Abstract
Objective:
Predicting risk of posttraumatic stress disorder (PTSD) in the acute care setting is challenging given the pace and acute care demands in the emergency department (ED) and the infeasibility of using time-consuming assessments. Currently, no accurate brief screening for long-term PTSD risk is routinely used in the ED. One instrument widely used in the ED is the 27-item Immediate Stress Reaction Checklist (ISRC). The aim of this study was to develop a short screener using a machine learning approach and to investigate whether accurate PTSD prediction in the ED can be achieved with substantially fewer items than the IRSC.
Method:
This prospective longitudinal cohort study examined the development and validation of a brief screening instrument in two independent samples, a model development sample (N = 253) and an external validation sample (N = 93). We used a feature selection algorithm to identify a minimal subset of features of the ISRC and tested this subset in a predictive model to investigate if we can accurately predict long-term PTSD outcomes.
Results:
We were able to identify a reduced subset of 5 highly predictive features of the ISRC in the model development sample (AUC = 0.80), and we were able to validate those findings in the external validation sample (AUC = 0.84) to discriminate non-remitting vs. resilient trajectories.
Conclusion:
This study developed and validated a brief 5-item screener in the ED setting, which may help to improve the diagnostic process of PTSD in the acute care setting and help ED clinicians plan follow-up care when patients are still in contact with the healthcare system. This could reduce the burden on patients and decrease the risk of chronic PTSD.
Keywords: Acute trauma, Posttraumatic stress disorder, Emergency department, Machine learning, Screener
1. Introduction
Traumatic injury resulting in hospitalization leads to a range of adverse physical and mental health outcomes, often resulting in costly healthcare utilization and significant functional impairment and disability [1,2]. Posttraumatic stress disorder (PTSD) is one common reaction to trauma and has a major economic burden on society, with more than $23 billion in excess costs due to PTSD in the United States in 2018 alone [3]. Importantly, PTSD remains underdiagnosed by healthcare providers, and standard practices to connect patients to trauma-specific behavioral health services remain uncommon in medical settings serving civilians [4,5]. Given the commonality of patients seeking medical care in emergency department (ED) settings following trauma exposure, screening, and identifying individuals at risk for developing PTSD in this setting are particularly important.
The development of PTSD following trauma in acute trauma patients is high with 6–29% showing rates of PTSD at 12-months post-injury [6,7]. Yet, because not everyone who visits the ED following trauma exposure will develop PTSD, there is a clear need for early screening to identify those most at risk. The American College of Surgeons Committee on Trauma recommends mental health screening in trauma patients to identify those at risk [8]. Barriers to a systematic screening of acute stress symptoms could be overcome by using a screening tool that is brief and designed for use with acute stress symptoms. Data supports that it is feasible to screen trauma patients within 24 h of trauma and individuals surveyed for PTSD symptoms are more likely to then utilize services [9]. While numerous prospective studies have now created PTSD risk algorithms for patients based on varied electronic medical data and psychological symptom measures [10-14], most of these studies use varied measures of acute stress symptoms that are often lengthy and would be difficult to integrate into a busy ED setting. Early intervention studies conducted in the ED have reported decreased PTSD and depression symptoms following early intervention [15] and been associated with possibly mitigating a genetic risk for PTSD [16], but the inability to predict risk in the ED for the development of PTSD negated the effects of early intervention [17]. Thus, the need for an efficient measure to predict the risk for PTSD is huge.
At present, the only research available on brief (5 items or less) PTSD screening in the acute post-trauma period is with measures validated for use following the 4-week requirement for PTSD symptoms and has not been developed specifically for use in the acute stress period directly following the traumatic event. The most widely used PTSD screening tool is the PC-PTSD-5 [18], a validated 5-item measure of PTSD symptoms that can be used in diverse trauma-exposed populations and has been used to predict chronic PTSD in acute trauma patients [19]. However, given what we know about the differences between acute stress response and PTSD, it is not clear that the items identified in a PTSD screening tool would be equally effective as a screener for potential PTSD development in the acute aftermath of trauma.
The Immediate Stress Response Checklist (ISRC) is a 27-item, validated verbal questionnaire designed to evaluate the key features of acute stress responses in the immediate post-trauma period [20]. The ISRC is not designed to provide a diagnosis but can be used to determine what acute stress symptoms are present and has strong potential to serve as a valuable tool to screen for PTSD risk in ED patients. However, a 27-item measure is far too lengthy to be used as a screening tool in the ED. Schultebraucks, Shalev [10] utilized the ISRC along with data obtained in electronic medical records to create a predictive algorithm for who is at greatest risk for the development of chronic PTSD following acute trauma exposure. Study findings indicated certain ISRC symptoms together with electronic health records data were particularly valuable in the prediction model, namely “I felt like I was not there, like I was not part of what was going on” (ISRC item 6), “I felt confused” (ISRC item 7), “I get upset when something reminds me of what happened” (ISRC item 26) and “I feel hyper or like I can’t stay still” (ISRC item 27). While this study was helpful in suggesting potential items relevant for a screener, it was not conducted to directly develop a screener based on the ISRC questionnaire alone and to identify an essential subset of ISRC items that accurately distinguish different PTSD trajectories.
1.1. Purpose of the study
Accurate risk identification for the development of PTSD in trauma patients accessing acute trauma center or ED services is critical to provide tailored resources and mitigate risk through early intervention strategies with at-risk patients. Utilizing data from patients’ immediate stress reaction response, the current study 1) created a brief version of the ISRC that best predicts later development of PTSD (up to 12 months post-trauma) using a prospective cohort of trauma survivors from a level 1 trauma center and 2) examined the generalizability of this brief screener using an independently collected prospective cohort of trauma survivors from another level 1 trauma center as an external validation sample. Given the critical clinical need to treat patients with non-remitting PTSD, accurate assessment of an individual’s risk for chronic PTSD is necessary for effective prevention and treatment allocation. Early detection is key for reducing long-term disability and distress related to PTSD symptoms following acute trauma exposure as it could lead to early diagnosis and management of these cases, and therefore could help reduce the occurrence of the disorder.
2. Methods
2.1. Participants and procedure
The first sample (n = 253) of ED patients was prospectively enrolled from 2012 to 2017 at the Marcus Trauma Center of the Grady Memorial Hospital and was used for model development (discovery sample). A second independent sample (n = 93) of ED patients was prospectively enrolled from 2012 to 2016 at Bellevue Hospital Center and was used for external validation of the predictive model (validation sample).
The participants were approached based on information of the ED trauma surgery discharge rounds or the team’s rounding sheet. Potential eligible patients were contacted by the study personnel in the ED and our inclusion and exclusion criteria were assessed. See Schultebraucks, Shalev [10] for details on the patient flow for study recruitment.
All participants had experienced a traumatic event (e.g., life-threatening accident, sexual or physical assault) satisfying the DSM-5 trauma criterion A of PTSD (American Psychiatric Association, 2013). Additional inclusion criteria were the ability to give informed consent, age between 18 and 65 years (Grady sample) or 18–70 years of age (Bellevue sample), residence in the United States , and fluency in speaking English (Grady sample) or English, Spanish, and Mandarin (Bellevue sample). Current intoxication, suicidal ideation or suicide attempts in the past 3 months, a history of schizophrenia, psychosis or mania, Glasgow Coma Scale score < 15, respiratory distress, or medical instability, were exlusion criteria for the Grady sample. For the Bellevue sample, exclusion criteria were risk for ongoing traumatic exposure (e.g., domestic violence), evidence of homicidal/suicidal behavior, custody of police or Department of Correction, current or past psychotic symptoms, open head injury, survivors in coma or evidence of traumatic brain injury indicated by a Glasgow Coma Scale score < 13 or no reliable access to email or telephone. All enrolled patients were treated and diagnostically examined as usual.
In line with the most recent version of the Declaration of Helsinki, both studies were approved by their respective research ethics oversight committee, the Emory Institutional Review Board, and the Grady Hospital Research Oversight Committee (Grady study) and the ethics committee of New York University (Bellevue study). All participants signed informed consent. Participants were compensated $50 for their time and involvement per visit.
2.2. Outcome measure
For model development, the outcome measure was the modified PTSD Symptom Scale (mPSS) [21] prospectively collected at 1, 3, 6, and 12-months after ED discharge at Grady Memorial Hospital. The outcome measure used for the external validation of the predictive model was measured with the PTSD Checklist for DSM-5 (PCL -5) [22]. Data were prospectively collected at ED admission, within 7 days thereafter (phone screen interview), and at 1, 3, 6, and 12-months after ED admission at Bellevue Hospital, NYC. We used latent growth mixture modeling (LGMM) to identify heterogeneous trajectories of PTSD as described in previous studies [10,23-27]. Distinct LGMMs were calculated for the model development sample using the mPSS scores from 4 time points (1 month, 3 month, 6 month and 12 month after ED admission) and the PCL-5 score from 5 time points (7 days, 1 month, 3 month, 6 month and 12 month after ED admission) for the external validation sample. In this current study, we predicted ED patients on a longitudinal symptom trajectory of non-remitting PTSD symptoms compared to a resilient trajectory as determined by the LGMM to identify patients with the greatest need for preventive interventions.
2.3. Predictor variables
We included all 27 items of the ISRC [20] in our analysis. Participants were asked 15 items about what they were thinking/feeling when the potential traumatic event happened and 12 items about how they are feeling right now. They were asked to rate these on a 3-point Likert scale from 1 = “Not true”, to 1 = “Somewhat or sometimes true” to 2 “very or often true”.
2.4. Statistical analysis
2.4.1. Data pre-processing
Continuous variables were scaled and centered, and missing values were imputed using k-nearest neighbor estimation using the R package recipes.
2.4.2. Features selection
We used recursive features elimination (RFE) via the caret R package (rfFuncs) as a feature selection algorithm in the model development sample to identify a reduced feature subset of relevant items from the ISRC. RFE applies a backward selection process applying random forests to identify the most important features. We used 5 times 10fold cross-validation to guard against overfitting.
2.4.3. Model development and model evaluation
To examine the predicate accuracy of the reduced feature set, we applied extreme Gradient Boosting (XGB) [28] using ‘xgbTree’ in caret R package to examine the predictive accuracy using the reduced feature set. The data collected at the Grady Memorial Hospital in Atlanta was used for model development. We used 10-fold cross-validation on the training set to assess the bias-variance trade-off and to gauge the extent of potential “over-” or “underfitting” of the model [29]. We used sensitivity for hyperparameter tuning to select the model with the largest sensitivity in the model development sample.
To examine whether the reduced feature set can be used to identify patients at risk in a new, completely unseen sample, we tested the model on the date collected in the Bellevue Hospital in New York as an external validation sample. This is an important step to test the generalizability of this reduced feature set. All steps of data inspection preprocessing and analysis were performed using R version 4.1.1 in RStudio version 1.4.1717.
3. Results
The model development sample consisted of N = 253 trauma survivors (mean age 35.63 ± 12.57; 42.7% female; 3.6% Hispanic) who were resilient or developed non-remitting symptoms after ED admission. In the external validation sample we identified N = 93 trauma-survivors with non-remitting PTSD symptoms or who were resilient (mean age 37 ± 13.92; 30.1% were female; 28% were Hispanic).
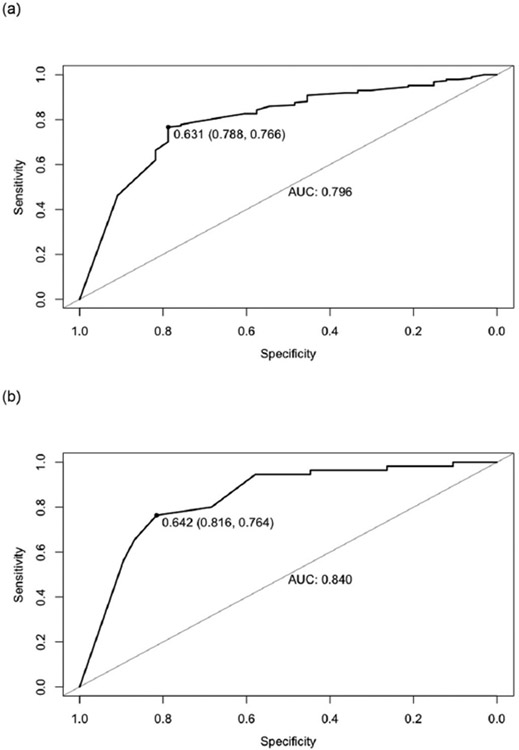
The five features (item 7, 9, 11, 24 and 27) that were identified as the most important ones for predicting our outcome of interest using the RFE algorithm are presented in Table 1. We were able to accurately discriminate non-remitting and resilient trajectories with a high discriminatory accuracy (Fig. 1) by using the reduced set of features presented in Table 1 in the model development sample (AUC = 0.80) as well as in the external validation set (AUC = 0.84). The short version of the ISRC based on our study findings are presented in Table 2.
Table 1.
Reduced set of features identified with the RFE algorithm.
| Outcome | 5 features selected with the RFE algorithm |
|---|---|
| Non-remitting vs. resilient trajectory | ISRC item 7: “I felt confused” ISRC item 9: “People like my family or friends seemed like strangers to me” ISRC item 11: “At times I was not sure where I was or what time it was” ISRC item 24: “I feel spacey or out of touch with the world around me” ISRC item 27: “I feel hyper or like I can’t stay still” |
Fig. 1.
Receiver operating characteristic (ROC curves) evaluating the predictive power in (a) the model development sample and (b) the external validation sample for distinguishing the non-remitting vs. resilient trajectory.
Table 2.
Immediate stress reaction checklist - short version based on study findings.
| Directions: When something bad or scary happens, people can have different thoughts or feelings. You’ve told us a little about what happened to you today/tonight. These items are about what you were thinking and feeling while this was happening. Tell me how true each one is for you. | |||
|---|---|---|---|
| WHILE IT WAS HAPPENING: | NOT TRUE |
SOMEWHAT or SOMETIMES TRUE |
VERY or OFTEN TRUE |
| 1. I felt confused. | 0 | 1 | 2 |
| 2. People like my family or friends seemed like strangers. | 0 | 1 | 2 |
| 3. At times I was not sure where I was or what time it was. | 0 | 1 | 3 |
| Directions: These next items are about how you are doing right now. Tell me how true each one is for you. | |||
| NOW | NOT TRUE |
SOMEWHAT or SOMETIMES TRUE |
VERY or OFTEN TRUE |
| 4. I feel spacey or out of touch with the world around me. | 0 | 1 | 2 |
| 5. I feel “hyper” or like I can’t stay still. | 0 | 1 | 2 |
4. Discussion
Our results demonstrate the development and validation of a brief 5-item screening instrument for long-term PTSD risk in the acute phase after a traumatic event, which demonstrates high discriminatory accuracy related to PTSD symptom trajectories prospectively over a 12-month follow-up. We identified a reduced subset of 5 items using a machine learning approach. A closer look at the items selected by the feature selection algorithm reveals a high consistency with the item set identified in Schultebraucks, Shalev [10]. Both peritraumatic dissociation [30] as well as hyperarousal [31,32] have been associated with an increased risk for PTSD in previous studies. The majority of the symptoms fit with components of peritraumatic dissociation, or the experience of emotional numbness, derealization, or depersonalization during or shortly after the trauma occurs [33]. Peritraumatic dissociation can disrupt the normal processing of the trauma, lead to more fragmented memories of the event, and ultimately increase the risk for the development of PTSD symptoms [34]. Furthermore, network analyses have shown that acute hyperarousal symptoms have been shown to be central in the occurrence of PTSD symptoms in the first days to weeks after trauma [35,36]. This underpins the relative diagnostic significance of these items. In addition, our results extend previous studies, as we are the first study to develop and validate a brief screener in the acute care setting, rather than 1 month after trauma as previous studies have done [19]. One of the greatest benefits of using a screener directly in the ED is that patients are still in contact with the healthcare system and clinicians can plan more individualized and targeted follow-up care, and screening measure could be used to identify and target patients at high risk for early intervention resources [15,17]).
4.1. Strength and limitation
Despite the relatively small sample size, this sample offers many advantages, such as the fact that it is a prospectively collected cohort, with data collected completely independently at two different sites to evaluate risk factors for PTSD. Having an external validation sample increases the generalizability of our findings, although it is recommended to further evaluate the brief 5-item screening instrument on a larger scale with a diverse population to further evaluate its predictive value.
4.2. Clinical implications
The development of a short screener for PTSD risk in the acute aftermath of a traumatic event is of high clinical relevance. It has been shown that existing early interventions are more likely to be successful if they are targeted to those who are at high risk for PTSD [37-40]. PTSD diagnosis 1 month after ED admission provides an accurate estimate of chronic PTSD [39], however, the ED is often the only contact of patients with the healthcare system and therefore provides a unique opportunity for risk assessment to prognosticate which patient need to be followed after the traumatic event for implementing early intervention strategies.
In the ED setting, time-consuming clinical interviews and psychometric assessments are often unfeasible, given the pace and acute care demands [41]. This is also evident from the fact that despite the significant psychological morbidity associated with potentially traumatic events, only 7% of EDs regularly screen for PTSD symptoms [42]. Our brief validated 5-item screening instrument has the potential to be used in EDs, even when EDs perennially face high patient volumes and overcrowding [43]. This provides a unique opportunity for our brief 5-item screening instrument to provide additional capacity to prevent downstream mental health consequences, even when ED clinicians are tasked with providing high-quality care for the most acute medical priorities. Additionally, our measure would be administered as a self-report scale and would not require specialist administration or staffing resources. Further examination is certainly needed to further evaluate the brief screener and its ability to discriminate different trajectories of PTSD symptomatology. Future research could also investigate if this brief measure generalizes to other acute trauma settings, such as in combat zones with military samples. Nonetheless, these findings support the claim that an accurate prognosis can be performed in a trauma center using a small subset of items, thereby potentially allowing to reduce the complexity of the diagnostic procedure and therefore increase the ecological validity.
5. Conclusion
The current study is the first to use a machine learning algorithm to identify a reduced subset of only 5 items of the ISRC for accurately predicting long-term PTSD in the ED, i.e., in the acute aftermath of the traumatic event. Those results are a crucial step toward improving PTSD prognostication, which could reduce the burden on patients and decrease the risk of chronic PTSD.
Acknowledgments
We acknowledge the entire Grady Trauma Project team for their contributions to data collection and management and are particularly grateful to our participants for their willingness to be a part of this project. The study was supported by K01MH102415 (I.R. Galatzer-Levy) and R01MH094757 (K.J. Ressler).
Disclosures
Dr. Rothbaum has or recently had funding from Wounded Warrior Project, National Science Foundation, Cohen Veteran Bioscience, Bob Woodruff Foundation, The Hidden Heroes Fund (an initiative of the Elizabeth Dole Foundation), Department of Defense Clinical Trial Grant No.W81XWH-10-1-1045, and McCormick Foundation. Dr. Rothbaum receives royalties from Oxford University Press, Guilford, APPI, and Emory University and received advisory board payments from Jazz Pharmaceuticals, Nobilis Therapeutics and Sophren. Dr. Rothbaum owns equity in Virtually Better, Inc. that creates virtual environments. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Dr. Maples-Keller has received funding and consulting payments from COMPASS Pathways, and receives support from the Wounded Warrior Project (WWP) and the Infinite Hero Foundation. Dr. Ressler has performed scientific consultation for Bioxcel, Bionomics, Acer, Takeda, and Jazz Pharma; serves on Scientific Advisory Boards for Sage and the Brain Research Foundation, and he has received sponsored research support from Brainsway and Alto Neuroscience. He receives research funding from the NIH. Dr. Galatzer-Levy received scientific advisory board funding in the past from GW Pharmaceuticals and salary and stock grants from Meta and Google. Furthermore, he received support from the National Institute of Mental Health (K01MH102415). Dr. Schultebraucks received support from the National Institute of Mental Health (R01MH129856) and the National Heart, Lung, and Blood Institute (R01HL156134). Dr. Powers received support from the National Center for Complementary & Integrative Health (K23AT009713). The remaining authors have nothing to disclose.
Footnotes
CRediT authorship contribution statement
K. Schultebraucks: Writing – original draft, Formal analysis, Supervision, Methodology, Visualization. J.S. Stevens: Project administration, Supervision, Writing – review & editing. V. Michopoulos: Project administration, Supervision, Writing – review & editing. J. Maples-Keller: Investigation, Supervision, Writing – review & editing. J. Lyu: Formal analysis. R.N. Smith: Writing – review & editing. B.O. Rothbaum: Writing – review & editing. K.J. Ressler: Funding acquisition, Supervision, Writing – review & editing. I.R. Galatzer-Levy: Funding acquisition, Project administration. A. Powers: Project administration, Supervision, Methodology, Writing – original draft, Writing – review & editing.
References
- [1].DiMaggio C, et al. Traumatic injury in the United States: in-patient epidemiology 2000–2011. Injury 2016;47(7):1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McFARLANE AC. The long-term costs of traumatic stress: intertwined physical and psychological consequences. World Psychiatry 2010;9(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Davis LL, et al. The economic burden of posttraumatic stress disorder in the United States from a societal perspective. J Clin Psychiatry 2022;83(3):40672. [DOI] [PubMed] [Google Scholar]
- [4].da Silva HC, et al. PTSD in mental health outpatient settings: highly prevalent and under-recognized. Brazilian J Psychiatry 2018;41:213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwartz AC, et al. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv 2005;56(2):212–5. [DOI] [PubMed] [Google Scholar]
- [6].Zatzick D, et al. A national US study of posttraumatic stress disorder, depression, and work and functional outcomes after hospitalization for traumatic injury. Ann Surg 2008;248(3):429–37. [DOI] [PubMed] [Google Scholar]
- [7].Bryant RA, et al. The psychiatric sequelae of traumatic injury. Am J Psychiatry 2010;167(3):312–20. [DOI] [PubMed] [Google Scholar]
- [8].Bulger EM, et al. Nationwide survey of trauma center screening and intervention practices for posttraumatic stress disorder, firearm violence, mental health, and substance use disorders. J Am Coll Surg 2022;234(3):274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].La Vonne AD, et al. Comparison of self-administered post traumatic stress disorder tool vs. researcher administered tool in the emergency department. J Natl Med Assoc 2018,110(l):18–22. [DOI] [PubMed] [Google Scholar]
- [10].Schultebraucks K, et al. A validated predictive algorithm of posttraumatic stress course following emergency department admission after a traumatic stressor. Nat Med 2020;26(7):1084–8. [DOI] [PubMed] [Google Scholar]
- [11].Schultebraucks K, et al. Forecasting individual risk for long-term posttraumatic stress disorder in emergency medical settings using biomedical data: a machine learning multicenter cohort study. Neurobiol Stress 2021;14:100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wafa M-H, et al. Identification of biopSychoSocial factors predictive of posttraUmatic stress disorder in patients admitted to the emergency department after a trauma (ISSUE): protocol for a multicenter prospective study. BMC Psychiatry 2019;19(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kravets V, et al. Early identification of post-traumatic stress disorder in trauma patients: development of a multivariable risk prediction model. Am Surg 2022;(18):31348221121549. [DOI] [PubMed] [Google Scholar]
- [14].Ziobrowski HN, et al. Development and validation of a model to predict posttraumatic stress disorder and major depression after a motor vehicle collision. JAMA Psychiat 2021;78(11):1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rothbaum BO, et al. Early intervention may prevent the development of PTSD: a randomized pilot civilian study with modified prolonged exposure. Biol Psychiatry 2012;72(11):957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rothbaum BO, et al. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J Clin Psychiatry 2014,75(12):1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maples-Keller JL, et al. Investigation of optimal dose of early intervention to prevent posttraumatic stress disorder: a multiarm randomized trial of one and three sessions of modified prolonged exposure. Depress Anxiety 2020;37(5):429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Prins A, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med 2016;31(10):1206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Manser SS, et al. Do screening and a randomized brief intervention at a level 1 trauma center impact acute stress reactions to prevent later development of posttraumatic stress disorder? J Trauma Acute Care Surg 2018;85(3):466–75. [DOI] [PubMed] [Google Scholar]
- [20].Fein JA, et al. Emergency department evaluation of acute stress disorder symptoms in violently injured youths. Ann Emerg Med 2001;38(4):391–6. [DOI] [PubMed] [Google Scholar]
- [21].Foa EB, et al. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. J Trauma Stress 1993;6(4):459–73. [Google Scholar]
- [22].Weathers FW, et al. The PTSD Checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at, http://www.ptsd.va.gov2013. [Google Scholar]
- [23].Hinrichs R, et al. Increased skin conductance response in the immediate aftermath of trauma predicts PTSD risk. Chronic Stress 2019;3. p. 2470547019844441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Michopoulos V, et al. Association of Prospective Risk for Chronic PTSD Symptoms With Low TNFα and IFNγ Concentrations in the Immediate Aftermath of Trauma Exposure. Am J Psychiatr 2019;177(1):58–65. [DOI] [PubMed] [Google Scholar]
- [25].Lori A, et al. Transcriptome-wide association study of post-trauma symptom trajectories identified GRIN3B as a potential biomarker for PTSD development. Neuropsychopharmacology 2021;46(10):1811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pencea I, et al. Emotion dysregulation is associated with increased prospective risk for chronic PTSD development. J Psychiatr Res 2020;121:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lalonde CS, et al. Sex differences in Peritraumatic inflammatory cytokines and steroid hormones contribute to prospective risk for nonremitting posttraumatic stress disorder. Chronic Stress 2021;5. p. 24705470211032208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System, in Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. San Francisco, California, USA: ACM; 2016. p. 785–94. [Google Scholar]
- [29].Cawley GC, Talbot NL. On over-fitting in model selection and subsequent selection bias in performance evaluation. J Mach Learn Res 2010,11(Jul):2079–107. [Google Scholar]
- [30].Lensvelt-Mulders G, et al. Relations among peritraumatic dissociation and posttraumatic stress: a meta-analysis. Clin Psychol Rev 2008;28(7):1138–51. [DOI] [PubMed] [Google Scholar]
- [31].Bryant RA, et al. A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. J Abnorm Psychol 2000;109(2):341. [PubMed] [Google Scholar]
- [32].Kearns MC, et al. Early interventions for PTSD: a review. Depress Anxiety 2012;29(10):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Breh DC, Seidler GH. Is peritraumatic dissociation a risk factor for PTSD? J Trauma Dissociation 2007;8(1):53–69. [DOI] [PubMed] [Google Scholar]
- [34].McCanlies EC, et al. Association of peritraumatic dissociation with symptoms of depression and posttraumatic stress disorder. Psychol Trauma Theory Res Pract Policy 2017;9(4):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bryant RA, et al. Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: a network analysis. JAMA Psychiat 2017;74(2):135–42. [DOI] [PubMed] [Google Scholar]
- [36].Greene T, et al. Dynamic networks of PTSD symptoms during conflict. Psychol Med 2018;48(14):2409–17. [DOI] [PubMed] [Google Scholar]
- [37].Shalev AY, Barbano AC. PTSD: risk assessment and early management. Psychiatr Ann 2019;49(7):299–306. [Google Scholar]
- [38].Vermetten E, Zhohar J, Krugers HJ. Pharmacotherapy in the aftermath of trauma; opportunities in the ‘golden hours’. Curr Psychiatry Rep 2014;16(7):455. [DOI] [PubMed] [Google Scholar]
- [39].Roberts NP, et al. Early psychological intervention following recent trauma: a systematic review and meta-analysis. Eur J Psychotraumatol 2019;10(1):1695486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sijbrandij M, et al. Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Lancet Psychiatry 2015;2(5):413–21. [DOI] [PubMed] [Google Scholar]
- [41].van der Mei WF, et al. Evaluating a screener to quantify PTSD risk using emergency care information: a proof of concept study. BMC Emerg Med 2020;20(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Love J, Zatzick D. Screening and intervention for comorbid substance disorders, PTSD, depression, and suicide: a trauma center survey. Psychiatr Serv 2014;65(7):918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Asplin BR, et al. A conceptual model of emergency department crowding. Ann Emerg Med 2003;42(2):173–80. [DOI] [PubMed] [Google Scholar]