Abstract
Primary hyperhidrosis is a disorder of profuse sweating which negatively influences a patient's quality of life and is caused because of over-activation of the sympathetic nervous system. It was believed that hyperhidrosis is a condition limited to only anxious individuals; however, this hypothesis is discredited now. It has been found that people with a positive family history of primary hyperhidrosis are likely to suffer from this condition, suggesting a strong genetic basis. Genetic analysis has revealed a dominant autosomal pattern of inheritance with a variable degree of penetrance and is a sex-independent trait. It is a heterogeneous condition both genetically and clinically as different studies revealed variable genetics and clinical factors. There are no proper criteria for diagnosis as it is not treated as disease by most affected persons. Various studies revealed opposing results in localizing disease gene loci, so further genetic research is needed to pinpoint genes responsible for causing this debilitating condition. Gene expression profiling of human anxiety-causing genes in hyperhidrotic sufferers will also help to devise new treatment modalities. This review highlights the current genetic studies on hyperhidrosis, which may prove to be helpful in understanding the molecular mechanism governing hyperhidrosis.
Keywords: Hyperhidrosis (HH), hyperhidrotic, primary hyperhidrosis (PH), primary palmer hyperhidrosis (PPH)
Introduction
Sweating is the physiological response of the body to excessive heat and physical stress, but for some people, it exceeds to what is known to be normal and becomes a debilitating problem.[1] It can be characterized as primary focal hyperhidrosis and secondary hyperhidrosis. Primary hyperhidrosis is the most common type and typically affects different body parts such as face, hands, feet, and armpits, whereas secondary hyperhidrosis develops because of medical conditions (underlying diseases) such as diabetes mellitus, endocrine disease, neurologic disease, and so on.[2] Sweating is of two types: (1) thermoregulatory and (2) emotional. Both these types of sweating are because of the altered physiological state of the human body.[3]
Hyperhidrosis poses an immense level of psychological stress, which may force patients into social isolation.[4] It adversely affects daily life activities of the patients, leading them to suffer from mild to serious psychological problems depending on the severity of their hyperhidrosis. However, the socio-environmental condition of a patient plays a significant role as well.[29]
The prevalence varies among different populations as it was estimated between 1.0% and 6.1%, with the highest prevalence ratio in European individuals as compared to various Asian populations.[5]
The exact origin of hyperhidrosis is still unclear. It is proposed to result from the over-activation of the sympathetic nerves.[6] Additionally, it seems to be related with secretory cell metabolism or neuro-hormonal defects, but without any visible abnormality in the structure of sweat glands.[7] The expression study of neuregulin-1 (Nrg-1) revealed an over-expression of Nrg-1 messenger ribonucleic acid in hyperhidrosis patients, which results in hyper-methylation of the axons of the sympathetic ganglia and seems to cause over-activation of the thoracic sympathetic system.[6]
The strong patterns of inheritance suggest the genetic basis of hyperhidrosis and therefore considered to be hereditary in nature. Family history of hyperhidrosis plays a fundamental role in the onset of hyperhidrosis.[8] Hyperhidrosis is a multi-factorial complex genetic disorder, and understanding the genetic patterns of inheritance may help researchers to apprehend the underlying mechanism of hyperhidrosis.
It is noted that affected persons do not care about its diagnosis and proper treatment measures. However, it adversely disturbs the routine life activities of an individual without sex discrimination. Therefore, primary health care workers could guide patients for proper management of this issue for betterment of daily healthy living.
A Genetic perspective
Hyperhidrosis is the disorder of the autonomic nervous system. Earlier, for decades, Riley–Day syndrome or familial dysautonomia (FD) was the only genetic disorder; however, with increasing awareness, the list of genetic disorders influencing the autonomic nervous system increased significantly. Understanding the genetic mechanism of autonomic disorders helps to understand the pathophysiological and molecular models of different diseases related to autonomic dysfunction.[9]
Hyperhidrosis is a genetically transmitted disorder. However, it is still unclear whether primary hyperhidrosis is a single gene defect or a multi-factorial disorder.[10] In a study conducted by Ro et al.,[10] out of 49 patients, 32 (65%) demonstrated a positive family history; however, the control group revealed no family history of hyperhidrosis. From the genetic analysis, Ro et al. argued that primary hyperhidrosis is hereditary in nature with a variable degree of penetrance and is a sex-independent condition.[10] Another study revealed a positive family history of hyperhidrosis in 62% of patients, and the trait had skipped a generation, signifying either autosomal dominant transmission with reduced disease penetrance or an autosomal recessive disease with a high disease allele frequency.[11]
To identify the disease locus, genome-wide DNA polymorphic markers were used, and haplotype detailed analysis revealed primary palmer hyperhidrosis (PPH) loci which are positioned at 14q11.2-q13 to an interval between D14S1070 and D14S990.[12] The putative gene mapped by Higashimoto et al.[12] may be the major gene, but because of the genetically heterogeneous nature, the existence of other gene loci surely modifies the expression patteren of the identified gene. Another contradictory study revealed a hyperhidrosis locus on chromosome 2q31.1,[13] providing strong evidence of different genetic variants.
A study investigated 410 patients, out of which 147 (36%) patients showed an autosomal dominant inheritance pattern,[8] which is lesser than what has been observed in previous studies;[14] variation in the sample size may be the reason behind the variability in results. No substantial difference has been detected between positive family history and negative family history in sweat volume, sex, and onset age.[8] In mouse models, aquaporin-5 gene (AQP-5) has been identified to play a vital role in sweating, which may provide a potential understanding into the underlying mechanism in pathophysiology of mamalian hyperhidrosis,[15] but opposing human studies revealed no significant differences in the expression of AQP-5 gene in patients with palmoplanter hyperhidrosis and healthy subjects,[16,17] suggesting other genetic mechanisms in the pathogenesis of hyperhidrosis [Table 1].
Table 1.
Genetic analysis to identify disease gene loci revealed in opposing studies indicating other unidentified genetic mechanisms governing primary hyperhidrosis
| References | No. of Subjects | Results |
|---|---|---|
| Higashimoto et al., 2006[12] | 11 families (42 affected and 40 unaffected members) | Palmer hyperhidrosis (PPH) loci which are positioned at 14q11.2-q13 to an interval between D14S1070 and D14S990 |
| Chen et al., 2015[13] | Six generation family with PHH (n=21) | Hyperhidrosis locus on chromosome 2q31.1 |
| Nakahigashi et al., 2013[16] | N=7 patients (4 males and 3 females) | AQP-5 genes do not play any significant role in PHH pathogenesis in humans as opposed to a mouse study by (Nejsum et al..) |
The cellular process in sweat glands is controlled by ITPR2 gene, which codes for 2 inositol 1,4,5-trisphosphate receptor (InsP3R2) and is associated with human disease known as anhidrosis (inability to sweat). InsP3R2 also mediates Ca2+ release, which is important for eccrine sweat production in both humans and mice. It has been shown that missense mutation (p.G2498S) may induce isolated anhidrosis, which is localized within the pore-forming region of the mice and lacks the functional InsP3R2, with compromised Ca2+ production, resulting in the decrease of sweat production, and holds a potential pharmacological target in the treatment of hyperhidrosis.[18]
FoxA1 gene, which codes for Forkhead transcription factor, plays a significant role in sweat regulation through bestrophin 2 anion channel and Na–K–Cl cotransporter. The study showed FoxA1 transcription factor to be an important factor that generates sweating capacity in mice. Knockdown of FoxA1 revealed complete anhidrosis, which was confirmed by iodine starch test on mice footpads. This anhidrosis is accompanied by down-regulation of Na–K–Cl cotransporter 1 (Nkcc1) and the Ca2+-activated anion channel bestrophin 2 (Best2).[19] These components can be pharmaceutical targets to reduce the symptoms of hyperhidrosis.
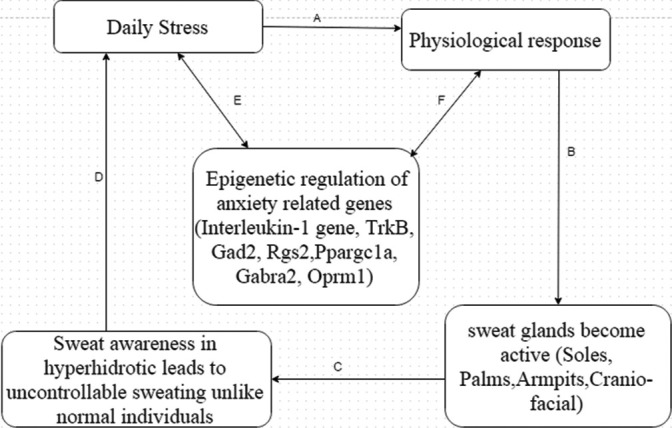
The environment influences the expression of certain genes.[20] In hyperhidrotic individuals, all experience the same level of social and enviormental stress which may cause varying levels of anxiety and constant anxiety may influence the expression of genes. Anxiety disorders are initiated by environmental stimuli in genetically susceptible individuals, and therefore, genetic research could present a great route to understand the molecular basis of the diseases. It has been extensively accepted that excessive sweating triggers anxiety[21] and in turn anxiety makes sweating worse and the sufferers of this disorder get caught up in hyperhidrosis sweat cycle [Figure 1].
Figure 1.
Hyperhidrosis sweat cycle: (a) Daily stress induces physiological response and sympathetic nervous system becomes active, (b) sweat glands become active (glands become more active in hyperhidrosis individuals due to physiological imbalance, (c) sweat awareness makes the situation worse for hyperhidrotic sufferers, and it becomes a primary stressor, (d) primary stressor may aggravate the daily stress, due to which the sufferers get caught up in hyperhidrosis sweat cycle, (e) daily chronic stress may negatively influence the expression pattern of certain human anxiety-related genes, and (f) changes in the epigenetics may impact the normal physiological homeostasis of the sufferers by influencing the autonomic nervous system
A study revealed that constant or chronic stress may influence the pattern of interleukin-1 gene expression, making it a potential cause of stress and heart diseases.[22]
Anxiety disorder is complex multi-factorial disease caused by a combination of environmental and genetic factors. Recent studies showed association of several genes with anxiety disorder.[23] Characterization of susceptible genes for anxiety disorders involves quantitative trait locus (QTL) mapping. The QTL mapping revealed human anxiety-causing genes, which are TrkB, Gad2, Rgs2, Ppargc1a, Gabra2, and Oprm1.[19]
Understanding the relationship between anxiety and hyperhidrosis-causing genes will help to devise better anxiolytics, which in turn helps in controlling the condition by breaking the vicious hyperhidrosis sweat cycle [Figure 1].
Genetic Marker Association
In a study conducted by Chihiro Endo et al.,[27] 2018, to explore the novel skin-related trait associations, three SNPs were discovered (rs56089838, rs1534480, and rs6500380) with alleles G/C, T/C, and G/A found in genes PPPICB, PLB1, and ABCC11, respectively. For too much phenotype with an extraordinary level of sweating, two novel SNPs were discovered, with one SNP residing at chromosome 2 at a distance of 28.82–29.05 Mb and the other one found on chromosome 16 at a distance of 48.26–48.45 Mb.
Genome-wide association studies of primary palmer parasympathetics in Japanese pointed out a genetic region (14q112-q13), but the region did not turn out to be associated. Findings of the genome studies revealed that the disease was associated with SNPs lying in two genes present on chromosome 2 and chromosome 16. Previous studies showed the weight of support for two genes (PLB1 and PPPICB) as candidates for their contribution toward sweating. PLBI encodes for enzyme PLB, which possesses phospholipase A1 and phospholipase A2 activities on an enzymatic level. PLB1 was primarily discovered in humans with its expression in epidermis and was presumed to have function by developing a skin barrier action by assisting breaking down of lipids to free fatty acids.[25,28] Moreover, on the basis of its contribution in developing acrosome exocytose in sperm, it is leeway in modulating secretory processes in conditions like sweating.
The study also hypothesizes that PPPICB eQTL SNP may influence production of sweat by adapting to the quantity of PPPICB existing and in turn swaying phosphorylation, the level of AQP5, and other proteins indispensable for sweat gland function.
One of the signals present on chromosome 16 covers the genetic region giving rise to two genes, ABCC11 and LONP2. Following the eQTL and linkage disequilibrium orientation analysis, the research ended up with the outcome that an already predicted missense SNP residing in ABCC11 (rs7822931) was the potential allele in five closely associated SNPs on that locus. The studied missense SNP was thought to be involved with wet versus dry earwax and body odor in previously conducted research. The study is the first ever to report and show the relation of SNP (rs7822931) with hyperhidrosis.[24]
Another study[26] was carried out to probe genetic polymorphism analysis (of SNPs) in patients with primary hyperhidrosis. To explore genetic variants, 21 cases and the same number of control DNA samples were analyzed with genotyping of 20 SNPs associated with butyrycholineestrase (BCHE) and cholinergic receptor (CHRNA7) genes. After analysis, patients with primary hyperhidrosis were found to have -116A allele (P-value = 0.15) for rs1126680 coupled with K-variant allele (P–value = 0.65) in rs1803274.
In short, 18 out of 20 candidate SNPs did not mark any affiliation with primary hyperhidrosis. However, only exclusive alliance with primary hyperhidrosis was observed in patients possessing both -116A and K-variants on BCHE gene.[26]
Future Directions
Further research is required to provide evidence characterizing the exact mechanism of pathogenesis and to identify the putative gene or network of genes causing primary hyperhidrosis. Targeted next generation sequencing and employing bioinformatics data analysis techniques could prove to be useful in characterizing the disease-causing genes and also increase our knowledge in understanding the mechanism of pathogenesis governing PHH. A better understanding of the condition will eventually lead to better management of the condition. More genome-wide association studies are needed to be conducted to screen out the associate genetic variants with this disease so that therapeutic strategies could be devised. Population genetic studies could be beneficial to see which alleles associated with this disease are population-specific. Studies should also be planned to figure out biomarkers for this disease and to see their potential use to determine commencement of the disease.
Key take-home message
| Primary hyperhidrosis is a link to dysregulation of the sympathetic nervous system which can be enhanced by physiological, emotional, and physical actions of a patient |
| There is a strong genetic basis related to primary hyperhidrosis along with other factors |
| Primary hyperhidrosis is an autosomal dominant condition with diverse penetrance patterns in different individuals |
| Heterogeneous condition with involvement of multiple factors |
| There are no proper diagnosis criteria for disorder |
| Adversely affects the healthy life of affected persons |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hamm H. Impact of hyperhidrosis on quality of life and its assessment. Dermatol Clin. 2014;32:467–76. doi: 10.1016/j.det.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol. 2011;64:690–5. doi: 10.1016/j.jaad.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Wohlrab J, Bechara FG, Schick C, Naumann M. Hyperhidrosis: A central nervous dysfunction of sweat secretion. Dermatol Ther. 2023;13:453–63. doi: 10.1007/s13555-022-00885-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber A, Heger S, Sinkgraven R, Heckmann M, Elsner P, Rzany B. Psychosocial aspects of patients with focal hyperhidrosis. Marked reduction of social phobia, anxiety and depression and increased quality of life after treatment with botulinum toxin A. Br J Dermatol. 2005;152:342–5. doi: 10.1111/j.1365-2133.2004.06334.x. [DOI] [PubMed] [Google Scholar]
- 5.Schote AB, Schiel F, Schmitt B, Winnikes U, Frank N, Gross K, et al. Genome-wide linkage analysis of families with primary hyperhidrosis. PLoS One. 2020;15:e0244565. doi: 10.1371/journal.pone.0244565. doi: 10.1371/journal.pone.0244565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu Y, Luo R, Li X, Lin M, Qiu M. Hypermyelination and overexpression of neuregulin-1 in thoracic sympathetic nerves in patients with primary palmar hyperhidrosis. J Clin Neurosci. 2012;19:1651–3. doi: 10.1016/j.jocn.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Bovell DL, Clunes MT, Elder HY, Milsom J, Jenkinson DM. Ultrastructure of the hyperhidrotic eccrine sweat gland. Br J Dermatol. 2001;145:298–301. doi: 10.1046/j.1365-2133.2001.04351.x. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita N, Tamada Y, Kawada M, Mizutani K, Watanabe D, Matsumoto Y. Analysis of family history of palmoplantar hyperhidrosis in Japan. J Dermatol. 2009;36:628–31. doi: 10.1111/j.1346-8138.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- 9.Axelrod FB. Genetic autonomic disorders. https://doi.org/10.1016/j.spen.2012.120.002. Semin Pediatr Neurol. 2013;20:3–11. doi: 10.1016/j.spen.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Ro KM, Cantor RM, Lange KL, & Ahn SS. Palmar hyperhidrosis: Evidence of genetic transmission. J Vasc Surg. 2002;35:382–6. doi: 10.1067/mva.2002.119507. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann H, Saadia D, Polin C, Hague S, Singleton A, Singleton A. Primary hyperhidrosis--evidence for autosomal dominant inheritance. Clin Auton Res. 2003;13:96–8. doi: 10.1007/s10286-003-0082-x. [DOI] [PubMed] [Google Scholar]
- 12.Higashimoto I, Yoshiura K, Hirakawa N, Higashimoto K, Soejima H, Totoki T, et al. Primary palmar hyperhidrosis locus maps to 14q11.2-q13. Am J Med Genet A. 2006;140:567–72. doi: 10.1002/ajmg.a.31127. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Lin M, Chen X, Cao Z, Tan Z, Xiong W, et al. A novel locus for primary focal hyperhidrosis mapped on chromosome 2q31.1. Br J Dermatol. 172:1150–3. doi: 10.1111/bjd.13383. [DOI] [PubMed] [Google Scholar]
- 14.Shih CJ, Wang YC. Thoracic sympathectomy for palmar hyperhidrosis: Report of 457 cases. Surg Neurol. 1978;10:291–6. [PubMed] [Google Scholar]
- 15.Nejsum LN, Kwon T-H, Jensen UB, Fumagalli O, Frøkiaer J, Krane CM, et al. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci U S A. 2002;99:511–6. doi: 10.1073/pnas.012588099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakahigashi K, Nomura T, Miyachi Y, Kabashima K. Normal immunostaining pattern for aquaporin-5 in the lesions of palmoplantar hyperhidrosis. Case Rep Dermatol. 2013;5:61–3. doi: 10.1159/000349908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Sorbo F, Brancati F, De Joanna G, Valente EM, Lauria G, Albanese A. Primary focal hyperhidrosis in a new family not linked to known loci. Dermatology (Basel, Switzerland) 2011;223:335–42. doi: 10.1159/000334936. [DOI] [PubMed] [Google Scholar]
- 18.Klar J, Hisatsune C, Baig SM, Tariq M, Johansson ACV, Rasool M. Abolished InsP3R2 function inhibits sweat secretion in both humans and mice. J Clin Investig. 2014;124:4773–80. doi: 10.1172/JCI70720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokolowska E, Hovatta I. Anxiety genetics-findings from cross-species genome-wide approaches. Biol Mood Anxiety Disord. 2013;3:9. doi: 10.1186/2045-5380-3-9. doi: 10.1186/2045-5380-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JK, & Kim SC. Environmental effects on gene expression phenotype have regional biases in the human genome. Genetics. 2007;175:1607–13. doi: 10.1534/genetics.106.069047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross KM, Schote AB, Schneider KK, Schulz A, Meyer J. Elevated social stress levels and depressive symptoms in primary hyperhidrosis. PloS One. 2014;9:e92412. doi: 10.1371/journal.pone.0092412. doi: 10.1371/journal.pone.0092412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlereth T, Dittmar JO, Seewald B, Birklein F. Peripheral amplification of sweating--a role for calcitonin gene-related peptide. J Physiol. 2006;576:823–32. doi: 10.1113/jphysiol.2006.116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hovatta I, Barlow C. Molecular genetics of anxiety in mice and men. Ann Med. 2008;40:92–109. doi: 10.1080/07853890701747096. [DOI] [PubMed] [Google Scholar]
- 24.Endo C, Johnson TA, Morino R, Nakazono K, Kamitsuji S, Akita M, et al. Genome-wide association study in Japanese females identifies fifteen novel skin-related trait associations. Sci Rep. 2018;8:8974. doi: 10.1038/s41598-018-27145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maury E, Prévost MC, Nauze M, Redoulès D, Tarroux R, Charvéron M, et al. Human epidermis is a novel site of phospholipase B expression. Biochem Biophys Res Commun. 2002;295:362–9. doi: 10.1016/s0006-291x(02)00657-5. [DOI] [PubMed] [Google Scholar]
- 26.Simes BC, Moore JP, Brown TC, Rushforth TJ, Bookout AL, Richardson CL. Genetic polymorphism analysis of patients with primary hyperhidrosis. Clin Cosmet Investig Dermatol. 2018;11:477–83. doi: 10.2147/CCID.S176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henning MAS, Thorlacius L, Ibler KS, Jemec GBE. How to diagnose and measure primary hyperhidrosis: A systematic review of the literature. Clin Auton Res. 2021;31:511–28. doi: 10.1007/s10286-021-00794-6. [DOI] [PubMed] [Google Scholar]
- 28.Bradshaw RA, Dennis EA. United States of America: Academic Press; 2009. Handbook of Cell Signaling. [Google Scholar]
- 29.Henning MA, Pedersen OB, Jemec GB. Genetic disposition to primary hyperhidrosis: a review of literature. Arch Dermatol Res. 2019;311:735–40. doi: 10.1007/s00403-019-01966-1. doi: 10.1007/s00403-019-01966-1. [DOI] [PubMed] [Google Scholar]