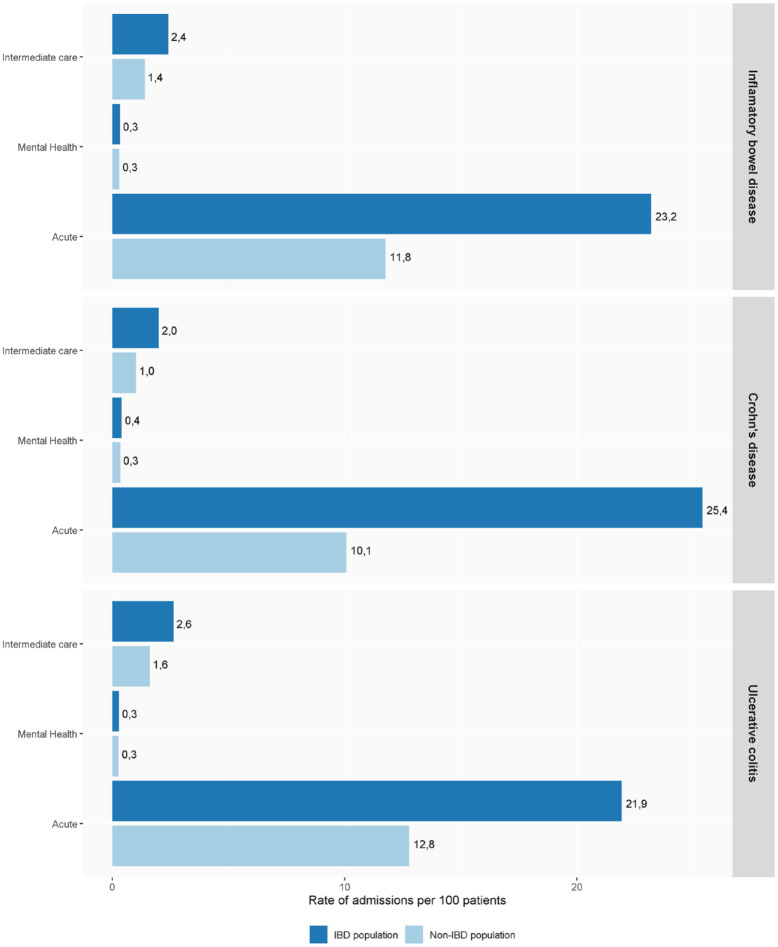
Hospitalization rates in patients with IBD were twice those of the general population (rates per 100 patients of 23.3 versus 11.8; p < 0.05) and of those in intermediate care hospitals (2.4 versus 1.4; p < 0.05). Regarding mental health hospitalization, the use of resources was similar in patients with and without IBD (0.3 versus 0.2 and 0.3, respectively; p < 0.05). See Figure 2 for details.
Figure 2.
Healthcare resource distribution of IBD; CD and UC in 2020.
CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Sensitivity analysis
In 2019 the prevalence of IBD per 100,000 inhabitants was 471.5 (172.9 for CD and 298.5 for UC). Patients with IBD had a global healthcare expenditure of 167M€, more than three times the rate in the non-IBD group (51M€). This means that an expenditure of 116M€ is attributable to IBD. The figures for 2020 were similar. The highest health cost corresponded to pharmacy (95M€), followed by hospitalization (27M€), outpatient care (19M€), primary health care (12M€), and emergency room care (7M€). The data for the 2 years were similar except for a moderate rise in the cost of hospitalization in 2019.
The average annual expenditure cost per patient was 4500€ for IBD and 1400€ for non-IBD, which means that an expenditure of 3000€ per patient was attributable to IBD. This cost also differed between the forms of the disease, at 3553€ for UC and 6021€ for CD. Pharmacy was the variable with the highest per capita cost (total 2562€; CD 3888€, UC 1740€), followed by hospitalization (total 715.5€; CD 795€, UC 669€). Variables with lower costs were outpatient care, primary health care, and emergency room expenses (see Supplemental Table 4 and Supplemental Figures 4 and 5 for details).
Discussion
The present study shows that healthcare expenditure is markedly higher in IBD patients than in their non-IBD counterparts. The average expenditure per patient was approximately 3.4 times higher than in the non-IBD population, representing around 4200€ per patient and year. Pharmacological treatment accounted for more than 60% of the costs. Although patients with IBD presented a moderately higher risk of mortality and more comorbidities than the non-IBD population, comorbidities do not seem to have a major impact on costs.
High healthcare resources use and costs in IBD have also been observed in other countries. In the United States in 2014, Metha 29 estimated direct and indirect costs ranging between 14.6B$ and 31.6B$. In a longitudinal retrospective cohort of 52,782 IBD patients attended privately or with Medicare insurance between 2007 and 2016, Park et al. found the annual cost of IBD patients in United States to be more than three times higher than those of matched non-IBD patients ($22,987 versus $6956: separate data for CD and UC were not provided). Costs were higher in both pediatric and elderly IBD populations than in the middle age group. Moreover, the cost in the first year after diagnosis was very high (>$25,000). The major drivers of costs were hospitalization (40%), emergency room visits (18%), and pharmacotherapy (12%). 11 In a study of 9056 CD and 10,364 UC patients performed in 2003–2004, Kappelman et al. 12 reported mean annual costs of $8265 and $5066 for CD and UC, respectively. The major driver of cost was hospitalization. The per capita cost in our study – 4200€ per patient – was markedly lower than in these US studies just mentioned, probably due to the notable differences in the characteristics of the health systems in the two study sites. 11
Studies elsewhere in Europe have reported similar expenditures to ours. In the Epi-IBD group study, which includes 20 European countries plus Israel, the mean cost of IBD patient per year was 2609€ (8058€ for CD and 2088€ for UC). Interestingly, higher costs were observed during the first 5 years. 30 The Danish study by Lo et al. followed 513 IBD patients from 2003 to 2015. Although the study does not provide a direct estimation of cost per patient in the whole group, the data provided for the different subgroups of patients ranged from 4300€ to 6200€ for CD and from 2200€ to 4000€ per patient and year, figures within the range of the data observed in our study. 31
van Linschoten et al. 32 compared the cost of IBD between continents, reporting considerable variations. In Asian countries, for example, the costs are generally lower. In South Korea, Kim et al. 33 found that the healthcare costs of IBD rose from 23M$ during 2010 to 50M$ during 2014, with a cost per patient of 572$ for IBD (300$ for UC and 1197$ for CD); in India, Kamat et al. 34 reported an annual cost of 778$ for UC and 950$ for CD.
Whereas previous work has attributed the highest proportion of expenditure to hospitalization11,12,35 our data suggest that there is currently a shift in IBD-related costs. For instance, the introduction of biologics has increased the pharmacological cost, which is currently the highest expense. By contrast, hospitalization shows a clear decrease. Our results are in line with the Costs Of Inflammatory bowel disease in the Netherlands (COIN study), which found that healthcare costs are mainly due to medication and that anti-TNFα therapy is the most expensive, while hospitalization and surgery accounted for only a small part of the costs. 36 An interesting unanswered question is whether the total cost has evolved, that is, whether it has risen, fallen, or remained stable.
Indirect costs, such as time lost from work and decreased productivity, could not be estimated in our study due to the characteristics of the database and the source of the data. Although important, these factors have been evaluated only in a few studies. 37 In this regard, employees with IBD have been reported to be 2.5 times more likely to receive short-term disability benefits.38,39 In other studies, by contrast, the differences in indirect costs between CD, UC, and the non-IBD population were non-significant. 31
Regarding comorbidities in IBD, the recent review by Argollo et al. 40 reported an increase in the risk of comorbidities in IBD, including cardiovascular disease, neuropsychological disorders, and metabolic syndrome. Our data are similar, with cardiac diseases, neoplasia, arthritis, and chronic obstructive pulmonary disease being the most frequent comorbidities.
This study has certain limitations. First, the CHSS database includes only patients who use the public health system. This means that, conceivably, some patients with mild disease might not have been recorded. The public system is used by more than 80% of the population of Catalonia; its rate of use is, however, much higher, since the remaining 20% of non-users include healthy individuals who do not use the system but have access to it in case of need. Furthermore, as IBD is a chronic disease and pharmacological treatments are relatively expensive, few private insurance policies cover expensive biologic treatments and most patients requiring biological drugs are controlled in the public system. Thus, the dispensing of biologicals is almost 100% public.
Further limitations include the fact that this study underestimates the real healthcare expenditures of this disease, as we were unable to estimate indirect costs. In addition, data for 2020 may be biased by the COVID-19 pandemics, although the sensitivity analysis comparing the data with those of 2019 showed a similar total expenditure and distribution of costs. Moreover, probably only a portion of differences in expenditures between IBD patients and controls are due to the IBD itself; as mentioned, IBD patients have more comorbidities and may have extra-intestinal manifestations, which may also increase the patients’ expenses. Another limitation is that it is not possible to exactly determine the cost of outpatient medications for the social security system because in Catalonia there is a co-payment for these medications and the payment percentage by the patients changes according their socioeconomic status.
As noted above, the evolution of total cost over time and the impact of new therapies should be further analyzed in future studies. This study evaluates only 1 year and it would be interesting to assess the trends in healthcare expenditure over a longer period. Few data are available on this issue, but the study by Click et al. 41 showed that total health care expenditure in the United States nearly doubled between 1998 and 2015. It might be of interest to compare the absolute cost of pharmacotherapy across countries and the proportion that drugs represent inside the total cost per patient. Costs of healthcare vary substantially depending on gross domestic product, but pharmaceutical companies try to keep prices consistent across countries. Thus, the burden that drugs may represent as compared to the overall costs of healthcare in low-income countries may divert investment from other forms of healthcare, leading to inequities in the use of resources and in access to effective treatments.
Furthermore, our data provide an accurate estimation of the direct cost of IBD. Taking into account data on cost estimation and the probable increases in the incidence and prevalence of IBD and in the use of biological drugs shown in previous studies,13,14 our data may be very useful for planning IBD care in the years to come.
In conclusion, per capita healthcare expenditure in IBD patients in 2020 in Catalonia was approximately 3.4 times higher than in the non-IBD population. IBD patients also presented a higher prevalence of comorbidities (1.5–2 higher) and greater use of healthcare resources than the population without IBD.
Supplemental Material
Supplemental material, sj-png-1-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Supplemental material, sj-png-2-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Supplemental material, sj-png-3-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Supplemental material, sj-png-4-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Supplemental material, sj-png-5-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Acknowledgments
We thank Michael Maudsley for his help with the English.
Footnotes
ORCID iDs: Belen Garcia-Sagué  https://orcid.org/0000-0001-6501-1227
https://orcid.org/0000-0001-6501-1227
Xavier Calvet  https://orcid.org/0000-0002-6278-9663
https://orcid.org/0000-0002-6278-9663
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Eduard Brunet-Mas, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain; CIBERehd, Instituto de Salud Carlos III, Madrid, Spain.
Belen Garcia-Sagué, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain.
Emli Vela, Unitat d’Informació i Coneixement, Servei Català de la Salut, Generalitat de Catalunya, Barcelona, Spain Digitalization for the Sustainability of the Healthcare System (DS3), Bellvitge Biomedical Research Institute (IDIBELL), Barcelona, Spain.
Luigi Melcarne, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain.
Laura Patricia Llovet, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain.
Caridad Pontes, Digitalization for the Sustainability of the Healthcare System (DS3), Bellvitge Biomedical Research Institute (IDIBELL), Barcelona, Spain; Àrea Assistencial, Servei Català de la Salut, Generalitat de Catalunya.
Pilar García-Iglesias, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain.
Anna Puy, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain.
Sergio Lario, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain; CIBERehd, Instituto de Salud Carlos III, Madrid, Spain.
Maria Jose Ramirez-Lazaro, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain; CIBERehd, Instituto de Salud Carlos III, Madrid, Spain.
Albert Villoria, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona Sabadell, Catalunya, Spain; CIBERehd, Instituto de Salud Carlos III, Madrid, Spain.
Johan Burisch, Gastrounit, Medical Division, University Hospital Copenhagen – Amager and Hvidovre, Hvidovre, Denmark; Copenhagen Center for Inflammatory Bowel Disease in Children, Adolescents and Adults, University Hospital Copenhagen – Amager and Hvidovre, Hvidovre, Denmark.
Gilaad G. Kaplan, Departments of Medicine and Community Health Sciences, University of Calgary, Calgary, AB, Canada
Xavier Calvet, Servei d’Aparell Digestiu, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Departament de Medicina, Universitat Autònoma de Barcelona Sabadell, Catalunya, Spain; CIBERehd, Instituto de Salud Carlos III, Madrid, Spain; Departament de Medicina, Universitat Autònoma de Barcelona, Sabadell, Spain.
Declarations
Ethics approval and consent to participate: Local ethics committee of the Hospital Universitari Parc Taulí in Sabadell (CEIm 2022/5038). Owing to the use of an electronic database as the data source and the irreversible anonymization of the data extracted, patient-informed consent was not required for this study.
Consent for publication: Not applicable.
Author contributions: Eduard Brunet-Mas: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Belen Garcia-Sagué: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Emli Vela: Data curation; Formal analysis; Methodology; Resources; Writing – review & editing.
Luigi Melcarne: Writing – review & editing.
Laura Patricia Llovet: Writing – review & editing.
Caridad Pontes: Writing – review & editing.
Pilar García-Iglesias: Writing – review & editing.
Anna Puy: Writing – review & editing.
Sergio Lario: Writing – review & editing.
Maria Jose Ramirez-Lazaro: Writing – review & editing.
Albert Villoria: Writing – review & editing.
Johan Burisch: Writing – review & editing.
Gilaad G. Kaplan: Writing – review & editing.
Xavier Calvet: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
AV has served as a speaker and consultant from for MSD and Abbvie. EB-M has served as a speaker and consultant for Janssen and Chiesi. LM has served as a speaker and consultant from for MSD, Abbvie, Janssen, Takeda, Pfizer, and Tillots. BG-S, EV, LPL, CP, PG-I, AP, SL, and MJR-L have no conflicts of interest to declare. JB reports personal fees from AbbVie, grants and personal fees from Janssen-Cilag, personal fees from Celgene, grants and personal fees from MSD, personal fees from Pfizer, grants and personal fees from Takeda, grants and personal fees from Tillots Pharma, personal fees from Samsung Bioepis, grants and personal fees from Bristol Myers Squibb, grants from Novo Nordisk, personal fees from Pharmacosmos, personal fees from Ferring, personal fees from Galapagos. GGK has received honoraria for speaking or consultancy from Abbvie, Janssen, Pfizer, Amgen, and Takeda. He has received research support from Ferring, Janssen, Abbvie, GlaxoSmithKline, Merck, and Shire. He has been a consultant for Gilead. He shares ownership of a patent: Treatment of Inflammatory Disorders, Autoimmune Disease, and PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 September 2018. XC has received grants for research from Abbott, MSD, and Vifor, and fees for advisory board services from Abbott, MSD, Takeda, and Vifor. He has also given lectures for Abbott, MSD, Takeda, Shire, and Allergan.
Availability of data and materials: For ethical/privacy reasons the data cannot be shared publicly because they come from a population registry supervised by the Catalan Health Surveillance System and can only be accessed from CatSalut. The data will be shared on reasonable request made to the corresponding author.
References
- 1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009; 361: 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohn’s Colitis 2013; 7: 322–337. [DOI] [PubMed] [Google Scholar]
- 3. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017; 390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 4. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012; 380: 1590–1605. [DOI] [PubMed] [Google Scholar]
- 5. Golovics PA, Mandel MD, Lovasz BD, et al. Inflammatory bowel disease course in Crohn’s disease: is the natural history changing? World J Gastroenterol 2014; 20: 3198–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013; 145: 996–1006. [DOI] [PubMed] [Google Scholar]
- 7. He Y, Mao R, Yuan G, et al. The hospitalization burden of inflammatory bowel disease in China: a nationwide study from 2013 to 2018. Therap Adv Gastroenterol 2022; 15: 17562848221102307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohn’s Colitis 2022; 16: 2–17. [DOI] [PubMed] [Google Scholar]
- 9. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohn’s Colitis 2020; 14: 4–22. [DOI] [PubMed] [Google Scholar]
- 10. Burisch J, Zhao M, Odes S, et al. The cost of inflammatory bowel disease in high-income settings: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2023; 8: 458–492. [DOI] [PubMed] [Google Scholar]
- 11. Park KT, Ehrlich OG, Allen JI, et al. The cost of inflammatory bowel disease: an initiative from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis 2020; 26: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology 2008; 135: 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brunet E, Roig-Ramos C, Vela E, et al. Prevalence, incidence and mortality of inflammatory bowel disease in Catalonia: a population-based analysis. Ann Med 2018; 50: 613–619. [DOI] [PubMed] [Google Scholar]
- 14. Brunet E, Vela E, Melcarne L, et al. Time trends of Crohn’s disease in Catalonia from 2011 to 2017. Increasing use of biologics correlates with a reduced need for surgery. J Clin Med 2020; 9: 2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farré N, Vela E, Clèries M, et al. Real world heart failure epidemiology and outcome: a population-based analysis of 88,195 patients. PLoS One 2017; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cancio JM, Vela E, Santaeugènia S, et al. Long-term impact of hip fracture on the use of healthcare resources: a population-based study. J Am Med Dir Assoc 2019; 20: 456–461. [DOI] [PubMed] [Google Scholar]
- 17. Vela E, Carot-Sans G, Clèries M, et al. Development and validation of a population-based risk stratification model for severe COVID-19 in the general population. Sci Rep 2022; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Population on 1 January. Provinces. IDESCAT: web de la estadıstica ofical de Catalunya. Generalitat de Catalunya, Institud de Estadıstica de Catalunya, https://www.idescat.cat/pub/?id=aec&n=245&lang=es (2020). [Google Scholar]
- 19. Bilal U, Cainzos-Achirica M, Cleries M, et al. Socioeconomic status, life expectancy and mortality in a universal healthcare setting: an individual-level analysis of >6 million Catalan residents. Prev Med (Baltimore) 2019; 123: 91–94. [DOI] [PubMed] [Google Scholar]
- 20. Monterde D, Vela E, Clèries M, et al. Multimorbidity as a predictor of health service utilization in primary care: a registry-based study of the Catalan population. BMC Fam Pract 2020; 21: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vela E, Clèries M, Monterde D, et al. Performance of quantitative measures of multimorbidity: a population-based retrospective analysis. BMC Public Health 2021; 21: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monterde D, Vela E, Clèries M. Los grupos de morbilidad ajustados: nuevo agrupador de morbilidad poblacional de utilidad en el ámbito de la atención primaria. Aten Primaria 2016; 48: 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miquel M, Clèries M, Vergara M, et al. Economic burden of cirrhosis in Catalonia: a population-based analysis. BMJ Open 2018; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Espin ID, Vela E, Pauws S, et al. Proposals for enhanced health risk assessment and stratification in an integrated care scenario. BMJ Open 2016; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vela E, Clèries M, Alberto V, et al. Population-based analysis of the healthcare expenditure in Catalonia (Spain): what and who consumes more resources? Gac Sanit 2019; 33: 24–31. [DOI] [PubMed] [Google Scholar]
- 26. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 27. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 2007; 85: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care 2016; 22: s51–60. [PubMed] [Google Scholar]
- 30. Burisch J, Vardi H, Schwartz D, et al. Health-care costs of inflammatory bowel disease in a pan-European, community-based, inception cohort during 5 years of follow-up: a population-based study. Lancet Gastroenterol Hepatol 2020; 5: 454–464. [DOI] [PubMed] [Google Scholar]
- 31. Lo B, Vind I, Vester-Andersen MK, et al. Direct and indirect costs of inflammatory bowel disease: ten years of follow-up in a danish population-based inception cohort. J Crohn’s Colitis 2020; 14: 53–63. [DOI] [PubMed] [Google Scholar]
- 32. van Linschoten RCA, Visser E, Niehot CD, et al. Systematic review: societal cost of illness of inflammatory bowel disease is increasing due to biologics and varies between continents. Aliment Pharmacol Ther 2021; 54: 234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim JW, Lee CK, Rhee SY, et al. Trends in health-care costs and utilization for inflammatory bowel disease from 2010 to 2014 in Korea: a nationwide population-based study. J Gastroenterol Hepatol 2018; 33: 847–854. [DOI] [PubMed] [Google Scholar]
- 34. Kamat N, Ganesh Pai C, Surulivel Rajan M, et al. Cost of illness in inflammatory bowel disease. Dig Dis Sci 2017; 62: 2318–2326. [DOI] [PubMed] [Google Scholar]
- 35. Rao BB, Click BH, Koustroubakis IE, et al. The cost of Crohn’s disease: varied healthcare expenditure patterns across distinct disease trajectories. Inflamm Bowel Dis 2017; 23: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Der Valk ME, Mangen MJJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut 2014; 63: 72–79. [DOI] [PubMed] [Google Scholar]
- 37. Bloomfeld RS, Bickston SJ. Financial toxicity in people with inflammatory bowel disease. Inflamm Bowel Dis 2021; 27: 1170–1171. [DOI] [PubMed] [Google Scholar]
- 38. Cohen R, Skup M, Ozbay AB, et al. Direct and indirect healthcare resource utilization and costs associated with ulcerative colitis in a privately-insured employed population in the US. J Med Econ 2015; 18: 447–456. [DOI] [PubMed] [Google Scholar]
- 39. Gibson TB, Ng E, Ozminkowski RJ, et al. The direct and indirect cost burden of Crohn’s disease and ulcerative colitis. J Occup Environ Med 2008; 50: 1261–1272. [DOI] [PubMed] [Google Scholar]
- 40. Argollo M, Gilardi D, Peyrin-Biroulet C, et al. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol Hepatol 2019; 4: 643–654. [DOI] [PubMed] [Google Scholar]
- 41. Click B, Lopez R, Arrigain S, et al. Shifting cost-drivers of health care expenditures in inflammatory bowel disease. Inflamm Bowel Dis 2020; 26: 1268–1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-png-1-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Supplemental material, sj-png-2-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Supplemental material, sj-png-3-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Supplemental material, sj-png-4-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology
Supplemental material, sj-png-5-tag-10.1177_17562848231222344 for Economic impact of inflammatory bowel disease in Catalonia: a population-based analysis by Eduard Brunet-Mas, Belen Garcia-Sagué, Emli Vela, Luigi Melcarne, Laura Patricia Llovet, Caridad Pontes, Pilar García-Iglesias, Anna Puy, Sergio Lario, Maria Jose Ramirez-Lazaro, Albert Villoria, Johan Burisch, Gilaad G. Kaplan and Xavier Calvet in Therapeutic Advances in Gastroenterology