Abstract
Background:
Among patients with peripheral artery disease (PAD), depression is diagnosed in 17–25% and negatively impacts wound healing, quality of life, and survival. We hypothesized that depression is underdiagnosed in patients with PAD. Additionally, given the associations between depression and mortality in PAD patients, there is an increased need to investigate the strength of this relationship. The present analysis includes 2 studies to address the following aims: (1) Investigation of the prevalence of concomitant PAD and depression in a cohort from the Southeastern United States, and (2) Examination of the association between depression and all-cause mortality in a cohort of Canadian patients with PAD.
Methods:
STUDY 1: From June–August 2022, the Patient Health Questionnaire Module 9 (PHQ-9) was administered to all patients seeking PAD-related care including medical, wound/podiatric, or vascular interventional/surgical treatment, in the University of North Carolina-Chapel Hill Vascular, Wound, and Podiatry clinics. The PHQ-9 assesses symptoms over 2 weeks and is scored 0–27, with higher scores indicating increasingly severe depression. Demographics, primary diagnosis, depression history, and antidepressant prescription were determined through chart review. We compared the proportion of positive depression screenings (PHQ-9 ≥ 5) to known depression. Among those treated for depression, the PHQ-9 score severity was evaluated. T-tests and χ2 tests were used to compare means and proportions. STUDY 2: From July 2015 to October 2016, the Geriatric Depression Scale Short Form was administered to adult patients with PAD undergoing revascularization. The Geriatric Depression Scale Short Form is a self-report measure of depression with a score >5 consistent with depression. The prevalence of depression was determined; primary outcome was all-cause mortality at 6 months.
Results:
STUDY 1: In 104 PAD patients (mean age 66.6 ± 11.3 years, 37% female), 37% of respondents scored ≥5 on the PHQ-9 survey, indicating at least mild depression. Only 18% of PAD patients had a history of depression, demonstrating a significant difference between the PHQ-9 findings and documented medical history. While depression was underdiagnosed in both men and women, men were more likely to have unrecognized depression (chi-squared statistic = 35.117, df = 1, P< 0.001). Among those with a history of depression, 74% had a current prescription for antidepressant medication, but 57% still had an elevated PHQ-9 score indicating possible undertreatment. STUDY 2: In 148 patients (mean age 70.3 ± 11.0 years, 39% female) the prevalence of screened depression was 28.4%, but only 3.3% had a documented history of depression suggesting significant underdiagnosis. Patients with depression were significantly more likely to die within 6 months of revascularization (9.5% vs. 0.9%; odds ratio 1.48, 95% confidence interval: 1.08 to 2.29). There was no association between depression and risk of length of stay, reintervention, or readmission.
Conclusions:
Depression is underdiagnosed and undertreated among patients with PAD, which has grave consequences as it is associated with 1.5 times the odds of mortality within 6 months of revascularization. There is a critical need for more robust screenings and comprehensive mental health treatment for patients with concomitant depression and PAD.
INTRODUCTION
Peripheral artery disease (PAD) is a common vascular condition with significant effects on morbidity and mortality. In its most severe form, it can lead to ischemic ulcers or gangrene,1 and chronic limb-threatening ischemia remains a leading cause of lower-limb amputation in the United States and Canada.2,3 While patient-centered outcomes, such as the impact of chronic vascular disease on independence, functional status, and mood, have been understudied, there are reports that the quality of life among patients with chronic limb-threatening ischemia is poor.4
The prevalence of depression in the general U.S. population is 8.4%, with chronic diseases being associated with higher rates of depression.5 There is substantial evidence to support the burden of depression in cardiac disease,6,7 and patients with depression undergoing coronary bypass surgery have an increased risk of death, postoperative cardiac events, and readmission.8–10 On the whole, the risk of depression in patients with PAD may even exceed that of coronary disease,11,12 but its prevalence has been poorly defined in the literature, with rates of concomitant PAD and depression ranging from 3–48%.13 Although the role of depression in PAD treatment and outcomes is not yet fully understood, several studies have found an increased risk of mortality in PAD patients with depression, ranging from 17–24% greater than the 11.3% risk among their nondepressed peers.14–16 Age at PAD diagnosis, female gender, and symptom severity have been associated with higher rates of depression.17,18 Other mediating factors may include social support, family income, alcohol consumption, and hypertension.11,19,20
It is essential to understand the prevalence of depression in patients with PAD. Appropriate screening and mental health treatment are imperative to achieve better treatment outcomes within this population. Furthermore, given the associations between depression and mortality in PAD patients, there is an increased need to investigate the strength of this relationship. The present analysis includes 2 studies to address the current gap in the literature: (1) An investigation of the prevalence of concomitant PAD and depression in a cohort from the Southeastern United States, and (2) The examination of the association between depression and all-cause mortality in a cohort of Canadian patients with PAD undergoing revascularization.
STUDY 1: DEPRESSION IS UNDERDIAGNOSED IN PATIENTS WITH PAD
Methods
Study Design.
From June to August 2022, the Patient Health Questionnaire Module 9 (PHQ-9) screening was offered to all patients seeking PAD-related care, including medical, wound/podiatric, or vascular interventional/surgical treatment, in the University of North Carolina-Chapel Hill Vascular, Wound, and Podiatry clinics. Patients were allowed to opt out of the mental health survey, and a total of 28 patients declined to participate. The survey was read aloud to patients with vision or literacy challenges. Patients completed the questionnaire, we accessed the electronic health record to manually extract demographic data, medications, comorbidities, history of depression, and details of their PAD symptoms, including Rutherford score and wound details. This study was approved by the institutional review board at the University of North Carolina at Chapel Hill.
Depression Screening Test.
The PHQ-9 assesses depression symptoms and is routinely used as a screening tool due to its high sensitivity and specificity across multiple patient populations (88% and 88%, respectively).21 The PHQ-9 surveys symptoms over the past 2 weeks and consists of 9 questions scored from 0 to 3, with 3 indicating greater frequency and intensity of symptoms (see Supplementary Materials). The total is therefore scored from 0–27, with higher scores indicating greater symptom severity. Scores were recorded directly in the electronic health record. The clinician was notified if patients scored ≥15, indicating moderately severe symptoms. These patients were offered mental health resources and connected with primary care providers for follow-up assessments and support.
Participants.
In the 2-month study period, 106 adult patients attended clinic appointments for evaluation and treatment of PAD. Two new patients who presented for evaluation of PAD were excluded due to no evidence of arterial disease on hemodynamic testing.
Statistical Analysis.
Among the final 104 participants, we compared the proportion of positive screenings (PHQ-9 ≥ 5) to known depression history using a χ2 test. Among those previously treated for depression, the percentage of PHQ-9 scores ≥5 severity was calculated. T-tests and χ2 tests were used to compare means and proportions. All analyses were conducted using SAS 9.4 statistical software (Cary, NC).
Results
The cohort of 104 patients with PAD was 37% female, 35% non-White, and had an average age of 66.6 ± 11.3 years (Table I). The average time lived with PAD was 4.2 ± 3.1 years and an average Rutherford score of 2.7 ± 2.0. Women with PAD were more likely than men to have a history of depression [34% vs. 9%, Difference (95% CI) = 25% (9%, 42%)].
Table I.
Study 1 Demographics and PHQ-9 questionnaire data
| Total (n = 104) | Prior depression (n = 19) | No prior depression (n = 85) | Difference (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 66.6 ± 11.3 | 64.7 ± 12.5 | 67.0 ± 11.1 | −2.3 (−8.0, 3.4) |
| Sex (female) | 38 (36.5%) | 13 (68.4%) | 25 (29.4%) | 39.0% (14.3%, 57.4%) |
| Race | ||||
| White/Caucasian | 68 (65.4%) | 14 (73.7%) | 54 (63.5%) | 10.2% (−14.2%, 28.2%) |
| Black/African American | 33 (31.7%) | 5 (26.3%) | 28 (32.9%) | 6.6% (−17.6%, 24.5%) |
| Other | 3 (2.9%) | 0 (0.0%) | 3 (3.5%) | 3.5% (−13.5%, 9.8%) |
| Hypertension | 95 (91.3%) | 16 (84.2%) | 79 (92.9%) | 8.7% (−4.0%, 30.8%) |
| Dyslipidemia | 91 (87.5%) | 16 (84.2%) | 75 (88.2%) | 4.0% (−9.4%, 26.4%) |
| Obesity | 47 (45.2%) | 13 (68.4%) | 34 (40.0%) | 28.4% (3.6%, 47.3%) |
| Diabetes | 69 (66.3%) | 13 (68.4%) | 56 (65.9%) | 2.5% (−21.7%, 21.9%) |
| Coronary artery disease | 51 (49.0%) | 10 (52.6%) | 41 (48.2%) | 4.4% (−19.0%, 26.9%) |
| Cerebrovascular disease | 21 (20.2%) | 3 (15.8%) | 18 (21.2%) | 5.4% (−17.6%, 19.6%) |
| Chronic obstructive pulmonary disease | 23 (22.1%) | 6 (31.6%) | 17 (20.0%) | 11.6% (−7.3%, 35.1%) |
| Congestive heart failure | 32 (30.8%) | 6 (31.6%) | 26 (30.6%) | 1.0% (−18.3%, 25.1%) |
| Smoker | ||||
| Current | 25 (24.0%) | 7 (36.8%) | 18 (21.2%) | 15.6% (−4.6%, 38.9%) |
| Former | 54 (51.9%) | 8 (42.1%) | 46 (54.1%) | 12.0% (−12.1%, 33.5%) |
| Never | 25 (24.0%) | 4 (21.1%) | 21 (24.7%) | 3.6% (−20.1%, 19.7%) |
| Wound | 49 (47.1%) | 10 (52.6%) | 39 (45.9%) | 6.7% (−16.7%, 29.2%) |
| Revascularization | 78 (75.0%) | 14 (73.7%) | 64 (75.3%) | 1.6% (−16.1%, 25.4%) |
For all PAD patients, the average PHQ-9 score was 4.4 ± 4.4 (Table II). Across all PAD patients, 37% of respondents scored ≥5 on the PHQ-9 survey, indicating at least mild depression. Only 18% of patients with PAD had a history of depression, demonstrating a significant difference between the PHQ-9 findings and documented medical history [Difference (95% CI) = 18% (6%, 30%)].
Table II.
Study 1 depression and PHQ-9 scores
| Total (n = 104) | Prior depression (n = 19) | No prior depression (n = 85) | Difference (95% CI) | |
|---|---|---|---|---|
|
| ||||
| PHQ-9 categories | ||||
| Minimal (0–4) | 66 (63.5%) | 9 (47.4%) | 57 (67.1%) | 19.7% (−3.7%, 41.7%) |
| Mild (5–9) | 27 (26.0%) | 7 (36.8%) | 20 (23.5%) | 13.3% (−7.0%, 36.7%) |
| Moderate (10–14) | 9 (8.7%) | 2 (10.5%) | 7 (8.2%) | 2.3% (−8.6%, 23.6%) |
| Moderate-severe (15–19) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0% (−16.8%, 4.3%) |
| Severe (20+) | 2 (1.9%) | 1 (5.3%) | 1 (1.2%) | 4.1% (−2.7%, 23.5%) |
| Average PHQ-9 score | 4.4 ± 4.4 | 6.2 ± 4.9 | 3.9 ± 4.2 | 2.3 (0.1, 4.5) |
| Antidepressant prescription | 27 (26.0%) | 14 (73.7%) | 13 (15.3%) | 58.4% (34.1%, 74.1%) |
While depression was underdiagnosed in both men and women, men were more likely to have unrecognized depression. The positive depression screening to history of depression ratio was 3.8 and 1.2 for men and women, respectively (chi-squared statistic = 35.117, df = 1, P < 0.001). Among PAD patients with a history of depression, 74% had a current prescription for antidepressant medication, and 57% had an elevated PHQ-9 score.
STUDY 2: DEPRESSION IS ASSOCIATED WITH MORTALITY IN PAD PATIENTS
Methods
Study Design.
This was a subanalysis from the FRailty Assessment in Lower Extremity Arterial Disease prospective study,22 and protocols were approved by the Jewish General Hospital (Montreal, QC, Canada). Data were collected at 2 tertiary centers in Montreal, Quebec, Canada. Questionnaires were available in English and French languages. The methods and primary results of the primary study were recently reported22; briefly, patients completed a preprocedural assessment of frailty, disability, comorbidity, cognitive function, and mood and subsequently completed a follow-up interview at 6 and 12 months.
Depression Screening Tool.
Depressive symptoms were evaluated with the 15-item Geriatric Depression Scale Short Form (GDS-SF). A GDS-SF score of ≥5 is indicative of clinically relevant depression (see Supplementary Materials). The GDS-SF is a validated screening instrument for depression and has a sensitivity of 72% and a specificity of 78%.23 The GDS-SF was administered at baseline, 6 months and 12 months postprocedure. Patients were stratified according to their screened depression status based on the GDS-SF.
Participants.
Adult patients undergoing endovascular or open interventions for PAD were consecutively enrolled between October 2015, and August 2016. Patients with PAD with Rutherford class III or higher were recruited. Patients were excluded from the study if they had emergency surgery or unstable vital signs, severe neuropsychiatric impairment, or a prohibitive language barrier.
Statistical Analysis.
In this substudy of FRailty Assessment in Lower Extremity Arterial Disease, the prevalence, correlates, and prognostic impact of depressive mood symptoms were examined. The primary end point studied was all-cause mortality at 6 months. The secondary outcomes were readmission, vascular reintervention, and length of stay. Continuous variables were summarized with the sample mean and standard deviation. Depression status was analyzed as a continuous variable (GDS-SF scores of 0–15 points). Univariate analysis was performed to assess the association of all relevant clinical covariates with the study end points. Multivariable logistic regression was used to estimate the association between 6-month all-cause mortality, reintervention, and vascular readmission with the predictor variable of screened depression using the GDS-SF. Multivariable linear regression was used to estimate the association between length of stay and our predictor variable. Regression models were adjusted for baseline covariates, including age, sex, body mass index, surgical approach, and the revised cardiac risk index. Survival curves were generated with the Kaplane – Meier method. Statistical analyses were performed with the R studio software package (version 0.99.491, Boston, MA) and STATA software package (version 14.1, College Station, Texas).
Results
The cohort consisted of 148 patients undergoing endovascular or open interventions for PAD, with a mean age of 70.3 ± 11.0 and 39% females (Table III). Patients received either an endovascular intervention (54.7%) or an open intervention (45.3%), and there was no clinically relevant difference in the proportion who had depression in each group (27.2% and 29.9% for endovascular interventions and open interventions). Overall, the prevalence of positive depression screening was 28.4% (N = 42), whereas the documented prevalence in the clinical chart was only 3.3%
Table III.
Study 2 baseline characteristics of cohort of patients stratified by depression status
| Total N = 148 | Depressed N = 42 (28.4%) | Not depressed N = 106 (71.6%) | P value | |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
| Age (years) | 70.3 ± 11.0 | 69.5 ± 11.2 | 70.6 ± 11.0 | 0.5861 |
| Male (%) | 90 (60.8%) | 25 (59.5%) | 65 (59.6%) | 0.5381 |
| Endovascular (%) | 81 (54.7%) | 22 (52.4%) | 59 (55.7%) | 0.7919 |
| Open (%) | 67 (45.3%) | 20 (47.6%) | 47 (44.3%) | 0.8053 |
| Patient Characteristics | ||||
| Critical limb ischemia (%) | 65 (43.9%) | 24 (54.1%) | 41 (38.7%) | 0.2314 |
| Smoking (%) | 111 (75%) | 34 (81.0%) | 77 (72.6%) | 0.3472 |
| Hypertension (%) | 106 (71.6%) | 33 (78.6%) | 73 (68.9%) | 0.3058 |
| Dyslipidemia (%) | 92 (62.2%) | 29 (69.0%) | 63 (59.4%) | 0.3797 |
| Coronary artery disease (%) | 45 (30.4%) | 14 (33.3%) | 31 (29.2%) | 0.7844 |
| Chronic kidney disease (%) | 14 (9.5%) | 7 (16.7%) | 7 (6.6%) | 0.5703 |
| Cerebrovascular disease (%) | 11 (7.4%) | 4 (10.0%) | 7 (6.6%) | 0.8474 |
| Chronic obstructive pulmonary disease (%) | 20 (13.5%) | 6 (14.3%) | 14 (13.2%) | 0.9488 |
At 6 months, 4 deaths (9.5%) were observed in the depressed group compared to 1 death (0.9%) in the nondepressed group (Table IV, Fig. 1). On multivariable analysis, the association between depression and6-monthall-causemortality was statistically significant (odds ratio [OR] 1.48, 95% confidence interval [CI]: 1.08 to 2.29). Depression did not significantly predict vascular reintervention (OR 1.11, 95% CI: 0.99 to 1.26), readmission (OR 1.16, 95% CI 0.96 to 1.39), or length of stay (OR 1.16, 95% CI 0.96 to 1.39).
Table IV.
Outcome variables
| Outcome variables | Total N = 148 | Depressed N = 42 (28.3%) | Not depressed N = 106 (71.6%) |
|---|---|---|---|
|
| |||
| Primary end point | |||
| All-cause mortality at 6 months (%) | 5 (3.4%) | 4 (9.5%) | 1 (0.9%) |
| Secondary end points | |||
| Length of stay (days) | 3.1 [0–52] | 4.9 [0–52] | 2.6 [0–49] |
| Reintervention (%) | 50 (33.8%) | 16 (38.1%) | 34 (32.1%) |
| Readmission (%) | 46 (31.1%) | 15 (35.7%) | 31 (29.2%) |
Fig. 1.
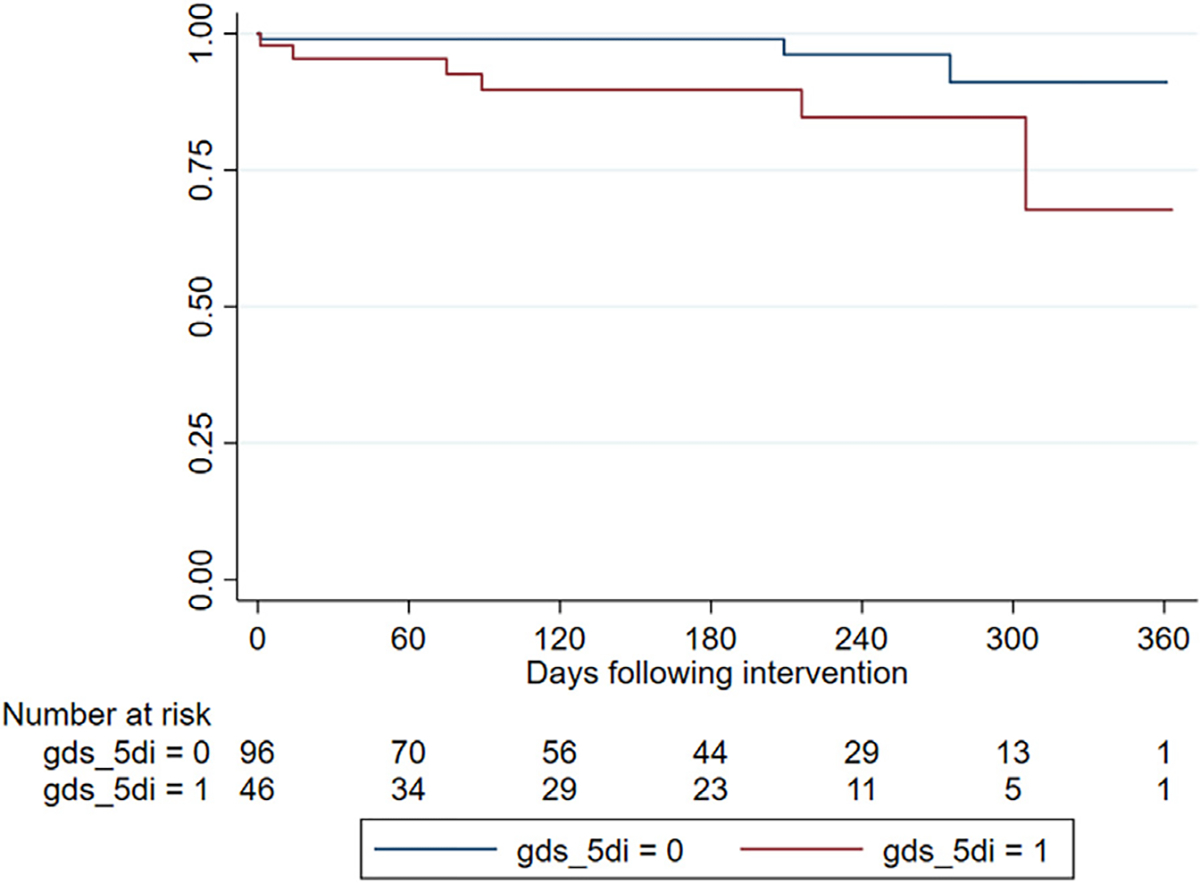
Kaplan–Meier Survival Curve. Kaplan–Meier Survival Curve of PAD patients undergoing open or endovascular interventions. Patients with concomitant depression have a worse survival rate at 6 months compared to patients who do not suffer from depression.
DISCUSSION
The results of these 2 studies demonstrate that depression is underdiagnosed among patients with PAD in diverse cohorts and that the presence of depression was significantly associated with mortality. Regardless of the depression screening tool used, depression was frequently identified in patients undergoing treatment for PAD. Depression is underdiagnosed by up to 19–25%. Our results suggest that depression underdiagnosis occurs across different centers and regions, considering that our work was conducted in 2 different countries with distinct PAD population characteristics. Together, these results indicate that the underdiagnosis of depression holds true across the spectrum of PAD disease severity and is consistent between the PHQ-9 and GDS-S scales and across American and Canadian cohorts.
Furthermore, our results indicate that even among patients with known depression, 57% still had elevated depression symptom scores, suggesting that these patients are undertreated. This is of particular significance because the results of the second study indicate that among Canadian adults presenting for PAD intervention, depression is associated with increased all-cause mortality. This supports prior research, which found that, among U.S. veterans, a comorbid diagnosis of depression at the time of PAD diagnosis was associated with a 17% greater mortality risk over 5 years than patients without depression.14 Additionally, a recent meta-analysis foundthat depression wasassociatedwitha24% increase in mortality, making it a significant clinical factor in PAD management.15
These findings indicate that identifying and treating depression is a crucial target to improve the care for patients with PAD. In addition to increased mortality, depression has been associated with a decline in patients’ quality of life: Depressed patients have shorter pain-free and total walking distances and experience more significant annual declines in functioning relative to their nondepressed counterparts.24,25 Depression severity is also associated with higher inflammation in PAD patients,26 and depressed patients have a greater risk of recurrent and debilitating symptoms.27 Therefore, it is imperative to intervene in PAD patients with depressive symptoms to improve quality of life and reduce mortality risk.
Past research has found an increased risk of depression among women with PAD.17 Similarly, our study found that women were more likely to have a history of depression; however, we found that men were more likely to be underdiagnosed with depression. These results suggest the importance of considering the role of depression in all patients presenting with PAD and may be an important avenue for future research to investigate the influence of gender on depression symptom presentation within this population. This is especially salient given the scarcity of research on the underdiagnosis of depression in men and even fewer investigations into depression in men with PAD.
Several studies have demonstrated that treating depression in PAD patients is feasible. Garnefski et al. found that adjustments in cognitive coping strategies were related to improvement in depressive symptoms in a cohort of patients with PAD.28 Similarly, a small trial of a cognitive-behavioral self-help program for patients with depression and PAD found significantly improved depression symptom scores at posttest and follow-up.29 These findings indicate that treating depression is a reachable target in patients with PAD and should be an important component of the care plan. Given our findings of underdiagnosed depression in PAD patients, a collaborative approach between vascular and primary care providers may benefit the identification and treatment of depression in the setting of PAD.
There are several limitations to consider. The first is that the PHQ-9 and GDS-S are screening tools rather than diagnostic tools. While both are well-studied and widely used to assess depression symptom severity, elevated scores are not necessarily diagnostic of major depressive disorder or other psychiatric illnesses. While the generalizability of results is improved by assessing both cohorts, the datasets could not be combined to improve the power of the study since different depression screening tools are used at each of the institutions. Additionally, the patient populations served in North Carolina and Quebec are heterogenous with different access to health insurance, public health policies, and ultimately different mental health screening protocols and procedures. Nonetheless, the reproducibility of our findings in these heterogenous settings is compelling evidence that a relationship exists between depression and PAD. Both studies are limited by modest sample size; however, it is noteworthy that both study sites found similar results indicating underdiagnosis of depression, despite the differences between the methodologies. Regarding the findings on depression and mortality, the number of total deaths (5) was low within the 6 months and this association may have been strengthened with more data at the 1-year and 3-year benchmark. Additionally, we did not specifically assess how depression impacts limb loss and amputation-free survival, which may be of interest in future analysis as limb preservation is an important clinical target in PAD. Finally, due to small sample size, we do not have enough power to report on the association between severity of depression and severity of PAD; however, this is an important avenue for future research and intervention.
CONCLUSIONS
Depression is underdiagnosed and undertreated among patients with PAD and is a significant predictor of all-cause mortality among PAD patients undergoing revascularization. This may carry significant implications for the success of complex treatment strategies, outcomes, disease progression, and quality of life. Our findings indicate a strong need for more robust screenings and comprehensive mental health treatment for patients with PAD and depression.
Supplementary Material
Funding sources:
This work was supported by the Carolina Medical Student Research Program (CMSRP).
Footnotes
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.1016/j.avsg.2023.03.002.
REFERENCES
- 1.Hardman RL, Jazaeri O, Yi J, et al. Overview of classification systems in peripheral artery disease. Semin Intervent Radiol 2014;31:378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquina PF, Miller M, Carvalho AJ, et al. Special considerations for multiple limb amputation. Curr Phys Med Rehabil Rep 2014;2:273–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain MA, Al-Omran M, Salata K, et al. Population-based secular trends in lower-extremity amputation for diabetes and peripheral artery disease. Can Med Assoc J 2019;191:E955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell RJ, Choudhry N, Conte M, et al. Factors associated with lower preoperative quality of life in patients with chronic limb-threatening ischemia in the BEST-CLI trial. J Vasc Surg 2022;76:1642–50. [DOI] [PubMed] [Google Scholar]
- 5.Wells KB, Golding JM, Burnam MA. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry 1988;145:976–81. [DOI] [PubMed] [Google Scholar]
- 6.Haschke A, Hutter N, Baumeister H. Indirect costs in patients with coronary artery disease and mental disorders: a systematic review and meta-analysis. Int J Occup Med Environ Health 2012;25:319–29. [DOI] [PubMed] [Google Scholar]
- 7.Damen NL, Versteeg H, Boersma E, et al. Depression is independently associated with 7-year mortality in patients treated with percutaneous coronary intervention: results from the RESEARCH registry. Int J Cardiol 2013;167:2496–501. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003;362:604–9. [DOI] [PubMed] [Google Scholar]
- 9.Connerney I, Sloan RP, Shapiro PA, et al. Depression is associated with increased mortality 10 years after coronary artery bypass surgery. Psychosom Med 2010;72:874–81. [DOI] [PubMed] [Google Scholar]
- 10.Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. J Geriatr Cardiol 2012;9:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda Y, Mok Y, Mathews L, et al. Psychosocial factors and subsequent risk of hospitalizations with peripheral artery disease: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2021;329:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiner Ž, De Sutter J, Ryden L, et al. Peripheral arterial disease and intermittent claudication in coronary heart disease patients. Int J Cardiol 2021;322:227–32. [DOI] [PubMed] [Google Scholar]
- 13.Brostow DP, Petrik ML, Starosta AJ, et al. Depression in patients with peripheral arterial disease: a systematic review. Eur J Cardiovasc Nurs 2017;16:181–93. [DOI] [PubMed] [Google Scholar]
- 14.Arya S, Lee S, Zahner GJ, et al. The association of comorbid depression with mortality and amputation in veterans with peripheral artery disease. J Vasc Surg 2018;68:536–545.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scierka LE, Mena-Hurtado C, Ahmed ZV, et al. The association of depression with mortality and major adverse limb event outcomes in patients with peripheral artery disease: a systematic review and meta-analysis. J Affect Disord 2022;320:169–77. [DOI] [PubMed] [Google Scholar]
- 16.Agnelli G, Belch JJF, Baumgartner I, et al. Morbidity and mortality associated with atherosclerotic peripheral artery disease: a systematic review. Atherosclerosis 2020;293:94–100. [DOI] [PubMed] [Google Scholar]
- 17.Smolderen KG, Spertus JA, Vriens PW, et al. Younger women with symptomatic peripheral arterial disease are at increased risk of depressive symptoms. J Vasc Surg 2010;52:637–44. [DOI] [PubMed] [Google Scholar]
- 18.Smolderen KG, Hoeks SE, Pedersen SS, et al. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med 2009;14:297–304. [DOI] [PubMed] [Google Scholar]
- 19.Aragão JA, de Andrade LGR, Neves OMG, et al. Anxiety and depression in patients with peripheral arterial disease admitted to a tertiary hospital. J Vasc Bras 2019;18:e20190002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolderen KG, Plomondon ME, Armstrong EJ, et al. Depression and long-term prognostic outcomes following peripheral endovascular interventions in the VA Healthcare System. Vasc Med 2018;23:454–60. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drudi LM, Ades M, Mancini R, et al. Frailty assessment in older adults undergoing interventions for peripheral arterial disease. J Vasc Surg 2019;70:1594–1602.e1. [DOI] [PubMed] [Google Scholar]
- 23.Lesher EL, Berryhill JS. Validation of the geriatric depression scale–short form among inpatients. J Clin Psychol 1994;50:256–60. [DOI] [PubMed] [Google Scholar]
- 24.Ruo B, Liu K, Tian L, et al. Persistent depressive symptoms and functional decline among patients with peripheral arterial disease. Psychosom Med 2007;69:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott MM, Greenland P, Guralnik JM, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med 2003;18:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez NV, Ramirez JL, Khetani SA, et al. Depression severity is associated with increased inflammation in veterans with peripheral artery disease. Vasc Med 2018;23: 445–53. [DOI] [PubMed] [Google Scholar]
- 27.Cherr GS, Wang J, Zimmerman PM, et al. Depression is associated with worse patency and recurrent leg symptoms after lower extremity revascularization. J Vasc Surg 2007;45:744–50. [DOI] [PubMed] [Google Scholar]
- 28.Garnefski N, Grol M, Kraaij V, et al. Cognitive coping and goal adjustment in people with Peripheral Arterial Disease: relationships with depressive symptoms. Patient Educ Couns 2009;76:132–7. [DOI] [PubMed] [Google Scholar]
- 29.Garnefski N, Kraaij V, Wijers E, et al. Effects of a cognitive-behavioral self-help program on depressed mood for people with peripheral arterial disease. J Clin Psychol Med Settings 2013;20:186–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


