Abstract
Background & aims:
PUFA intake is associated with reduced cardiovascular and all-cause mortality in the general population; however, evidence about this association in older adults is controversial. The objective of this study was to evaluate the relationship between PUFA intake and serum concentration, and the association of these variables with all-cause and cardiovascular mortality.
Methods:
in this cohort study, we selected 927 community dwelling adults aged ≥65 years enrolled in the InCHIANTI study from 1998 to 2000 and followed-up for 9 years. The association between PUFA intake and serum concentration was evaluated using scatterplot and Pearson correlation test; all-cause and cardiovascular mortality was analyzed using the Kaplan-Meier method and Cox regressions adjusted for potential confounders.
Results:
mean age of the population was 75 years (SD 7.3), 55% were women. There was no association between overall PUFAs, linolenic and linoleic acid intake and their serum concentration. There was no association between quartiles (Q) of PUFA intake and all-cause mortality: compared to Q1 of PUFA intake, the adjusted HR (95% CI) for overall mortality were: 1.05 (0.74–1.50) in Q2, 1.10 (0.76–1.58) in Q3, and 0.98 (0.68–1.41) in Q4; this lack of association was confirmed for cardiovascular mortality. Compared to Q1, participants in the fourth quartile of PUFA serum concentration had lower risk of all-cause mortality (adjusted HR [95%CI]: Q2 1.10 [0.79–1.53], Q3 0.84 [0.60–1.19], Q4 0.66 [0.44–0.995]), no association was found for cardiovascular mortality.
Conclusions:
In our sample of community-dwelling older adults, PUFA intake is not associated with PUFA serum concentration. Interventions to modulate PUFA concentration based on dietary intake may not be effective in preventing mortality in this population.
Keywords: Aged, Diet, Polyunsaturated fatty acids, Mortality, Linolenic acid, Linoleic acid
1. Introduction
Both PUFA intake and PUFA serum concentration may have a role in the prevention of cardiovascular disease (CVD) and death [1], and this effect is likely to be mediated by PUFA serum concentration. Indeed, observational studies in adult populations found an inverse association between PUFA intake and CVD mortality and all-cause mortality [2–5]. Assessing PUFA dietary intake has two major drawbacks: it is inherently imprecise, dependent on the questionnaire used, and it is only a hypothetical proxy of the PUFA serum concentration, but it has the advantage of being less expensive and more easily available; furthermore, it is a modifiable factor [6]. However, clinical trials on PUFA supplementation showed discordant results [4].
In older adults, studies on the relationship between PUFA and mortality have provided discordant results. In a relatively small population, Solfrizzi et al. did not find a relationship between dietary PUFA intake and mortality [7], while Mozaffarian et al. showed a statistically significant inverse correlation between serum ω−3 PUFA serum concentration and all-cause and CVD mortality [8]; similar results were found by Wu et al. for ω−6 PUFA concentration [9]. Mozaffarian et al. and Wu et al. evaluated the association between PUFA intake and serum concentration using food-frequency questionnaires performed three years before the blood sampling, thus being not representative of the PUFA intake at the time of PUFA serum concentration assessment. Thus, it is not clear if the discordant results reported in the literature may be due to a weak relationship between PUFA intake and PUFA serum concentration or to biases in the available studies.
We speculated that the lack of association between PUFA intake and mortality in older adults might be underpinned by a weak relationship between PUFA intake and PUFA serum concentration in this population.
The objective of this study was firstly to evaluate the correlation between dietary intake of PUFA and PUFA serum concentration, and then to evaluate whether PUFA intake, serum concentration or both were associated with all-cause and cardiovascular mortality in a sample of community-dwelling older adults.
2. Materials and methods
2.1. Data source and study design
We analyzed data from the longitudinal InCHIANTI study [10]. The baseline study was supported by the Italian Ministry of Health and partly supported by the US National Institute on Aging. After obtaining informed consent, participants were randomly selected from the populations of two town areas in Tuscany, Italy; baseline data collection started in September 1998 and was completed in March 2000. The interviews of the eligible participants were performed at home by trained study researchers and were followed by a physical examination at the study clinic and by a laboratory analysis. Follow-up visits were scheduled at 3, 6, and 9 years. The procedures followed were in accordance with the Helsinki Declaration of 1975 as revised in 1983.The Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol. There was no reward for participation to the study.
2.2. Sample selection
From 1453 participants included in the InCHIANTI study, 1155 were aged 65 years or older; of these, 1139 had estimated PUFA dietary intake at baseline. We selected only those having the PUFA serum concentration evaluated at the same baseline assessment (N: 927) (Supplementary Fig. 1).
2.3. Definition of exposure and outcome
PUFA dietary intake was estimated using the EPIC questionnaire [11]. This questionnaire investigates the intake frequency over the previous year of 236 specific foods, along with the average size of the servings, selected from a range as shown in photographs. The information derived from the questionnaire is automatically converted into data on energy, micro- and macronutrient intake by a specifically designed software. The EPIC nutritional assessment has been successfully validated in an older adult population, by comparing the dietary intake estimated by this method with the dietary intake estimated by a direct method of measuring, weighing and recording of seven day-food consumptions [12]. For the present analyses we used total PUFA intake, and linoleic and linolenic acid (g/day) (respectively, the essential and most represented ω−6 and ω−3 in the diet).
Blood samples for PUFA serum concentration were collected in the morning, after the participants had been fasting for at least 8 h. Measurement of individual fatty acid was performed using gaschromatography (Hewlett-Packard, Palo Alto, CA). Total PUFAs were calculated by summing C18:2 n-6 cis, C18:3 n-3 cis, C20:2 n-6 cis, C20:3 n-9 cis, C20:4 n-6 cis, C20:5 n-3 cis and C22:6 n-3 cis fatty acids and expressed in mg/l. The median time between food questionnaire and blood sample was 26 days (Supplementary Fig. 2).
We considered mortality for all causes and cardiovascular mortality (ICD-9 codes from 410 to 440.9 and from 444 to 444.91 [13]) as outcome measures; these data were collected from mortality registers after a 9-year follow-up and were available for all participants.
2.4. Analytic approach
We standardized PUFA for total lipid intake (PUFA intake) or total fatty acid weight (PUFA serum concentration). The characteristics of the study sample were reported using descriptive statistics (mean and standard deviation for continuous variables, proportion for categorical variables), according to quartiles of PUFA intake. We included associated diseases (e.g. hypertension, diabetes), cigarette smoke (pack-years), blood pressure, total cholesterol, estimated glomerular filtration rate (eGFR), evaluated through the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, total caloric and alcohol intake (EPIC Questionnaire). Finally, we considered years of education and physical activity.
The cross-sectional correlation between PUFA intake and PUFA serum concentration was evaluated using a scatterplot with LOESS interpolation and the Pearson coefficient. Mortality risk across quartiles of PUFA intake and serum concentration was calculated using the Kaplan-Meier method. The relationship between quartiles of PUFA intake and all-cause mortality and cardiovascular mortality was estimated using Cox regressions and reported as hazard ratios (HRs) and 95% confidence intervals (CIs). The models were then adjusted for potential confounders, selected on the basis of the clinical significance, prior knowledge, and results of the univariate analysis. To explore the different role of demographic and clinical variables, we first adjusted for age and sex, and then for the other potential confounders (education, CKD-EPI, pack/year, hypertension, diabetes, BMI, caloric intake/body weight, alcohol and oleic acid consumption). The same analysis was performed for PUFA serum concentration. As sensitivity analysis, we also evaluated unstandardized PUFA intake and serum concentration; the results were virtually unchanged and therefore not reported.
All analyses were performed using R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria) [14].
3. Results
3.1. General results
The mean age of the population was 75 years (SD 7.3), 55% were female. Cut-off values for quartiles of PUFA intake/lipid intake ratio (Q1-Q2-Q3-Q4) were 0.100, 0.107, and 0.117. Patients in Q3 were younger compared to the others (73.8 years vs. more than 75 years in the other quartiles). There were no differences across quartiles in sex, BMI, eGFR, and total cholesterol serum concentration. Total caloric intake, protein, lipids, and mono-unsaturated fatty acids intake decreased across quartiles, while there were no differences in total carbohydrates intake. The linoleic/linolenic acid ratio increased across quartiles (I quartile: 5.2, SD 0.7 to IV quartile: 6.7, SD 1.4, P < 0.001). The prevalence of comorbidities did not change according to PUFA intake, with the exception of diabetes, that was more frequent in the IV quartile (19% vs. 7% in the I quartile, P = 0.002) (Table 1).
Table 1.
General characteristics of the population according to total PUFA/total lipid intake quartiles.
| Characteristics | I quartile (0.072–0.100) | II quartile (0.101–0.107) | III quartile (0.108–0.117) | IV quartile (0.118–0.248) | P |
|---|---|---|---|---|---|
| N = 232 | N = 232 | N = 232 | N = 231 | ||
| Age | 75.4 (7.5) | 75.1 (7.6) | 73.8 (6.8) | 75.7 (7.4) | 0.030 |
| Female sex | 59 | 51 | 54 | 58 | 0.360 |
| Education (years) | 5.7 (3.7) | 5.3 (3.1) | 5.2 (3) | 5.3 (3.5) | 0.35 |
| BMI (Kg/mq) | 27.2 (3.7) | 27.3 (3.7) | 27.7 (3.9) | 27.4 (4.7) | 0.644 |
| eGFR (CKD-EPI) (ml/min/1.73m2) | 70.5 (13.8) | 71.2 (14.2) | 71.6 (14.7) | 70.2 (14.8) | 0.714 |
| Total cholesterol (mg/dl) | 217.1 (43.9) | 215.2 (39.7) | 217.4 (37.5) | 214.8 (40.1) | 0.864 |
| Total Kcal intake | 1997.8 (602.6) | 1960.1 (563.1) | 1937.4 (544.6) | 1779.6 (519.6) | <0.001 |
| Total Protein (g/day) | 78 (22.2) | 76.5 (19.9) | 76.6 (20.6) | 69.5 (18.1) | <0.001 |
| Total lipids (g/day) | 69.4 (21.3) | 68 (19.7) | 64 (19.3) | 56.2 (17.5) | <0.001 |
| Total glucids (g/day) | 253.5 (86.5) | 246.6 (77) | 253 (78.5) | 242.2 (78.2) | 0.356 |
| Linoleic (ω−6)/Linolenic (ω−3) acid ratio | 5.2 (0.7) | 5.7 (0.8) | 5.9 (0.8) | 6.7 (1.4) | <0.001 |
| MUFA (g/day) | 33.6 (11.5) | 34.8 (10.5) | 32.6 (11) | 27.8 (9.2) | <0.001 |
| Smoke (pack/year) | 12.2 (20.7) | 13.1 (22.8) | 12 (19.8) | 12.6 (19.8) | 0.94 |
| Reduced physical activity | 66 | 63 | 64 | 66 | 0.892 |
| Hypertension | 59 | 56 | 64 | 65 | 0.190 |
| Diabetes | 7 | 13 | 13 | 19 | 0.002 |
| COPD | 6 | 12 | 8 | 10 | 0.125 |
| Metabolic Syndrome | 22 | 17 | 22 | 27 | 0.059 |
| Gait speed (m/s) | 1 (0.3) | 1 (0.3) | 1 (0.3) | 1 (0.3) | 0.167 |
| Serum lipid reducing agents | 0.03 (0.17) | 0.03 (0.16) | 0.04 (0.19) | 0.05 (0.20) | 0.459 |
Abbreviations: COPD: chronic obstructive pulmonary disease; CKD-EPI: chronic kidney disease epidemiology collaboration; eGFR: estimated glomerular filtration rate; MUFA: monounsaturated fatty acids.
We found no relationship in individual ω−3 and ω−6 PUFA serum concentration across quartiles of PUFA intake, except for a slight increase in eicosapentaenoic acid concentration in the II and III quartile (Supplementary Table 1).
The median follow-up time was 9 years, with a cumulative follow-up time of 6957 years. Over the follow-up time, 318 participants died, with an incidence rate of 4.57/100 person-year (95% CI 4.09–5.09) and a cumulative risk of 34.4% (95% CI 31.2–37.4); there were 114 cardiovascular deaths, with an incidence rate of 1.64/100 person-year (95% CI 1.36%–1.95%) and a cumulative risk of 16.9% (95% CI 11.5%–22%).
3.2. Association between PUFA intake and PUFA serum concentration
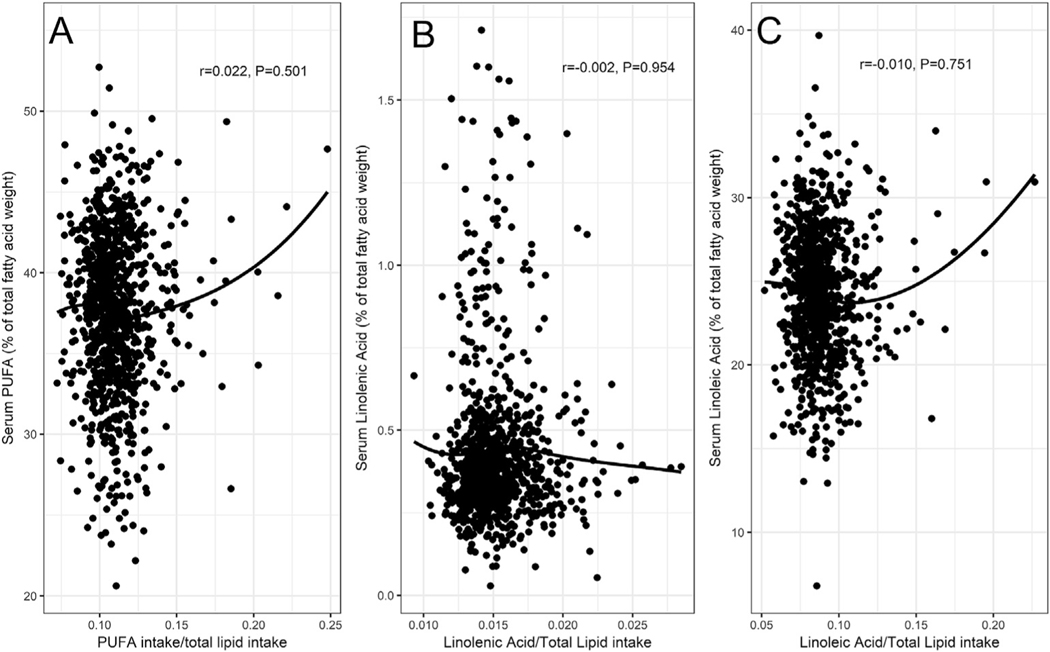
As shown in Fig. 1, panel A, we found no association between PUFA intake and PUFA serum concentration (r = 0.022, P = 0.501). Results did not change after stratification by sex (male: r: 0.028, P = 0.526; female: r: 0.005, P = 0.924), or by age (age <80 years: r: −0.002, P = 0.960; age ≥ 80 years: r: 0.066, P = 0.324) (data not shown). Similar results were found for the association between linolenic and linoleic acid intake and serum concentration (r −0.002, P = 0.954 and r = −0.010, P = 0.751) (Fig. 1, panels B and C, respectively).
Fig. 1.
Correlation between intake and serum concentration of overall PUFAs (panel A), linolenic (ω−3) (panel B) and linoleic acid (ω−6) (panel C).
3.3. PUFA intake and mortality
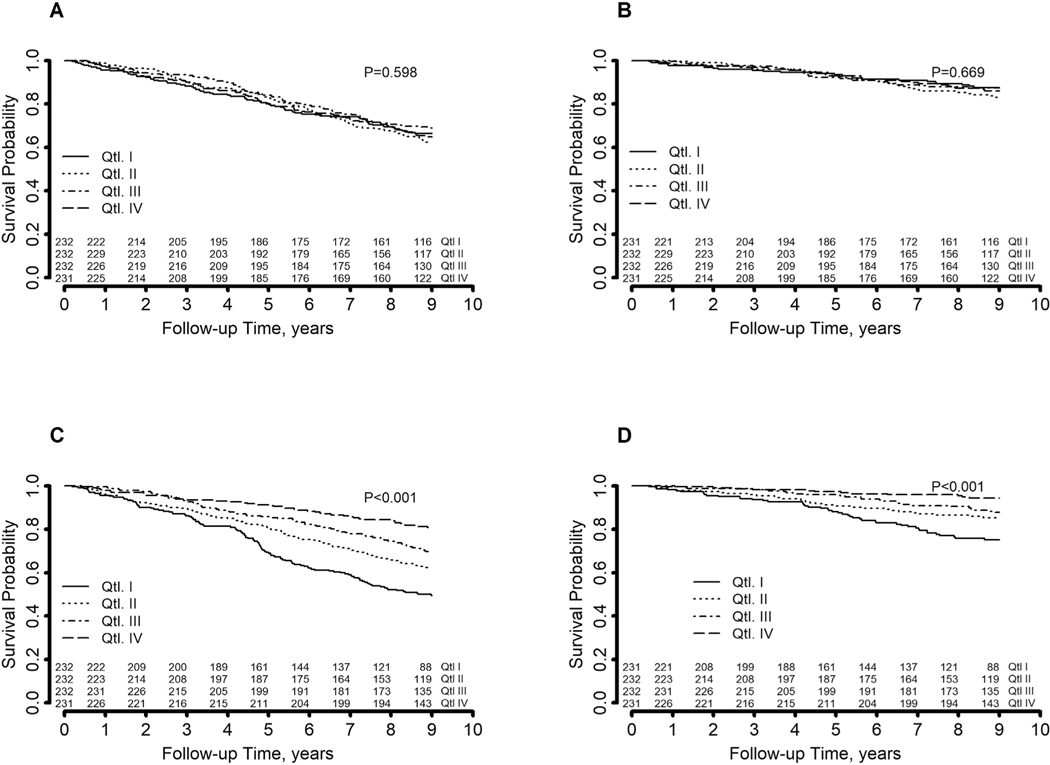
Kaplan-Meier curves showed no differences in both all-cause and cardiovascular mortality across quartiles of PUFA intake/total lipid intake (Fig. 2, panels A and B).
Fig. 2.
Kaplan-Meyer curves of overall and cardiovascular mortality by quartiles of PUFA intake (panels A and B, respectively) and quartiles of PUFA serum concentration (panels C and D, respectively).
Similar results were found for linoleic and linolenic intake (Supplementary Figs. 3 and 4, respectively). The lack of association between PUFA intake quartiles and all-cause mortality was confirmed in Cox regression models adjusted for age, sex, education, CKD-EPI, cigarette packs/year, hypertension, diabetes, BMI, caloric intake/body weight, alcohol and oleic acid consumption with HR 1.05 (95% CI 0.74–1.50), 1.10 (0.76–1.58), and 0.98 (0.68–1.41) in Q2, Q3, and Q4, respectively. The corresponding adjusted HR (95% CI) for cardiovascular mortality were 1.22 (0.66–2.25), 1.40 (0.76–2.59), and 0.98 (0.51–1.86) (Table 2). The lack of association with all-cause and cardiovascular mortality was confirmed also for quartiles of linolenic and linoleic acid intake (Supplementary Table 2).
Table 2.
HR for all-cause mortality and cardiovascular mortality according to PUFA/total lipid intake, using the I quartile as the reference one.
| Quartiles | All-cause mortality |
Cardiovascular mortality |
||
|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HRa (95% CI) | Crude HR (95% CI) | Adjusted HRa (95% CI) | |
| II quartile | 1.10 (0.81–1.49) | 1.05 (0.74–1.50) | 1.34 (0.80–2.24) | 1.22 (0.66–2.25) |
| III quartile | 0.89 (0.64–1.22) | 1.10 (0.76–1.58) | 1.03 (0.60–1.78) | 1.40 (0.76–2.59) |
| IV quartile | 1.03 (0.76–1.41) | 0.98 (0.68–1.41) | 1.11 (0.65–1.90) | 0.98 (0.51–1.86) |
Abbreviations: HR: hazard ratio.
Models adjusted for age, sex, education, body mass index, estimated glomerular filtration rate (CKD-EPI), caloric intake/body weight, smoke, hypertension, diabetes, alcohol and oleic acid consumption.
3.4. PUFA serum concentration and mortality
Kaplan-Meier curves showed a significant decrease in both all-cause and cardiovascular mortality across increasing quartiles of PUFA serum concentration (Fig. 2, panels C and D, respectively). However, in Cox-regression models adjusted for potential confounders, the association with all-cause mortality was evident only for Q4 (adjusted HR [95%CI]: Q2 1.10 [0.79–1.53], Q3 0.84 [0.60–1.19], Q4 0.66 [0.44–0.995], P for trend 0.028). The association was also evident for cardiovascular mortality (crude HR [95% CI]: Q2 0.57 [0.36–0.90], Q3 0.44 [0.27–0.72], Q4 0.20 [0.11–0.38]), but did not reach statistical significance after adjustment for potential confounders (adjusted HR [95%CI]: Q2 1.14 [0.66–1.99], Q3 0.91 [0.52–1.59], Q4 0.62 [0.30–1.29], P for trend 0.213) (Table 3).
Table 3.
HR for all-cause mortality and cardiovascular mortality according with PUFA serum concentration (% of total fatty acid weight), using the I quartile as the reference one.
| Quartiles | All-cause mortality |
Cardiovascular mortality |
||
|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HRa (95% CI) | Crude HR (95% CI) | Adjusted HRa (95% CI) | |
| II quartile | 0.66 (0.50–0.88) | 1.10 (0.79–1.53) | 0.57 (0.36–0.90) | 1.14 (0.66–1.99) |
| III quartile | 0.50 (0.37–0.67) | 0.84 (0.60–1.19) | 0.44 (0.27–0.72) | 0.91 (0.52–1.59) |
| IV quartile | 0.30 (0.21–0.42) | 0.66 (0.44–0.995) | 0.20 (0.11–0.38) | 0.62 (0.30–1.29) |
Abbreviations: HR: hazard ratio.
Models adjusted for age, sex, education, body mass index, estimated glomerular filtration rate (CKD-EPI), caloric intake/body weight, smoke, hypertension, diabetes, alcohol and oleic acid consumption.
Kaplan-Meier curves documented a significant decrease in mortality across increasing quartiles of linolenic and linoleic serum concentration (Supplementary Figs. 1 and 2), but these results did not reach statistical significance after adjustment for potential confounders (Supplementary Table 3).
4. Discussion
In a sample of community-dwelling older adults, we did not find an association between PUFA intake and PUFA serum concentration. There was no association between PUFA intake and mortality, while an inverse relationship was evident between PUFA serum concentration and all-cause mortality, possibly with a threshold effect as indicated by the more evident reduction in risk in the highest quartile of PUFA serum concentration.
To the best of our knowledge, data on the association between PUFA intake and PUFA serum concentration have never been reported in a cohort representative of the general population of older adults in which PUFA intake and serum concentration have been estimated within a narrow time window: Huang et al. found an association between linoleic acid and docosahexaenoic acid serum concentration and intake, that was less evident for other PUFAs, but it was estimated in a sample of men with kidney failure [15]. Studying a cohort of 60-year-old adults from Sweden, Laguzzi et al. found an association between fish intake and eicosapentaenoic and docosahexaenoic acid serum concentration, and between vegetable fat intake and linoleic acid and total PUFA serum concentration. However, the authors used a non-validated food questionnaire, that included only a few food items [16]. This association was also studied in a sample of older adults free from cardiovascular disease in the Cardiovascular Health Study (CHS): Wu et al. found a non-linear relationship between linoleic (ω−6) acid intake and serum concentration, that was more evident when intake of linoleic acid was <8% of total daily energy [9]; in the same cohort, Mozaffarian et al. found an association between ω−3 PUFA intake and serum concentration, with a stronger association for ω−3 PUFA intake <400 mg/day [8]. However, in the CHS the food questionnaire was administrated about three years before the serum assays, thus it might be not representative of the PUFA intake at the moment of the blood sampling, and the association found between PUFA intake and serum concentration might have been subject to bias. In our sample, the average PUFA dietary intake was similar to that reported in the above-mentioned studies; however, we did not find an association with PUFA serum concentration. These discrepancies might be related to different sample characteristics (e.g. community dwelling older adults vs. older men with kidney failure).
The lack of association between PUFA intake and serum concentration in older adults might be related to the variability of the different processes involved in the absorption and metabolism of linoleic and linolenic acid, such as protein-mediated enterocyte PUFA uptake [17], or senescence-related epigenetic modifications of genes associated with lipid metabolism [18]. These epigenetic modifications are expected to have less influence on the relationship between dietary intake and serum concentration of linoleic and linolenic acid, because they are at the beginning of the metabolic pathway of PUFA synthesis. However, our data indicate that no association is present even for these two PUFA precursors.
Regarding the association between PUFA intake and mortality, our results are in line and extend those previously reported by Solfrizzi et al. in a sample of 278 older adults followed for 8.5 years, where no association was found between total PUFA intake and mortality for all causes [19]. Our data support their results in a larger cohort, also for linoleic and linolenic acid intake, and not only for all-cause mortality, but also for cardiovascular mortality. It must be noted that in our sample, participants with the higher PUFA/total lipid intake also had a reduced energy and nutrient intake. Thus, in this group the high PUFA/total lipid ratio seems to be due to a decrease in the denominator rather than to an increase in the numerator. These participants, however, are no different compared those in the other quartiles with respect to other health status indicators. Thus, it is unlikely that this unbalance may have biased our results.
With respect to the association between PUFA serum concentration and mortality, in the CHS Wu et al. found an inverse relationship between linoleic (ω−6) acid and all-cause and CVD mortality [9]; similar results were found also for ω−3 PUFAs [8]. Our results confirm the inverse association between overall PUFA serum concentration and all-cause, but not cardiovascular mortality, where only a trend in reduction of mortality was evident; furthermore, we did not find an association between linolenic and linoleic acid and mortality. These partially contrasting results might be related to the different characteristics of our population, since we did not exclude patients affected by cardiovascular diseases, and the sex distribution of our sample was more representative of the general population, but also to the smaller sample size of our population with consequent fewer events.
Our study has many strengths: to the best of our knowledge, this is the first study on this topic estimating PUFA intake and serum concentration within a short period of time in a relatively large sample of community dwelling older adults. We used a food questionnaire for the estimation of the PUFA intake in the previous years validated in a similar population, thus providing reliable dietary information [11] and we did not use exclusion criteria, thus we provide data on a sample of “real life” older adults population. Finally, our study could help to clarify the discordant results of studies on PUFA dietary intake and serum concentration and mortality in older people.
On the other hand, we had a relatively small sample size, with consequent small number of events. We had information only on overall PUFA, linolenic and linoleic acid intake, therefore we could not study the association between dietary intake and serum concentration of all the individual fatty acids. However, linolenic and linoleic acid are respectively the most represented ω−3 and ω−6 PUFA in the diet of our sample and have the advantage of being less influenced by lipid metabolism compared to other PUFA. Finally, information on PUFA supplementation was not available.
In conclusion, our results indicate that older people in the highest quartile of PUFA serum concentration have lower all-cause mortality risk. Nonetheless, we found no association between intake and serum concentration, suggesting that interventions to modulate PUFA concentration based on dietary intake may not be effective on mortality in this population.
Supplementary Material
Footnotes
Statement of authorship
DL, RAI, and CP had the original idea for this study and drafted the manuscript; LF and SB participated in the InCHIANTI study design and conduction and revised the manuscript for important intellectual content; DL and CP analyzed data; DL had primary responsibility for final content. All authors read and approved the final manuscript.
Conflict of interest
The authors have no conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2019.02.030.
References
- [1].Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr 2001;20:5–19. [DOI] [PubMed] [Google Scholar]
- [2].Wan Y, Zheng J, Wang F, Li D. Fish, long chain omega-3 polyunsaturated fatty acids consumption, and risk of all-cause mortality: a systematic review and dose-response meta-analysis from 23 independent prospective cohort studies. Asia Pac J Clin Nutr 2017;26:939–56. [DOI] [PubMed] [Google Scholar]
- [3].Guasch-Ferré M, Babio N, Martínez-González MA, Corella D, Ros E, Martín-Peláez S, et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr 2015;102:1563–73. [DOI] [PubMed] [Google Scholar]
- [4].Micha R, Pen~alvo JL, Cudhea, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 2017;317:912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med 2016;176:1134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024–33. [DOI] [PubMed] [Google Scholar]
- [7].Harris WS, Luo J, Pottala JV, Espeland MA, Margolis KL, Manson JE, et al. Red blood cell polyunsaturated fatty acids and mortality in the Women’s Health Initiative Memory Study. J Clin Lipidol 2017;11:250–9. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, et al. Plasma phospholipid long-chain ω−3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med 2013;158:515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wu JHY, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS, et al. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation 2014;130:1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–25. [DOI] [PubMed] [Google Scholar]
- [11].Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol 1997;26(Suppl. 1):S152–60. [DOI] [PubMed] [Google Scholar]
- [12].Bartali B, Turrini A, Salvini S, Lauretani F, Russo CR, Corsi AM, et al. Dietary intake estimated using different methods in two Italian older populations. Arch Gerontol Geriatr 2004;38:51–60. [DOI] [PubMed] [Google Scholar]
- [13]. https://www.cdc.gov/nchs/icd/icd9cm.htm.
- [14].R Core Team. R. A language and environment for statistical computing [internet]. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available from: https://www.R-project.org/. [Google Scholar]
- [15].Huang X, Sjögren P, Cederholm T, Ärnlöv J, Lindholm B, Risérus U, et al. Serum and adipose tissue fatty acid composition as biomarkers of habitual dietary fat intake in elderly men with chronic kidney disease. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc 2014;29:128–36. [DOI] [PubMed] [Google Scholar]
- [16].Laguzzi F, Alsharari Z, Risérus U, Vikstro€m M, Sjögren P, Gigante B, et al. Cross-sectional relationships between dietary fat intake and serum cholesterol fatty acids in a Swedish cohort of 60-year-old men and women. J Hum Nutr Diet Off J Br Diet Assoc 2016;29:325–37. [DOI] [PubMed] [Google Scholar]
- [17].Wang TY, Liu M, Portincasa P, Wang DQ-H. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest 2013;43:1203–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Malavolta M, Moccheggiani M. Molecular basis of nutrition and aging 1st edition. 2016. [Google Scholar]
- [19].Solfrizzi V, Dintrono A, Colacicco A, Capurso C, Palasciano R, Capurso S, et al. Unsaturated fatty acids intake and all-causes mortality: a 8.5-year follow-up of the Italian Longitudinal Study on aging. Exp Gerontol 2005;40:335–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.