Summary
Background
Reference intervals of thyroid-stimulating hormone (TSH) and free thyroxine (FT4) are statistically defined by the 2·5th–97·5th percentiles, without accounting for potential risk of clinical outcomes. We aimed to define the optimal healthy ranges of TSH and FT4 based on the risk of cardiovascular disease (CVD) and mortality.
Methods
This systematic review and individual participant data (IPD) meta-analysis identified eligible prospective cohorts through the Thyroid Studies Collaboration, supplemented with a systematic search via Embase, MEDLINE (Ovid), Web of science, the Cochrane Central Register of Controlled Trials, and Google Scholar from January 2011 to October 2022. We included cohorts that collected TSH and/or FT4, cardiovascular outcomes and/or mortality for adults. The primary outcome was a composite outcome including CVD events (coronary heart disease, stroke, and heart failure) and all-cause mortality. Secondary outcomes included CVD events, all-cause mortality, and CVD mortality. We performed one-step (cohort-stratified Cox models) and two-step (random-effects models) meta-analyses adjusting for age, sex, smoking, systolic blood pressure, diabetes mellitus, and total cholesterol. The study protocol was registered with PROSPERO CRD42017057576.
Findings
We identified 3935 studies, of which 53 cohorts fulfilled the inclusion criteria and 26 cohorts agreed to participate. We included IPD on 134 346 participants with a median age of 59 years. There was a J-shaped association of FT4 with the composite outcome and secondary outcomes, with the 20th- 40th percentiles of FT4 (median 13·5–14·8 pmol/L) conveying the lowest risk. Compared to the 20th- 40th percentiles, the age- and sex-adjusted HR (95% CI) for FT4 in the 80th-100th percentiles was 1·20 (1·11–1·31) for the composite outcome, 1·34 (1·20–1·49) for all-cause mortality, 1·57 (1·31–1·89) for CVD mortality and 1·22 (1·11–1·33) for CVD events. In individuals aged ≥70 years, ten-year absolute risk of composite outcome increased over 5% for women with FT4 >85th percentile (median 17·6 pmol/L), and men with FT4 >75th percentile (16·7 pmol/L). Nonlinear associations were identified for TSH, with the 60th-80th percentiles of TSH (median 1·90–2·90 mIU/L) associated with the lowest risk of CVD and mortality. Compared to the 60th −80th percentiles, the age- and sex-adjusted HR (95% CI) of TSH in the 0th-20th percentiles was 1·07 (1·02–1·12) for the composite outcome, 1·09 (1·05 to 1·14) for all-cause mortality, and 1·07 (0·99–1·16) for CVD mortality.
Interpretation
There was a J-shaped association of FT4 with CVD and mortality. Low concentrations of TSH were associated with a higher risk of all-cause mortality and CVD mortality. The 20th–40th percentiles of FT4 and the 60th-80th percentiles of TSH could represent the optimal healthy ranges of thyroid function based on the risk of CVD and mortality, with more than 5% increase of ten-year composite risk identified for FT4 >85th percentile in women and men aged over 70 years. We propose a feasible approach to establish the optimal healthy ranges of thyroid function, allowing for better identification of individuals with a higher risk of thyroid-related outcomes.
Funding
None
Introduction
Reference ranges are used to interpret the results of biomarkers and assist clinical decision making. There are usually two ways to determine reference ranges.1 One way is to define the reference intervals by the 2·5th to 97·5th percentiles according to the distribution in the apparently healthy population. This has been applied to many biomarkers used in the clinic, including thyroid-stimulating hormone (TSH) and free thyroxine (FT4). However, these reference intervals do not account for the risk of adverse clinical outcomes. Several studies have shown that differences of thyroid function within the reference intervals are related to an increased risk of atrial fibrillation,2 stroke,3 heart failure, and mortality.4 Therefore, current reference intervals may fail to identify individuals with a higher risk of disease who could potentially benefit from intervention.
Another approach to define reference ranges is to establish clinical decision limits, based on outcomes from clinical studies. Results from clinical studies rather than statistically defined reference intervals are increasingly used to optimize clinical-decision making.1 For example, the upper limit of BMI is defined by the risk of mortality and related diseases,5 which would be much higher if defined by the 97·5th percentile, considering the high prevalence of obesity worldwide. Another example is that more stringent treatment thresholds for low-density lipoprotein cholesterol have been applied to patients with a high risk of cardiovascular disease due to the benefits shown by intensive lipid-lowering therapy in randomized controlled trials (RCTs).6 Clinical decision limits are usually based on thresholds applied in RCTs, indicating the benefits from interventions. However, there is a lack of long-term RCTs to determine the clinical decision limits for thyroid function. Alternatively, results from large observational studies can provide practical evidence. Most guidelines of subclinical hypothyroidism (elevated TSH combination with FT4 within the reference range) recommend treatment when TSH >10 mIU/L,7,8 given the increased risk of cardiovascular disease (CVD)9,10 as well as progression to overt hypothyroidism.11 However, TSH concentrations lower than currently used treatment thresholds are already associated with a higher risk of fatal coronary heart disease (CHD).9 Moreover, FT4 has been implicated in stronger associations with clinical parameters compared to TSH.12 Higher FT4 concentrations with TSH within the reference range may be associated with increased all-cause mortality, particularly in older age.13,14 Applying an epidemiological approach, we could assess the risk of adverse clinical consequences analysing TSH and FT4 as continuous variables. Thus, the optimal healthy ranges for TSH and FT4 would be defined as being associated with the lowest risk. Although thyroid hormones have pleiotropic effects, the cardiovascular system is one of the most studied and important targets. Exploring the optimal healthy ranges of thyroid function based on the risk of CVD and mortality could be seen as a proof of concept before extending to other clinical outcomes and establishing the proper clinical decision limits. This has already been undertaken within the Rotterdam Study, but those analyses only focused on CVD events in a single population.15 Optimal healthy ranges can be particularly relevant for thyroid function, with levothyroxine as one of the most commonly prescribed drugs worldwide and an estimated 7% of the U.S. population having an active prescription.16
We aimed to evaluate the association between thyroid function (i.e., TSH and FT4 concentrations) and the composite risk of CVD and mortality with individual-participant data (IPD) meta-analyses, and to further identify the age- and sex-specific optimal healthy ranges according to the risk estimates.
Methods
Search strategy and selection criteria
This IPD meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participant Data. According to our registered protocol on PROSPERO (CRD42017057576), we identified studies mainly through the Thyroid Studies Collaboration (TSC),9 a consortium of cohorts recruited through a systematic search (from 1950 through 2010). We additionally conducted a systematic literature search of the Embase, MEDLINE (Ovid), Web of science, the Cochrane Central Register of Controlled Trials, and Google Scholar from January 1st, 2011 through February 12th, 2017 with no language restrictions to identify potentially eligible cohorts. We further updated the systematic search to October 13th, 2022 to gain insight into which cohorts we have potentially missed. The search strategies used are described in appendix pp 4–5. We included prospective population-based cohorts with TSH and/or FT4 measured in adults as well as CVD events and/or mortality recorded. We excluded cohorts that included solely 1) pregnant women, 2) individuals with overt thyroid diseases, and 3) individuals with CVD. Eligibility for inclusion was assessed by four reviewers (AD, YX, LC, TIMK) independently with any disagreement resolved by a fifth independent reviewer (RPP). We assessed study quality and risk of bias with the Newcastle-Ottawa Scale.17
Data analysis
We invited all eligible cohorts to collaborate and provide IPD on demographics, TSH, FT4, thyroid peroxidase antibodies (TPOAb), history of CVD and risk factors (smoking, diabetes mellitus, systolic blood pressure, total cholesterol, and anthropometrics), medication use mostly defined by the Anatomical Therapeutic Chemical (ATC) codes (thyroid-altering medication including amiodarone, antithyroid drugs, thyroid hormone replacement, glucocorticoids, iodine; lipid-lowering and antihypertensive medication), CVD events, CVD mortality, and all-cause mortality. We confirmed that participants characteristics and results obtained from each cohort were consistent with previous publications. If any discrepancies were found, we contacted the primary study investigators to clarify and solve any differences. Each cohort was approved by local ethics committees and had obtained informed consent from participants. Formal ethical approval for this project was exempted by the medical ethics committee of Erasmus University Medical Center Rotterdam (MEC-2022-0237, 16-05-2022).
The primary outcome was a composite outcome, defined as the first occurrence of CVD events (CHD, stroke, and heart failure) or all-cause mortality following study entry. CVD events, CVD mortality, and all-cause mortality were assessed separately as secondary outcomes. In the primary analysis of the composite outcome, only cohorts providing all the elements of the composite outcome were included. However, we deployed a sensitivity analysis to include all cohorts with available information on CHD events and all-cause mortality, since they are the main components of the composite outcome. Detailed definitions of CVD events and CVD mortality are provided in appendix pp 8-10.
A detailed description of statistical analyses is provided in the appendix pp 5–7. In brief, TSH and FT4 concentrations were transformed into cohort-specific percentiles to harmonize the data ascertained through different assays between the cohorts. We performed a one-step IPD meta-analysis as our main analysis, applying cohort-stratified Cox proportional hazards models with different baseline hazard functions for each cohort.18 Given the prior knowledge that both insufficient and excess circulating thyroid hormones are deleterious to the cardiovascular system, potential nonlinear association was expected and was assessed with restricted cubic splines with three knots at the 10th, 50th, and 90th percentiles. The percentiles of TSH and FT4 with the lowest hazard ratio of outcomes were recorded and used as reference values to depict the associations.
As a validation, we additionally conducted a two-step IPD meta-analysis, pooling estimates from the individual cohorts with a random-effects model (DerSimonian and Laird19). Based on the percentiles with the lowest risk (the 60th-80th percentiles of TSH, the 20th-40th percentiles of FT4) identified in one-step meta-analyses, we categorized TSH and FT4 into quintiles and selected the fourth quintile of TSH and the second quintile of FT4 as the reference groups. The I-square statistic20 was used to assess the heterogeneity across cohorts and publication bias was evaluated by funnel plots and Egger’s test.21
All the analyses were adjusted for age and sex in the first model. We additionally adjusted for other traditional cardiovascular risk factors, including smoking, systolic blood pressure, diabetes mellitus, and total cholesterol in the second model,9 also took the availability of data in most cohorts into account. We conducted the multi-level multiple imputation for missing covariates value (0%−100%) in the second model with five imputed datasets in both one-step and two-step meta-analyses.22,23 Exact number and the proportion of missing covariates for each cohort is shown in appendix pp 11–12. Given potential mediation effects of most covariates, data imputation and reduced sample size in the second model, the results of the first model are presented as the main results.
To explore the source of heterogeneity and identify potential effect modification, we extended the one-step meta-analyses with interaction terms and conducted prespecified subgroup analyses. Subgroup analyses were performed on age (<70 and ≥70 years24; 18–49 years, 50–69 years, 70–79 years, and ≥80 years), sex, race (White, non-White), and iodine status (as defined by Iodine Global Network25 using data that was the closest to the entry time and information provided by cohorts). We calculated ten-year absolute risk estimates with stratification by both age and sex to further quantify the effect estimates (i.e., hazard ratios [HRs]).
We conducted several sensitivity analyses to test the robustness of our results, including additional adjustments for antihypertensives, lipid-lowering medication and body mass index, restricting analyses to euthyroid participants (either defined by reference intervals used by each cohort according to the assay or the 2·5th-97·5th percentiles of each cohort), using log-transformed TSH and FT4 or standardized TSH and FT4, using repeated measurements of thyroid function, excluding participants with thyroid-altering medication or positive TPOAb, excluding cohorts not using a third generation assay, excluding participants with history of CVD and/or diabetes, including only studies with formal adjudication procedures for CVD outcomes and including cohorts with available data on CHD events and mortality in the analysis of composite outcome (i.e., less well defined composite outcome). All the analyses were done using SPSS (version 26) and R statistical software (version 4·1·0, packages rms, smoothHR, micemd, meta, forestplot).
Results
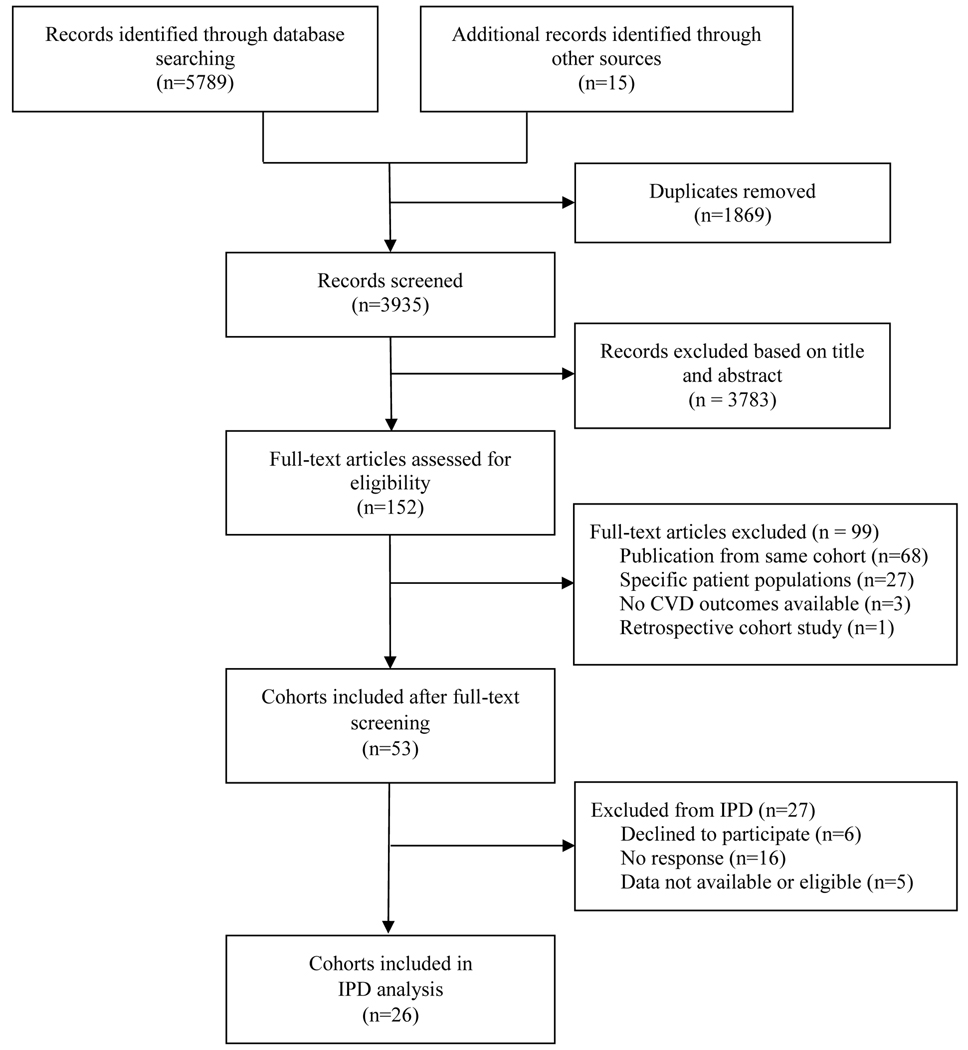
We identified 3935 studies after removing duplicates, of which 3783 were excluded based on the review of titles and abstracts and 99 after the full-text screening, resulting in 53 potentially eligible cohorts. With more than two-year of efforts, 26 prospective cohorts with sufficient data agreed to participate in this IPD meta-analysis (figure 1). Further update of the systematic search identified six additional cohorts. The included cohorts were from Europe (n=15), Brazil (n=2), USA (n=5), Japan (n=1), Australia (n=2), and Iran (n=1), which provided data on 134 346 participants with TSH available (103 407 participants with FT4 available) with a median (IQR) follow-up of 11·5 (7·7–14·9) years. The entry year of included cohorts ranged from 1974 to 2012. The median age of participants from each cohort ranged from 38 to 85 years, with an overall median age of 59 (range 18–106) years at baseline (table 1 and appendix pp 11–12). The corresponding values of percentiles of TSH and FT4 were provided in appendix pp 13–15. The composite outcome was available in thirteen cohorts, with 18 173 (30·3%) events among 59 907 participants. All cohorts provided information on all-cause mortality (32 943 [24·5%] events in 134 344 persons), and twenty-four cohorts also reported CVD mortality (10 076 [8·2%] events in 122 939 persons). Thirteen cohorts reported CVD events (12 212 [20·4%] events in 59 907 persons), and eight of them used formal adjudication procedures.
Figure 1:
PRISMA flow chart of included cohorts
Table:
Characteristics of included studies and their participants
| Study, year (Reference) | n | Country | Median age (range)* | Women | Thyroid medication at baseline† | Thyroid medication during follow-up‡ | TSH, mIU/L, median (IQR) | FT4, pmol/L, mean (SD) | Follow-up time, median (IQR) | Person-Years |
|---|---|---|---|---|---|---|---|---|---|---|
| Bari Study, 2006–201826 | 338 | Italy | 67 (22–93) | 78 (23·1%) | 23 (6·8%) | 63 (18·6%) | 1·75 (1·14–3·00) | 16·4 (3·2) | 7·3 (2·6–11·3) | 2326 |
| InCHIANTI Study, 1998–201027 | 1213 | Italy | 71 (21–102) | 684 (56·4%) | 33 (2·7%) | 56 (4·6%) | 1·32 (0·84–2·00) | 18·6 (4·6) | 9·1 (7·6–9·3) | 9695 |
| Pisa Cohort§, 1992–201628 | 9067 | Italy | 68 (18–98) | 3165 (34·9%) | 541 (6·0%) | NA | 1·60 (0·93–2·60) | 12·3 (4·9) | 6·3 (3·8–9·3) | 58153 |
| Belfrail Study, 2008–201429 | 545 | Belgium | 84 (80–102) | 343 (62·9%) | 52 (9·5%) | 25 (4·6%) | 1·19 (0·74–1·76) | 11·9 (2·6) | 4·9 (3·1–5·2) | 2233 |
| Leiden 85-plus Study, 1997–200930 | 558 | the Netherlands | 85 (NA) | 370 (66·3%) | 21 (3·8%) | 39 (7·0%) | 1·82 (1·16–2·90) | 14·6 (2·7) | 5·4 (2·7–8·6) | 3204 |
| NBS, 2000–201531 | 6677 | the Netherlands | 57 (18–98) | 3575 (53·5%) | 119 (1·8%) | 193 (2·9%) | 1·37 (0·92–2·00) | 13·6 (2·6) | 13·6 (13·3–13·9) | 83911 |
| PREVEND Study (NC1), 1997–201613 | 2703 | the Netherlands | 46 (28–75) | 1389 (51·4%) | NA | 72 (2·7%) | 1·36 (0·95–1·93) | 12·8 (2·4) | 18·9 (13·5–19·1) | 42255 |
| PREVEND Study (NC2), 2001–201613 | 4554 | the Netherlands | 53 (32–80) | 2253 (49·2%) | 90 (2·0%) | 140 (3·1%) | 1·59 (1·1–12·33) | 15·6 (2·4) | 13·9 (11·3–14·3) | 56337 |
| PROSPER trial, 1997–201232 | 5796 | Scotland, Ireland, and the Netherlands | 75 (69–83) | 2996 (51·7%) | 259 (4·5%) | 349 (6·0%) | 1·88 (1·2–12·76) | NA | 3·3 (3·0–3·5) | 18637 |
| Rotterdam Study I-III, 1997–201533 | 9681 | the Netherlands | 63 (46–106) | 5499 (56·8%) | 308 (3·2%) | 530 (5·5%) | 1·90 (1·28–2·77) | 15·7 (2·3) | 8·8 (7·0–14·4) | 100255 |
| SHIP-START Study, 1997–201934 | 3849 | Germany | 49 (20–81) | 1970 (51·2%) | 247 (6·4%) | 572 (14·9%) | 0·81 (0·53–1·19) | 14·4 (2·3) | 19·6 (18·5–20·4) | 68339 |
| Heinz Nixdorf Recall Study, 2000–201835 | 4316 | Germany | 60 (45–76) | 2171 (50·3%) | NA | NA | 1·26 (0·83–1·84) | 17·0 (3·1) | 13·9 (10·4–15·5) | 55034 |
| EPIC-Norfolk Study, 1995–201036 | 13393 | UK | 59 (40–78) | 7340 (54·8%) | 490 (3·7%) | NA | 1·70 (1·20–2·50) | 12·5 (3·9) | 13·4 (12·6–14·3) | 171664 |
| Birmingha m Study, 1988–199937 | 1191 | UK | 68 (60–94) | 681 (57·2%) | 0 (NA) | 53 (4·5%) | 1·60 (1·00–2·50) | NA | 10·2 (5·5–10·6) | 9626 |
| Whickham Survey¶, 1972–199238 | 2691 | UK | 46 (18–93) | 1433 (53·3%) | 110 (4·1%) | 90 (3·3%) | 2·10 (1·00–3·20) | 8·4 (2·0) | 19·0 (15·0–20·0) | 43832 |
| VIVIT Cohort, 1999–201839 | 1717 | Austria | 65 (27–88) | 585 (34·1%) | NA | NA | 1·58 (0·97–2·35) | NA | 11·0 (7·6–12·2) | 18152 |
| ELSA-Brasil Study, 2008–201840 | 13625 | Brazil | 51 (35–74) | 7397 (54·3%) | 1038 (7·6%) | 1190 (8·7%) | 2·01 (1·39–2·92) | 15·5 (2·6) | 9·2 (8·7–9·5) | 122800 |
| Japanese-Brazilian Thyroid Study, 1999–200741 | 1110 | Brazil | 57 (30–92) | 591 (53·2%) | 0 (NA) | NA | 1·40 (0·82–2·46) | 14·2 (6·3) | 7·3 (7·0–7·5) | 7789 |
| Health, ABC Study, 1997–201242 | 2799 | USA | 74 (69–81) | 1434 (51·2%) | 278 (9·9%) | 469 (16·8%) | 2·14 (1·37–3·23) | NA | 11·9 (7·5–12·3) | 28085 |
| MrOS Study, 2000–202143 | 1602 | USA | 73 (65–99) | 0 (NA) | 123 (7·7%) | 213 (13·3%) | 2·07 (1·38–3·10) | 12·7 (2·1) | 13·8 (8·3–19·0) | 20828 |
| Cardiovascular Health Study, 1994–201744 | 4000 | USA | 74 (64–98) | 2362 (59·1%) | 403 (10·1%) | 764 (19·1%) | 2·13 (1·37–3·33) | 15·7 (3·1) | 11·8 (6·7–17·8) | 48939 |
| NHANES, (1999, 2001, 2007, 2009, 2011)201545 | 12174 | USA | 48 (18–85) | 6087 (50·9%) | 733 (6·2%) | NA | 1·54 (1·04–2·28) | 10·4 (2·1) | 7·7 (5·6–8·8) | 99616 |
| NHANES III, 1988–201546 | 15945 | USA | 43 (18–90) | 8478 (52·2%) | 458 (3·3%) | NA | 1·50 (1·00–2·24) | NA | 22·4 (15·7–24·6) | 311471 |
| TTS, 1997-201847 | 5763 | Iran | 38 (20–90) | 3392 (58·9%) | 177 (3·1%) | 358 (6·2%) | 1·61 (0·96–2·66) | 15·9 (5·2) | 18·1 (15·5–18·5) | 97911 |
| Nagasaki Adult Health Study, 1984–199848 | 2830 | Japan | 57 (38–92) | 1723 (60·9%) | 46 (1·6%) | 7 (0·2%) | 2·90 (2·10–3·90) | 18·4 (5·9) | 13·0 (12·3–13·6) | 34333 |
| Health in Men Study, 2001–201214 | 4106 | Australia | 76 (70–89) | 0 (NA) | 139 (3·4%) | NA | 1·99 (1·40–2·85) | 16·1 (2·5) | 8·7 (7·4–9·6) | 32910 |
| Busselton Health Study, 1981–200149 | 2103 | Australia | 51 (18–90) | 1042 (49·5%) | 28 (1·3%) | 47 (2·2%) | 1·45 (0·98–2·09) | 16·3 (4·2) | 20·0 (19·3–20·0) | 37219 |
| Overall | 1343 46 | 26 cohorts | 59 (18–106) | 67023 (49·9%) | 5722 (4·6%) | 5230 (7·2%) | 1·66 (1·07–2·56) | 14·3 (4·0) | 11·5 (7·7–14·9) | 15855 54 |
TSH=Thyroid stimulating hormone; FT4=free thyroxine; T4=Thyroxine. InCHIANTI=Invecchiare in Chianti; NBS=Nijmegen Biomedical Study; PREVEND=Prevention of Renal and Vascular Endstage Disease; PROSPER=Prospective Study of Pravastatin in the Elderly at Risk; SHIP=Study of Health in Pomerania; EPIC=European Prospective Investigation of Cancer; VIVIT cohort= a cohort from Vorarlberg Institute for Vascular Investigation and Treatment; Health ABC study=Health, Aging, and Body Composition Study; NHANES=National Health and Nutrition Examination Survey; MrOS=Osteoporotic Fractures in Men Study; TTS=Tehran Thyroid Study.
Participants <18 years of age were excluded.
Participants with missing information on thyroid medication at baseline: Belfrail 2, MrOS 87, NBS 138, RS 295, Whickham 2, TTS 69, ELSA-Brasil 16.
Participants with missing information on thyroid medication during follow-up: Belfrail 139, Birmingham 1026, CHS 12, MrOS 217, RS 89, Whickham 1624, TTS 2702, ELSA-Brasil 974, PREVEND 969.
Excluded patients with acute coronary syndrome or severe illness.
Whickham survey performed a first-generation assay for TSH and total T4 assays instead of free T4 assays.
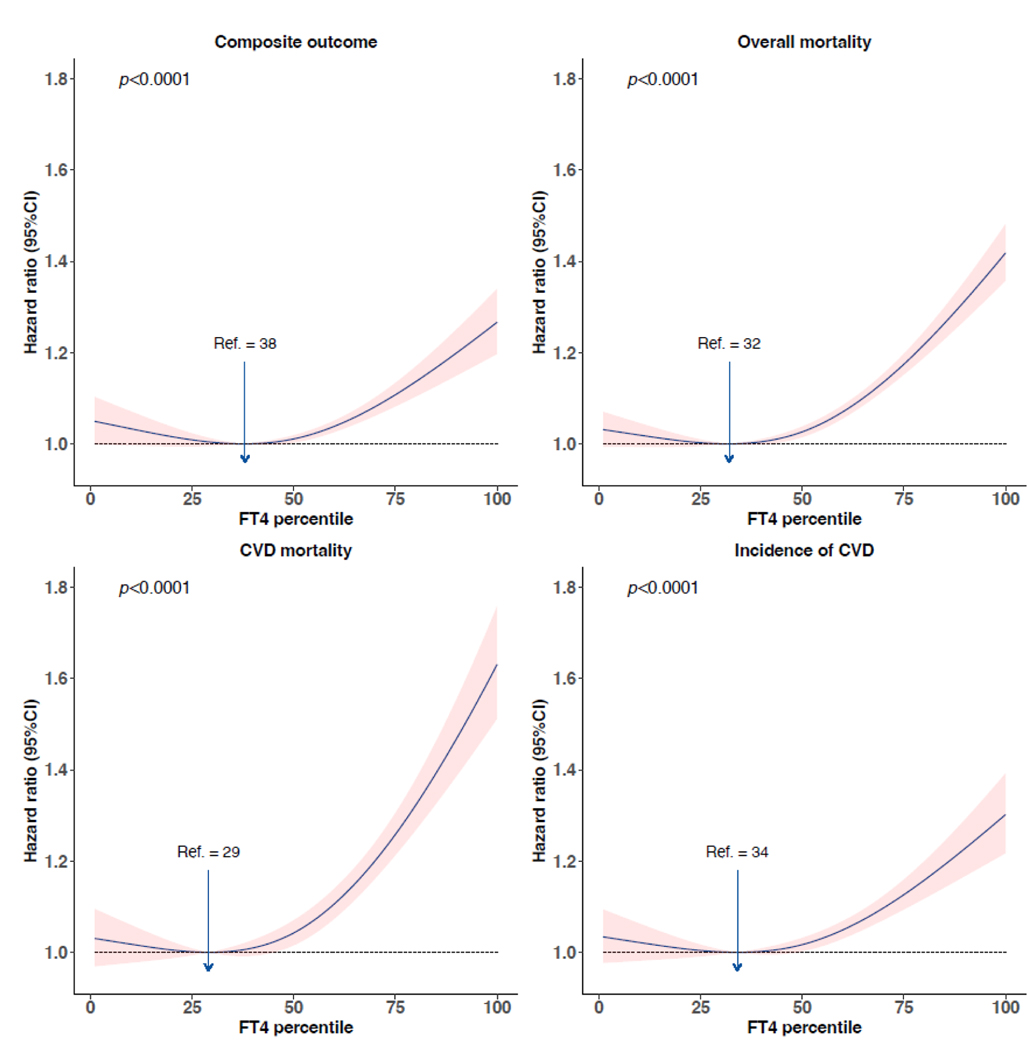
We plotted HRs generated from the one-step meta-analyses using the FT4 percentile with the lowest HR as a reference. There was a consistent J-shaped association between FT4 and the composite outcome as well as secondary outcomes. Overall, FT4 between the 20th (median value of the 20th percentile in all cohorts 13·5 [IQR 11·2–13·9] pmol/L) and 40th percentiles (median 14·8 [12·3–15·0] pmol/L) conferred the minimum risk of the composite outcome, all-cause mortality, CVD mortality, and CVD events. Above the 50th percentile of FT4 (median 15·3 [12·7–15·8] pmol/L), the risk of each outcome increased with increasing FT4 in a largely linear manner (figure 2). Further adjustment with covariates in the second model did not change the results significantly (appendix p 18).
Figure 2:
Association of FT4 percentiles with composite outcome, all-cause mortality, CVD mortality, and CVD events. Hazard ratios were plotted against FT4 percentiles taking the FT4 percentile with the lowest hazard ratio as the reference. Hazard ratios were adjusted for age and sex. The shaded band represents the 95% confidence interval. FT4=free thyroxine. CVD=cardiovascular disease. P denotes the p value for the association of exposure with the outcome. No. of events/No. of participants: composite outcome, 14904/51081 (29.2%); all-cause mortality, 21873/94295 (23.2%); CVD mortality, 6866/82926 (8.3%); CVD events, 9990/51081 (19.6%).
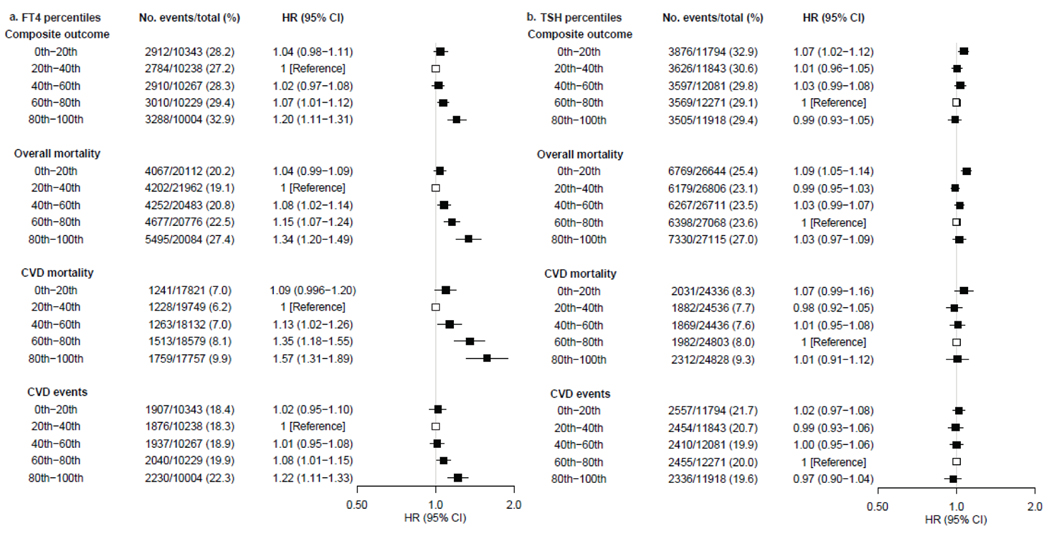
In concordance with one-step meta-analyses, a J-shaped trend of FT4 was identified across the quintiles in two-step analyses. Compared to the 20th-40th percentiles, the age- and sex-adjusted HR of FT4 in the 80th-100th percentiles was 1·20 (95% CI 1·11–1·31) for the composite outcome, 1·34 (1·20–1·49) for all-cause mortality, 1·57 (1·31–1·89) for CVD mortality and 1·22 (1·11–1·33) for CVD events (figure 3a). The effect sizes attenuated slightly after additional adjustment with the second model (appendix p 19). I2 values ranged from 0% to 82%. No relevant publication bias was identified by funnel plots or Egger’s tests (appendix pp 38–41).
Figure 3:
Association of quintiles of TSH and FT4 with the composite outcome, all-cause mortality, CVD mortality, and CVD events. Hazard ratios were adjusted for age and sex. TSH=thyroid stimulating hormone. FT4=free thyroxine. CVD=cardiovascular disease.
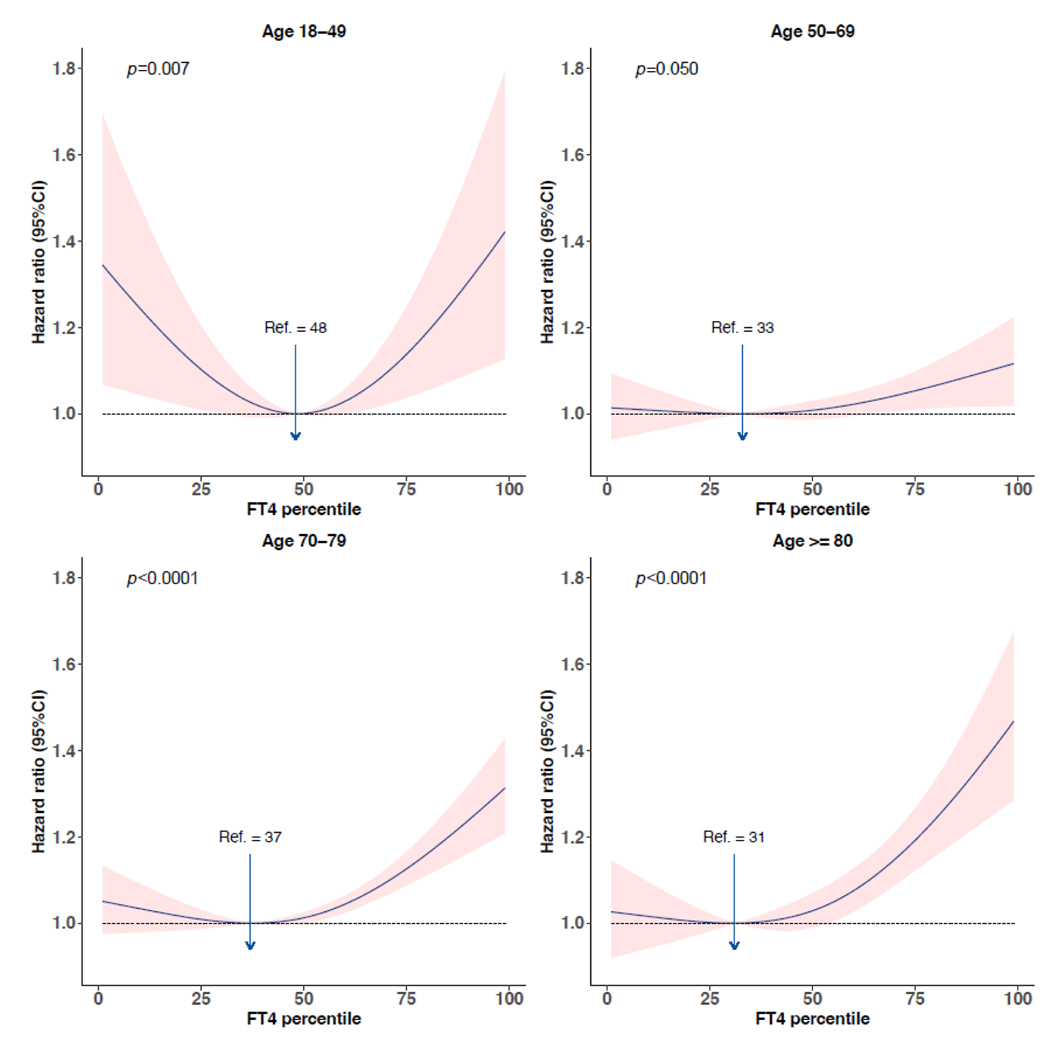
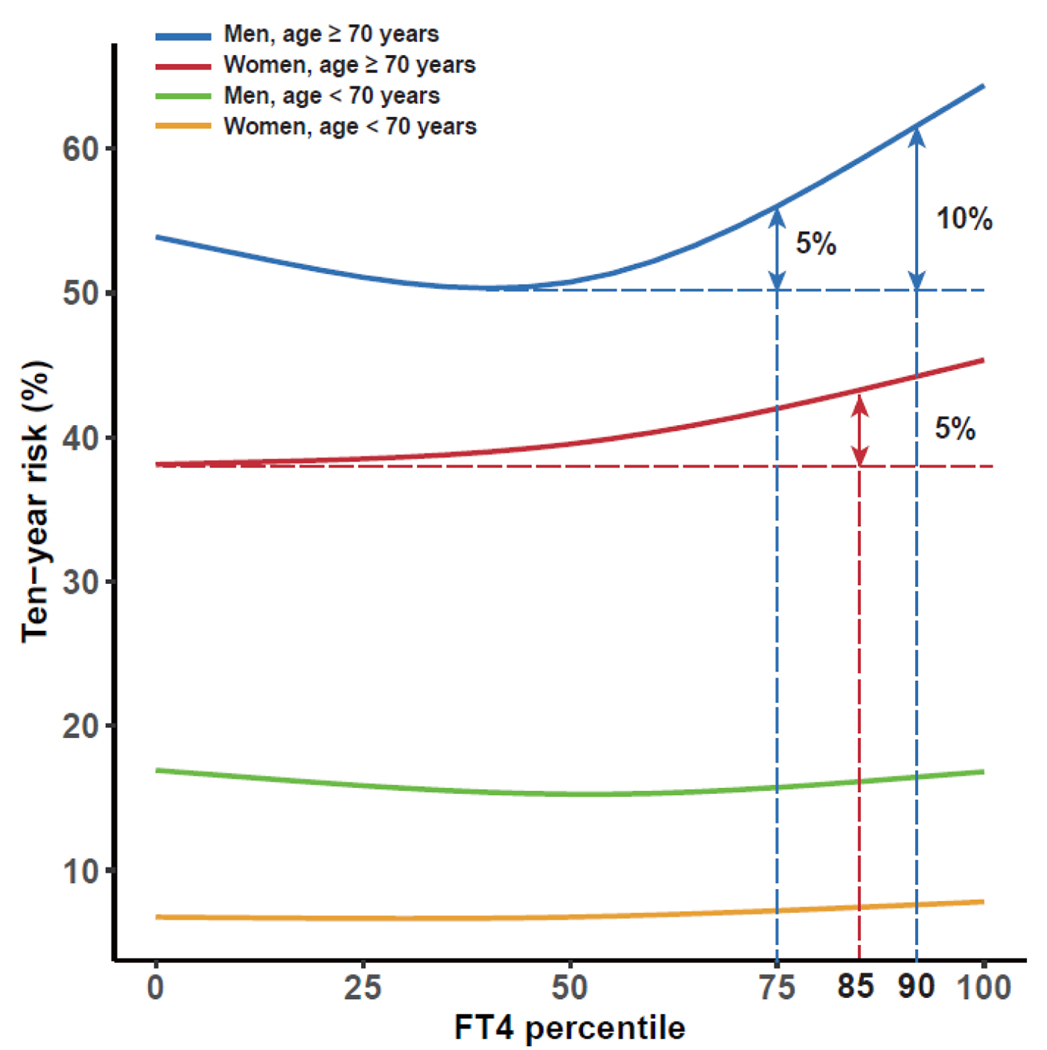
Significant interaction terms between age and FT4 were identified for the composite outcome, CVD mortality, and CVD events (p for interaction=0·0034, 0·025, 0·011 respectively). A nonlinear association of FT4 with the composite outcome and secondary outcomes was identified for all age-stratified analyses (appendix p 20). Compared with younger participants, the concentration of FT4 conveying the lowest risk was slightly lower for participants aged ≥70 years. The increased risk of composite outcome and CVD events with increasing FT4 was more prominent among individuals ≥70 years. Stratified analyses with more age groups also demonstrated a more pronounced effect of FT4 as age increased over the age of 50. A U-shaped association was identified for individuals aged 18–49 years (figure 4). The risk of composite outcome and all-cause mortality was differential between men and women (p for interaction 0·10, 0·042 respectively), with lower concentrations of FT4 associated with the lowest risk among women (appendix p 21). Among individuals ≥70 years, the ten-year absolute risk estimate increased more than 5% for women with FT4 above the 85th percentile (median 17·6 [IQR 15·0–18·3] pmol/L), and more than 5% and 10% for men with FT4 >75th (16·7 [14·0–17·4] pmol/L) and >90th percentile (18·4 [16·0–19·1] pmol/L) respectively (figure 5). There were no differences in the association of thyroid function and outcomes of interest between different races, although we only had a few studies that included non-White participants (appendix p 22). No substantial differences in the associations were identified across different iodine status (appendix p 23).
Figure 4:
Association between FT4 percentiles and composite outcome stratified by age categories. Hazard ratios were plotted against FT4 percentiles taking the FT4 percentile with the lowest hazard ratio as reference. Hazard ratios were adjusted for age and sex. The shaded band represents the 95% confidence interval. FT4=free thyroxine. P denotes the p value for the association of exposure with the outcome. No. of events/No. of participants: age 18–49, 889/14659 (6.1%); age 50–69, 5374/22176 (24.2%); age 70–79, 6391/11007 (58.1%); age ≥80, 2250/3239 (69.5%).
Figure5:
Absolute ten-year risk of the composite outcome by FT4 percentiles stratified by age and sex. FT4=free thyroxine. No. of events/No. of participants: women aged <70 years 2659/20401 (13.0%), men aged <70 years 3604/16434 (21.9%), women aged ≥70 years 3720/5828 (63.8%), men aged ≥70 years 4921/8418 (58.5%).
Nonlinear associations were identified for TSH, with low concentrations of TSH (below the median concentration) associated with a higher risk of the composite outcome, all-cause mortality, and CVD mortality. A trend of a somewhat higher risk of CVD mortality and all-cause mortality was implied for high concentrations of TSH. The 95% CI for the association between low TSH concentrations and CVD included one (appendix p 24). However, additional adjustment for other cardiovascular risk factors in the second model attenuated the association between high concentrations of TSH and CVD mortality (appendix p 25). Overall, TSH between the 60th (median 1·90 [IQR 1·68–2·25] mIU/L) and 80th percentiles (2·90 [2·41–3·32] mIU/L) was associated with the lowest risk of CVD and mortality.
Two-step meta-analyses showed that the 0th to 20th percentiles of TSH was associated with a higher risk of composite outcome (age- and sex-adjusted HR 1·07 [95% CI 1·02–1·12]), mortality (1·09 [1·05–1·14]), and CVD mortality (1·07 [0·99–1·16]) compared to the 60th to 80th percentiles. The association of high concentrations of TSH with all-cause mortality and CVD mortality was not supported by two-step meta-analyses (figure 3b). The effect sizes remained similar after additional adjustment with the second model (appendix p 26). I2 values ranged from 0% to 50% with no relevant publication bias identified by funnel plots or Egger’s tests (appendix pp 42–45 ).
There was no significant interaction between TSH and age in the association with any of the outcomes (p for interaction 0·77, 0·55, 0·25, 0·26, respectively) (appendix p 27). Interaction between TSH and sex was identified on the association with all-cause mortality and CVD mortality (p for interaction=0·073, 0·057), with a U-shaped association indicated for men (appendix p 28). There was no significant interaction between TSH and race (appendix p 29). No substantial differences in the associations were identified across different iodine status (appendix p 30).
Restricting the analyses to euthyroid participants either defined by the reference intervals used by each cohort according to the assay or the 2·5th-97·5th percentile defined per cohort showed similar results to our main analyses (appendix p 31). Excluding thyroid medication users and excluding participant with positive TPOAb did not meaningfully change the risk estimates. Using repeated measurements of thyroid function test, log-transformed TSH and FT4 or standardized TSH and FT4 and other sensitivity analyses, did not reveal different results (appendix pp 32–37).
Discussion
In this IPD meta-analysis, there was a consistent J-shaped association of FT4 with an increased risk of composite outcome and secondary outcomes, independent of traditional cardiovascular risk factors. Low concentrations of TSH were associated with an increased risk of composite outcome. FT4 between the 20th and 40th percentiles (median 13·5–14·8 pmol/L) and TSH between the 60th to 80th percentiles (1·90–2·90 mIU/L) were associated with the lowest risk of CVD and mortality. In individuals aged over 70 years, the ten-year risk of the composite outcome increased over 5% in women with FT4 >85th percentile (median 17·6 pmol/L), and in men with FT4 >75th (16·7 pmol/L) and 90th percentile (18·4 pmol/L), the risk increased by more than 5% and 10% respectively.
In contrast to TSH, the association of FT4 with CVD and mortality was consistent and pronounced. This discrepancy in the association of TSH or FT4 on CVD and mortality has been demonstrated in previous studies, where FT4 showed evident associations with atrial fibrillation, sudden cardiac death, atherosclerotic events, and all-cause mortality, without corresponding findings for TSH.2,13,14,33,50 A recent meta-analysis on associations of thyroid function with various clinical parameters also indicated that compared to TSH, FT4 had stronger associations with clinical outcomes, including mortality, CVD, and dementia.12 Nevertheless, these findings have not yet led to a change on the current paradigm of diagnosis, prognosis or treatment of thyroid disorders. TSH represents the effect of thyroid hormone on the pituitary,51 while FT4 is converted to bioactive hormone, triiodothyronine (T3), which has direct actions on the end-organs. In the cardiomyocyte, T3 binds to thyroid hormone receptor, regulating genes expression and influences the myocardial contractility. T3 also modulates the ion channels and alters cardiac chronotropy through thyroid hormone receptor independent effects.52 TSH is considered as the most sensitive biomarker for thyroid disease due to the complex, non-linear relationship between TSH and FT4,53 but within the general population, as our study and other studies imply,12 the circulating concentrations of the actual hormones may be more relevant to other clinical consequences.
In concordance with the associations of FT4, low concentrations of TSH were associated with a higher risk of the composite outcome. However, this was mainly driven by the association with all-cause mortality and CVD mortality. Our finding of higher mortality risk with lower TSH is in line with a previous IPD meta-analysis of Collet et al, that indicated an increased risk of all-cause mortality and CHD mortality among participants with subclinical hyperthyroidism.54 Our study implied that high concentrations of TSH were associated with a somewhat increased risk of all-cause and CVD mortality, but this was not further validated by two-step meta-analyses or adjustment with other cardiovascular risk factors (for CVD mortality). Moreover, no statistically significant association of TSH with CVD events was identified. This is partially in line with a previous IPD analysis, which demonstrated no overall significant association of subclinical hypothyroidism with CHD events, CHD mortality, and all-cause mortality, but a higher risk of CHD events when TSH >10 mIU/L and a higher risk of CHD mortality when TSH >7 mIU/L.9 There could be several explanations for this discrepancy. First, we conducted a different approach to analyse the association continuously across the full range of thyroid function assuming a continuum of risk, instead of categorization of TSH. Of note, TSH >7 mIU/L correspond to the 97th to 100th percentiles among our study population, which represents a group of individuals with relatively extreme conditions, while our study focused more on risk estimates among the general population. Therefore, the results from those two different approaches are not directly comparable. Second, our study included a broader definition of CVD and CVD mortality while the previous study only included CHD events and CHD mortality.9 Third, our study included more cohorts and longer follow-up time for the cohorts that do overlap between the two studies. This is relevant as temporal changes have occurred that may have resulted in alterations in the effect of high TSH on CVD risk, including changes in iodine status, aging populations and increased efforts for cardiovascular risk management. The latter is of importance as cardiovascular risk management targets dyslipidemia, which in turn is thought to be one of the mechanisms though which hypothyroidism can cause CVD.55
We demonstrated that the association between FT4 and the risk of CVD and mortality differed by age and sex. More prominent associations, lower optimal healthy ranges, and higher increase of absolute risk across the range of FT4 were indicated for individuals older than 70 years. There could be several explanations but might suggest that progressive decline in cardiac reserve and reduced ability of cardioprotection during aging may render elderly individuals more vulnerable to higher thyroid hormone concentrations leading to a higher susceptibility to CVD.56 This aligns with guidelines for (subclinical) hypothyroidism that recommend more conservative strategies and low levothyroxine starting dose for the elderly.7,8 Compared to men, women had lower optimal healthy ranges of FT4, this may partially be explained by the lower FT4 concentrations among women, as well as the difference in set-points.57 Because of the high background risk of CVD and mortality among men and older participants, FT4 had a greater impact on the absolute risk estimates of older men, with over 10% increased ten-year risk of composite outcome. These findings suggest age- and sex-specific strategies for thyroid disease could be necessary.
Optimal healthy ranges are not synonymous to but could be seen as a step towards improving the definition of reference ranges and hence aiding clinical decision making. Before going forward, however, there are still several challenges. Besides the need for RCTs to inform the clinical decision limits, first, it is yet to be decided whether clinical decision limits should be established based on increase in relative risk or absolute risk estimates. Despite the ongoing debate on which risk estimates should be used,58 absolute risk estimates are held to reflect the absolute benefit with reduction of the risk and has been increasingly used in CVD management in general.59 Second, with evidence of continuous risk (i.e., no clear thresholds), it is difficult to decide what magnitude of increased risk is acceptable to establish thresholds. Our study identified an over 5% increased ten-year risk of the composite outcome within the reference interval of FT4, which is comparable to the increase of CVD risk across the range of cholesterol in individuals above 70 years.60 Besides, there are more thyroid function related challenges. For example, we have studied the optimal healthy ranges in the context of CVD. However, thyroid hormones are pleiotropic and variations of thyroid function are associated with multiple relevant clinical outcomes, including bone,61 cancer,62 and non-alcoholic fatty liver disease.63 Which outcomes should be included in order to define clinical decision limits are still up for debate. Furthermore, in our study, we describe age- and sex-specific optimal healthy ranges for TSH and FT4 in relation to CVD and mortality. Whether age- and sex-specific thresholds should be applied needs to be addressed, with additional evidence on the risk differences by age and sex with other thyroid-relevant outcomes needed. Nevertheless, our study provides a feasible approach, paving the way for future studies. This collective information can be used to inform future RCTs to provide evidence for more tailored clinical decision limits.
Levothyroxine (LT4) has become the second most common medication prescribed in the United States as well as in England.64,65 Part of the rise in usage can be attributed to a declining treatment threshold in TSH.66 Around 30% of individuals with thyroid function within the reference interval and 40% with mild to moderate subclinical hypothyroidism were prescribed with levothyroxine in 2018.16 Due to no clear benefit and uncertainty on potential harms, a new published guideline based on evidence from RCTs recommends against thyroid hormone therapy for subclinical hypothyroidism unless TSH >20 mIU/L.67 Our findings argue in favour of “low euthyroid status”, implicating the potential risk of CVD and mortality that could accompany the LT4 therapy, especially for elderly individuals. This may further raise the concerns about overtreatment of LT4 among the elderly. Although we found no differences in our results when excluding levothyroxine users, we did not conduct subgroup analysis on participants with levothyroxine, due to insufficient sample size. Among athyreotic patients, higher FT4 might be required to achieve appropriate levels of triiodothyronine.68 Therefore, whether our results are also applicable to all patients with thyroid hormone therapy would need to be addressed in studies specifically designed for those patients.
Our study is strengthened by using IPD to avoid biases from aggregate data and to standardize the definition of outcomes and variables used.69 We used a composite outcome to incorporate the pleiotropic effect of thyroid hormone, although alternative approaches provided no substantial changes in estimates. We performed one-step IPD meta-analyses as our main analyses, allowing the exploration of nonlinear associations and the optimal healthy ranges. We converted TSH and FT4 into percentiles to harmonize the data from different cohorts and increase the generalizability. The results were robust for log-transformation and standardization of TSH and FT4. We used repeated measurements of thyroid function to further validate the results obtained from a single measurement. This study also has several limitations. First, the majority of included participants were White from Europe and the USA, and our results may therefore not be generalizable to other countries and non-White individuals. Second, due to power issues amongst others, we were not able to investigate the association in individuals taking thyroxine or those with a history of CVD. Studies that are specifically designed for these subgroups are needed. Third, we identified additional cohorts with the updated systematic search through October 2020 that we were not able to include in our IPD due to the considerable time needed to acquire data.70 Nevertheless, the number of potential participants in the additional six cohorts identified was fairly limited (n=15 196) had we been able to include all. Fourth, given the observational study design, we cannot rule out the unmeasured confounding and residual confounding due to measurement error and time-varying confounding. Moreover, HRs may be limited by the built-in bias.71 Finally, T3 was not included in this study due to unavailability in most cohorts, therefore the conversion of T4 to T3 remains unexplored. Further studies are warranted to explore the potential additional merit of T3 measurement in the association with CVD.
In summary, our IPD meta-analysis of 134 346 participants from 26 cohorts indicated a J-shaped association of FT4 with CVD and mortality. Lower TSH was associated with an increased risk of all-cause and CVD mortality. FT4 between the 20th and 40th percentiles (median 13·5–14·8 pmol/L) and TSH between the 60th and 80th percentiles (median 1·90–2·90 mIU/L) were identified to be the optimal healthy ranges defined by the risk of CVD and mortality. Beyond the optimal healthy ranges, more than 5% increase of ten-year risk of the composite outcome was implied for women with FT4 >85th percentile (median 17·6 pmol/L), and over 5% and 10% increase for men with FT4 >75th (16·7 pmol/L) and 90th percentile (18·4 pmol/L) respectively. These thresholds are lower than the currently applied thresholds for diagnosis and treatment of subclinical thyroid dysfunction. This risk-based analysis could be a step towards refining the reference ranges of thyroid function test and facilitate identification of individuals with a higher risk of thyroid-related outcomes.
Supplementary Material
Research in context
Evidence before this study
Reference intervals of thyroid-stimulating hormone (TSH) and free thyroxine (FT4) are statistically defined by the 2·5th-97·5th percentiles, without accounting for potential risk of clinical outcomes. Therefore, current reference intervals may fail to identify individuals with a higher risk of disease who could potentially benefit from intervention. We identified eligible prospective cohorts through the Thyroid Studies Collaboration, supplemented with a systematic search via Embase, MEDLINE, Web of science, the Cochrane Central Register of Controlled Trials, and Google Scholar from January 2011 to October 2022. We included cohorts that collected TSH and/or FT4, cardiovascular outcomes and/or mortality for adults.
Added value of this study
With individual participant data from 134 346 participants, our study showed a J-shaped association of FT4 with CVD events, all-cause mortality, and CVD mortality, as a composite outcome or individual outcomes, with the 20th-40th percentiles (median 13·5–14·8 pmol/L) of FT4 conveying the lowest risk. Among individuals ≥70 years, the ten-year absolute risk estimate increased more than 5% for women with FT4 >85th percentile (median 17·6 pmol/L), and over 5% and 10% for men with FT4 >75th (16·7 pmol/L) and >90th percentile (18·4 pmol/L) respectively. Low concentrations of TSH were associated with a higher risk of all-cause mortality and CVD mortality, with the 60th to 80th percentiles (median 1·90–2·90 mIU/L) associated with the lowest risk.
Implications of all the available evidence
Our study indicated a J-shaped association of FT4 with CVD and mortality. Low concentrations of TSH were associated with a higher risk of all-cause mortality and CVD mortality. Based on the risk estimates, the optimal healthy ranges of thyroid function may lie within 20th–40th percentiles of FT4 and the 60th-80th percentiles of TSH. In men and women aged above 70 years, FT4 >85th percentile concurred with an over 5% increase of ten-year composite risk of CVD and mortality. This threshold is lower than the currently applied threshold for diagnosis and treatment of subclinical thyroid dysfunction. Further studies are warranted specifically in patients using thyroid hormone supplementation.
Acknowledgements
The Thyroid Studies Collaboration (TSC, http://www.thyroid-studies.org/) is funded by a grant from the Swiss National Science Foundation (SNSF 32003B_200606) to Prof. Rodondi. Health, Aging, and Body Composition Study was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
The Health In Men Study was funded by Project Grants from the National Health and Medical Research Council of Australia, and assays of TSH and FT4 by research grants from the Fremantle Hospital Medical Research Foundation, Fremantle Hospital, Western Australia, and the Ada Bartholomew Medical Research Trust, University of Western Australia.
The Osteoporotic Fractures in Men (MrOS) Study is supported by the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, R01 AG066671, and UL1 TR002369. https://mrosonline.ucsf.edu The EPIC (European Prospective Investigation of Cancer)-Norfolk Study has received funding from the Medical Research Council (MR/N003284/1, MC-UU_12015/1 and MC_UU_00006/1) and Cancer Research UK (C864/A14136).
The Cardiovascular Health Study (CHS) is supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629, R01AG032317, and K24 AG 042765 from the National Institute on Aging (NIA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A full list of principal Cardiovascular Health Study investigators and institutions can be found at https://chs-nhlbi.org/.
The Heinz Nixdorf Recall Study (HNR) was supported by the Heinz Nixdorf Foundation. Parts of the study were also supported by the German Research Council (DFG) [DFG project: EI 969/2-3, ER 155/6-1;6-2, HO 3314/2-1;2-2;2-3;4-3, INST 58219/32-1, JO 170/8-1, KN 885/3-1, PE 2309/2-1, SI 236/8-1;9-1;10-1], the German Ministry of Education and Science [BMBF project: 01EG0401, 01GI0856, 01GI0860, 01GS0820_WB2-C, 01ER1001D, 01GI0205], the Ministry of Innovation, Science, Research and Technology, North Rhine-Westphalia (MIWFTNRW). Additional support was provided by the DFG SFB/TR 296 LOCOTACT.
The Longitudinal Study of Adult Health (ELSA-Brasil was funded by the Ministry of Health and the Ministry of Science and Technology and by FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo).
The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the US Department of Energy (DOE). This publication was supported by RERF Research Protocol A10-08. The views of the authors do not necessarily reflect those of the two governments.
The InCHIANTI study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and by the U.S. National Institute on Aging (contracts N01-AG-916413, N01-AG-5-0002, and N01-AG-821336, and grant R01-AG-027012).
The PREVEND study was supported by The Dutch Kidney Foundation which supported the infrastructure of the PREVEND program from 1997 to 2003 (Grant E.033). The University Medical Center Groningen supported the infrastructure from 2003 to 2006. The Netherlands Heart Foundation provided support for studies on HDL metabolism (Grant 2001.005)
The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research Network of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg-West Pomerania.
The Birmingham study was supported by the Associazione Italiana per la Ricera sul Cancro, the United Birmingham Hospitals Endowment Fund, and the Royal College of General Practitioners Research Foundation.
The BELFRAIL study [B40320084685] was funded by an unconditional grant from the Fondation Louvain (Belgium). The Fondation Louvain is the support unit of the Université Catholique de Louvain in charge of developing education and research projects of the university by collecting gifts from corporate, foundations and alumni.
The PREVEND study was supported by The Dutch Kidney Foundation which supported the infrastructure of the PREVEND program from 1997 to 2003 (Grant E.033). The University Medical Center Groningen supported the infrastructure from 2003 to 2006. The Netherlands Heart Foundation provided support for studies on HDL metabolism (Grant 2001.005).
NPR received funding from Dutch Cardiovascular Alliance (DCVA) grant IN CONTROL II (2018-27). YX was supported by a scholarship from the China Scholarship Council (202006010054). RGJW was supported by the Novo Nordisk Foundation Challenge Programme (NNF17OC0027812).
The authors thank all the participants and staff of the cohort studies contributing to the TSC. We acknowledge Wichor M Bramer from Erasmus MC Medical Library for developing and updating the search strategies.
Footnotes
Data sharing: Our data protection agreements with the TSC and participating cohorts do not allow us to share individual-level data from these cohorts to third parties.
Declaration of interests TIMK reports personal fees from IBSA, Meck, Berlin-Chemie, and Quidel. TIMK is unpaid co-chair of the ATA guidelines on thyroid and pregnancy. BBY reports grants from National Health and Medical Research Council, Fremantle Hospital Medical Research Foundation and Ada Bartholomew Medical Research Trust. NR reports a grant from Swiss National Science Foundation. SR reports a grant for an investigator initiated trial by Merck KGaA, manufacturer of levothyroxine and speaker fees from Merck KGaA, Abbott Pharmaceuticals Ltd., IBSA Ltd., makers of levothyroxine. JWJ reports research grants from and/or was speaker (with or without lecture fees) on a.o. (CME accredited) meetings sponsored/supported by Abbott, Amarin, Amgen, Athera, Biotronik, Boston Scientific, Dalcor, Daiichi Sankyo, Edwards Lifesciences, GE Healthcare Johnson and Johnson, Lilly, Medtronic, Merck-Schering-Plough, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi Aventis, the Netherlands Heart Foundation, CardioVascular Research the Netherlands (CVON), the Netherlands Heart Institute and the European Community Framework KP7 Programme. DCB reports research grants from NIH. DF reports a research grant from DFG SFB TR 296 “LOCOTACT”. RGJW reports a research grant from the Novo Nordisk Foundation Challenge Programme (NNF17OC0027812). All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Ozarda Y, Sikaris K, Streichert T, Macri J, intervals ICoR, Decision L. Distinguishing reference intervals and clinical decision limits - A review by the IFCC Committee on Reference Intervals and Decision Limits. Crit Rev Clin Lab Sci 2018; 55(6): 420–31. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner C, da Costa BR, Collet TH, et al. Thyroid Function Within the Normal Range, Subclinical Hypothyroidism, and the Risk of Atrial Fibrillation. Circulation 2017; 136(22): 2100–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaker L, Baumgartner C, den Elzen WP, et al. Thyroid Function Within the Reference Range and the Risk of Stroke: An Individual Participant Data Analysis. J Clin Endocrinol Metab 2016; 101(11): 4270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab 2015; 100(3): 1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med 1999; 341(6): 427–34. [DOI] [PubMed] [Google Scholar]
- 6.Scott MG, Neil JS, Alison LB, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 139(25): e1082–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012; 22(12): 1200–35. [DOI] [PubMed] [Google Scholar]
- 8.Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur Thyroid J 2013; 2(4): 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. Jama 2010; 304(12): 1365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 2012; 126(9): 1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 2004; 291(2): 228–38. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald SP, Bean NG, Falhammar H, Tuke J. Clinical Parameters Are More Likely to Be Associated with Thyroid Hormone Levels than with Thyrotropin Levels: A Systematic Review and Meta-Analysis. Thyroid 2020; 30(12): 1695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groothof D, Flores-Guerrero JL, Nolte IM, et al. Thyroid function and risk of all-cause and cardiovascular mortality: a prospective population-based cohort study. Endocrine 2021; 71(2): 385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeap BB, Alfonso H, Hankey GJ, et al. Higher free thyroxine levels are associated with all-cause mortality in euthyroid older men: the Health In Men Study. Eur J Endocrinol 2013; 169(4): 401–8. [DOI] [PubMed] [Google Scholar]
- 15.Chaker L, Korevaar TIM, Rizopoulos D, et al. Defining Optimal Health Range for Thyroid Function Based on the Risk of Cardiovascular Disease. J Clin Endocrinol Metab 2017; 102(8): 2853–61. [DOI] [PubMed] [Google Scholar]
- 16.Brito JP, Ross JS, El Kawkgi OM, et al. Levothyroxine Use in the United States, 2008–2018. JAMA Intern Med 2021; 181(10): 1402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed October 15 2022).
- 18.de Jong VMT, Moons KGM, Riley RD, et al. Individual participant data meta-analysis of intervention studies with time-to-event outcomes: A review of the methodology and an applied example. Res Synth Methods 2020; 11(2): 148–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–88. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–58. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109): 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolani S, Debray TP, Koffijberg H, van Buuren S, Moons KG. Imputation of systematically missing predictors in an individual participant data meta-analysis: a generalized approach using MICE. Stat Med 2015; 34(11): 1841–63. [DOI] [PubMed] [Google Scholar]
- 23.Vincent A, Ian RW, Shahab J, et al. Multiple Imputation for Multilevel Data with Continuous and Binary Variables. Statistical Science 2018; 33(2): 160–83. [Google Scholar]
- 24.Peeters RP. Subclinical Hypothyroidism. N Engl J Med 2017; 376(26): 2556–65. [DOI] [PubMed] [Google Scholar]
- 25.The Iodine Global Network. Global scorecard of iodine nutrition in 2015–2020 in the general population based on school-age children (SAC). https://www.ign.org/cm_data/ (accessed 9th January 2023).
- 26.Iacoviello M, Guida P, Guastamacchia E, et al. Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des 2008; 14(26): 2686–92. [DOI] [PubMed] [Google Scholar]
- 27.Ceresini G, Marina M, Lauretani F, et al. Relationship Between Circulating Thyroid-Stimulating Hormone, Free Thyroxine, and Free Triiodothyronine Concentrations and 9-Year Mortality in Euthyroid Elderly Adults. J Am Geriatr Soc 2016; 64(3): 553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iervasi G, Molinaro S, Landi P, et al. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med 2007; 167(14): 1526–32. [DOI] [PubMed] [Google Scholar]
- 29.Vaes B, Pasquet A, Wallemacq P, et al. The BELFRAIL (BFC80+) study: a population-based prospective cohort study of the very elderly in Belgium. BMC Geriatr 2010; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meuwese CL, Gussekloo J, de Craen AJ, Dekker FW, den Elzen WP. Thyroid status and renal function in older persons in the general population. J Clin Endocrinol Metab 2014; 99(8): 2689–96. [DOI] [PubMed] [Google Scholar]
- 31.van de Ven AC, Netea-Maier RT, de Vegt F, et al. Associations between thyroid function and mortality: the influence of age. Eur J Endocrinol 2014; 171(2): 183–91. [DOI] [PubMed] [Google Scholar]
- 32.Nanchen D, Gussekloo J, Westendorp RG, et al. Subclinical thyroid dysfunction and the risk of heart failure in older persons at high cardiovascular risk. J Clin Endocrinol Metab 2012; 97(3): 852–61. [DOI] [PubMed] [Google Scholar]
- 33.Bano A, Chaker L, Mattace-Raso FUS, et al. Thyroid Function and the Risk of Atherosclerotic Cardiovascular Morbidity and Mortality: The Rotterdam Study. Circ Res 2017; 121(12): 1392–400. [DOI] [PubMed] [Google Scholar]
- 34.Ittermann T, Haring R, Sauer S, et al. Decreased serum TSH levels are not associated with mortality in the adult northeast German population. Eur J Endocrinol 2010; 162(3): 579–85. [DOI] [PubMed] [Google Scholar]
- 35.Schmermund A, Möhlenkamp S, Stang A, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J 2002; 144(2): 212–8. [DOI] [PubMed] [Google Scholar]
- 36.Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3): 404–10. [DOI] [PubMed] [Google Scholar]
- 37.Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285): 861–5. [DOI] [PubMed] [Google Scholar]
- 38.Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab 2010; 95(4): 1734–40. [DOI] [PubMed] [Google Scholar]
- 39.Saely CH, Leiherer A, Muendlein A, et al. High plasma omentin predicts cardiovascular events independently from the presence and extent of angiographically determined atherosclerosis. Atherosclerosis 2016; 244: 38–43. [DOI] [PubMed] [Google Scholar]
- 40.Aquino EM, Barreto SM, Bensenor IM, et al. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): objectives and design. Am J Epidemiol 2012; 175(4): 315–24. [DOI] [PubMed] [Google Scholar]
- 41.Sgarbi JA, Matsumura LK, Kasamatsu TS, Ferreira SR, Maciel RM. Subclinical thyroid dysfunctions are independent risk factors for mortality in a 7.5-year follow-up: the Japanese-Brazilian thyroid study. Eur J Endocrinol 2010; 162(3): 569–77. [DOI] [PubMed] [Google Scholar]
- 42.Waring AC, Rodondi N, Harrison S, et al. Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol (Oxf) 2012; 76(6): 911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials 2005; 26(5): 569–85. [DOI] [PubMed] [Google Scholar]
- 44.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. Jama 2006; 295(9): 1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue K, Ritz B, Brent GA, Ebrahimi R, Rhee CM, Leung AM. Association of Subclinical Hypothyroidism and Cardiovascular Disease With Mortality. JAMA Netw Open 2020; 3(2): e1920745. [DOI] [PubMed] [Google Scholar]
- 46.Inoue K, Tsujimoto T, Saito J, Sugiyama T. Association Between Serum Thyrotropin Levels and Mortality Among Euthyroid Adults in the United States. Thyroid 2016; 26(10): 1457–65. [DOI] [PubMed] [Google Scholar]
- 47.Tohidi M, Derakhshan A, Akbarpour S, et al. Thyroid Dysfunction States and Incident Cardiovascular Events: The Tehran Thyroid Study. Horm Metab Res 2018; 50(1): 37–43. [DOI] [PubMed] [Google Scholar]
- 48.Imaizumi M, Akahoshi M, Ichimaru S, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab 2004; 89(7): 3365–70. [DOI] [PubMed] [Google Scholar]
- 49.Walsh JP, Bremner AP, Bulsara MK, et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 2005; 165(21): 2467–72. [DOI] [PubMed] [Google Scholar]
- 50.Chaker L, van den Berg ME, Niemeijer MN, et al. Thyroid Function and Sudden Cardiac Death: A Prospective Population-Based Cohort Study. Circulation 2016; 134(10): 713–22. [DOI] [PubMed] [Google Scholar]
- 51.Larsen PR. Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med 1982; 306(1): 23–32. [DOI] [PubMed] [Google Scholar]
- 52.Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 2017; 14(1): 39–55. [DOI] [PubMed] [Google Scholar]
- 53.Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Lim EM, Walsh JP. The relationship between TSH and free T₄ in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013; 98(7): 2936–43. [DOI] [PubMed] [Google Scholar]
- 54.Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med 2012; 172(10): 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su X, Peng H, Chen X, Wu X, Wang B. Hyperlipidemia and hypothyroidism. Clin Chim Acta 2022; 527: 61–70. [DOI] [PubMed] [Google Scholar]
- 56.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin 2012; 8(1): 143–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaker L, Korevaar TI, Medici M, et al. Thyroid Function Characteristics and Determinants: The Rotterdam Study. Thyroid 2016; 26(9): 1195–204. [DOI] [PubMed] [Google Scholar]
- 58.Chapelle N, Martel M, Barkun AN, Bardou M. Relative risk rather than absolute risk reduction should be preferred to sensitise the public to preventive actions. Gut 2022; 71(6): 1045–6. [DOI] [PubMed] [Google Scholar]
- 59.Jackson R. Guidelines on preventing cardiovascular disease in clinical practice. BMJ 2000; 320(7236): 659–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.group SOw, collaboration ESCCr. SCORE2-OP risk prediction algorithms: estimating incident cardiovascular event risk in older persons in four geographical risk regions. European Heart Journal 2021; 42(25): 2455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aubert CE, Floriani C, Bauer DC, et al. Thyroid Function Tests in the Reference Range and Fracture: Individual Participant Analysis of Prospective Cohorts. J Clin Endocrinol Metab 2017; 102(8): 2719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan SR, Chaker L, Ruiter R, et al. Thyroid Function and Cancer Risk: The Rotterdam Study. J Clin Endocrinol Metab 2016; 101(12): 5030–6. [DOI] [PubMed] [Google Scholar]
- 63.Bano A, Chaker L, Plompen EP, et al. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: The Rotterdam Study. J Clin Endocrinol Metab 2016; 101(8): 3204–11. [DOI] [PubMed] [Google Scholar]
- 64.Kane SP. Levothyroxine, ClinCalc DrugStats Database, Version 2022.08. August 24, 2022. ClinCalc: https://clincalc.com/DrugStats/Drugs/Levothyroxine. (accessed January 9, 2023. [Google Scholar]
- 65.Prescription Cost Analysis - England, 2018. [PAS] March 28, 2019. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed January 9 2023).
- 66.Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med 2014; 174(1): 32–9. [DOI] [PubMed] [Google Scholar]
- 67.Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ 2019; 365: l2006. [DOI] [PubMed] [Google Scholar]
- 68.Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA 2008; 299(7): 769–77. [DOI] [PubMed] [Google Scholar]
- 69.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340: c221. [DOI] [PubMed] [Google Scholar]
- 70.Veroniki AA, Ashoor HM, Le SPC, et al. Retrieval of individual patient data depended on study characteristics: a randomized controlled trial. J Clin Epidemiol 2019; 113: 176–88. [DOI] [PubMed] [Google Scholar]
- 71.Hernán MA. The hazards of hazard ratios. Epidemiology 2010; 21(1): 13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.