Abstract
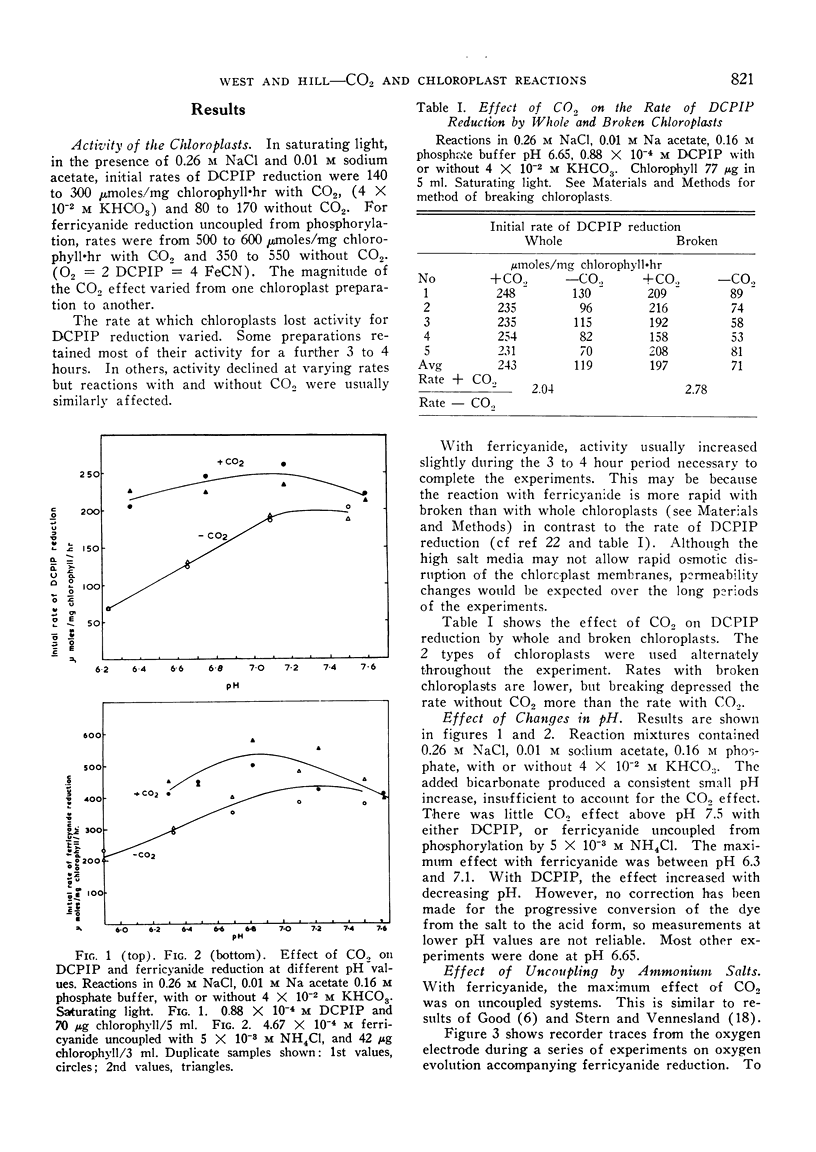
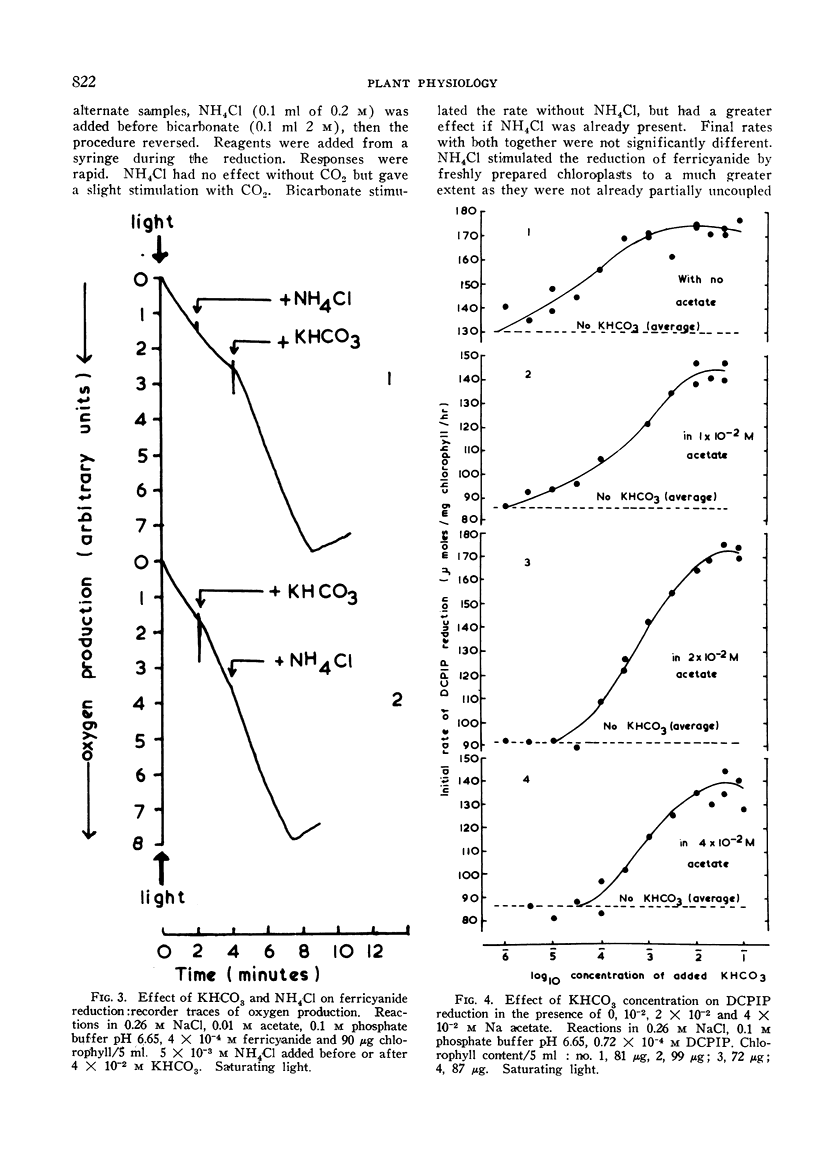
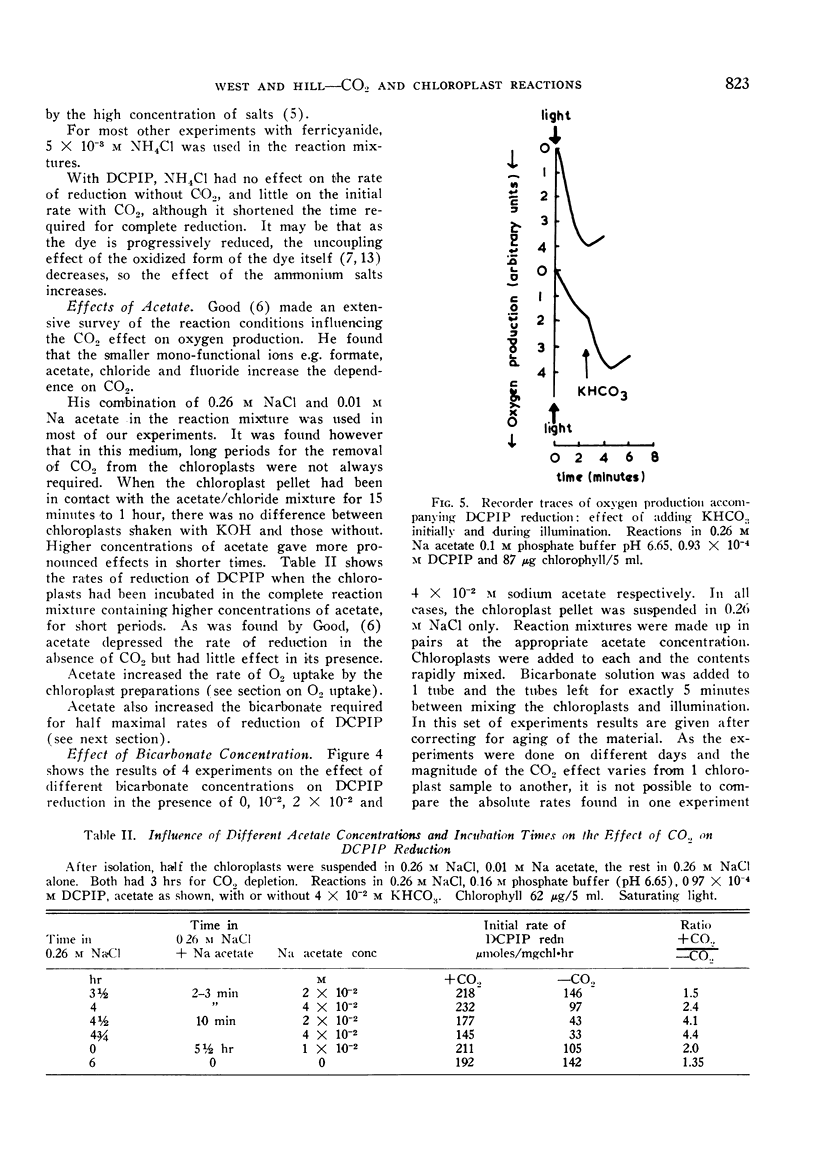
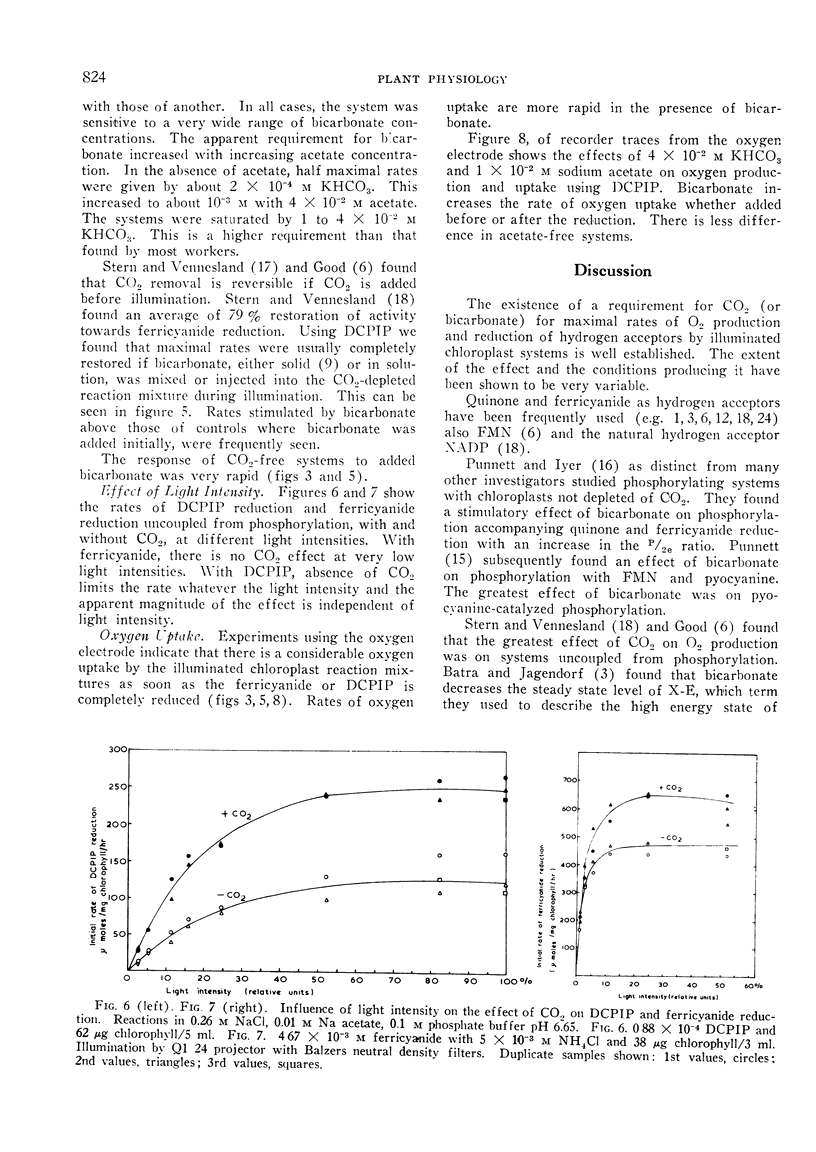
Pea chloroplasts isolated in salt media show decreased rates of 2:6 dichlorophenolindophenol (DCPIP) and ferricyanide reduction when depleted of CO2 at pH values below 7.5. The greatest effect of CO2 was on uncoupled systems. The incorporation of 10−2, 2 × 10−2 and 4 × 10−2 m sodium acetate into the reaction mixtures progressively increased the bicarbonate concentration required for half maximal rates of reduction of DCPIP. The reaction was saturated by bicarbonate concentrations of 1 to 4 × 10−2 m. With both DCPIP and ferricyanide, the addition of bicarbonate to illuminated chloroplast systems depleted of CO2 gave very rapid increases in the rates of reduction. Bicarbonate also stimulated oxygen uptake by the illuminated chloroplasts when added hydrogen acceptors had been reduced. There was no effect of bicarbonate on ferricyanide reduction at low light intensities, but with DCPIP reduction, the apparent magnitude of the effect was independent of light intensity. This suggests that DCPIP reacts with the chloroplast electron transport chain at a site nearer to a photochemical stage than does ferricyanide. It also suggests that CO2 has at least 2 sites of action.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Brown A. H., Mayne B. C. Stimulation of the Hill reaction by carbon dioxide. Plant Physiol. 1961 Mar;36(2):202–207. doi: 10.1104/pp.36.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra P. P., Jagendorf A. T. Bicarbonate effects on the Hill reaction and photophosphorylation. Plant Physiol. 1965 Nov;40(6):1074–1079. doi: 10.1104/pp.40.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle F. P. Some Factors Involved in Oxygen Evolution From Triturated Spinach Leaves. Science. 1948 Oct 1;108(2805):359–360. doi: 10.1126/science.108.2805.359. [DOI] [PubMed] [Google Scholar]
- GOOD N. E. Uncoupling of the Hill reaction from photophosphorylation by anions. Arch Biochem Biophys. 1962 Mar;96:653–661. doi: 10.1016/0003-9861(62)90352-1. [DOI] [PubMed] [Google Scholar]
- GROMET-ELHANAN Z., AVRON M. THE ROLE OF INDOPHENOL DYES IN PHOTOREACTIONS OF CHLOROPLASTS. Biochemistry. 1964 Mar;3:365–373. doi: 10.1021/bi00891a011. [DOI] [PubMed] [Google Scholar]
- Good N. E. Carbon Dioxide & the Hill Reaction. Plant Physiol. 1963 May;38(3):298–304. doi: 10.1104/pp.38.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnett T. Influence of Growth Conditions on the Enhancement of Photophosphorylation by Carbon Dioxide. Plant Physiol. 1965 Nov;40(6):1283–1284. doi: 10.1104/pp.40.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennesland B., Turkington E. O. The relationship of the Hill reaction to photosynthesis: studies with fluoride-poisoned blue-green algae. Arch Biochem Biophys. 1966 Sep 26;116(1):153–161. doi: 10.1016/0003-9861(66)90023-3. [DOI] [PubMed] [Google Scholar]
- Walker D. A. Correlation between Photosynthetic Activity and Membrane Integrity in Isolated Pea Chloroplasts. Plant Physiol. 1965 Nov;40(6):1157–1161. doi: 10.1104/pp.40.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]


