Abstract
Gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-10 (IL-10) production by liver, spleen, lung, peripheral blood mononuclear cells (MNC), and peritoneal exudate cells (PEC) in experimental bacterial peritonitis was examined by cecum ligation and puncture (CLP) (with an 18-gauge needle) of BALB/c mice. MNC of organs were cultured for 18 h, and cytokine levels in supernatants were examined. Cytokines contained in peritoneal lavage fluid were regarded as those produced by PEC. Only liver MNC and PEC produced substantial amounts of IFN-γ, and PEC were the main source of IL-10, especially 12 h after CLP. As reflected by the cytokine production by liver MNC and PEC, serum IFN-γ and IL-10 levels were elevated after CLP. C57BL/6 (B6) mice and BALB/c nude mice showed a similar pattern of cytokine production. TNF-α levels in culture supernatants, peritoneal lavage fluid, and sera were not significantly elevated compared to those of sham-operated mice. In vivo depletion of NK cells of B6 mice with anti-asialo GM1 or anti-NK1.1 antibody greatly decreased IFN-γ levels in liver MNC culture supernatants and sera, suggesting that liver NK cells are IFN-γ producers. On the other hand, plastic-adherent PEC macrophages are the major IL-10 producers. Mice subjected to a cecum ligation and cut procedure (which have a more severe peritonitis) showed much higher IFN-γ and IL-10 levels than those subjected to CLP, while mice subjected to CLP with a smaller (22-gauge) needle showed low levels of these cytokines. These findings show that liver NK cells and PEC macrophages are important for the production of proinflammatory and anti-inflammatory cytokines in bacterial peritonitis.
Recent reports indicate that livers of adult mice and humans contain not only a large population of NK cells but also many T cells with NK-cell markers (12, 24, 29, 30, 34). In addition, livers of adult mice contain c-kit+ pluripotent stem cells (35, 40) that give rise to multilineage cells, indicating that adult liver is still an important hematopoietic and immunocompetent organ. It has been recently demonstrated in mice that lipopolysaccharide (LPS) (endotoxin) activates liver NK cells and NK1.1+ T cells via interleukin-12 (IL-12) production from Kupffer cells (33). IL-12 and gamma interferon (IFN-γ) produced by NK cells as well as NK1.1+ T cells are responsible for inducing antitumor cytotoxicity of these cells (12, 23, 33, 34). Further, the liver is well known as an organ which produces C-reactive protein in inflammations (13).
Several researchers examined cytokine production in mice subjected to cecum ligation and puncture (CLP mice) because CLP mice can be a model of severe bacterial infection and bacterial peritonitis (2, 6, 38). IFN-γ is a representative of the T helper type 1 (Th1) cytokines produced by NK cells, conventional T cells, and NK1.1+ T cells (1, 18, 23, 36). On the other hand, IL-10 is one of the representatives of Th2 cytokines produced by macrophages and T cells (5, 7, 8, 14, 19, 20). Th1 cytokines trigger an inflammatory immune response, and Th2 cytokines counterregulate Th1 cytokines (5, 7, 19, 26) and inhibit inflammation beyond the maximum phase into the recovery phase. However, there has been no report in which the production of IFN-γ and IL-10 in mice with bacterial peritonitis was systematically examined. The present study is designed to clarify the organs and leukocyte populations responsible for the production of these cytokines in CLP mice. We demonstrate that liver NK cells and macrophages from peritoneal exudate cells (PEC) produce IFN-γ and IL-10, respectively, and systemic levels of these cytokines are likely to reflect cytokine production from these cells, suggesting that these cells play a role in modulating inflammation of bacterial peritonitis.
MATERIALS AND METHODS
Mice.
Male BALB/c mice, BALB/c nu/nu mice, and C57BL/6 (B6) mice 6 to 8 weeks of age were purchased from Japan SLC Inc., Hamamatsu, Japan. Mice were fed under specific-pathogen-free conditions.
CLP and CLC procedures.
CLP was performed essentially as previously described (2). Briefly, after intraperitoneal pentobarbital anesthetization, the anterior abdominal walls of the mice were shaved and a small incision was made to expose the cecum, which was ligated at its base with 3.0 silk. The cecum was punctured through once with an 18-gauge needle, and a small volume of feces was placed on the exterior. In some experiments, the CLP was made with a 22-gauge needle. The cecum was then returned to the peritoneal cavity, and the abdomen was closed (CLP procedure). Sham-operated mice underwent the same procedure without ligation and puncture of the cecum. In other experiments (cecal ligation and cut [CLC] procedure), after ligation of the cecum at its base, the cecum was cut and resected at the end. This procedure produced a more severe peritonitis in the mice.
Isolation of MNC.
Under ether anesthesia, mice were bled from the subclavian artery and vein to obtain sera. The livers were removed from the mice. Hepatic mononuclear cells (MNC) were prepared essentially as previously described (33). Briefly, the liver was passed through stainless steel mesh and suspended in Hanks balanced salt solution. After one washing, liver MNC were isolated from hepatocytes, nuclei of hepatocytes, and Kupffer cells with an osmolarity- and pH-adjusted 33% Percoll solution (Sigma, St. Louis, Mo.) containing 100 U of heparin per ml (centrifuged at 500 × g for 15 min at room temperature). The pellet was resuspended in erythrocyte lysis solution (0.17 mM NH4Cl, 0.01 mM EDTA, 0.1 M Tris, pH 7.3) and then washed twice in 10% fetal calf serum (FCS)–RPMI 1640 medium. The spleen was pressed through a stainless steel mesh, and MNC were obtained after lysing of erythrocytes. Peripheral blood MNC were obtained from heparinized blood by Ficol-Hypaque density gradient centrifugation. MNC (2.5 × 106) were incubated for 18 h in 1 ml of 10% FCS–RPMI (penicillin and streptomycin included) for 18 h in 24-well flat-bottomed plates, and supernatants were subjected to enzyme-linked immunosorbent assay (ELISA).
For lung MNC, lung was minced, suspended in 15 ml of medium containing 0.05% collagenase (Wako, Tokyo, Japan) and 0.01% trypsin inhibitor (Sigma), and shaken for 20 min in a 37°C water bath. Thereafter, lung tissues were pressed through stainless steel mesh, and MNC were obtained with a heparin-containing Percoll solution as described above.
Collection of PLF.
To examine cytokine production by PEC, 2 ml of phosphate-buffered saline (PBS) was intraperitoneally injected into CLP mice, a small incision was made in the abdominal wall, and peritoneal lavage fluid (PLF) was collected with a syringe. After centrifugation, supernatants of PLF were pooled for ELISA.
Isolation of plastic-adherent PEC and nonadherent PEC.
PLF was obtained by injection of 2 ml of 10% FCS–RPMI 1640 after CLP and passed through nylon mesh. PLF was then incubated in a 24-well flat-bottomed plate for 2 h at 37°C in 5% CO2. After gentle pipetting to collect nonadherent cells, adherent cells and nonadherent cells were subjected to culture for 8 h and culture supernatants were subjected to ELISA.
Isolation of Kupffer cells.
The liver was minced, treated with 5 ml of Dispase (1,000 U/ml) for 2 h at 37°C, and washed twice in PBS. Hepatocytes were partly removed by centrifugation at 500 rpm (125 × g) for 5 min. After lysing of erythrocytes, hepatic MNC were suspended in 10 ml of 10% FCS–RPMI 1640 and incubated in collagen-coated plastic dishes (Falcon) for 2 h at 37°C in 5% CO2, and then nonadherent cells were gently removed and adherent Kupffer cells were obtained with a cell scraper.
In vivo cell depletion.
Monoclonal mouse anti-NK1.1 antibody (Ab) (PK136) (200 μg/mouse) or polyclonal rabbit anti-asialo GM1 (anti-AGM1) Ab (50 μg/mouse) was injected into B6 mice twice a week before CLP in order to eliminate NK-type cells (37). Polyclonal rabbit anti-AGM1 Ab was purchased from Wako. The PK136 hybridoma was grown in our laboratory. Depletion of NK1.1+ T cells was confirmed not only by direct determination (37) but also by the deletion of Vα14 T-cell receptor mRNA (which is specific for NK1.1+ T cells [37]).
Assays for serum IFN-γ, tumor necrosis factor alpha (TNF-α), and IL-10 levels.
Cytokine levels were evaluated by using the cytokine-specific ELISA commercially available from Endogen, Inc. (Boston, Mass.).
Statistical analysis.
Differences between groups were analyzed by the Mann-Whitney U test. Differences were considered significant if P was <0.05.
RESULTS
Liver MNC produce IFN-γ, and PEC produce IL-10.
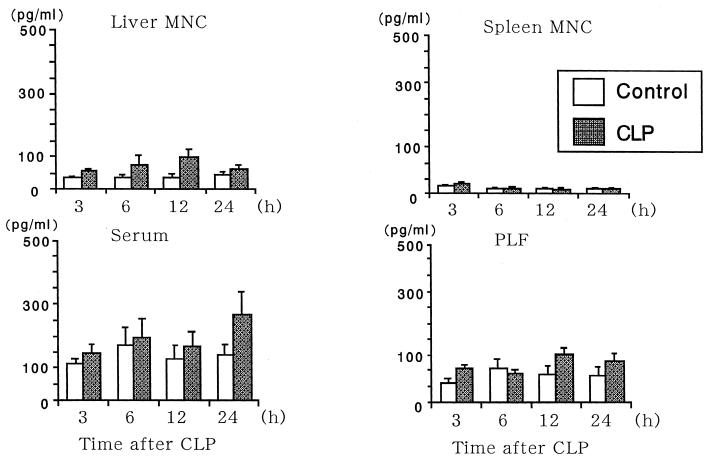
Liver MNC of BALB/c mice, especially those from mice 12 h after CLP, produced a large amount of IFN-γ in vitro, while splenocytes did not (Fig. 1). Serum IFN-γ levels in CLP mice also reached a peak 12 h after CLP. PEC also produced a substantial amount of IFN-γ (Fig. 1). However, TNF-α levels had a tendency to increase after CLP, but the increases were not statistically significant compared to results for sham operations (Fig. 2). PEC produced a large amount of IL-10, and liver MNC produced a small but significant amount of IL-10, while spleen MNC did not produce a significant amount of IL-10 (Fig. 3). Serum IL-10 also greatly increased (Fig. 3). Although serum IL-10 levels were still high 24 h after CLP, the levels approached basal levels by 48 h after CLP (not shown). These results were confirmed further with B6 mice. Data from five B6 mice are shown in Fig. 4.
FIG. 1.
Time course of IFN-γ production by MNC and PEC (PLF) and levels of IFN-γ in serum. Four to eight individual mice of each group (at least two independent experiments) were examined at the indicated time points. Data are means ± standard errors.
FIG. 2.
Time course of TNF-α production by MNC and levels of TNF-α in serum. Four to eight individual mice of each group were examined at the indicated time points. Data are means ± standard errors.
FIG. 3.
Time course of IL-10 production by MNC and levels of IL-10 in serum. Four to eight individual mice of each group were examined at the indicated time points. Data are means ± standard errors.
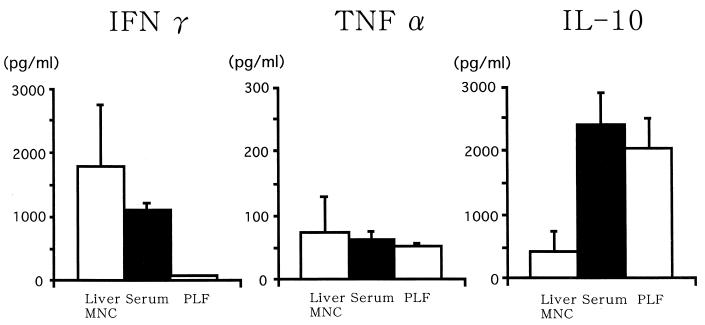
FIG. 4.
Cytokine production by MNC and serum cytokine levels for B6 mice 12 h after CLP. Data are means and standard errors.
Nude mice also produce IFN-γ and IL-10.
Liver MNC of athymic BALB/c nude mice also produced IFN-γ, and PEC produced IL-10 (Fig. 5), suggesting that thymus-derived T cells are not the major IFN-γ and IL-10 producers. However, in contrast to the case for normal mice, serum IFN-γ levels were elevated even in nude mice that had the sham operation and did not significantly differ from those in CLP mice.
FIG. 5.
Cytokine production by MNC and serum cytokine levels for athymic BALB/c nu/nu mice 6 h after CLP. Five individual mice were examined. Data are means and standard errors.
Serum IFN-γ and IL-10 levels depend on the severity of peritonitis.
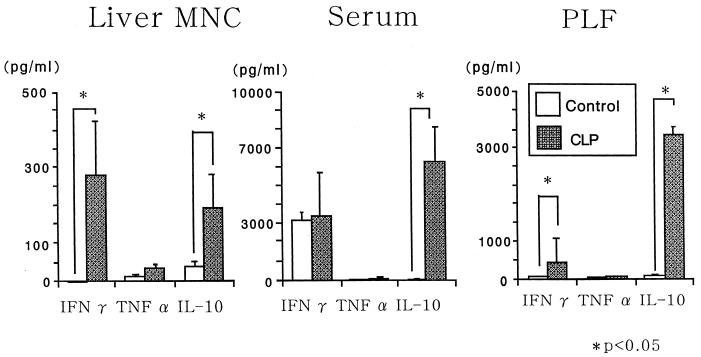
We first compared CLP mice with CLC mice. Since some CLC mice died within 12 h after CLC and most CLC mice died within 24 h, we compared the groups of mice at 6 h after CLP or CLC. CLC mice showed significantly higher serum IFN-γ and IL-10 levels than CLP mice (Fig. 6). Although IFN-γ production by liver MNC of CLC mice was also higher than that by liver MNC of CLP mice, IL-10 levels in PLF were not significantly different between CLP mice and CLC mice (Fig. 6). Subsequently, we compared cytokine production by CLP mice with the puncture made by an 18-gauge needle with that by CLP mice with the puncture made by a 22-gauge needle at 12 h after CLP. As expected, MNC of CLP mice with the puncture made by a 22-gauge needle produce small amounts of cytokines (Fig. 7).
FIG. 6.
Comparison of cytokine levels in CLP mice and CLC mice. Six individual BALB/c mice of each group were examined 6 h after the operation. Data are means and standard errors.
FIG. 7.
Comparison of cytokine levels in BALB/c CLP mice with a large-diameter puncture (18-gauge needle [18G]) and with a small-diameter puncture (22-gauge needle). Four individual mice of each group were examined. Data are means and standard errors.
Liver NK cells are responsible for IFN-γ production, but NK-cell depletion did not affect mouse mortality.
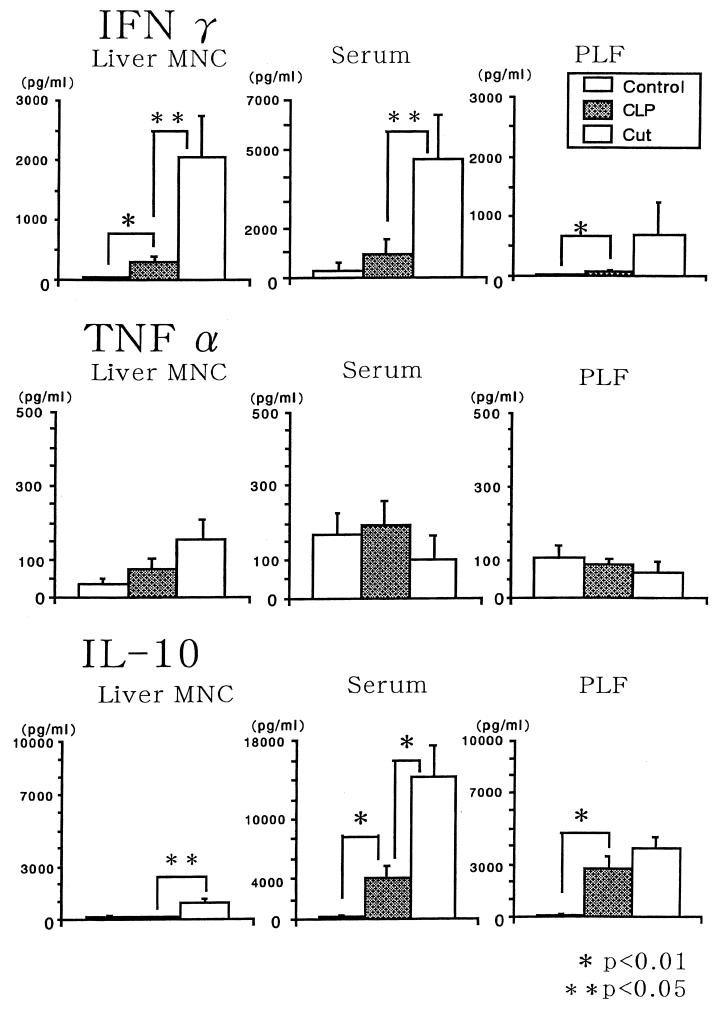
We recently reported that anti-AGM1 Ab treatment of B6 mice in vivo depletes only liver NK cells, while anti-NK1.1 Ab treatment depletes both liver NK cells and NK1.1+ T cells with intermediate T-cell receptor (37). Since NK1.1 can be detected in only some strains of mice, such as B6 and B10 mice, and most other strains lack NK1.1 antigen (Ag) (no Ab is available to detect a counterpart of NK1.1 Ag), we used B6 mice. Because both NK cells and NK1.1+ T cells produce IFN-γ by NK1.1 Ag cross-linking (1) and NK1.1+ T cells produce a larger amount of IFN-γ than do NK cells by the stimulation of IL-12 (23), we tried to determine which type of cells in the livers of CLP mice produce IFN-γ. The results showed that either anti-AGM1 Ab or anti-NK1.1 Ab pretreatment of B6 CLP mice similarly decreased IFN-γ levels in liver MNC supernatants and sera (Fig. 8), suggesting that liver NK cells but not NK1.1+ T cells are the main IFN-γ producers in this model. Together with the results for nude mice (Fig. 4), this suggested that NK cells but neither conventional T cells nor NK1.1+ T cells are the main IFN-γ producers. However, the survival rate of BALB/c CLP mice depleted of NK cells by anti-AGM1 Ab treatment was not significantly different from that of control BALB/c CLP mice; within 48 h, 6 of 22 CLP mice and 6 of 20 NK-cell-depleted CLP mice died. On the other hand, six CLP mice with the puncture made by a 22-gauge needle were monitored, and all were alive after 48 h.
FIG. 8.
Effect of anti-AGM1 Ab or anti-NK1 Ab pretreatment in vivo on IFN-γ and IL-10 production in B6 mice. Mice were intraperitoneally injected twice with either Ab (or with PBS as a control) before CLP (4 and 2 days before CLP), and cytokine levels in liver MNC supernatants and sera were examined 12 h after CLP. Five individual mice of each group were examined. Data are means and standard errors.
Plastic-adherent PEC are producers of IL-10.
Since PEC of CLP mice contain a large population of granulocytes and smaller populations of lymphocytes and macrophages (not shown), we examined which subset produces IL-10. At 5 h after CLP, PLF was obtained, both plastic-adherent PEC and nonadherent PEC were cultured for 8 h, and IL-10 levels in culture supernatants were examined. The results show that adherent PEC produce a larger amount of IL-10 than do nonadherent PEC (Fig. 9), indicating that PEC macrophages are a main population responsible for IL-10 production. In contrast, plastic-adherent liver MNC (Kupffer cells) did not produce IL-10 (not shown), suggesting that IL-10 in liver MNC culture supernatants was produced by T cells.
FIG. 9.
Plastic-adherent PEC are the main producers of IL-10. At 5 h after CLP, PLF was obtained, adherent and nonadherent cells were cultured in 1 ml of 10% FCS–RPMI for 8 h, and IL-10 levels in supernatants were examined. Data are means ± standard errors for four individual CLP mice.
Peripheral blood MNC and lung MNC of CLP mice do not produce significant amounts of cytokines.
Levels of cytokine production by peripheral blood MNC and lung MNC were examined. Neither produced significant amounts of IFN-γ, TNF-α, or IL-10, although lung MNC produced a small amount of IL-10 (Table 1).
TABLE 1.
Cytokine production by lung and peripheral blood MNCa
| MNC | Expt no. | Concn (pg/ml)
|
||
|---|---|---|---|---|
| IFN-γ | TNF-α | IL-10 | ||
| Lung | ||||
| Control | 1 | 9.3 | 12.5 | 85.1 |
| 2 | 4.4 | 12.2 | 252.7 | |
| CLP | 1 | 0 | 14.3 | 30.5 |
| 2 | 9.0 | 4.7 | 180.4 | |
| Peripheral blood (CLP)b | 1 | 0 | 17.2 | 0 |
| 2 | 0 | 18.4 | 0 | |
| 3 | 0 | 19.8 | 0 | |
| 4 | 0 | 20.6 | 0 | |
Mice were examined 12 h after CLP.
Control peripheral blood MNC were not examined.
DISCUSSION
In the present study, we demonstrated that liver NK cells produce a Th1-type cytokine, IFN-γ; that PEC macrophages produce a Th2-type cytokine, IL-10; and that both play an important role in the cytokine cascade of experimental bacterial peritonitis. In contrast, splenocytes, lung MNC, and peripheral blood MNC did not produce significant amounts of these cytokines. Although TNF-α production by MNC of the organs tested had a tendency to increase after CLP, the increases were not statistically significant compared to results for control mice. The amounts of IFN-γ and IL-10 correlate with the severity of inflammation, whereas it was also found that reduction of IFN-γ production by NK-cell depletion resulted in an augmentation of IL-10 production, suggesting that IFN-γ and IL-10 cross-regulate each other. However, NK-cell depletion did not significantly affect the mortality of CLP mice.
NK cells produce IFN-γ when stimulated with IL-12 (1, 18, 25) or IL-18 (IFN-γ-inducing factor) (25), both of which are produced by monocyte lineage cells stimulated with bacterial Ags or bacterial superantigens (25, 36). Thus, IFN-γ is important not only for antitumor immunity but also for antibacterial immunity (36). However, IFN-γ together with TNF-α is also reportedly crucial for endotoxin-induced lethal shock syndrome, known as the generalized Shwartzman reaction, suggesting that uncontrolled exaggeration of this innate response sometimes causes multiorgan failure or shock (23, 28, 36, 41). On the other hand, IL-10 is reported to antagonize Th1 cytokines (including IFN-γ) and counterregulate inflammation of bacterial infection (5, 7, 8, 14, 19, 20, 38). IL-10 is also reported to be important to decrease lethality in mouse endotoxemia and bacterial infection (including CLP), because exogenous and endogenous IL-10 increases resistance in these mice (14, 19, 39).
Our results are consistent with a previous report that the severity of inflammation in CLP mice depends on the diameter of the needle used for CLP (39). Nevertheless, in contrast to our present results, that report demonstrated that the serum IL-10 concentration was greater in the group with the small-diameter cecal puncture than in those with intermediate- and large-diameter punctures (39). The reason for this discrepancy is unknown at present. However, it was reported that the elevation of serum IL-10 was associated with the development of sepsis in trauma patients (31), and we also recently found that patients with severe bacterial peritonitis with septic shock or multiorgan failure have higher levels of IL-10 than patients with less severe bacterial peritonitis (27).
Although IFN-γ and IL-10 normally antagonize each other (5, 7, 19), our findings indicate that both Th1 and Th2 cytokines could be produced simultaneously in acute inflammations. In addition, it should be noted that although IL-10 is usually considered an anti-inflammatory cytokine in bacterial infections, a recent study showed that IL-10 enhanced macrophage colony-stimulating factor-induced growth and functions of macrophages, including phagocytosis and H2O2 production (11). These findings suggest that IL-10 is protective against bacterial infection not only because it is an anti-inflammatory cytokine but also because it can be positively involved in inflammation. A possibility that the role of IL-10 in bacterial infections or its interaction with proinflammatory cytokines could be more complex than previously expected is also raised.
Of interest is that IL-10 is produced mainly in situ by PEC macrophages, whereas IFN-γ is produced mainly by liver NK cells. Since we recently reported that mouse Kupffer cells produce IL-12 after intraperitoneal LPS injection (33), bacterial peritonitis may induce production of IFN-γ from liver NK cells by stimulating Kupffer cells. It was also recently reported that intravenous Escherichia coli injection induces elevation of serum IL-12, IFN-γ, and IL-10 in baboons (15). Since Kupffer cells do not produce IL-10 in CLP mice, it seems likely that bacterial stimulation differentially stimulates monocyte lineage cells, PEC macrophages, and Kupffer cells. In an earlier study (38), the failure to detect plasma IFN-γ in CLP mice was probably a function of the source of the ELISA kit.
Splenocytes and MNC of organs other than the liver do not produce the cytokines tested. It is possible that bacterial Ags or components may be preferentially brought to the liver, because approximately 70% of monocyte lineage cells of mammals reside in the liver as Kupffer cells (4, 9, 21). In fact, most bacteria that enter the bloodstream are trapped by Kupffer cells and are thereby removed from the blood (4, 9, 21). In addition, substantial amounts of bacterial Ags, including LPS or peptidoglycan polysaccharides, are continuously brought from the intestine to the liver (16, 17, 22). It was reported that LPS priming of lymphocytes or leukocytes augments their response to subsequent bacterial stimulation (17, 32). Thus, it is conceivable that liver MNC are already presensitized and respond immediately and vigorously to bacterial infection. Of course, we do not deny that splenocytes can produce cytokines; it is known that splenocytes respond to mitogens and produce cytokines, and splenocytes stimulated with LPS or bacterial superantigens in vitro produce a substantial amount of IFN-γ (10). These findings, however, suggest that in vitro experiments sometimes do not reflect immunological events occurring in vivo. We propose that liver is a peculiar organ which is prepared to promptly trigger Th1 immune response. The production of C-reactive protein from hepatocytes (13) in inflammation supports our proposal.
We recently found that liver NK1.1+ T cells are more potent IFN-γ producers than NK cells after in vivo IL-12 stimulation (23). However, the present results suggest that NK cells are the main IFN-γ producers in bacterial peritonitis, because both anti-AGM1 Ab and anti-NK1.1 Ab similarly inhibit IFN-γ production. It is suggested that different Ags or factors may preferentially stimulate distinct lymphocyte populations in the liver to produce IFN-γ. Our results also revealed that conventional thymus-derived T cells are not the main IFN-γ producers in bacterial peritonitis.
Although liver NK cells seemed to be important to induce the Th1 immune response in bacterial peritonitis, NK-cell depletion in CLP mice did not significantly affect mouse mortality. This finding suggests that cells other than NK cells (including macrophages and granulocytes) and cytokines other than IFN-γ are important for the first defense against bacterial peritonitis, although NK-cell depletion could not completely suppress serum IFN-γ. However, it was reported that IFN-γ is important for mouse resistance to Listeria infection (3), and it has been recently demonstrated by use of IFN-γ receptor-deficient mice that IFN-γ is essential for the protection of mice in an experimental peritonitis model similar to CLP (42). Although results with gene-mutated mice should be interpreted carefully, it seems likely that IFN-γ has a protective role in peritonitis. It is also possible that NK cells and their Th1 cytokines may generate bacterial Ag-specific Th1 T-cell clones and produce more effective antibacterial immunity against rechallenge with the same bacteria or bacterial Ags.
TNF-α was not elevated significantly in CLP and CLC mice. The mortality of CLP mice was not affected by anti-TNF-α Ab (6) or in TNF-α receptor-deficient mice (42). Since it was also reported that IL-10 inhibits TNF-α production in endotoxemia (8, 19), the possibility is raised that a large amount of IL-10 in CLP or CLC mice may inhibit TNF-α production. Liver MNC of CLP mice with the puncture made by a 22-gauge needle produced only a small amount of IFN-γ, probably because liver MNC do not respond to more localized infections.
For nude mice, serum IFN-γ levels in mice with sham operations were not significantly different from those in CLP mice despite the fact that IFN-γ production by liver MNC showed a clear disparity. Although the exact reason for this discrepancy is unclear, NK cells and extrathymic T cells in organs other than the liver of thymus-deficient mice may somehow be activated to compensate for the lack of thymus-derived T cells and produce IFN-γ even when stimulated by a sham operation.
IL-10 levels in PLF were not significantly different for CLP mice and CLC mice. It can be speculated that a large amount of IL-10 produced by PEC macrophages of CLC mice may rapidly shift into circulation and that the dilution of the IL-10 in the peritoneal cavity by PBS in the process of obtaining PLF may mask the difference.
The present study identifies the source of increased IFN-γ and IL-10 in mice with bacterial peritonitis.
REFERENCES
- 1.Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker C C, Chaudry I H, Gaines H O, Baue A E. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 3.Bancroft G J, Schreiber R D, Unanue E R. A T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 4.Benacerraf B, Sebestyen M M, Schlossman S. A quantitative study of the kinetics of blood clearance of P32 labeled Escherichia coli and staphylococci by the reticuloendotherial system. J Exp Med. 1959;110:27–48. doi: 10.1084/jem.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Waal Malefyt R, Abrams J, Bennett B, Figdor C, de Vries J E. IL-10 inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskandari M K, Bolgos G, Miller C, Nguyen D T, DeForge L E, Remick D G. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 7.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 8.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenbeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory S H, Barczynski L K, Wing E J. Effector function of hepatocytes and Kupffer cells in the resolution of systemic bacterial infection. J Leukoc Biol. 1992;51:421–424. doi: 10.1002/jlb.51.4.421. [DOI] [PubMed] [Google Scholar]
- 10.Habu, Y., and S. Seki. Unpublished data.
- 11.Hashimoto S, Yamada M, Motoyoshi K, Akagawa K S. Enhancement of macrophage colony-stimulating factor-induced growth and differentiation of human monocytes by interleukin-10. Blood. 1997;89:315–321. [PubMed] [Google Scholar]
- 12.Hashimoto W, Takeda K, Anzai R, Ogasawara K, Sakihara H, Sugiura K, Seki S, Kumagai K. Cytotoxic NK1.1 Ag+ αβ T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995;154:4333–4340. [PubMed] [Google Scholar]
- 13.Haubrich W S, Schaffner F, Berk J E. Bockus gastroenterology. Vol. 3. Philadelphia, Pa: W. B. Sanders Co.; 1995. p. 1871. [Google Scholar]
- 14.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen P M, van der Pouw Kraan T C T M, de Jong I W, van Mierlo G, Wijdenes J, Chang A A, Aarden L A, Taylor Jr F B, Hack C E. Release of interleukin-12 in experimental Escherichia coli septic shock in baboons: relation to plasma levels of interleukin-10 and interferon-γ. Blood. 1996;87:5144–5151. [PubMed] [Google Scholar]
- 16.Lichtman S N, Wang J, Schwab J H, Lemasters J J. Comparison of peptidoglycan-polysaccharide and lipopolysaccharide stimulation of Kupffer cells to produce tumor necrosis factor and interleukin-1. Hepatology. 1994;19:1013–1022. [PubMed] [Google Scholar]
- 17.Lichtman S N, Keku J, Schwab J H, Sartor R B. Evidence for peptidoglycan absorption in rats with experimental small bowel bacterial overgrowth. Infect Immun. 1991;59:555–562. doi: 10.1128/iai.59.2.555-562.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Chow J M, Gri G, Garra G, Gerosa F, Wolf S F, Dzialo R, Trincheri G. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gerard C, Delvaux A, de Groote D, Abramowicz D, Velu T, Goldman M. IL-10 controls IFN-γ and TNF production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–1171. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 20.Moore K W, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 21.Moulder J W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985;49:298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolan J P. Intestinal endotoxins as mediators of hepatic injury: an idea whose time has come again. Hepatology. 1989;10:887–891. doi: 10.1002/hep.1840100523. [DOI] [PubMed] [Google Scholar]
- 23.Ogasawara K, Takeda K, Hashimoto W, Shirai K, Okuyama R, Yanai N, Obinata M, Kumagai K, Takada H, Hiraide H, Seki S. Involvement of NK1+ T cells and their IFN-g production in the generalized Shwartzman reaction. J Immunol. 1996;160:3522–3527. [PubMed] [Google Scholar]
- 24.Ohteki T, Okuyama R, Seki S, Abo T, Sugiura K, Kusumi A, Ohmori T, Watanabe H, Kumagai K. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J Immunol. 1992;149:1562–1570. [PubMed] [Google Scholar]
- 25.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature (London) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 26.Okura Y, Yamamoto T, Goto S, Inomata T, Hirono S, Hanawa H, Feng L, Wilson C B, Kihara I, Izumi T, Shibata A, Aizawa Y, Seki S, Abo T. Characterization of cytokine and iNOS mRNA expression in situ during the course of experimental autoimmune myocarditis in rats. J Mol Cell Cardiol. 1997;29:491–502. doi: 10.1006/jmcc.1996.0293. [DOI] [PubMed] [Google Scholar]
- 27.Ono, S., S. Aosasa, S. Osada, and H. Mochizuki. Unpublished data.
- 28.Ozman L, Pericin M, Hakimi J, Chizzonite R A, Wysocka M, Trinchieri G, Gately M, Garotta G. Interleukin 12, interferon γ, and tumor necrosis factor α are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–916. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh M, Seki S, Hashimoto W, Ogasawara K, Kobayashi T, Kumagai K, Matsuno S, Takeda K. Cytotoxic γδ or αβ T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886–3892. [PubMed] [Google Scholar]
- 31.Sherry R M, Cue J I, Goddard J K, Parramore J B, DiPiro J T. Interleukin-10 is associated with the development of sepsis in trauma patients. J Trauma. 1996;40:613–616. doi: 10.1097/00005373-199604000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Smedly L A, Tonnesen M G, Sandhaus R A, Haslett C, Guthrie L A, Johnston R B, Jr, Henson P M, Worthen G S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986;77:1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi M, Ogasawara K, Takeda K, Hashimoto W, Sakihara H, Kumagai K, Anzai R, Satoh M, Seki S. LPS induces NK1.1+ αβ T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol. 1996;156:2436–2442. [PubMed] [Google Scholar]
- 34.Takeda K, Seki S, Ogasawara K, Anzai R, Hashimoto W, Sugiura K, Takahashi M, Satoh M, Kumagai K. Liver NK1.1+ CD4+ αβ T cells activated by IL-12 as a major effector in inhibition of experimental tumor metastasis. J Immunol. 1996;156:3366–3373. [PubMed] [Google Scholar]
- 35.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996;2:198–204. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 36.Trinchieri G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 37.Tsukahara A, Seki S, Iiai T, Moroda T, Watanabe H, Suzuki S, Tada T, Hiraide H, Hatakeyama K, Abo T. Mouse liver T cells: their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301–309. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 38.van der Poll T, Marchant A, Buurman W A, Berman L, Keogh C V, Lazarus D D, Nguyen L, Goldman M, Moldawer L L, Lowry S F. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995;155:5397–5401. [Google Scholar]
- 39.Walley K R, Lukas N W, Stanford T J, Strieter R M, Kunkel S. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe H, Miyaji C, Seki S, Abo T. c-kit+ stem cells and thymocyte precursors in the liver of adult mice. J Exp Med. 1996;184:687–693. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wysocka M, Kubin M, Vieria L Q, Ozman L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 42.Zantl N, Uebe A, Neumann B, Wagner H, Siewert J R, Holzman B, Heidecke C D, Pfeffer K. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun. 1998;66:2300–2309. doi: 10.1128/iai.66.5.2300-2309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]