Abstract
Pathogenic Salmonella species initiate infection of a host by inducing their own uptake into intestinal epithelial cells. An invasive phenotype is conferred to this pathogen by a number of proteins that are components of a type III secretion system. During the invasion process, the bacteria utilize this secretion system to release proteins that enter the host cell and apparently interact with unknown host cell components that induce alterations in the actin cytoskeleton. To investigate the role of secreted proteins as direct modulators of invasion, we have evaluated the ability of Salmonella typhimurium to enter mammalian cells that express portions of the Salmonella invasion proteins SipB and SipC. Plasma membrane localization of SipB and SipC was achieved by fusing carboxyl- and amino-terminal portions of each invasion protein to the intracellular carboxyl-terminal tail of a membrane-bound eukaryotic receptor. Expression of receptor chimeras possessing the carboxyl terminus of SipB or the amino terminus of SipC blocked Salmonella invasion, whereas expression of their chimeric counterparts had no effect on invasion. The effect on invasion was specific for Salmonella since the perturbation of uptake was not extended to other invasive bacterial species. These results suggest that Salmonella invasion can be competitively inhibited by preventing the intracellular effects of SipB or SipC. In addition, these experiments provide a model for examining interactions between bacterial invasion proteins and their host cell targets.
Salmonella infections continue to be an important health concern in both developed and undeveloped countries (14). These pathogenic bacteria possess many virulence determinants which they use to establish infection of a host. For example, lipopolysaccharide provides protection against host killing mechanisms (52, 54, 56). Additionally, the bacteria possess a set of genes that encode a type III secretion system which enables the bacteria to penetrate the membrane of host cells (1, 24, 27, 29, 35, 38, 42). Other determinants, including a virulence plasmid, permit these bacteria to survive and grow within the host lymphatic system (16, 17, 28, 30–33, 53, 58).
The ability to invade mammalian cells is critical to the ability of Salmonella to initiate infection of a host (39). Studies using microscopy and immunofluorescence to examine the internalization event have found that the cytoskeleton of the host is dramatically rearranged during entry (20, 27, 40) and depends on the polymerization of actin (15, 19, 26). Recent work from several laboratories has identified the genes responsible for Salmonella invasion (3, 12, 23, 42, 47). These genes reside on 35 kb of contiguous DNA that comprise pathogenicity island 1 (46) and maps to centisome 63 of the chromosome. The proposed functions of these gene products include transcriptional regulation of the invasion genes, protein secretion, and activation of host cell uptake pathways.
Numerous studies have identified proteins that are secreted into the extracellular media during the growth of invasive Salmonella typhimurium (34, 35, 42, 50). Two of these secreted invasion proteins, SipB and SipC, are likely the candidate proteins that interact directly with eukaryotic cell targets because they are essential for invasion (41), are the major secreted invasion proteins (50), and are translocated into the host cell in association with bacterial invasion (11). Furthermore, purified recombinant IpaC, the Shigella homologue of SipC (29), can activate cellular kinase activity and promote cellular uptake of noninvasive Shigella flexneri (44).
While it is clear that SipB and SipC have important roles in Salmonella internalization, the molecular details of their functions are unclear. We have explored the possibility of expressing SipB and SipC in cells sensitive to Salmonella invasion as a potential method for characterizing an interaction between these two invasion proteins and eukaryotic cells. Portions of SipB or SipC were fused to a plasma membrane-bound receptor to facilitate expression in mammalian cells. Surprisingly, expression of distinct portions of either SipB or SipC specifically blocked the ability of invasive Salmonella to penetrate cells.
MATERIALS AND METHODS
Construction of invasion protein chimera cDNA plasmids.
Chromosomal DNA fragments from S. typhimurium SL1344 (60) invasion genes encoding SipB and SipC were amplified by PCR such that a BspE1 site was engineered into the 5′ end while an XhoI or NotI site was engineered into the 3′ end. PCR products were cloned and amplified in pGEM-T (Promega). Cloned invasion gene sequences were then ligated into pCR3 (Invitrogen) containing platelet-activating factor (PAF) receptor (PAFR) sequences (kindly provided by Rory Fisher, The University of Iowa), using a BspE1 site in the 3′ end of the PAFR cDNA and an XhoI or NotI site in the downstream region of the pCR3 multiple cloning site. Oligonucleotide primers used for sequencing and PCR were synthesized by the University of Iowa DNA Core Facility. Forward and reverse primer sequences are, respectively, 5′-TCCGGATGGTAAATGACGCAAGTAGC-3′ and 5′-GTTCGTTTCCTCGAGTTAGCGCGTCTG-3′ for the amino terminus of SipB, 5′-TCCGGAAGAAATCGGCTGAGTTCCAGG-3′ and 5′-ATGTCGACTTATGCGCGACTCTGGCGCA-3′ for the carboxyl terminus of SipB, 5′-TCCGGATGTTAATTAGTAATGTGGGAA-3′ and 5′-CATTCTCGAGCCCCTTTTATTTCCAGTT-3′ for the amino terminus of SipC, and 5′-TCCGGATGAATGCGTTGTCCGGTAGTA-3′ and 5′-ATGTCGACTTAAGCGCGAATATTGCCTG-3′ for the carboxyl terminus of SipC. Sequences were verified by automated fluorescent dideoxynucleotide sequencing by the University of Iowa DNA Core Facility.
Cell culture and transfections.
Baby hamster kidney (BHK) cells (American Type Culture Collection) were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum at 37°C in a 5% CO2 humidified atmosphere. Cells were split every 3 to 4 days via trypsinization. Cells were transfected by using lipofection or electroporation, with similar results. Electroporation was performed as described previously (48) in 0.4-cm cuvettes, using 3.8 × 106 cells, 25 μg of DNA, 220 V, 950 μF, and 90 ms. Lipofection was performed in 24-well tissue culture dishes as described previously (8), using 1 μg of DNA per well and 5 μl of Lipofectamine (GIBCO-BRL) per μg of DNA. Human embryonic kidney (HEK) and COS-7 cells were maintained and transfected in a similar fashion except that 0.5 μg of DNA per well was used in the transfection cocktail. For BHK cell dose-response experiments, total plasmid DNA was maintained at 1 μg per well by the addition of insertless pCR3 vector.
Invasion assays.
Bacterial invasion, as determined by a gentamicin resistance assay (40), was assessed 48 to 54 h after transfection by inoculating 106 to 107 bacteria per well. Bacteria were incubated with transfected cells for 60 min. Cells were then incubated for 90 min with culture media containing gentamicin (50 μg/ml) to eliminate extracellular bacteria. Percent invasion was normalized based on the percent invasion observed in cells transfected with the PAFR cDNA. S. typhimurium SL1344 (60), Listeria monocytogenes 10403S (4), enteropathogenic Escherichia coli (EPEC) JPN15/pMAR7 (2), and E. coli HB101 expressing invasin (plasmid kindly provided by Ralph Isberg, Tufts University) were the invasive strains used in these studies.
Evaluation of ligand binding and receptor signaling in transfected cells.
BHK cell transfectants were examined for the ability to bind [3H]WEB 2086 and to accumulate inositol phosphates (IP) in response to PAF. Intact cell binding of [3H]WEB 2086 was assessed at 25°C by a previously described method (49) in which saturation binding data were transformed to a Scatchard plot for the determination of receptor expression and affinity. PAF-induced IP accumulation was determined by chloroform-methanol extraction and ion-exchange chromatography as described previously (8).
RESULTS
Expression of Salmonella invasion proteins in BHK cells.
Several groups have identified SipB and SipC as the major invasion proteins secreted by invasive Salmonella (34, 35, 42, 50), while another group documented the translocation of these two proteins into the host cell in association with invasion (11). Since it is unclear if invasion protein translocation precedes or follows bacterial invasion, we wished to evaluate the role of SipB and SipC as intracellular effectors of invasion. Therefore, we explored the feasibility of expressing SipB and SipC in cells as a means of evaluating their potential intracellular invasion-determining activities.
Since SipB and SipC are prokaryotic proteins, it is difficult to ensure their plasma membrane expression in eukaryotic cells. To ensure that they reach this putative site of action, we individually fused portions of either SipB or SipC to the seven-transmembrane-spanning guanine nucleotide-binding protein-coupled PAFR. This fusion-based approach was chosen because previous studies in our laboratory revealed that transfection of plasmids expressing SipB or SipC from the cytomegalovirus promoter had no effect on Salmonella invasion (data not shown). Fusions of invasion proteins to a receptor were used, as ligand-binding and agonist-induced second-messenger studies could verify the expression of the acceptor portion of the fusion protein. The PAFR was chosen since 40 of 46 amino acids from the intracellular carboxyl terminus of this protein can be removed without diminishing receptor expression. Additionally, a constitutively active PAFR is generated when the receptor is truncated at this site in the intracellular carboxyl-terminal tail (55). Based on this signaling property of the PAFR, failure of invasion protein fusion at this site should result in a constitutively active PAFR. Since none of the PAFR-invasion protein fusions were constitutively active (data not shown), the intracellular carboxyl-terminal tail of the PAFR was exploited as a site for expressing heterologous peptide sequences from SipB and SipC. Therefore, using this membrane anchor approach to express SipB and SipC in eukaryotic cells, we could evaluate their intracellular effects on Salmonella invasion.
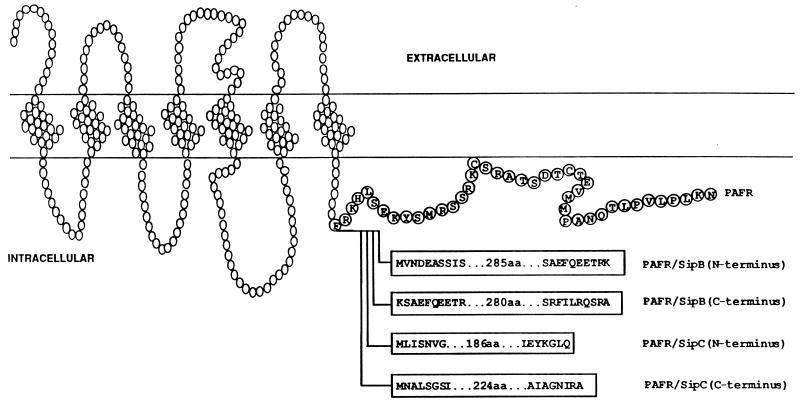
Since both SipB and SipC are larger than the PAFR, invasion protein domains were fused to avoid potential steric hindrances imposed by fusion of the full-length invasion proteins. Additionally, previous studies have revealed that protein domains can be useful experimental tools when fused to other proteins or when individually expressed (discussed in reference 8). Therefore, we prepared cDNAs encoding PAFR fusions to peptide sequences derived from the amino terminus of SipB (PAFR/BN), the carboxyl terminus of SipB (PAFR/BC), the amino terminus of SipC (PAFR/CN), and the carboxyl terminus of SipC (PAFR/CC) (Fig. 1). Specifically, DNAs encoding secreted invasion protein domains were cloned onto the 3′ end of the coding region of a PAFR cDNA plasmid. Plasmid DNA was transfected into BHK cells. BHK cells were chosen because they are sensitive to Salmonella invasion, possess no endogenous PAFRs, and, unlike PAFR-expressing cells, do not accumulate IP second messengers in response to PAF (6–9). To assess receptor expression, studies were undertaken to evaluate ligand binding and PAF-induced IP accumulation in BHK cell transfectants. Ligand binding of intact cells was determined by using the PAFR antagonist WEB 2086 (13). The evaluation of receptor signaling and expression revealed that the PAFR and all four PAFR-invasion protein chimeras were expressed at similar levels (data not shown).
FIG. 1.
Structures of PAFR-invasion protein chimeras. Open circles correspond to the PAFR backbone; blocks in the carboxyl-terminal tail contain letters corresponding to the amino- and carboxyl-terminal residues of the four invasion protein segments that were fused to the PAFR to generate the chimeric receptors. The introduction of invasion protein sequences following F301 resulted in a deletion of 40 amino acids (aa), represented by encircled letters, from the carboxyl-terminal tail of the PAFR.
Attenuation of Salmonella invasion by transfection of cDNAs encoding PAFR-invasion protein chimeras.
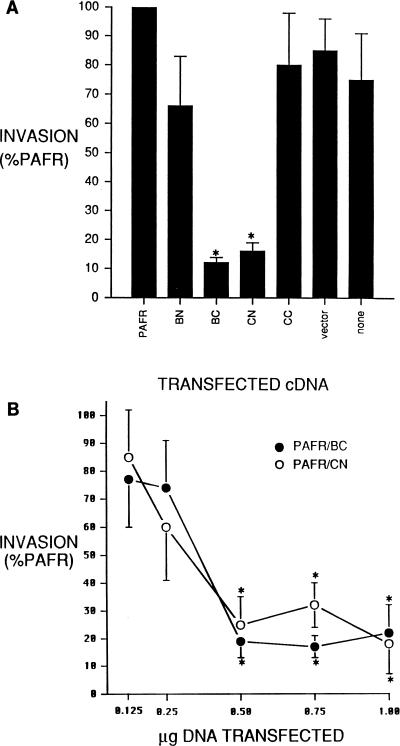
The ability of S. typhimurium to invade was evaluated in BHK cells transfected with cDNAs encoding one of the four PAFR-invasion protein chimeras. As presented in Fig. 2A, BHK cells transfected with cDNA encoding PAFR/BC or PAFR/CN were significantly less susceptible to Salmonella invasion than were PAFR transfectants. The invasion of Salmonella was decreased by 88% ± 5% and 84% ± 6% in PAFR/BC and PAFR/CN transfectants, respectively. However, no significant difference in invasion was detected in BHK cells transfected with PAFR/BN or PAFR/CC cDNA. Additionally, no synergistic or enhancing effects on invasion were detected in BHK cells simultaneously transfected with two, three, or four of the chimeric cDNAs (data not shown). To assess whether the effect was specific to BHK cells, we performed similar transfection-based experiments with HEK293 and COS-7 cells. An analogous pattern of invasion inhibition was observed when HEK293 or COS-7 cells were transfected with PAFR/BC or PAFR/CN cDNA (data not shown). As depicted in Fig. 2B, the levels of inhibition mediated by PAFR/BC and PAFR/CN were dependent on the amount of cDNA introduced into cells, as transfection of 0.5, 0.75, and 1 μg of DNA/well significantly inhibited invasion (68% ± 8% to 83% ± 4% inhibition versus PAFR controls), whereas no inhibition was detected following transfection of 0.125 or 0.25 μg of DNA/well. The latter response is consistent with cotransfection results (not shown) in which no inhibition was detected in cells cotransfected with 0.25 μg of DNA of all four constructs per well.
FIG. 2.
Attenuation of Salmonella invasion by transfection of cDNAs encoding PAFR-invasion protein chimeras. (A) Effect of transfecting PAFR-invasion protein fusions on the invasion of S. typhimurium SL1344. BHK cells were transfected with insertless pCR3 vector (vector) or pCR3 containing a cDNA encoding the PAFR, PAFR/BN (BN), PAFR/BC (BC), PAFR/CN (CN), or PAFR/CC (CC). Invasion was also assessed in untransfected BHK cells (none). Cells were transfected, invasion assays were performed, and results were analyzed as described in Materials and Methods. Data represent the mean ± standard error of the mean from three separate transfections assayed simultaneously and repeated at least twice with similar results. Statistical significance was determined by analysis of variance with Scheffe’s F test for multiple comparisons. ∗, P < 0.05 versus PAFR transfectants. (B) Effects of transfecting increasing amounts of cDNAs encoding PAFR/BC or PAFR/CN. Total plasmid DNA was maintained at 1 μg/well by the addition of insertless pCR3 vector. Cells were transfected, invasion assays were performed, and results were analyzed as described in Materials and Methods. Data presented are standardized based on invasion data obtained from cells transfected with an equal dose of the PAFR cDNA. Invasion data derived from PAFR transfectants was statistically indistinct at all transfection doses. Dose-response data represents the mean ± standard error of the mean from three separate transfections assayed simultaneously and repeated at least twice with similar results. Statistical significance was determined by Student’s paired t test. ∗, P < 0.05 versus result from an equal dose of PAFR cDNA.
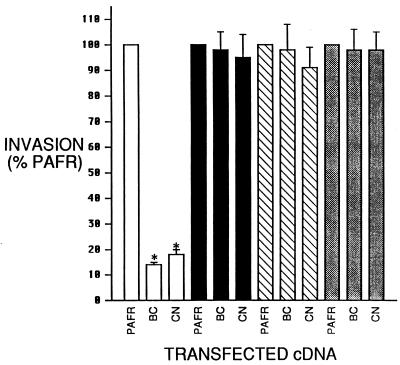
Relationship between MOI and Salmonella invasion in BHK cells expressing PAFR/BC or PAFR/CN.
We also assessed the relationship between the inhibition of invasion and the number of bacteria in the invasion assays, i.e., the multiplicity of infection (MOI). Despite exponential increases in MOI, BHK cells transfected with cDNAs encoding PAFR/BC or PAFR/CN were uniformly resistant to Salmonella invasion compared with BHK cells transfected with the PAFR cDNA (Fig. 3). The extent of inhibition did not vary with the MOI and ranged from 86% ± 6% (PAFR/CN, MOI of 10) to 91% ± 2% (PAFR/BC, MOI of 1).
FIG. 3.
Relationship between MOI and Salmonella invasion in BHK cells expressing PAFR/BC or PAFR/CN. Cells were transfected with 1 μg of plasmid DNA per well as described for Fig. 2A; invasion was assessed and analyzed as described for Fig. 2B. Bacteria were added at 5 × 105, 5 × 106, or 5 × 107/well to assess invasion at an MOI of 1 (open bars), 10 (black bars), or 100 (hatched bars), respectively. Data presented are standardized based on the percent invasion obtained from cells transfected with the PAFR cDNA and exposed to the same number of bacteria. Unstandardized invasion data derived from PAFR transfectants ranged from 7.4% ± 0.4% (MOI of 1) to 11.5% ± 0.6% (MOI of 100). Data represents the mean ± standard error of the mean from three separate transfections assayed simultaneously and repeated at least twice with similar results. Statistical significance among all responses was determined by analysis of variance with Scheffe’s F test for multiple comparisons. ∗, P < 0.05 versus PAFR transfectants.
Specificity of the PAFR/BC- or PAFR/CN-mediated inhibition of Salmonella invasion of BHK cell transfectants.
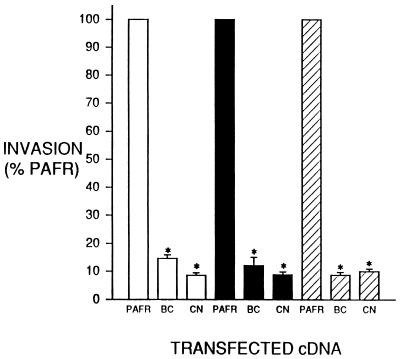
To evaluate the specificity of the inhibition of Salmonella invasion in cells expressing PAFR/BC or PAFR/CN, we examined the effect of expressing these two hybrid receptors on the invasion of Listeria and EPEC and bacterial internalization mediated by the Yersinia invasin gene. Previous work has demonstrated that Listeria (22, 45), EPEC (5, 51), and bacteria expressing invasin (36, 37) invade by mechanisms different from that ascribed to Salmonella. The transient transfection system used for Salmonella invasion assays was employed to measure the effect of PAFR/BC or PAFR/CN on the invasion of these three other invasive organisms. Transfection of cDNAs encoding PAFR/BC and PAFR/CN, which inhibit Salmonella invasion 86% ± 7% and 87% ± 6%, respectively, of the level for the PAFR control, had no effect on bacterial entry mediated by Listeria, EPEC, or invasin (Fig. 4).
FIG. 4.
Specificity of the PAFR/BC- or PAFR/CN-mediated inhibition of Salmonella invasion of BHK cell transfectants. Shown are effects of transfecting the PAFR/BC (BC) or PAFR/CN (CN) cDNA on the invasion of Salmonella (open bars), Listeria (filled bars), E. coli expressing invasin (hatched bars), and EPEC (gray bars). Cells were transfected by electroporation, and invasion assays were performed as described for Fig. 2B at an MOI of 10. Data presented are based on the percentage of bacterium-specific invasion observed in cells transfected with an equal dose of the PAFR cDNA. Data represents the mean ± standard error of the mean from three separate transfections assayed simultaneously and repeated at least twice with similar results. Statistical significance was determined by Student’s paired t test. ∗, P < 0.05 versus PAFR transfectants.
DISCUSSION
The data presented above indicate that cellular expression of portions of Salmonella-secreted invasion proteins results in specific inhibition of Salmonella invasion. This effect on invasion was dose dependent and specific for Salmonella. The significance of these results is underscored by other studies documenting an inhibition of Salmonella invasion. Many groups have inhibited Salmonella invasion by exposing tissue culture cells to the actin polymerization inhibitor cytochalasin D (15, 19, 25). Recent work has shown that Salmonella entry can be reduced by expressing a dominant negative CDC42 protein in COS-1 cells, thus implicating this molecule as a mediator of invasion (10).
The precise mechanisms underlying the inhibition of invasion presented in our study are unknown, although there are several possible explanations. First, the introduction of Salmonella invasion protein domains into tissue culture cells may activate host cell processes that generally prevent the uptake of particles. This does not appear likely since PAFR/BN and PAFR/CC did not affect invasion, although their inability to do so may be hindered by biophysical constraints imposed on these two proteins but not imposed on PAFR/BC and PAFR/CN. Our finding that uptake of three unrelated invasive bacteria was unaffected by the expression of PAFR/BC and PAFR/CN also argues against this explanation. Additionally, since transfected and untransfected cells appeared normal based on cytoskeletal staining experiments (not shown), PAFR/BC and PAFR/CN expression did not perturb normal actin rearrangement distribution.
A second explanation is that a region of the invasion protein portion of the hybrid receptor may be located extracellularly and sterically interfere with the ability of Salmonella to enter cells. Likewise, invasion protein sequences could traverse the membrane and function as a tethered antagonist for extracellular invasion protein receptors. This phenomenon is plausible since both SipB and SipC may possess putative membrane-spanning domains (42). However, increasing the MOI 100-fold had no effect on the inhibition of Salmonella entry whereas invasion was restored when transfection-mediated receptor expression was diminished by decreasing the dose of transfected cDNA. As the relationship between chimeric receptors and invasive bacteria does not directly correlate with the inhibition of invasion, it appears unlikely that the invasion protein sequences prevent invasion through a direct interaction with the bacteria or by binding to an extracellular invasion protein receptor.
A third explanation for the invasion-inhibiting activities of PAFR/BC and PAFR/CN is that the two invasion protein sequences interact with proteins capable of regulating the integrity of the cytoskeleton. That is, the invasion protein segments interact with the cellular target of the invasion proteins or with native invasion proteins translocated by the bacteria. This leads to a model in which the full-length invasion proteins activate a signal that induces cytoskeletal changes whereas the incomplete invasion proteins behave as weak partial agonists/competitive antagonists. This type of partial protein-based antagonism has been previously described with coexpression of a catecholamine receptor and peptides corresponding to its intracellular domains (43). Similarly, a peptide corresponding to the ligand-binding domain of the thyroid hormone receptor was able to compete with the thyroid hormone receptor for a transcriptional corepressor molecule (57). Extrapolation of these two competitive sequestration models to our results suggests that SipB and SipC interact with intracellular regulators of the cytoskeleton, consistent with studies demonstrating the translocation of these two proteins into the cell during bacterial invasion (11). Therefore, it is possible that the carboxyl terminus of SipB and the amino terminus of SipC retain binding sites for the proteins to which the full-length invasion proteins bind during the entry process. Alternatively, it is possible that the carboxyl terminus of SipB serves as a chaperone for SipC or that the amino terminus of SipC serves as a chaperone for SipB; that is, PAFR/BC sequesters wild-type secreted SipC or PAFR/CN sequesters wild-type secreted SipB. This latter possibility is supported by the notion that chaperone proteins involved in type III secretion can facilitate effector translocation (21) or prevent effector interactions with secretion apparatus proteins (59) by binding to secreted proteins. Additionally, the inhibitory chaperone phenomenon is supported by our finding that the fused invasion protein sequences do not appear to disturb cellular functions. In summary, while the exact mechanisms underlying the inhibition of invasion observed in PAFR/BC and PAFR/CN transfectants are unclear, our studies indicate that SipB and SipC serve as intracellular effectors of invasion.
The entrance of Salmonella, or any invasive organism, into eukaryotic cells represents a focus for studying and preventing the pathogenic effects of the bacteria (18). For Salmonella, SipB and SipC represent a direct line of communication between the bacteria and its target cell. In support of this premise is our finding that expression of portions of SipB and SipC at the cellular membrane significantly perturbs Salmonella invasion. This method of invasion inhibition may have implications for the pharmacotherapeutic prevention of Salmonella pathogenesis at the initial site of bacterium-host contact. Additionally, the fusion of Salmonella invasion proteins to a membrane-bound receptor may serve as a useful model for studying the molecular actions of other bacterial invasion proteins.
ACKNOWLEDGMENTS
This work was supported by grant A138268 from the National Institutes of Health to B.D.J. S.A.C. was a recipient of an Infectious Diseases Postdoctoral training grant fellowship (AI 07343-09) from the National Institutes of Health via the University of Iowa Department of Internal Medicine, Division of Infectious Diseases.
We especially thank Rory Fisher for the contribution of reagents used in these studies and for reading the manuscript. We also thank John Harty for reading the manuscript, Tom Fahlen for advice on invasion assays, and Joanna Klein and Alana Latzke for ancillary technical support. In addition, we thank Rebecca Wilson for the Listeria strain used in this study.
REFERENCES
- 1.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 2.Baldini M M, Kaper J B, Levine M M, Candy D C A, Moon H W. Plasmid-mediated adhesion of enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2:534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- 3.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 5.Cantey J R, Moseley S L. HeLa cell adherence, actin aggregation, and invasion by nonenteropathogenic Escherichia coli possessing the eae gene. Infect Immun. 1991;59:3924–3929. doi: 10.1128/iai.59.11.3924-3929.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson S A, Chatterjee T K, Fisher R A. Lack of constitutive activation or inactivation of the platelet-activating factor receptor by glutamate substitution of alanine 230. Recept Signal Transduct. 1996;6:111–120. [PubMed] [Google Scholar]
- 7.Carlson S A, Chatterjee T K, Murphy K P, Fisher R A. Mutation of a putative amphipathic alpha-helix in the third intracellular domain of the platelet-activating factor receptor disrupts receptor/G protein coupling and signaling. Mol Pharmacol. 1998;53:451–458. doi: 10.1124/mol.53.3.451. [DOI] [PubMed] [Google Scholar]
- 8.Carlson S A, Chatterjee T K, Fisher R A. The third intracellular domain of the platelet-activating factor receptor is a critical determinant in receptor coupling to phosphoinositide phospholipase C-activating G proteins. Studies using intracellular domain minigenes and receptor chimeras. J Biol Chem. 1996;271:23146–23153. doi: 10.1074/jbc.271.38.23146. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee T K, Eapen A K, Fisher R A. A truncated form of RGS3 negatively regulates G protein-coupled receptor stimulation of adenylyl cyclase and phosphoinositide phospholipase C. J Biol Chem. 1997;272:15481–15487. doi: 10.1074/jbc.272.24.15481. [DOI] [PubMed] [Google Scholar]
- 10.Chen L M, Hobbie S, Galan J E. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 11.Collazo C M, Galan J E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 12.Collazo C M, Zierler M K, Galan J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 13.Dent G, Ukena D, Sybrecht G W, Barnes P J. [3H]WEB 2086 labels platelet activating factor receptors in guinea pig and human lung. Eur J Pharmacol. 1989;169:313–316. doi: 10.1016/0014-2999(89)90029-0. [DOI] [PubMed] [Google Scholar]
- 14.Edelman R, Levine M M. Summary of an international workshop on typhoid fever. Rev Infect Dis. 1986;8:329–349. doi: 10.1093/clinids/8.3.329. [DOI] [PubMed] [Google Scholar]
- 15.Elsinghorst E A, Baron L S, Kopecko D J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 17.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. . (Review.) [DOI] [PubMed] [Google Scholar]
- 19.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 20.Francis C L, Starnbach M N, Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992;6:3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 21.Fritz-Lindsten E, Rosqvist R, Johansson L, Forsberg A. The chaperone-like protein YerA of Yersinia pseudotuberculosis stabilizes YopE in the cytoplasm but is dispensable for targeting to the secretion loci. Mol Microbiol. 1995;16:635–647. doi: 10.1111/j.1365-2958.1995.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 22.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 23.Galan J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galán J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia del-Portillo F, Pucciarelli M G, Jefferies W A, Finlay B B. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J Cell Sci. 1994;107:2005–2020. doi: 10.1242/jcs.107.7.2005. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-del Portillo F, Pucciarelli M G, Jefferies W A, Finlay B B. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J Cell Sci. 1994;107:2005–2020. doi: 10.1242/jcs.107.7.2005. [DOI] [PubMed] [Google Scholar]
- 27.Ginocchio C, Pace J, Galán J E. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc Natl Acad Sci USA. 1992;89:5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groisman E A, Chiao E, Lipps C J, Heffron F. Salmonella typhimurium phoP virulence genes is a transcriptional regulator. Proc Natl Acad Sci USA. 1989;86:7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groisman E A, Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993;12:3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gulig P A, Caldwell L, Chiodo V A. Identification, genetic analysis, and DNA sequence of a 7.8 kilobase virulence region of the Salmonella typhimurium virulence plasmid. Mol Microbiol. 1992;6:1395–1411. doi: 10.1111/j.1365-2958.1992.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 31.Gulig P A, Curtiss R., III Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987;55:2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulig P A, Danbara H, Guiney D G, Lax A J, Norel F, Rhen M. Molecular analysis of spv virulence genes of the salmonella virulence plasmids. Mol Microbiol. 1993;7:825–830. doi: 10.1111/j.1365-2958.1993.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 33.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 34.Hermant D, Menard R, Arricau N, Parsot C, Popoff M Y. Functional conservation of the Salmonella and Shigella effectors of entry into epithelial cells. Mol Microbiol. 1995;17:781–789. doi: 10.1111/j.1365-2958.1995.mmi_17040781.x. [DOI] [PubMed] [Google Scholar]
- 35.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 36.Isberg R R, Leong J M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 37.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 38.Jones B D, Falkow S. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun. 1994;62:3745–3752. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones B D, Falkow S. Typhoid fever: host immune response and Salmonella virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 40.Jones B D, Paterson H F, Hall A, Falkow S. Salmonella typhimurium induces membrane ruffling by a growth factor receptor independent mechanism. Proc Natl Acad Sci USA. 1993;90:10390–10394. doi: 10.1073/pnas.90.21.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaniga K, Trollinger D, Galan J E. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol. 1995;177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaniga K, Tucker S, Trollinger D, Galan J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luttrell L M, Ostrowski J, Cotecchia S, Kendall H, Lefkowitz R J. Antagonism of catecholamine receptor signaling by expression of cytoplasmic domains of the receptors. Science. 1993;259:1453–1457. doi: 10.1126/science.8383880. [DOI] [PubMed] [Google Scholar]
- 44.Marquart M E, Picking W L, Picking W D. Soluble invasion plasmid antigen C (IpaC) from Shigella flexneri elicits epithelial cell responses related to pathogen invasion. Infect Immun. 1996;64:4182–4187. doi: 10.1128/iai.64.10.4182-4187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 46.Mills D M, Bajaj V, Lee C A. A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol Microbiol. 1995;15:749–759. doi: 10.1111/j.1365-2958.1995.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 47.Miras I, Hermant D, Arricau N, Popoff M Y. Nucleotide sequence of iagA and iagB genes involved in invasion of HeLa cells by Salmonella enterica subsp. enterica ser. Typhi. Res Microbiol. 1995;146:17–20. doi: 10.1016/0923-2508(96)80267-1. [DOI] [PubMed] [Google Scholar]
- 48.Paquereau L, Le Cam A. Electroporation-mediated gene transfer into hepatocytes: preservation of a growth hormone response. Anal Biochem. 1992;204:147–151. doi: 10.1016/0003-2697(92)90154-y. [DOI] [PubMed] [Google Scholar]
- 49.Parent J L, Le Gouille C, de Brum-Fernandes A J, Rola-Pleszczynski M, Stankova J. Mutations of two adjacent amino acids generate inactive and constitutively active forms of the human platelet-activating factor receptor. J Biol Chem. 1996;271:7949–7955. doi: 10.1074/jbc.271.14.7949. [DOI] [PubMed] [Google Scholar]
- 50.Penheiter K L, Mathur N, Giles D, Fahlen T, Jones B D. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer’s patches. Mol Microbiol. 1997;24:697–709. doi: 10.1046/j.1365-2958.1997.3741745.x. [DOI] [PubMed] [Google Scholar]
- 51.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaio M F, Rowland H. Bactericidal and opsonizing effects of normal serum on mutant strains of Salmonella typhimurium. Infect Immun. 1985;49:647–653. doi: 10.1128/iai.49.3.647-653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shea J E, Hensel M, Gleeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stinavage P, Martin L E, Spitznagel J K. O antigen and lipid A phosphoryl groups in resistance of Salmonella typhimurium LT-2 to nonoxidative killing in human polymorphonuclear neutrophils. Infect Immun. 1989;57:3894–3900. doi: 10.1128/iai.57.12.3894-3900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takano T, Honda Z, Sakanaka C, Izumi T, Kameyama K, Haga K, Haga T, Kurokawa K, Shimizu T. Role of cytoplasmic tail phosphorylation sites of platelet-activating factor receptor in agonist-induced desensitization. J Biol Chem. 1994;269:22453–22458. [PubMed] [Google Scholar]
- 56.Terakado N, Ushijima T, Samejima T, Ito H, Hamaoka T, Murayama S, Kawahara K, Danbara H. Transposon insertion mutagenesis of a genetic region encoding serum resistance in an 80 kb plasmid of Salmonella dublin. J Gen Microbiol. 1990;136:1833–1838. doi: 10.1099/00221287-136-9-1833. [DOI] [PubMed] [Google Scholar]
- 57.Tong G X, Jeyakumar M, Tanen M R, Bagchi M K. Transcriptional silencing by unliganded thyroid hormone receptor beta requires a soluble corepressor that interacts with the ligand-binding domain of the receptor. Mol Cell Biol. 1996;16:1909–1920. doi: 10.1128/mcb.16.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williamson C M, Pullinger G D, Lax A J. Identification of an essential virulence region on Salmonella plasmids. Microb Pathog. 1988;5:469–473. doi: 10.1016/0882-4010(88)90008-3. [DOI] [PubMed] [Google Scholar]
- 59.Woestyn S, Sory M P, Boland A, Lequenne O, Cornelius G R. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol Microbiol. 1996;20:1261–1271. doi: 10.1111/j.1365-2958.1996.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 60.Wray C, Sojka W J. Experimental Salmonella typhimurium in calves. Res Vet Sci. 1978;25:139–143. [PubMed] [Google Scholar]