Abstract
Background:
Given high rates of early complications and non-reversibility, refined targeting is necessitated for magnetic resonance-guided focused-ultrasound (MRgFUS) thalamotomy for essential tremor (ET). Selection of lesion location can be informed by considering optimal stimulation area from deep brain stimulation (DBS).
Methods:
118 patients with ET that received DBS (39) or MRgFUS (79) of the ventral intermediate nucleus (VIM) underwent stimulation/lesion mapping, probabilistic mapping of clinical efficacy, and normative structural connectivity analysis. The efficacy maps were compared, which depict the relationship between stimulation/lesion location and clinical outcome.
Results:
Efficacy maps overlap around the VIM ventral border and encompass the dentato-rubro-thalamic tract. While the MRgFUS map extends inferiorly into the posterior subthalamic area, the DBS map spreads inside the VIM antero-superiorly.
Conclusion:
Comparing the efficacy maps of DBS and MRgFUS suggests a potential alternative location for lesioning, more antero-superiorly. This may reduce complications, without sacrificing efficacy, and individualize targeting.
Introduction
Essential tremor (ET) is the most common movement disorder, affecting approximately 1% of the world’s population.1 The pathophysiology of ET involves a network comprising the cerebellum, thalamus, and motor cortex, which is interconnected by the Guillain-Mollaret triangle, prelemniscal, and cortico-pontine tracts.2 A lesion in any component of this network diminishes tremor.3 In medically-refractory cases, interventions aimed at modulating this network, namely deep brain stimulation (DBS) and magnetic resonance-guided focused ultrasound (MRgFUS) thalamotomy, have been effective at achieving tremor relief.4
In the past decade, interest has shifted from targeting the ventral intermediate (VIM) nucleus or posterior subthalamic area (PSA), to targeting white matter tracts of networks involved in ET, such as the dentato-rubro-thalamic tract (DRTT).5 Despite studies of the optimal location of DBS stimulation6,7 and MRgFUS lesioning8,9, further refinement in targeting methods for MRgFUS thalamotomy is necessitated given its non-negligible rates of early complications as well as the lack of reversibility and titratability compared to DBS.9
To identify alternative locations for lesioning, we compare DBS and MRgFUS efficacy maps that depict the relationship between target location and clinical outcome. We hypothesize that the overlap between these efficacy maps indicates the most relevant area for lesioning. Also potentially relevant is the area of the DBS efficacy map that does not overlap with the MRgFUS efficacy map, a region thought to represent careful maximization of motor benefits while minimizing unwanted side effects through DBS titration. It may therefore be considered that modification of MRgFUS target to more align with the efficacious region in DBS may improve motor outcomes and reduce side effects. These potential areas may help individualize MRgFUS lesion targeting.
Methods
Patient Population
This study was approved by our institutional research ethics board (University Health Network ID: #15-9777, #NCT02252380) using patient populations previously published.6,8,10 Baseline and postoperative Clinical Rating Scale for Tremor (CRST) scores were collected for each patient to measure clinical improvement as a percentage improvement from baseline.11 Included patients had dominant hand tremor medically refractory to two trials of full-dose therapeutic medication and experienced substantial disability in the performance of at least two daily activities.
Surgical Targeting
Image Acquisition, Lead Localization and Lesion Segmentation
The method used for image acquisition, lead localization, and lesion segmentation have been previously described by our group and others (Supplemental Materials).6,8
Statistical analysis
Probabilistic voxel-wise efficacy maps providing insight into spatial patterns of response to treatment were generated as previously described.12 Briefly, each transformed volume of activated tissue (VTA) or lesion was weighted by clinical improvement (percent improvement from baseline) and voxel-wise mean improvement computed by averaging the weighted values. Using unweighted frequency maps (n-map), denoting the number of VTAs or lesions overlapping at a given voxel, the raw average maps were thresholded to include only voxels with a minimum of 10% VTA/lesion overlap. These maps were further thresholded using a Wilcoxon signed-rank test (P<0.05, at each voxel). In a similar fashion, to identify voxels that, within each group, were associated with above/below average outcomes, the clinical improvement scores were z transformed for both groups of patients, and average voxel efficacy maps were calculated as described above.
Structural connectivity analysis was performed as previously described (Supplemental Materials).6,12 All streamlines touched by each lesion or VTA were identified. Next, unweighted frequency maps were generated to identify shared streamlines implicated in each treatment group, denoting the number of VTAs or lesions touching a given streamline. Group tractograms of shared streamlines were thresholded at 75%. Lastly, the streamlines common to both treatment groups were identified.
Results
Included for analysis were 118 patients with ET: 39 treated with unilateral DBS and 79 with unilateral MRgFUS. Demographics, summary improvement metrics, and side effects are detailed in Table 1. Side effects from our VIM-MRgFUS cohort are reported and compared to VIM-DBS side effects reported in the literature.13 Overall side-effect rates are similar except in gait impairment, in which 50.6% of MRgFUS subjects report permanent or transient impairment compared to 8.2% in DBS (Table 1).
Table 1:
Demographics of the two cohorts of Essential Tremor patients treated with either DBS or MRgFUS
| Cohort | Number of patients | Age in years (mean ± SD) | Sex (percent female) | Disease duration in years (mean ± SD) | Pre - operative CRST (mean ± SD) | Follow up in years (mean ± SD) | Clinical improvement at follow up (mean ± SD) | Side-effects (procedure and programming related) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Sensory | Gait impairment | Speech | DBS-specific# | ||||||||
| VIM-DBS | 33 | 65.6 ± 9.4 | 33.3% | 28.8 ± 18.1 | 58.7 ± 11.3 | 1.2 ± 1.1 | 39.7 ± 22.6% | 78.8%* | 6.5%* | 8.2%* | 8.3%* | 15%* |
| VIM-MRgFUS | 79 | 71.1 ± 9.0 | 32.9% | 35.4 ± 18.4 | 57.7 ± 15.8 | 1.1 ± 0.2 | 36.5 ± 25% | 47 (59.5%) | 10 (12.7%) | 40 (50.6%) | 4 (5.1%) | - |
Abbreviations: CRST: Clinical rating scale for tremor; DBS: deep brain stimulation; MRgFUS: magnetic resonance guided focused ultrasound; SD: standard deviation; VIM: ventral intermediate nucleus of the thalamus; # DBS specific side-effects: Lead problems, infection, intracranial hemorrhage, seizures, edema, mental deficits, death;
as reported in the systematic review by Giordano et al. 2020 (doi:10.1136/jnnp-2020-323216)
Simulation and Lesion Location
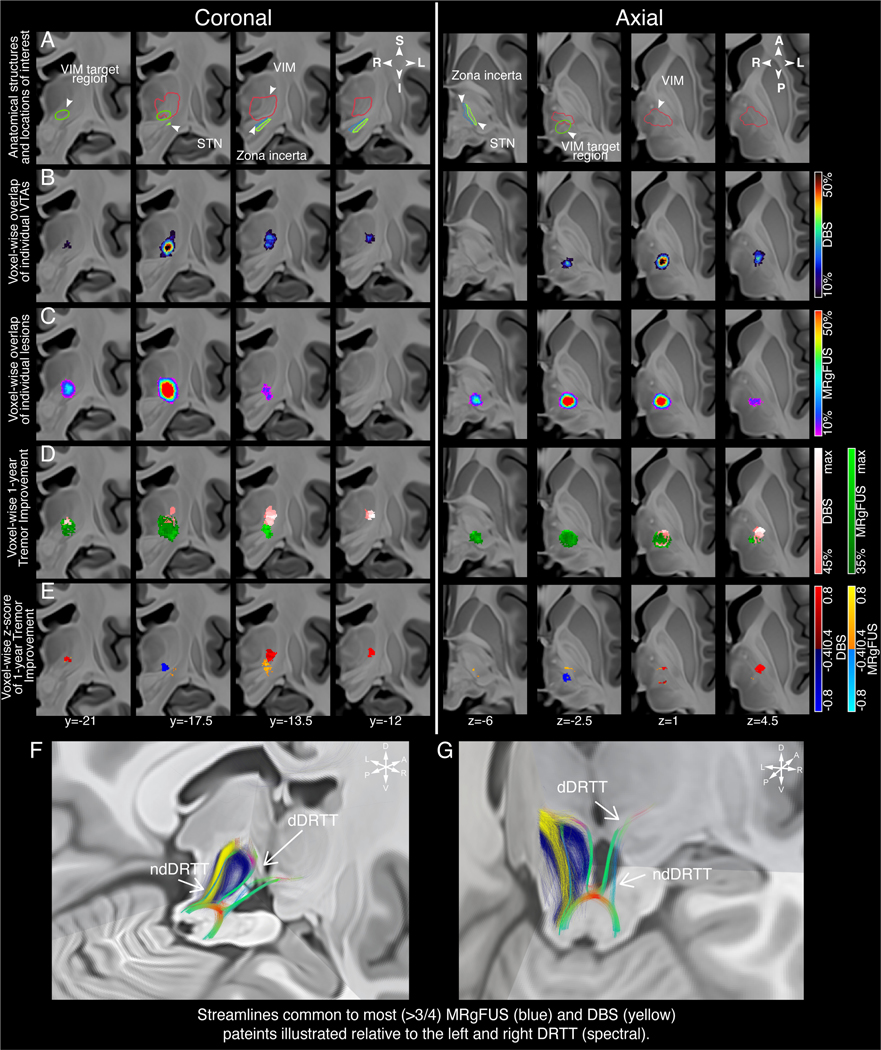
Figure 1A depicts the location of the VIM target region and surrounding structures of interest. DBS stimulation location at the time of follow-up is shown thresholded at a minimum of 10% voxel overlap between subjects (Figure 1B). It is apparent that stimulation remains mainly restricted to the VIM, with extension antero-superiorly towards the ventro-oralis posterior (VOp) nucleus of the thalamus, and frequent involvement of the non-decussating DRTT (ndDRTT) based on DTI analysis (Figure 1F). There is little infiltration of the VTAs inferiorly towards the prelemniscal radiations (Raprl) or zona incerta (ZI) of the posterior subthalamic area (PSA). Conversely, in MRgFUS lesioning (Figure 1C), lesions also encompass the bulk of the VIM, however, more frequently extend inferolateral into the region of the PSA, ZI, and both the decussating DRTT (dDRTT) and ndDRTT (Figure 1F).
Figure 1: Stimulation/lesion localization and clinical response mapping.
Coronal images are presented from posterior to anterior and axial images from inferior to superior (labelled in MNI coordinates). A) Depiction of the VIM target region surrounding structures of interest. B) Localization of DBS stimulation on clinically optimal settings at the time of follow-up and C) MRgFUS lesions identified on peri-postoperative imaging. The VTAs and MRgFUS lesions for individual patients are represented as percent overlap and thresholded at a minimum of 10% overlap. D) Probabilistic maps denoting areas of stimulation/lesioning associated with clinical improvement are depicted and thresholded at a minimum of 35%/45% improvement for data visualization. E) Z-scores depicting voxels that produce above-mean (hot spots, warm colors) and below-mean (cold spots, cold colors) clinical improvement for each intervention. F and G) Tractography streamlines from >75% of DBS VTAs (yellow) and MRgFUS lesions (blue) are visualized relative to the DRTT. Localization, probabilistic maps, and tractography streamlines are projected on coronal slices of a 100μm resolution, single-echo multi-flip fast low-angle shot 7T brain in Montreal Neurological Institute (MNI) 152 space.16 MNI coordinates for each slice are displayed. Abbreviations: DBS, deep brain stimulation; DRTT, dentatorubrothalamic tract; ndDRTT, non-decussating dentatorubrothalamic tract; dDRTT, decussating dentatorubrothalamic tract; MRgFUS, magnetic resonance-guided focused ultrasound; STN, subthalamic nucleus; VIM, ventral intermediate nucleus; VTA, volume of tissue activated.
Probabilistic Efficacy Maps and Structural Connectivity
Figure 1D demonstrates voxels at which intervention tended to produce clinical improvement greater than 35% or 45% (chosen for data visualization). For DBS, the best improvement is seen antero-superiorly in the VIM encompassing the ndDRTT, whereas the pattern of improvement for MRgFUS follows closely to the area of lesioning, extending inferolateral into the PSA and ZI, and involving both the dDRTT and ndDRTT. Z-scores were also computed to demonstrate voxels that produce above-mean (“hot spots”) and below-mean (“cold spots”) clinical improvement for each intervention (Figure 1E). For interventions with DBS, hot spots were located superolateral in the anterior portion of VIM at the level of the ndDRTT and cold spots inferomedial and posterior in the ventral VIM. For MRgFUS the hot spot was inferior compared to that for DBS and straddles both sides of the border between the VIM and PSA, with a second area posterior in the VIM.
Discussion
In this study, we present a combined probabilistic efficacy map of ET patients treated with either thalamic DBS or MRgFUS thalamotomy. 118 patients were assessed using contemporary neuroimaging approaches and paired with clinical follow-up data at 1 year. We identified that clusters of maximal improvement for both treatment modalities overlap around the VIM ventral border. While the MRgFUS map extends inferiorly into the PSA, the DBS map spreads inside the VIM antero-superiorly. Unsurprisingly these maps encompass the DRTT considerably, confirming previous work, which suggests that the optimal tremor target courses along the fibers of the DRTT.5,7 Furthermore, we see here that engagement of only the ndDRTT is sufficient for symptom benefit (Figure 1F). Whether lesioning of the dDRTT in addition to ndDRTT contributes to side effects in MRgFUS remains to be validated but serves as hypothesisgenerating for future studies.
It may be expected that the efficacy maps of the two modalities would substantially overlap as all patients had a similar target coordinate regardless of intervention type (similar x and y; z = 0 for DBS electrode tip, z = +2mm for MRgFUS). However, the efficacy maps only overlap at the VIM ventral border to some degree and extend in opposite directions. This may be interpreted in two ways: (1) The ET sweet spot is a dorso-ventrally traversing tract rather than a specific region/nucleus/coordinate, and/or (2) a more anterior and dorsal DBS stimulation site is chosen to avoid sensory side effects due to stimulation of the inferior/posterior located sensory relay nucleus and its afferent fibers. It also cannot be ruled out that the mechanism of action of DBS and MRgFUS may differ, resulting in different efficacy maps.
The DBS efficacy map can inform MRgFUS thalamotomy targeting as DBS programming is titrated to achieve optimal clinical benefit and minimize adverse events.14 This principle was validated at our center where more superior and slightly anterior MRgFUS lesions reduce adverse effects and provide a greater midline and ipsilateral tremor improvement.8,9 This modified targeting was also tested while treating the second side in patients by moving the target 1 mm more dorsally and 0.2–0.3 mm more anteriorly compared to conventional target coordinates. We achieved an adverse effect rate comparable to the first treated side even though a higher rate was expected according to historical radiofrequency thalamotomy experiences.15 Of note, this was a pilot trial in a small cohort and was not aiming to directly compare both targeting methods in terms of adverse effects. Our findings suggest personalized thalamotomy targeting: where a modified targeting approach aiming antero-superiorly in VIM could be utilized for at-risk patients. More personalized targeting should await future trials which investigate closely the difference in efficacy and risk of side effects between the two targeting approaches.
Limitations of this work include a retrospective cohort of patients treated with conventional DBS systems, lacking new directional stimulation technology able to shape the field of stimulation more precisely and better refine the optimal target location. Furthermore, all procedures performed generally followed the same protocol and technique, limiting the variation in lesion and stimulation location. Additionally, unrecognized confounders between the different treatment populations may have introduced bias, such as selection bias towards older individuals for MRgFUS treatment. Although the target was the nearly same for both DBS implantation and MRgFUS lesioning, due to the radially expanding lesions created in MRgFUS and linearly extending contact locations along the trajectory of the DBS electrode, DBS stimulation could not be achieved to the same inferior extent as the MRgFUS lesions. Therefore, due to lack of adequate coverage in our sample a comparison of treatment efficacy in this region, the PSA, could not be made. Furthermore, without further investigation it is unlikely that we’ll see direct targeting of the PSA with MRgFUS due to the risk of irreversible side effects.
Conclusions
Treatment for ET with DBS or MRgFUS produces efficacy maps encompassing the DRTT and depict the relationship between target location and clinical outcome. Comparing the efficacy maps of both modalities suggests that MRgFUS targeting which is more antero-superiorly to standard target location may show similar efficacy but possibly reduce the rate of gait impairment seen with current VIM-MRgFUS targeting. Future prospective studies should compare this method to conventional targeting.
Supplementary Material
Acknowledgments
We would like to acknowledge the contribution and support of Amelia Mesich, Nicole Owsicki, and Sarah Varughese.
Funding Sources
The study was supported by the RR Tasker Chair in functional Neurosurgery at University Health Network(A.M.L.), a Tier 1 Canada Research Chair in Neuroscience (A.M.L.), and the Canadian Institutes of Health Research (B.S.). This work was supported by the Canadian Institutes of Health Research Banting Fellowship (#471913) awarded to J.G. and by the University of Toronto and University Health Network Chair in Neuromodulation awarded to A.F.
Financial Disclosures and Conflicts of Interest
A.M.L. is a consultant to Abbott, Boston Scientific, Insightec and Medtronic and Scientific Director at Functional Neuromodulation.
S.K.K. holds honoraria, speakers fees and/or indirect support Abbott/Boston/Medtronic.
A.F. holds honoraria, speakers fees and/or indirect support Abbott/Boston/Medtronic.
References
- 1.Welton T, Cardoso F, Carr JA, et al. Essential tremor. Nature Reviews Disease Primers 2021 7:1. 2021;7(1):1–17. doi: 10.1038/S41572-021-00314-W [DOI] [PubMed] [Google Scholar]
- 2.Raethjen J, Deuschl G. The oscillating central network of Essential tremor. Clin Neurophysiol. 2012;123(1):61–64. doi: 10.1016/J.CLINPH.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 3.Dupuis MJM, Evrard FLA, Jacquerye PG, Picard GR, Lermen OG. Disappearance of essential tremor after stroke. Movement Disorders. 2010;25(16):2884–2887. doi: 10.1002/MDS.23328 [DOI] [PubMed] [Google Scholar]
- 4.Elias WJ, Lipsman N, Ondo WG, et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med. 2016;375(8):730–739. doi: 10.1056/NEJMOA1600159 [DOI] [PubMed] [Google Scholar]
- 5.Fox MD, Deuschl G. Converging on a Neuromodulation Target for Tremor. Ann Neurol. 2022;91(5):581–584. doi: 10.1002/ANA.26361 [DOI] [PubMed] [Google Scholar]
- 6.Elias GJB, Boutet A, Joel SE, et al. Probabilistic Mapping of Deep Brain Stimulation: Insights from 15 Years of Therapy. Ann Neurol. Published online November 30, 2020. doi: 10.1002/ana.25975 [DOI] [PubMed]
- 7.Nowacki A, Barlatey S, Al-Fatly B, et al. Probabilistic Mapping Reveals Optimal Stimulation Site in Essential Tremor. Ann Neurol. 2022;91(5):602–612. doi: 10.1002/ANA.26324 [DOI] [PubMed] [Google Scholar]
- 8.Boutet A, Ranjan M, Zhong J, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018;141(12):3405–3414. doi: 10.1093/BRAIN/AWY278 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto K, Sarica C, Elias GJB, et al. Ipsilateral and axial tremor response to focused ultrasound thalamotomy for essential tremor: clinical outcomes and probabilistic mapping. J Neurol Neurosurg Psychiatry. Published online October 22, 2022:jnnp-2021–328459. doi: 10.1136/JNNP-2021-328459 [DOI] [PubMed]
- 10.Kapadia AN, Elias GJB, Boutet A, et al. Multimodal MRI for MRgFUS in essential tremor: post-treatment radiological markers of clinical outcome. J Neurol Neurosurg Psychiatry. 2020;91(9):921–927. doi: 10.1136/JNNP-2020-322745 [DOI] [PubMed] [Google Scholar]
- 11.Sarica C, Fomenko A, Iorio-Morin C, et al. Letter to the Editor. Clinical Rating Scale for Tremor: a needed clarification. J Neurosurg. 2021;136(3):932–933. doi: 10.3171/2021.7.JNS211783 [DOI] [PubMed] [Google Scholar]
- 12.Germann J, Elias GJB, Neudorfer C, et al. Potential optimization of focused ultrasound capsulotomy for obsessive compulsive disorder. Brain. 2021;144(11):3529–3540. doi: 10.1093/BRAIN/AWAB232 [DOI] [PubMed] [Google Scholar]
- 13.Giordano M, Caccavella VM, Zaed I, et al. Comparison between deep brain stimulation and magnetic resonance-guided focused ultrasound in the treatment of essential tremor: a systematic review and pooled analysis of functional outcomes. J Neurol Neurosurg Psychiatry. 2020;91(12):1270–1278. doi: 10.1136/JNNP-2020-323216 [DOI] [PubMed] [Google Scholar]
- 14.Picillo M, Lozano AM, Kou N, Munhoz RP, Fasano A. Programming Deep Brain Stimulation for Tremor and Dystonia: The Toronto Western Hospital Algorithms. Brain Stimul. 2016;9(3):438–452. doi: 10.1016/J.BRS.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 15.Iorio-Morin C, Yamamoto K, Sarica C, et al. Bilateral Focused Ultrasound Thalamotomy for Essential Tremor (BEST-FUS Phase 2 Trial). Movement Disorders. 2021;36(11):26532662. doi: 10.1002/MDS.28716 [DOI] [PubMed] [Google Scholar]
- 16.Edlow BL, Mareyam A, Horn A, et al. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data. 2019;6(1). doi: 10.1038/S41597-019-0254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.