Abstract
Objective
To investigate the trends of 1-year mortality and neonatal morbidities in preterm infants with serious congenital heart disease (CHD).
Study design
This cohort study used a population-based administrative dataset of all liveborn infants of 26-36 weeks gestational age with serious CHD born in California between 2011 and 2017. We assessed 1-year mortality and major neonatal morbidities (ie, retinopathy of prematurity, bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage grade >2, and periventricular leukomalacia) across the study period and compared these outcomes with those in infants without CHD.
Results
We identified 1921 preterm infants with serious CHD. The relative risk (RR) of death decreased by 10.6% for each year of the study period (RR, 0.89; 95% CI, 0.84-0.95), and the RR of major neonatal morbidity increased by 8.3% for each year (RR, 1.08; 95% CI, 1.02-1.15). Compared with preterm neonates without any CHD (n = 234 522), the adjusted risk difference (ARD) for mortality was highest at 32 weeks of gestational age (9.7%; 95% CI, 8.3%-11.2%), that for major neonatal morbidity was highest at 28 weeks (21.9%; 95% CI, 17.0%-26.9%), and that for the combined outcome was highest at 30 weeks (26.7%; 95% CI, 23.3%-30.1%).
Conclusions
Mortality in preterm neonates with serious CHD decreased over the last decade, whereas major neonatal morbidities increased. Preterm infants with a gestational age of 28-32 weeks have the highest mortality or morbidity compared with their peers without CHD. These results support the need for specialized and focused medical neonatal care in preterm neonates with serious CHD.
In neonates with congenital heart disease (CHD), advances in surgical techniques allow surgeons to perform congenital heart surgery in smaller and more preterm infants. In the past, mortality of preterm infants born at <32 weeks of gestation with CHD was high,1 but recent studies have shown a higher rate of survival.2 Although a decrease in mortality is encouraging, it is important to also take neonatal morbidities into account. Major neonatal morbidities—bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and necrotizing enterocolitis (NEC)—are strong predictors for longer-term health and neurodevelopmental outcomes.3 Advances in neonatal medical care have decreased the incidence of major neonatal morbidities in preterm infants without other congenital malformations, although significant morbidities remain a concern for infants born at <28 weeks of gestational age.4 Data on the burden of neonatal morbidity in preterm neonates with CHD are sparse. Our group has shown that the prevalence of neonatal morbidities is higher in neonates with CHD compared with their peers of the same gestational age without CHD even in early term and term infants,5 although this was based on a population sample from more a decade ago and did not specifically look at preterm neonates.
The aim of the present study was to investigate the trends in 1-year mortality and burden of neonatal morbidities in preterm infants born at <37 weeks of gestational age with and without CHD in a contemporary (2011-2017) population-based cohort. We hypothesized that the increased survival of preterm neonates with CHD would be offset by an increase in major neonatal morbidities.
Methods
The sample was drawn from all California live-born infants between 2011 and 2017. Birth and death certificates (up to age 12 months) from California Vital Statistics were linked to hospital discharge, emergency department, and ambulatory surgery records from the California Office of Statewide Health Planning and Development. The linkage algorithm included such variables as Social Security number (often missing), date of birth, sex, ZIP code, and race/ethnicity. In addition, discharge and admission dates and transfer status were used to build the sequence of records. The records were matched based on dates, transfer status, and 2 of the aforementioned variables. Among matched records based on the 2 variables, other variables were compared, and a “match score” was calculated. The final match was done for the records with the best match score. A subset of all records was reviewed manually to ensure correct application of the algorithm. The combined dataset included information on maternal and infant characteristics derived from birth and death certificates, as well as diagnosis and procedure codes available from hospital discharge records (birth hospitalization and readmissions) from birth to age 1 year. The diagnosis and procedure codes are based on the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9/10-CM). Gestational age was determined by best obstetric estimate from ultrasound and/or last menstrual period.
All linked live-born infants with a gestational age of 26-36 completed weeks were included in this study (n = 248 958). All infants with ICD-9-CM or ICD-10-CM diagnostic or procedure codes consistent with CHD present in the birth, transfer, or readmission records were identified. We used the framework proposed by the National Quality Forum (PCS-021-09; http://www.qualityforum.org/Home.aspx) and cross-referenced with ICD-9-CM and ICD-10-CM codes to correctly classify infants with multiple ICD codes. A pediatric cardiologist and a pediatric cardiac intensivist reviewed cases to ensure the correct classification of infants with multiple diagnostic or procedure codes. The final diagnosis was reached by consensus.
Serious CHD was defined according to the criteria suggested by Ewer et al as a congenital heart lesion that either required surgery or would have been expected (in the event of early death) to require surgery within the first year of life.6 We excluded infants with only minor CHD (mainly consisting of diagnoses of ventricular septal defect without procedure codes and codes for atrial septal defect). We further excluded neonates with ICD-9/10-CM codes consistent for major structural birth defects other than CHD and those consistent with major chromosomal anomalies or genetic syndromes (ie, ICD-9-CM 758; ICD-10-CM Q90-99). We considered a structural birth defect “major” for the purposes of exclusion if determined by clinical review to result in mortality or significant morbidity associated with that specific birth defect.7 The purpose of this exclusion was to define a cohort of infants with isolated serious CHD for determining the mortality and morbidity associated with CHD.
The outcomes assessed were 1-year mortality (determined by death certificate or death as the hospital discharge status) and major neonatal morbidity (determined from ICD-9-CM and ICD-10-CM codes). These included BPD (ICD-9-CM 770.7, ICD-10-CM P27.1), NEC (ICD-9-CM 777.5, ICD- 10-CM P77), IVH grade >2 (ICD-9-CM 772.13 and 772.14, ICD-10-CM P52.2), PVL (ICD-9-CM 779.7, ICD-10-CM P91.2), and ROP stage >2. To define ROP stage >2, we used diagnostic or procedure codes (ICD-9-CM 362.25-7 or 14.2, 14.5, 14.7, 14.9; ICD-10-CM H35.14-6 or any procedure code for surgery on the retina or choroid plexus).
Descriptive statistics are presented for maternal and infant characteristics of preterm infants with and without serious CHD. The χ2 test was used to compare proportions, and the rank-sum test was used to compare medians. Observed proportions are presented with 95% CIs.
To assess the live-born prevalence of serious CHD by gestational age, a Poisson regression model was fitted with serious CHD as the outcome and gestational age in weeks as the predictor to calculate relative risk (RR) and 95% CI. Similarly, to assess mortality, major neonatal morbidity, and the composite outcome of mortality or major neonatal morbidity over time, Poisson regression models were built for each of these outcomes, with year as a continuous predictor to calculate RR and 95% CI.
We compared preterm neonates with serious CHD and those without CHD by calculating gestational age–specific adjusted RR with 95% CI. For this purpose, we used logistic models for each of the binary outcomes. The models were adjusted for sex, z-score for birth weight, and multiple gestation, and an interaction term between isolated CHD and gestational age was fitted. To obtain the adjusted risk difference (ARD), the difference between predicted outcome probabilities of neonates with CHD and those without CHD was calculated by gestational age using the “margins” command in Stata (StataCorp)8 while keeping the covariates at their mean values.
All analyses were performed using Stata version 16.1 (StataCorp). The study was approved by the Committee for the Protection of Human Subjects of the California Health and Human Services Agency.
Results
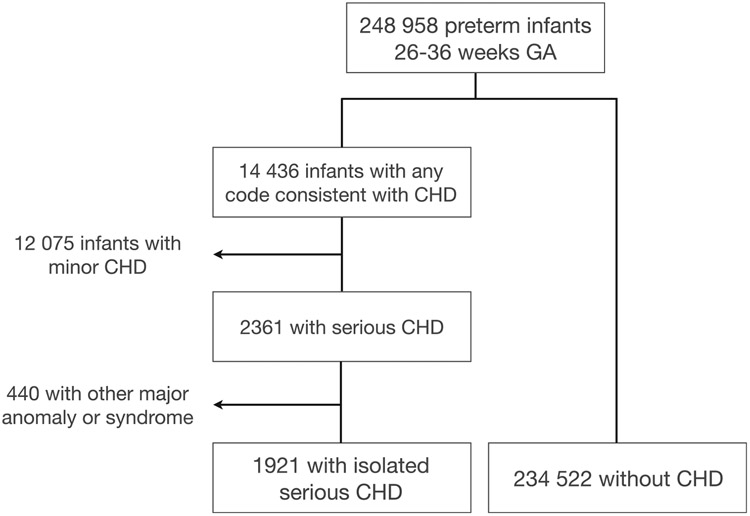
Out of 248 958 preterm infants born at 26-36 weeks of gestational age between 2011 and 2017, we identified 2361 infants with serious CHD (0.95%). Of these, 440 had another major birth anomaly or genetic syndrome and were excluded from the analysis, leaving 1921 neonates with isolated serious CHD (0.77%) (Figure 1; available at www.jpeds.com). The distribution of specific cardiac lesions in this cohort is provided in Table I (available at www.jpeds.com).
Figure 1.
Study population.
Table I.
Final diagnoses of preterm neonates with isolated serious CHD
| Diagnoses | Number | Percentage | Cumulative incidence |
|---|---|---|---|
| AV canal and TAPVR | 7 | 0.36 | 0.36 |
| Aortic stenosis | 31 | 1.61 | 1.98 |
| CoA | 106 | 5.52 | 7.50 |
| CoA/VSD | 57 | 2.97 | 10.46 |
| Complete or transitional AV canal | 160 | 8.33 | 18.79 |
| Complex left SV | 72 | 3.75 | 22.54 |
| Complex single ventricle | 38 | 1.98 | 24.52 |
| Cor triatriatum | 1 | 0.05 | 24.57 |
| DORV, not otherwise specified | 12 | 0.62 | 25.20 |
| Ebstein anomaly | 66 | 3.44 | 28.63 |
| HLHS | 156 | 8.12 | 36.75 |
| IAA or arch hypoplasia | 20 | 1.04 | 37.79 |
| IAA or arch hypoplasia + VSD | 12 | 0.62 | 38.42 |
| Malformation of tricuspid valve | 67 | 3.49 | 41.91 |
| PA/IVS | 28 | 1.46 | 43.36 |
| PAPVR | 15 | 0.78 | 44.14 |
| Pulmonary valve malformation | 23 | 1.20 | 45.34 |
| Pulmonary valve obstruction | 134 | 6.98 | 52.32 |
| TAPVR | 32 | 1.67 | 53.98 |
| TGA | 73 | 3.80 | 57.78 |
| TGA and VSD | 22 | 1.15 | 58.93 |
| TOF | 363 | 18.90 | 77.82 |
| Tausig Bing | 1 | 0.05 | 77.88 |
| Tricuspid atresia | 73 | 3.80 | 81.68 |
| Truncus arteriosus | 50 | 2.60 | 84.28 |
| VSD | 104 | 5.41 | 89.69 |
| VSD catheterization procedure | 185 | 9.63 | 99.32 |
| VSD/PAB | 8 | 0.42 | 99.74 |
| Corrected TGA | 5 | 0.26 | 100.00 |
| Total | 1921 | 100.00 |
AV, atrioventricular; CoA, coarctation of the aorta; DORV, double-outlet right ventricle; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; IVS, intact ventricular septum; PA, pulmonary atresia; PAB, pulmonary artery band; PAPVR, partial anomalous pulmonary venous return; SV, single ventricle; TAPVR, total anomalous pulmonary venous return; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
Maternal and infant characteristics in preterm neonates with and without isolated serious CHD are summarized in Table II (available at www.jpeds.com). Mothers of preterm infants with serious isolated CHD were older (P < .001) and more likely to be insured by public insurance (ie, Medi-Cal) (P = .013). Maternal education level did not differ between the 2 groups (P = .46). In terms of comorbidities, mothers of preterm infants with isolated serious CHD were more likely to be obese (P < .001) and to have hypertensive disorders of pregnancy (P < .001) and diabetes (P < .001). The causes of preterm birth did not differ between the groups with and without isolated serious CHD; the majority in both groups had preterm labor (P = .17). Infants with CHD were more likely to be born by cesarean delivery (P < .001) and more likely to be growth-restricted (P < .001). Multiple gestation pregnancy was not different between the 2 groups (P = .36) (Table II).
Table II.
Maternal and infant characteristics of preterm neonates with and without isolated serious CHD
| Characteristics | No serious CHD (N = 234 522) | Isolated serious CHD (N = 1921) | P value |
|---|---|---|---|
| Maternal race/ethnicity, n (%) | .036 | ||
| Non-Hispanic white | 58 372 (24.9) | 481 (25.0) | |
| Hispanic | 112 753 (48.1) | 910 (47.4) | |
| Non-Hispanic black | 16 416 (7.0) | 160 (8.3) | |
| Asian | 33 554 (14.3) | 245 (12.8) | |
| Other | 13 427 (5.7) | 125 (6.5) | |
| Maternal age, y, median (IQR) | 30.0 (25.0-35.0) | 31.0 (26.0-36.0) | <.001 |
| Parity, n (%) | |||
| Multiparous | 153 044 (65.3) | 1229 (64.0) | .24 |
| Nulliparous | 81 478 (34.7) | 692 (36.0) | |
| Insurance, n (%) | |||
| Private | 112 124 (50.9) | 871 (47.9) | .013 |
| Public | 108 302 (49.1) | 946 (52.1) | |
| Maternal education, n (%) | |||
| <12 y | 41 848 (17.8) | 352 (18.3) | .46 |
| 12y | 68 362 (29.1) | 578 (30.1) | |
| >12 y | 124 312 (53.0) | 991 (51.6) | |
| Maternal obesity, n (%) | |||
| Not obese | 98 267 (63.3) | 745 (58.0) | <.001 |
| Obese | 57 037 (36.7) | 540 (42.0) | |
| Hypertensive disorders, n (%) | |||
| None | 177 213 (75.6) | 1361 (70.8) | <.001 |
| Preexisting HTN, no PE | 5730 (2.4) | 63 (3.3) | |
| Preexisting HTN and PE | 9769 (4.2) | 119 (6.2) | |
| Gestational HTN, no PE | 10 303 (4.4) | 94 (4.9) | |
| Gestational HTN and PE | 31 507 (13.4) | 284 (14.8) | |
| Diabetes, n (%) | |||
| None | 191 185 (81.5) | 1456 (75.8) | <.001 |
| Gestational diabetes | 38 066 (16.2) | 381 (19.8) | |
| Preexisting diabetes | 5271 (2.2) | 84 (4.4) | |
| Placenta previa, n (%) | |||
| No placenta previa | 225 454 (96.1) | 1865 (97.1) | .031 |
| Placenta previa | 9068 (3.9) | 56 (2.9) | |
| Amniotic fluid volume, n (%) | |||
| Normal | 221 321 (94.4) | 1696 (88.3) | <.001 |
| Oligohydramnios | 9806 (4.2) | 131 (6.8) | |
| Polyhydramnios | 3395 (1.4) | 94 (4.9) | |
| Mode of delivery, n (%) | |||
| Vaginal delivery | 110 160 (47.0) | 661 (34.4) | <.001 |
| Cesarean | 124 362 (53.0) | 1260 (65.6) | |
| Reason for preterm birth, n (%) | |||
| Preterm labor | 112 194 (50.2) | 916 (49.0) | .17 |
| PROM | 59 660 (26.7) | 487 (26.1) | |
| Provider-initiated | 51 452 (23.0) | 465 (24.9) | |
| Gestational age, wk, median (IQR) | 35.0 (34.0-36.0) | 34.0 (32.0-36.0) | <.001 |
| Sex, n (%) | |||
| Male | 126 674 (54.0) | 1068 (55.6) | .17 |
| Female | 107 848 (46.0) | 853 (44.4) | |
| Birth weight, g, median (IQR) | 2475.0 (2077.0-2830.0) | 2121.0 (1525.0-2624.0) | <.001 |
| Birth weight z-score category, n (%) | |||
| AGA | 200 085 (85.3) | 1434 (74.6) | <.001 |
| SGA | 18 079 (7.7) | 332 (17.3) | |
| LGA | 16 358 (7.0) | 155 (8.1) | |
| Gestation, n (%) | |||
| Singleton birth | 186 091 (79.3) | 1508 (78.5) | .36 |
| Multiple birth | 48 431 (20.7) | 413 (21.5) |
AGA, appropriate for gestational age; HTN, hypertension; LGA, large for gestational age; PE, preeclampsia; PROM, premature rupture of membranes; SGA, small for gestational age.
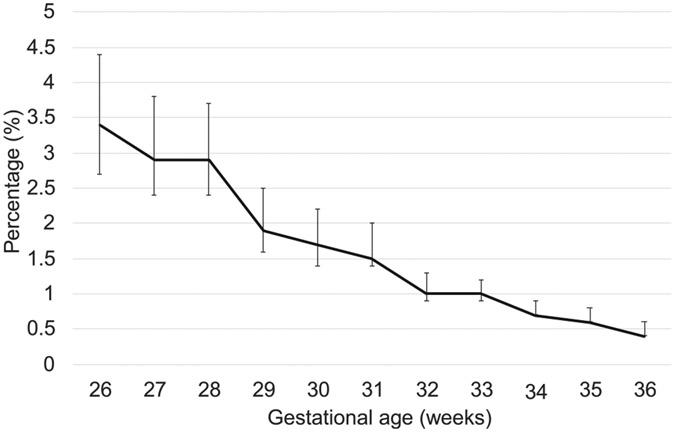
The prevalence of serious CHD was highest at 26 weeks (3.5%; 95% CI, 2.7%-4.4%) and lowest at 36 weeks (0.5%; 95% CI, 0.4%-0.6%) (Figure 2; available at www.jpeds.com). The RR of having serious CHD was decreased by 17.9% with each week of gestational age (RR, 0.82; 95% CI, 0.81-0.84).
Figure 2.
Prevalence of serious CHD by gestational age in preterm infants. The vertical bar represents 95% CI.
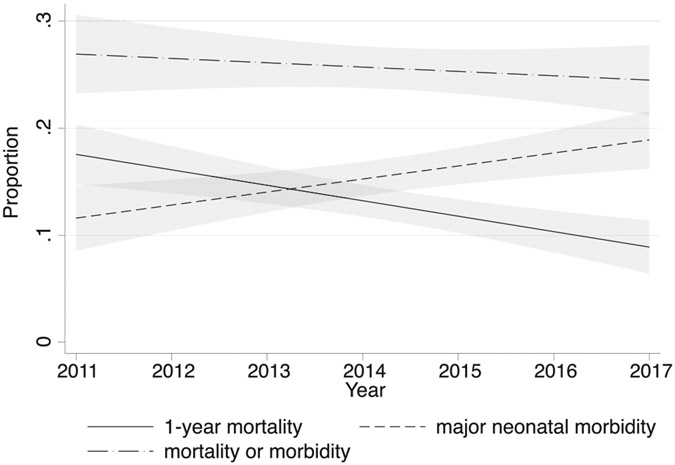
In the cohort of premature infants with CHD, mortality decreased from 19.1 % (95% CI, 14.7%-24.5%) in 2011 to 11.6% (95% CI, 8.5%-15.5%) in 2017 (Table III). The RR of death decreased by 10.6% for each year across the study period (RR, 0.89; 95% CI, 0.84-0.95; P < .001); however, the proportion of those with major neonatal morbidities increased from 12.4% (95% CI, 8.8%-17.1%) in 2011 to 21.6% (95% CI, 17.5%-26.4%) in 2017. The RR of major neonatal morbidity increased by 8.3% for each year across the study period (RR, 1.08; 95% CI, 1.02-1.15; P = .006). Thus, the proportion of preterm neonates with CHD with the composite outcome of mortality or major morbidity was similar in 2011 (28.2%; 95% CI, 23.0%-34.2%) to that in 2017 (29.2%; 95% CI, 24.5%-34.3%). There was no significant trend for this composite outcome from 2011 to 2017 (RR, 0.98; 95% CI, 0.94-1.03; P = .48) (Figure 3).
Table III.
Mortality and neonatal morbidity across years in preterm infants (<37 weeks of gestational age) with serious CHD
| Outcomes | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P value |
|---|---|---|---|---|---|---|---|---|
| Mortality | 19.1 (14.7-24.5) | 18.1 (13.6-23.6) | 13.2 (9.6-17.9) | 9.5 (6.3-14.1) | 11.8 (8.4-16.4) | 9.0 (6.4-12.4) | 11.6 (8.5-15.5) | <.001 |
| Any major neonatal morbidity | 12.4 (8.8-17.1) | 14.5 (10.5-19.8) | 14.7 (10.9-19.5) | 12.1 (8.5-17.0) | 13.7 (10.1-18.5) | 17.1 (13.6-21.4) | 21.6 (17.5-26.4) | .006 |
| Major neonatal morbidity in survivors only | 9.2 (6.2-13.4) | 11.9 (8.3-16.8) | 13.2 (9.6-17.9) | 9.1 (6.0-13.6) | 12.6 (9.1-17.2) | 13.5 (10.3-17.5) | 17.6 (13.9-22.1) | .01 |
| Mortality or major neonatal morbidity | 28.2 (23.0-34.2) | 30.0 (24.3-36.3) | 26.4 (21.4-32.1) | 18.6 (14.1-24.2) | 24.4 (19.6-30.0) | 22.5 (18.4-27.1) | 29.2 (24.5-34.3) | .48 |
| BPD | 8.8 (5.8-13.0) | 10.1 (6.8-14.8) | 7.5 (4.9-11.4) | 6.1 (3.6-10.0) | 9.5 (6.5-13.8) | 12.6 (9.6-16.5) | 14.9 (11.4-19.2) | .006 |
| NEC | 3.6 (1.9-6.8) | 2.2 (0.9-5.2) | 4.2 (2.3-7.4) | 4.7 (2.6-8.4) | 4.2 (2.3-7.4) | 3.9 (2.3-6.5) | 5.5 (3.5-8.5) | .17 |
| PVL or IVH | 1.6 (0.6-4.2) | 2.2 (0.9-5.2) | 2.3 (1.0-5.0) | 2.6 (1.2-5.7) | 1.1 (0.4-3.5) | 3.1 (1.7-5.5) | 3.6 (2.1-6.3) | .134 |
| ROP | 0.8 (0.2-3.1) | 1.3 (0.4-4.0) | 2.6 (1.3-5.5) | 1.7 (0.6-4.5) | 0.8 (0.2-3.0) | 0.6 (0.1-2.2) | 2.1 (1.0-4.4) | .92 |
Figure 3.
Linear trends for outcomes of preterm infants with serious CHD over time. Shaded area represents 95% CI.
Regarding specific morbidities, BPD was the only major morbidity that increased significantly over the study period (P = .006) (Table III). There was a trend toward increases in NEC and IVH/PVL that did not meet statistical significance.
In preterm neonates without CHD, mortality also decreased over the study period, from 1.2% (95% CI, 1.1%-1.4%) in 2011 to 1.0% (95% CI, 0.9%-1.1%) in 2017 (RR, 0.98, 95% CI, 0.96-0.99). However, in contrast to preterm neonates with CHD, the proportion of infants with major neonatal morbidities also decreased over the study period, from 2.0% (95% CI, 1.9%-2.2%) in 2011 to 1.5% (95% CI, 1.4%-1.6%) in 2017 (RR, 0.96; 95% CI, 0.94-0.97).
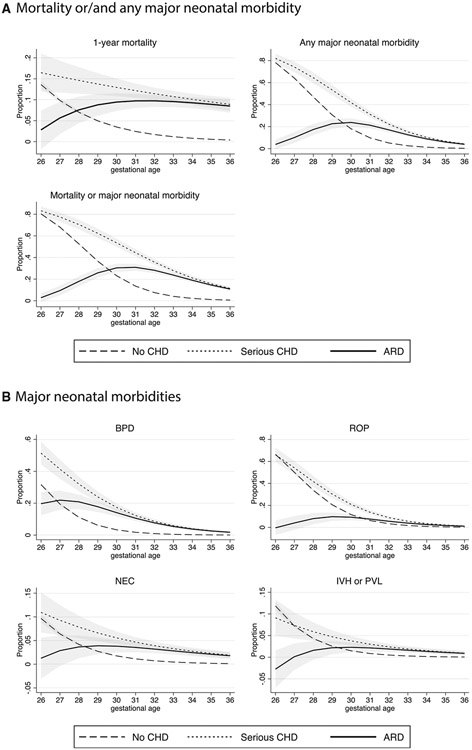
The mortality rate for infants with serious CHD was 24.4% (95% CI, 15.3%-37.3%) at 26 weeks of gestation and 11.2% (95% CI, 8.8%-14.1%) at 36 weeks of gestation (Table IV). The proportion of neonates with serious CHD who experienced any major neonatal morbidity was 62.7% (95% CI, 50.4%-74.6%) at 26 weeks of gestation and decreased to 4.8% (95% CI, 3.3%-7.0%) at 36 weeks of gestation (Table IV). Compared with neonates without serious CHD, the ARD for mortality was highest at 32 weeks of gestational age (9.7%; 95% CI, 8.3%-11.2%), that for major neonatal morbidity was highest at 28 weeks (21.9%; 95% CI, 17.0%-26.9%), and that for the composite outcome for mortality or major neonatal morbidity was highest at 30 weeks (26.7%; 95% CI, 23.3%-30.1%) (Table IV and Figure 4, A). Figure 4, B plots the predicted proportions and the ARDs of BPD, ROP, NEC, and IVH/PVL for neonates with CHD and those without serious CHD.
Table IV.
Mortality and major neonatal morbidity in preterm neonates with and without serious CHD
| Mortality/morbidities | No significant CHD, % (95% CI) | Significant CHD, % (95% CI) | ARD, % (95% CI) |
|---|---|---|---|
| Mortality | |||
| 26 wk | 15.9 (14.4-17.7) | 25.4 (15.3-37.3) | 2.8 (−1.8 to 7.5) |
| 27 wk | 9.6 (8.5-11.0) | 20.0 (11.9-31.7) | 5.7 (1.8-9.5) |
| 28 wk | 6.9 (6.0-7.9) | 10.8 (5.7-19.7) | 7.6 (4.4-10.8) |
| 29 wk | 4.1 (3.5-4.9) | 20.0 (11.9-31.7) | 8.8 (6.2-11.4) |
| 30 wk | 3.6 (3.1-4.2) | 9.5 (4.8-18.1) | 9.5 (7.3-11.6) |
| 31 wk | 2.4 (2.0-2.8) | 11.9 (7.0-19.6) | 9.7 (8.0-11.5) |
| 32 wk | 2.0 (1.7-2.2) | 12.1 (7.2-19.4) | 9.7 (8.3-11.2) |
| 33 wk | 1.4 (1.3-1.6) | 15.8 (11.0-22.1) | 9.6 (8.2-10.9) |
| 34 wk | 0.9 (0.8-1.0) | 9.1 (6.1-13.3) | 9.3 (7.9-10.6) |
| 35 wk | 0.6 (0.5-0.6) | 13.5 (10.4-17.4) | 8.9 (7.5-10.3) |
| 36 wk | 0.4 (0.4-0.5) | 11.2 (8.8-14.1) | 8.5 (6.9-10.1) |
| Major neonatal morbidities | |||
| 26 wk | 42.7 (40.5-45.0) | 62.7 (50.4-74.6) | 13.9 (7.2-20.5) |
| 27 wk | 32.6 (30.6-34.7) | 64.6 (52.1-75.4) | 19.3 (13.3-25.2) |
| 28 wk | 20.3 (18.9-21.9) | 43.4 (33.0-54.3) | 21.9 (17.0-26.9) |
| 29 wk | 13.6 (12.5-14.9) | 27.7 (18.0-40.0) | 21.7 (17.8-25.6) |
| 30 wk | 8.0 (7.3-8.8) | 25.0 (16.8-35.5) | 19.5 (16.5-22.4) |
| 31 wk | 4.4 (3.9-4.9) | 17.4 (11.3-25.8) | 16.3 (14.0-18.6) |
| 32 wk | 2.7 (2.4-3.0) | 11.2 (6.6-18.4) | 13.0 (11.1-14.8) |
| 33 wk | 1.3 (1.1-1.5) | 16.4 (11.5-22.7) | 10.0 (8.4-11.5) |
| 34 wk | 0.7 (0.6-0.8) | 13.8 (10.1-18.7) | 7.5 (6.1-8.8) |
| 35 wk | 0.4 (0.3-0.4) | 5.1 (3.3-7.9) | 5.5 (4.4-6.6) |
| 36 wk | 0.2 (0.1-0.2) | 4.8 (3.3-7.0) | 4.0 (3.0-5.0) |
| Mortality or morbidities | |||
| 26 wk | 52.4 (50.2-54.7) | 74.6 (62.7-83.7) | 11.8 (5.6-17.9) |
| 27 wk | 39.4 (37.3-41.5) | 69.2 (56.8-79.4) | 18.6 (13.0-24.2) |
| 28 wk | 25.6 (23.9-27.2) | 48.2 (37.5-59.0) | 23.7 (18.8-28.7) |
| 29 wk | 16.9 (15.6-18.2) | 40.0 (28.0-52.5) | 26.4 (22.3-30.5) |
| 30 wk | 10.8 (9.8-11.8) | 33.3 (24.0-44.2) | 26.7 (23.3-30.1) |
| 31 wk | 6.5 (5.9-7.1) | 26.6 (19.1-35.8) | 25.2 (22.5-27.9) |
| 32 wk | 4.4 (4.0-4.8) | 23.3 (16.4-31.9) | 22.7 (20.5-24.9) |
| 33 wk | 2.6 (2.4-2.9) | 28.1 (21.8-35.3) | 19.7 (17.7-21.7) |
| 34 wk | 1.6 (1.4-1.7) | 20.9 (16.4-26.4) | 16.7 (14.8-18.5) |
| 35 wk | 0.9 (0.8-1.0) | 17.0 (13.5-21.2) | 13.8 (12.0-15.6) |
| 36 wk | 0.6 (0.6-0.7) | 15.4 (12.6-18.7) | 11.3 (9.6-13.0) |
ARD was adjusted for sex, z-score for birth weight, and multiple gestation, with covariates kept at their mean values.
Figure 4.
Predicted adjusted outcomes of preterm infants with and without critical CHD. A, Mortality or/and any major neonatal morbidity. B, Major neonatal morbidities. The shaded area represents 95% CI.
Discussion
In this study of a contemporary population-based cohort of preterm neonates with serious CHD, we found an encouraging trend of declining mortality, from 19.1 % in 2011 to 11.6% in 2017. However, this is offset by a significant increase in major neonatal complications in this patient population. The proportion of major neonatal morbidity increased from 12.4% in 2011 to 21.6%, resulting in a RR increase of 8.3% for each year across the study period. Compared with peers without serious CHD of the same gestational age, the greatest discrepancy in mortality and morbidity was seen between 28 and 31 weeks of gestation.
It is well established that the prevalence of CHD is higher in preterm infants compared with their term counterparts. Chu et al reported a prevalence for severe CHD of 7.4 per 1000 in preterm neonates born between 25 and 32 weeks of gestation compared with 1.5/1000 in term births.9 Our study adds to this body of work by presenting the prevalence of CHD by week of gestational age. We found a linear relationship between the prevalence of CHD and gestational age; for each week increase in gestational age, the RR of CHD decreased by 17.9%. The reason for this increase with lower gestational age is not completely clear. In previous studies, women carrying a fetus with a CHD of any type had a 7-fold increase in the risk of early preeclampsia, which was attributed to the commonality of shared angiogenic imbalance in the mother and fetus.10,11 It could be speculated that this might at least contribute to an increased risk of extremely premature births in this patient population.
Mortality for CHD has continued to decrease over the last 3 decades12; however, large population-based studies investigating trends in mortality for CHD do not report mortality for preterm infants separately.13 In this study, we examined the trend in mortality for preterm neonates with CHD and found a decrease in mortality over time, with the RR of death decreasing by 10.6% for each year across the study period. This significant decrease in mortality is encouraging and may reflect advances in surgical techniques and perioperative care. It also may represent a shift in the dogma of congenital cardiac care, with more centers offering interventions for preterm infants with CHD in the current era, whereas previously cardiac interventions were not considered a viable option for extremely preterm infants.
However, these findings are offset by the increase in major neonatal morbidities across the study period. Major neonatal morbidities are well defined and extensively studied in the neonatal literature.4,14-16 They are strong surrogate markers for long-term health, neurodevelopmental outcomes, and quality of life.14 Given the challenges that even full-term infants with major CHD face in terms of neurodevelopment and quality of life,17 it is critical to focus on reducing morbidities in preterm neonates with CHD to prevent a compound effect of both preterm birth and CHD on long-term outcomes.
The concern about improving survival at the cost of increasing major neonatal morbidities has been a long-standing discussion among neonatologists, and the literature on this topic offers several important lessons. First, there is widespread consensus that the aim of neonatal care should be to focus on resuscitation of infants with a reasonable likelihood of an acceptable quality of life.16 Second, results of studies showing a decrease in mortality at the expense of increased morbidity have pointed out the importance of systematic approaches and interventions aimed at decreasing neonatal morbidity, including the adoption of consensus guidelines on how to manage respiratory distress syndrome or provide neuroprotective care.18 The result has been a sharp decline in neonatal complications among those without major congenital anomalies, with only the most immature infants being at considerable risk in the modern era.3,19 In the present study of preterm infants with CHD, the increase in major neonatal morbidity was related mainly to the observed increase in BPD. However, for each morbidity, the difference in incidence between infants with CHD and those without CHD was large and extended to later-preterm infants (gestational age 34-36 weeks).
It is reasonable to assume that some of the same interventions that were successful in preterm infants without CHD also might be beneficial in preterm infants with CHD if consistently applied in the preoperative and postoperative periods. In particular, respiratory management is critically important for neonates with CHD. In addition to cardiopulmonary bypass reducing pulmonary surfactant activity,20 these infants also often need prolonged respiratory support in the postoperative period. Evidence-based strategies to reduce lung injury of prematurity include less aggressive ventilation strategies, noninvasive respiratory support,21,22 minimally invasive surfactant therapy,23 and early administration of caffeine for central stimulation to facilitate weaning from the ventilator.24,25 Neurally adjusted ventilatory assist is another newer strategy to facilitate postextubation respiratory support and avoid reintubation,26 although currently there is no literature available on neonates with CHD and the benefit of this strategy. In addition, it has been shown that the range of partial pressure of oxygen variation is one of the main risk factors for ROP, and that fluctuations in oxygen administration should be avoided whenever feasible in this patient population.27 Although it appears that cardiac operations are technically feasible in preterm and small neonates with CHD and may result in survival, our findings illustrate the need to develop interdisciplinary clinical guidelines and evidence-based protocols specific to the premature infant with CHD to minimize morbidities and optimize long-term outcomes.
The data from our study suggest an inflection point in gestational age of 28-31 weeks during which medical changes in treatment can make the greatest difference. The ARD for major neonatal morbidity was highest at 28-31 weeks, ranging from 19.5% to 21.9% between those with CHD compared with peers of the same gestational age without CHD. A potential explanation for this finding might be that extremely immature neonates have a high risk of neonatal morbidity independent of CHD, and that preterm infants of gestational age >32 weeks are more resilient with fewer morbidities even if they have CHD. Although the implementation of evidenced-based neonatal interventions for preterm infants with CHD might benefit all age groups, the infants born at 28-31 weeks should be the primary target.
This study has several important limitations. First, case ascertainment of CHD and definitions of neonatal morbidities rely on ICD-9-CM and ICD-10-CM codes, and it is possible that we could have missed cases or morbidity outcomes if the ICD coding was incomplete. However, if this were the case, we would have expected to see nondifferential misclassification, which would bias our results toward the null. During the study period, coding was changed from ICD-9-CM to ICD-10-CM codes, which could have led to bias when assessing morbidities over time. However, given the opposite trends observed between premature infants without CHD and those with CHD, this bias seems unlikely. Regarding the type and timing of cardiac interventions, the lack of granularity of cardiac diagnoses, the complexity of cardiac procedure codes, and the lack of complete procedure codes for every case prevented us from performing analyses with regard to the type and timing of surgical repairs. Future studies should focus on the ideal surgical strategies in the preterm population with CHD, including the approach (ie, palliative vs complete) as well as timing of the first surgery. Along the same lines, we might have underestimated the incidence of severe ROP owing to missing ophthalmologic procedure codes in both groups.
Despite the limitations inherent in an administrative dataset, these data allowed us to study a population-based data sample, which is crucial in this patient population because CHD registries include only infants who were either treated in a cardiac intensive care unit or underwent surgery for CHD. Preterm infants who were treated in the intensive care nursery and never underwent surgery because of a palliative strategy would not be included in CHD registries. The other unique advantage is that this dataset allows us to compare outcomes in preterm neonates with CHD to their peers without CHD and to study other outcomes, such as major neonatal morbidities, that are not consistently captured in the CHD registries.
In California, mortality in preterm neonates with serious CHD decreased and major neonatal morbidity increased over the last decade. Preterm infants with a gestational age of 28-31 weeks have the highest morbidity rates compared with their peers of the same gestational age without CHD. Evidence-based strategies that are successfully used in very preterm infants without CHD in an attempt to reduce neonatal morbidity should be systematically implemented for all preterm neonates with CHD, to reduce the burden of neonatal morbidity in this patient population.
Glossary
- ARD
Adjusted risk difference
- BPD
Bronchopulmonary dysplasia
- CHD
Congenital heart disease
- ICD-9/10-CM
International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification
- IVH
Intraventricular hemorrhage
- NEC
Necrotizing enterocolitis
- PVL
Periventricular leukomalacia
- ROP
Retinopathy of prematurity
- RR
Relative risk
Footnotes
Funded by the California Preterm Birth Initiative. The authors declare no conflicts of interest.
References
- 1.Dees E, Lin H, Cotton RB, Graham TP, Dodd DA. Outcome of preterm infants with congenital heart disease. J Pediatr 2000;137:653–9. [DOI] [PubMed] [Google Scholar]
- 2.Desai J, Aggarwal S, Lipshultz S, Agarwal P, Yigazu P, Patel R, et al. Surgical interventions in infants born preterm with congenital heart defects: an analysis of the Kids’ Inpatient Database. J Pediatr 2017;191:103–9.e4. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steurer MA, Baer RJ, Keller RL, Oltman S, Chambers CD, Norton ME, et al. Gestational age and outcomes in critical congenital heart disease. Pediatrics 2017;140:e20170999. [DOI] [PubMed] [Google Scholar]
- 6.Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, Thangaratinam S, et al. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet 2011;378:785–94. [DOI] [PubMed] [Google Scholar]
- 7.Baer RJ, Norton ME, Shaw GM, Flessel MC, Goldman S, Currier RJ, et al. Risk of selected structural abnormalities in infants after increased nuchal translucency measurement. Am J Obstet Gynecol 2014;211:675. e1–19. [DOI] [PubMed] [Google Scholar]
- 8.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J 2012;12:308–31. [Google Scholar]
- 9.Chu PY, Li JS, Kosinski AS, Hornik CP, Hill KD. Congenital heart disease in premature infants 25-32 weeks’ gestational age. J Pediatr 2017;181:37–41.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auger N, Fraser WD, Healy-Profitós J, Arbour L. Association between preeclampsia and congenital heart defects. JAMA 2015;314:1588–98. [DOI] [PubMed] [Google Scholar]
- 11.Brodwall K, Leirgul E, Greve G, Vollset SE, Holmstrøm H, Tell GS, et al. Possible common aetiology behind maternal preeclampsia and congenital heart defects in the child: a cardiovascular diseases in Norway Project study. Paediatr Perinat Epidemiol 2015;30:76–85. [DOI] [PubMed] [Google Scholar]
- 12.Triedman JK, Newburger JW. Trends in congenital heart disease: the next decade. Circulation 2016;133:2716–33. [DOI] [PubMed] [Google Scholar]
- 13.Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990-2017. Medicine (Baltimore) 2020;99:e20593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Wassenaer-Leemhuis AG, Marlow N, Lees C, Wolf H, TRUFFLE investigators. The association of neonatal morbidity with long-term neurological outcome in infants who were growth restricted and preterm at birth: secondary analyses from TRUFFLE (Trial of Randomized Umbilical and Fetal Flow in Europe). Br J Obstet Gynaecol 2017;124:1072–8. [DOI] [PubMed] [Google Scholar]
- 15.Ambalavanan N, Carlo WA, Tyson JE, Langer JC, Walsh MC, Parikh NA, et al. Outcome trajectories in extremely preterm infants. Pediatrics 2012;130:e115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ancel PY, Goffinet F, Kuhn P, Langer B, Matis J, Hernandorena X, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort. JAMA Pediatr 2015;169:230–8. [DOI] [PubMed] [Google Scholar]
- 17.Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin Perinatol 2016;43:173–85. [DOI] [PubMed] [Google Scholar]
- 18.Sweet DG, Carnielli V, Greisen G, Hallman M, Özek E, te Pas A, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 update. Neonatology 2019;115:432–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HC, Liu J, Profit J, Hintz SR, Gould JB. Survival without major morbidity among very low birth weight infants in California. Pediatrics 2020;146:e20193865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griese M, Wilnhammer C, Jansen S, Rinker C. Cardiopulmonary bypass reduces pulmonary surfactant activity in infants. J Thorac Cardiovasc Surg 1999;118:237–44. [DOI] [PubMed] [Google Scholar]
- 21.Salvo V, Lista G, Lupo E, Ricotti A, Zimmermann LJI, Gavilanes AWD, et al. Comparison of three non-invasive ventilation strategies (NSIPPV/BiPAP/NCPAP) for RDS in VLBW infants. J Matern Fetal Neonatal Med 2018;31:2832–8. [DOI] [PubMed] [Google Scholar]
- 22.Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev 2016;6:CD001243. [DOI] [PubMed] [Google Scholar]
- 23.Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017;102:F17–23. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med 2006;354:2112–21. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 2007;357:1893–902. [DOI] [PubMed] [Google Scholar]
- 26.Lee BK, Shin SH, Jung YH, Kim EK, Kim HS. Comparison of NIV-NAVA and NCPAP in facilitating extubation for very preterm infants. BMC Pediatr 2019;19:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci 1995;36:2063–70. [PubMed] [Google Scholar]