Abstract
Inducible synthesis of nitric oxide (NO) by macrophages is an important mechanism of the host defense against intracellular infection in mice, but the evidence for significant levels of inducible NO production by human macrophages is controversial. Here we report that the human promyelocytic cell line HL-60, when differentiated to a macrophage-like phenotype, acquires the ability to produce substantial amounts of NO on stimulation with LPS or 1,25-dihydroxyvitamin D3 (1,25-D3) in the absence of activating factors such as gamma interferon. Expression of the inducible nitric oxide synthase (NOS2) was confirmed by sequencing of the reverse transcription-PCR product from stimulated HL-60 cells. Kinetic studies after lipopolysaccharide stimulation show that NOS2 mRNA levels rise within 3 to 6 h, that conversion of [14C]arginine to [14C]citrulline is maximal at 5 to 6 days, and that levels of reactive nitrogen intermediates stabilize at around 20 μM at 7 to 8 days. We find that 1,25-D3 acts to suppress the growth of Mycobacterium tuberculosis in these cells and that this effect is inhibited by NG-monomethyl-l-arginine, suggesting that vitamin D-induced NO production may play a role in the host defense against human tuberculosis.
A major area of immunological controversy is whether human macrophages possess the capacity to produce significant amounts of nitric oxide (NO) in response to infection (15). For mice there is abundant evidence that microbial and parasitic pathogens stimulate the high-output pathway of inducible NO synthesis in macrophages and that this constitutes an important arm of host defense (reviewed in references 9, 16, and 27). In contrast, human monocytes and macrophages generally fail to produce NO when stimulated in vitro with agents that induce a strong NO response in murine macrophages, such as lipopolysaccharide (LPS) plus gamma interferon. This has led several investigators to assert that human macrophages cannot generate antimicrobial concentrations of NO (15). However, a number of recent studies have documented NO release by human macrophages in vitro following unconventional stimuli such as infection with human immunodeficiency virus type 1 (5) or cross-linking of the surface receptor CD69 (14) or CD23 (41). Thus, there is an emerging view that human macrophages are capable of inducible NO synthesis but that this response may depend on factors such as the precise state of macrophage differentiation, complex stimuli, or tissue location.
An important question arising from these observations is the role of NO in the host defense against tuberculosis. For mice, studies conducted both in vivo and in vitro provide compelling evidence that macrophages can kill various mycobacterial species, including Mycobacterium tuberculosis, through an NO-dependent pathway (7, 23, 24, 33). The situation in humans is less clear. It has been observed that pulmonary alveolar macrophages from patients with tuberculosis express inducible nitric oxide synthase (NOS2) (27), but thus far there has been no evidence that human macrophages utilize NO to kill M. tuberculosis, although killing of other mycobacterial species has been described (28).
One way of addressing this problem is to search for NO-producing human macrophage-like cell lines that might ultimately provide clues to the regulation and function of inducible NO synthesis in vivo. The human promyelocytic cell line HL-60 has been extensively studied for its ability to differentiate into a variety of myelomonocytic phenotypes (11). Here we report on a line of HL-60 cells that, after a period of continuous culture at high ambient CO2 concentrations, adopted characteristic macrophage-like properties, including adherence, CD14 expression, the ability to phagocytose latex particles, and lack of nitroblue tetrazolium (NBT) reduction. LPS alone is sufficient to stimulate high levels of nitric oxide production by these cells, and several lines of evidence demonstrate that this is due to induction of the NOS2 gene. Unexpectedly, we find that 1,25-dihydroxyvitamin D3 (1,25-D3) is also a potent stimulus for NO production in this cell type, a result which is intriguing in view of the role played by vitamin D in the host defense against tuberculosis (35). Furthermore, we observe that 1,25-D3 acts to suppress the growth of M. tuberculosis in this macrophage-like HL-60 line, at least partly through an NO-dependent mechanism.
MATERIALS AND METHODS
Cell culture.
The human myeloblastic cell line HL-60 (ATCC CCL-240) was grown in Dulbecco modified Eagle medium (chosen for its low [<1 μM] nitrate content) supplemented with 10% heat-inactivated newborn calf serum, 2 mM l-glutamine, 10 IU of benzoylpenicillin per ml, 100 μg of streptomycin per ml, an extra 1 g of NaHCO3 per liter, and 1 g of d-glucose per liter. All materials were purchased from Sigma (Poole, United Kingdom). Cells were incubated at 37°C in a 7.5% CO2 atmosphere. Other conditions are described in Results.
Characterization.
Five million cells in 3 ml of medium were plated in six-well flat-bottomed plates, with or without 100 ng of LPS from Escherichia coli 0128:B12 (Sigma) per ml, for 24 h, after which the cells were resuspended and 100 μl of cell suspension per well was plated in 96-well flat-bottomed plates.
(i) Phagocytosis.
Five microliters of a 1:10 dilution of fluorescinated latex beads (5-μm diameter; Sigma) per well was added and left for 2 h. Cells were inspected by UV-light microscopy.
(ii) NBT reduction.
Five microliters of a 1-mg/ml solution of NBT (Sigma) in phosphate-buffered saline (PBS) per well was added and left for 2 h, after which the cells were inspected by microscopy. Cells reducing NBT normally contained blue-black granules.
(iii) Surface markers.
HL-60 cells (106 cells in PBS) were stained with either anti-human CD14 (Sigma), anti-human CD23 (Sigma), or an isotype control (Sigma) by an indirect immunofluorescence technique with a secondary antibody (goat anti-mouse immunoglobulin–fluorescein isothiocyanate conjugate from Sigma) as per the manufacturer’s instructions. Both FACScan analysis (Becton Dickinson, Oxford, United Kingdom) and microscopy were used to determine the number of positively stained cells.
(iv) NADPH-diaphorase.
A method described previously (18) was used. Briefly, culture medium was removed from the wells, and the cells were fixed in situ with 4% paraformaldehyde in PBS. The cells were washed and stained with NADPH-NBT buffer at 37°C for 45 min, after which they were washed and left in PBS for microscopy.
Stimulation assays.
Fifty microliters of cells in culture medium was added to 50 μl of medium containing stimulants in 96-well, flat-bottomed plates. After incubation at 37°C in 7.5% CO2 for various periods of time (specified in Results), the plate was wrapped in cling film and stored at −20°C until assayed for nitric oxide production. The stimulants used were phorbol myristic acetate (PMA), LPS from E. coli 0128:B12, 1,25-D3, ergocalciferol, and cholecalciferol, all purchased from Sigma. The vitamin D analogues were dissolved in dimethyl sulfoxide (DMSO) and stored at −70°C. NG-Methyl-l-arginine (l-NMMA) and NG-methyl-d-arginine (d-NMMA) were synthesized as described previously (30).
Time course experiments.
HL-60 cells (3 × 106) were plated in 24-well flat-bottomed plates in 2 ml of medium containing 100 ng of E. coli LPS per ml. Each day the contents of one well were harvested into a 2-ml centrifuge tube (BDH), while 10% of the medium was replaced with fresh medium (containing 100 ng of E. coli LPS per ml) in the remaining wells. Harvested cells were briefly centrifuged at 10,000 × g. The supernatant was stored at −20°C until assayed for NO, and the cells were dissolved in Tri-Reagent (Sigma) prior to RNA extraction for Northern blotting or reverse transcription-PCR (RT-PCR). Parallel cultures were set up in duplicate, using 1.5 × 105 cells in 100 μl of medium containing 100 ng of E. coli LPS per ml, in 96-well flat-bottomed plates for assay of NOS activity by arginine-to-citrulline conversion. As described above, 10% of the culture medium was replaced with fresh medium plus 100 ng of E. coli LPS per ml every 24 h. On the day before harvesting of cells, 250 nCi of l-[U-14C]arginine with a specific activity of 317 mCi/mmol (Amersham International PLC, Amersham, United Kingdom) was added to the well. The rate of arginine-to-citrulline conversion was assayed by high-pressure liquid chromatography (HPLC) as described below.
Measurement of reactive nitrogen intermediates (RNI) (nitrite plus nitrate).
Stock solutions of sodium nitrite and sodium nitrate (Sigma) at 100 mM in water were stored at 4°C stock solutions of nitrate reductase (Boehringer Mannheim, Lewes, United Kingdom) at 2.5 U/ml and a mixture of NADPH (Sigma) at 1.67 mg/ml plus flavin adenine dinucleotide (Boehringer Mannheim) at 0.05 mg/ml in water were stored at −20°C. Prior to use, 1 volume of nitrate reductase was mixed with 3 volumes of NADPH-flavin adenine dinucleotide (enzyme mixture). Griess reagent was prepared as previously described (34). The assay was performed in nonsterile 96-well flat-bottomed plates. The samples were divided between two plates (50 μl added to each), one for measuring nitrite and the other for measuring nitrate, each containing appropriate standards. Water (20 μl per well) was added to the nitrite plate, and enzyme mixture (20 μl per well) was added to the nitrate plate. All plates were incubated for 30 min at room temperature. Griess reagent (100 μl per well) was added and left for 5 min at room temperature, and then the optical densities (ODs) in all plates were read at 620 nm (reference) and 540 nm (test). Nitrite concentrations were calculated directly from the nitrite standard curve. To determine nitrate concentration, ODnitrite was subtracted from ODnitrate before comparison with the nitrate standard curve. Medium alone was used to calculate the assay background level, and this was subtracted from all data.
Measurement of arginine-to-citrulline conversion.
The measurement of arginine-to-citrulline conversion, which indicates the rate of NO synthesis, was performed as previously described (8). Briefly, 100 μl of a 10% trichloroacetic acid solution (Sigma) per microtiter well of cultured cells was added, the mixture was centrifuged at 10,000 × g for 5 min, and the supernatant (175 μl) was transferred to an HPLC autosampler vial (Sigma). Ten microliters of a 1:100 dilution of d-[3H]glucosamine (specific activity, 20 to 40 Ci/mmol; Amersham) per vial was added. Standards of 14C-labelled arginine and ornithine (both from Amersham) and citrulline (NEN-DuPont, Hounslow, United Kingdom) were used to determine elution times. Samples (150 μl of each) were analyzed on a 250- by 4-mm SCX300 cation-exchange column (Sigma), using a Beckman (High Wycombe, United Kingdom) HPLC system consisting of a 507e Autosampler, 128 dual pumps, 171 continuous-flow liquid-scintillation detector, and System Gold software version 6.4. Running conditions were as follows: buffer A, 0.01 M sodium citrate (Sigma), pH 2.2; buffer B, 0.15 M sodium citrate, pH 3.0; gradient of 100% A for min 0 to 11, 0 to 37% B for min 11 to 20, 37 to 100% B for min 20 to 25, 100% B for min 25 to 35, 100 to 0% B for min 35 to 36, and 100% A for min 36 to 45. The areas for the citrulline peaks were calculated and normalized against the glucosamine peak.
RT-PCR analysis of NOS1, NOS2, and NOS3 gene expression.
RNA was extracted with Tri-reagent according to the manufacturer’s instructions (Sigma) from HL-60 cells or from primary human umbilical endothelial cells (HUVECs), kindly provided by Kathy Makepeace. Reverse transcription was carried out with the RAP-PCR kit (Stratagene, Cambridge, United Kingdom) with oligo(dT)18 primer as per the manufacturer’s instructions. PCR amplification of cDNA was with 2 μl of cDNA, 1 μM each primer (see below), 1.8 mM MgCl2 (Boehringer Mannheim), 1× PCR buffer (Perkin-Elmer, Applied Biosystems, Warrington, United Kingdom), and 0.05 U of Taq-Gold (Perkin-Elmer) per μl in a total volume of 20 μl. Thermal cycling was as follows: 1 cycle of 94°C for 12 min and 55°C for 5 min; 35 cycles of 72°C for 2 min, 94°C for 1 min, and 55°C for 1 min; and 1 cycle of 72°C for 10 min. PCR products were separated on 1.2% agarose (Gibco, Paisley, United Kingdom) gels containing 1 μg of ethidium bromide (Sigma) per ml in 1× TAE buffer (Gibco) and viewed and photographed digitally under UV light with the Image Store 5000 system (UVP Life Sciences, Cambridge, United Kingdom). A 100-bp ladder (Gibco) was used for markers. NOS1 primers were 5′-GAATACCAGCCTGATCCCTGGAA-3′ and 5′-TCCAGGAGGGTGTCCACAGCGTG-3′ (599-bp product), based on the human GenBank sequence U17327 and analogous to the rat NOS1 primers described previously (19). NOS2 primers were 5′-TCCGAGGCAAACAGCACATTCA-3′ (31) and 5′-GATATCTTCGTGATAGCGCTTCTGGC-3′ (1,325-bp product). NOS3 primers were 5′-GTGATGGCGAAGCGAGTGAAG-3′ and 5′-CCGAGCCCGAACACACAGAAC-3′ (422-bp product) (31). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers were 5′-CCACCCATGGCAAATTCCATGGGA-3′ and 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ (598-bp product).
Northern analysis of NOS2 gene expression.
Total cellular RNA was prepared by using Tri-reagent (Sigma). RNA (10 μg per sample) was separated on a 1.4% agarose–1.8 M formaldehyde gel and transferred to a nylon membrane (Hybond N+; Amersham). This was hybridized with a 32P-labelled human NOS2 DNA fragment containing nucleotides 2813 to 3587. (This probe was generated by RT-PCR of RNA from MonoMac6 cells stimulated with LPS plus PMA, using primers 5′-CAAGTGGAAGTTCACCAACAGC-3′ and 5′-GATATCTTCGTGATAGCGCTTCTGGC-3′; the PCR product was cloned and found to match the published NOS2 gene sequence from human hepatocytes [Genbank accession no. L09210]). The membrane was then washed and exposed to X-ray film (Amersham), and the image was recorded digitally before analysis with Image Quant software (Molecular Dynamics Ltd., Kemsing, United Kingdom) without any enhancement. To allow for potential loading differences between lanes, the amount of NOS2 mRNA was compared with that of β-actin mRNA or with the total amount of RNA applied to the gel as determined by ethidium bromide staining.
M. tuberculosis experiments.
M. tuberculosis bacilli (strain H37Rv) were thawed from frozen stock, briefly sonicated, and incubated with HL-60hca (5 CFU/cell) at 37°C for 3 h in 500 μl of culture medium. The medium was the same as described above for HL-60 culture, except that ampicillin (100 μg/ml) was substituted for streptomycin and penicillin. The cells were washed three times in Dulbecco modified Eagle medium to remove noningested bacilli and resuspended at 106 cells/ml, and 2 ml was incubated in upright 25-cm2 tissue culture flasks in a humidified incubator at 37°C in a 5% CO2 atmosphere. Where appropriate, 1,25-D3 (2 × 10−8 M) and l-NMMA (1 mM) were added to the culture medium. After 6 days (immediately after maximal NO production [see Results]), the entire contents of each flask were harvested by centrifugation at 1,000 × g for 15 min, and the supernatants were collected, filter sterilized (0.22 μm), and frozen until assayed for RNI. The remaining cell pellets were lysed in water, and the viable bacilli were estimated by plating in serial dilutions on Middlebrook 7H10 agar supplemented with oleic acid, albumin fraction V, dextrose, and catalase (all from Difco Laboratories, East Molesey, United Kingdom). The plates were sealed in bags and incubated at 36°C for 2 to 3 weeks before M. tuberculosis CFU were enumerated with an Anderman colony counter. Results from individual experiments were expressed as a percentage of the CFU in unstimulated HL-60hca cells before calculation of means of data from several experiments. Nonparametric statistics (Wilcoxon matched-pairs signed rank test) were used to compare differences between groups.
RESULTS
Initial characterization of the HL-60hca cell line.
When HL-60 cells were cultured continuously at 7.5% CO2 in standard growth media without inducing factors, it was noted that a significant proportion of cells became adherent to the plastic tissue culture flask in the absence of any observable pH change to the medium when compared to cells kept at 5% CO2. After approximately eight cycles of selection by removal of nonadherent cells, it was found that the majority of cells were adherent and that this attribute was preserved after storage in liquid nitrogen. The cells became nonadherent within a week of reduction of the CO2 concentration to 5%, but adherence was restored within a few days of return to 7.5% CO2. We have termed this high-CO2 adherent line HL-60hca.
The following phenotypic properties were observed for both unstimulated and LPS-stimulated HL-60hca cells. They adhered to plastic. After 2 h of incubation with nonopsonized latex particles, phagocytosis was seen in >50% of cells. NBT reduction was observed in <5% of cells. As determined by fluorescence microscopy and by fluorescence-activated cell sorter analysis, they expressed surface CD14 but not CD23. LPS-stimulated cells, but not unstimulated cells, gave a positive NADPH-diaphorase reaction that was inhibited by 1 mM l-NMMA. Taken together with the published literature on HL-60 differentiation pathways (2, 3, 11), these results indicate that the HL-60hca cell line is essentially macrophage-like.
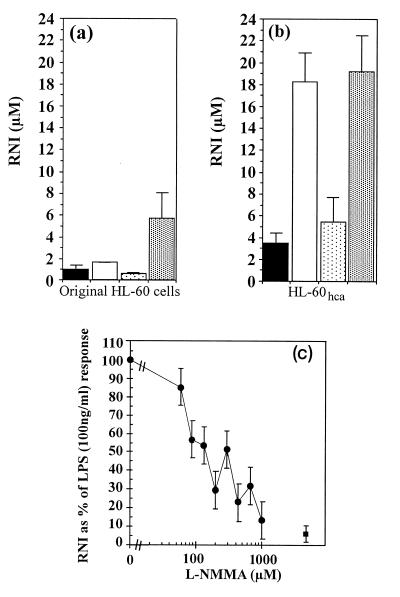
Results of initial experiments on RNI production by the parent HL-60 cells are shown in Fig. 1a. As previously described (40), stimulation with 100 ng of LPS per ml had little effect on its own, but in combination with 10 ng of PMA per ml it elicited 5.6 μM RNI after 2 days in culture. In contrast, HL-60hca cells produced 18.2 μM RNI after stimulation with LPS alone, and this response was not significantly boosted by the addition of PMA (Fig. 1b). The latter response was inhibited by l-NMMA (50% inhibitory concentration ∼150 μM) in a dose-dependent manner (Fig. 1c) but not by d-NMMA (data not shown). This result suggested that the rise in RNI levels was due to NOS activity.
FIG. 1.
RNI production by original HL-60 cells (a) and HL-60hca cells (b). Cells (105 in 100 μl of medium) were stimulated for 48 h with medium alone ( ), LPS (100 ng/ml) (□), PMA (10 ng/ml) (
), LPS (100 ng/ml) (□), PMA (10 ng/ml) ( ), or LPS (100 ng/ml) plus PMA (10 ng/ml) (
), or LPS (100 ng/ml) plus PMA (10 ng/ml) ( ). (c) Inhibitory effect of l-NMMA on RNI production by HL-60hca cells after 6 days of stimulation with LPS (100 ng/ml) (•). ■, background level of RNI production by unstimulated cells. Data are means ± SEMs for three experiments in each case.
). (c) Inhibitory effect of l-NMMA on RNI production by HL-60hca cells after 6 days of stimulation with LPS (100 ng/ml) (•). ■, background level of RNI production by unstimulated cells. Data are means ± SEMs for three experiments in each case.
Kinetics of nitric oxide production.
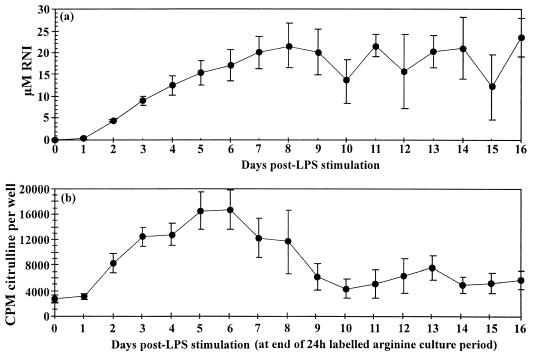
To assess the kinetics of the inducible response, 100 ng of LPS per ml was added to wells containing 3 × 106 HL-60hca cells in 2 ml of culture medium. To minimize the effect of nutrient starvation, 10% of the medium plus LPS was replaced each day, and estimates of cumulative RNI production were corrected accordingly (the amount of RNI removed each day was measured and added to the cumulative total). Supernatants were assayed for RNI by using Griess reagent. The accumulated level of RNI rose significantly after 2 days and reached a plateau of 20 μM at 7 to 8 days after stimulation (Fig. 2a). The daily rate of NO production is reflected in the rate of conversion of l-[14C]arginine to l-[14C]citrulline, which rose significantly within 2 days, reached a peak at 5 to 6 days, and fell to baseline levels by day 10 after stimulation (Fig. 2b). This decline did not appear to be attributable to loss of cell viability, since over 60% of cells were viable as determined by the trypan blue exclusion test when assessed on day 16.
FIG. 2.
(a) Accumulation of RNI with time in culture wells containing 3 × 106 HL-60hca cells in 2 ml of medium stimulated with LPS (100 ng/ml). Data represent means ± SEMs for nine experiments. (b) Daily rate of [14C]citrulline formation from [14C]arginine, using 1.5 × 105 cells per well containing 100 μl of medium and stimulated with 100 ng of LPS per ml. Results are means ± SEMs for six experiments.
Evidence for inducible NOS2 gene expression.
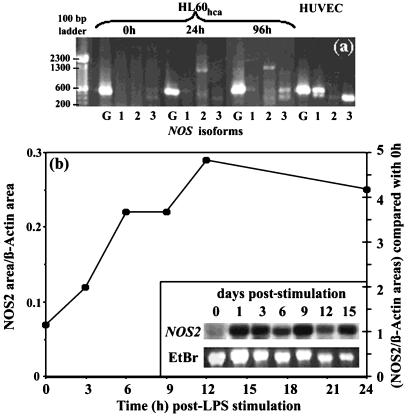
Taken together, these findings strongly suggest that HL-60hca cells possess inducible NOS activity, namely, that LPS stimulates arginine-to-citrulline conversion accompanied by generation of RNI (which is inhibitable by l-NMMA) and a positive NADPH-diaphorase reaction. To investigate this further, we used RT-PCR to examine expression of the genes for the three common NOS isoforms following LPS stimulation of HL-60hca cells. HUVECs were used as a positive control for the constitutive forms of NOS, i.e., neuronal NOS (the product of the NOS1 gene) and endothelial NOS (the product of the NOS3 gene). The authenticities of the PCR products assigned as NOS1 and NOS3 in HUVECs and of that assigned as NOS2 in HL-60hca cells were verified by cloning and sequencing of the respective PCR products. As shown in Fig. 3a, NOS2 expression was detected in HL-60hca cells at 24 and 96 h following LPS stimulation but was absent in unstimulated HL-60hca cells and in HUVEC controls. In contrast, NOS1 and NOS3 were constitutively expressed in HUVEC controls but not in HL-60hca cells, although a NOS3-like band was observed in HL-60hca cells at 96 h after LPS stimulation. To assess the kinetics of the inducible NOS2 response in HL-60hca cells, we performed a semiquantitative Northern analysis (Fig. 3b). A single band of 4.5 kb was observed and indicated that NOS2 mRNA expression starts to rise within 3 h (in agreement with the data of Linn et al. [22]), reaches a plateau by about 12 h, and is maintained for at least 16 days after LPS stimulation (Fig. 3b).
FIG. 3.
(a) RT-PCR for NOS1 (lanes 1), NOS2 (lanes 2), NOS3 (lanes 3), or a GAPDH control (lanes G) with RNA extracted from HL-60hca cells stimulated with LPS (100 ng/ml) for 0, 24, or 96 h and from unstimulated HUVEC controls. (b) Northern analysis of NOS2 gene expression in HL-60hca cells after various durations of stimulation with LPS (100 ng/ml). Kinetics over the first 24 h are shown by the graph, representing the ratio of NOS2 mRNA to β-actin mRNA as quantitated on a digital image as described in text. Longer kinetics are depicted in the inset, which compares NOS2 mRNA (Northern blot) with total RNA stained with ethidium bromide (EtBr). The molecular size of the NOS2 band is approximately 4.5 kb.
Induction of NO production by vitamin D.
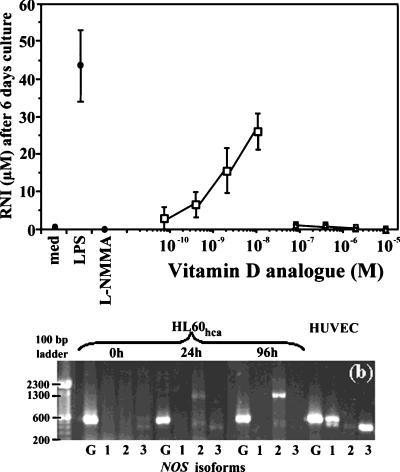
1,25-D3 has been previously used to differentiate HL-60 cells (11), and it has been reported that the combination of 1,25-D3 plus prostaglandin E1 stimulates NO production in this cell line (20). To examine the effect of vitamin D on NO production by HL-60hca cells, 2.5 × 105 cells in 100 μl of medium were stimulated for 6 days with 1,25-D3, ergocalciferol, or cholecalciferol at various doses. As shown in Fig. 4a, 1,25-D3 at nanomolar concentrations without other costimuli induced NO production in HL-60hca cells. With 10 nM 1,25-D3, the level of RNI production was approximately 60% of that seen in response to 100 ng of LPS per ml. In contrast, neither ergocalciferol nor cholecalciferol induced a significant RNI response when tested at concentrations as high as 10 μM. It is important to note that DMSO did not affect LPS-induced NO production when tested at the concentrations used to maintain vitamin D analogues in solution (data not shown) and that we were able to rule out LPS contamination of the 1,25-D3 by using several different batches of the compound and finding that it did not stimulate tumor necrosis factor production from HL-60hca and other macrophage cell lines, nor have we found 1-25 D3 alone to stimulate the parent cell line (data not shown).
FIG. 4.
(a) Induction of RNI in HL-60hca cells after 6 days of stimulation with 1,25-D3 (□), ergocalciferol (○), or cholecalciferol (▵). Filled circles denote experimental controls with medium alone, LPS (100 ng/ml), or LPS (100 ng/ml) plus l-NMMA (1 mM). Data points are means ± SEMs from four experiments. (b) RT-PCR for NOS1 (lanes 1), NOS2 (lanes 2), NOS3 (lanes 3), or a GAPDH control (lanes G) with RNA extracted from HL-60hca cells stimulated with 10−8 M 1,25-D3 LPS for 0, 24, or 96 h and from unstimulated HUVEC controls.
As part of the RT-PCR analysis described for Fig. 3a, we examined NOS1, NOS2, and NOS3 gene expression following stimulation with 1,25-D3. NOS2 mRNA was not detected in unstimulated cells but was weakly expressed at 24 h and strongly expressed at 96 h after stimulation. NOS1 and NOS3 gene expression was not detected following 1,25-D3 stimulation (Fig. 4b).
Effect of 1,25-D3 on M. tuberculosis in HL-60hca cells.
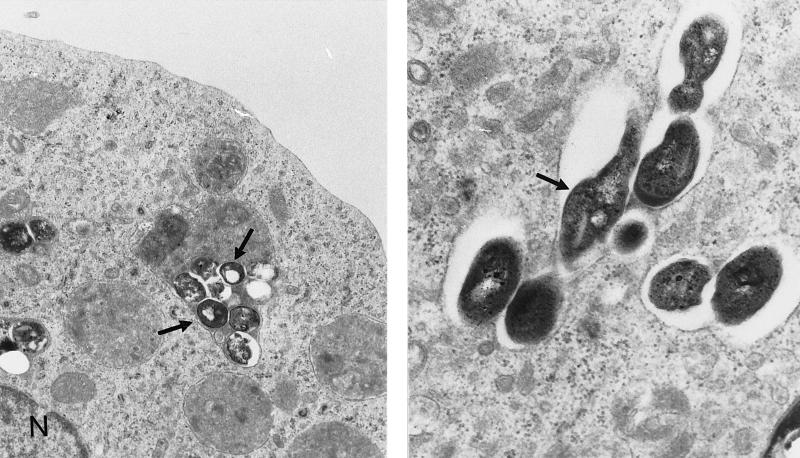
Electron microscopy demonstrated the ability of HL-60hca cells to ingest M. tuberculosis (Fig. 5). Light microscopy (Ziehl-Neelson stain) and fluorescence microscopy (auramine stain) indicated that on the order of 10% of cells were infected after 3 h of incubation at 37°C with 5 CFU/cell (data not shown). To test the hypothesis that NO induced by vitamin D might suppress the viability and/or growth of M. tuberculosis in these cells, approximately 2 × 106 HL-60hca cells infected with M. tuberculosis were cultured for 6 days in the presence of 1,25-D3 (20 nM), l-NMMA (1 mM), 1,25-D3 plus l-NMMA, or neither of these compounds. In a series of six experiments, the geometric mean number of bacilli recovered after 6 days from control cultures without 1,25-D3 or l-NMMA was 3.3 × 105 CFU (95% confidence interval, 0.8 × 105 to 14 × 105 CFU), and the mean RNI concentration was 2.2 μM (standard error of the mean [SEM], 0.7). Because of the variability between experiments, the number of CFU or the RNI concentration recovered from cells treated with 1,25-D3 or l-NMMA was analyzed as a percentage of the recovery from control cultures. As shown in Table 1, cultures containing 1,25-D3 showed a significant reduction in the number of bacilli recovered at 6 days (P = 0.02 by the Wilcoxon matched-pairs signed rank test) and a significant increase in the level of RNI (P = 0.015) compared to control cultures. Both of these effects of 1,25-D3 were inhibited by the addition of l-NMMA; i.e., recovered CFU were significantly higher (P = 0.03) and RNI levels were significantly lower (P = 0.02) in cultures treated with 1,25-D3 plus l-NMMA compared to those treated with 1,25-D3 alone. l-NMMA on its own had little effect on RNI levels or on the number of CFU recovered at 6 days, compared to control cultures.
FIG. 5.
Electron transmission micrograph of HL-60hca cells, demonstrating infection with M. tuberculosis after 3 days of culture. Magnifications, ×16,500 (left panel) and ×32,500 (right panel). N, HL-60hca cell nucleus. Arrows point to mycobacteria.
TABLE 1.
Effect of 1,25-D3 and l-NMMA on RNI production and recovery of M. tuberculosis from HL-60hca cells after 6 days of culture
| Stimulus (concn) | % RNIa | % CFUa |
P (Wilcoxon matched-pairs signed rank test)
|
|
|---|---|---|---|---|
| RNI | CFU | |||
| 1,25-D3 (20 μM) | 284 (90) | 53 (2) | 0.02b | 0.015b |
| l-NMMA (1 mM) | 104 (24) | 105 (24) | NSc | NSc |
| 1,25-D3 (20 μM) + l-NMMA (1 mM) | 110 (27) | 103 (35) | 0.02d | 0.03d |
Data shown are means (SEMs) from six experiments. Percentages are with respect to the values obtained for control cultures without 1,25-D3 or l-NMMA. These control cultures had a mean RNI level of 2.2 μM (SEM, 0.7) and a geometric mean recovery of M. tuberculosis of 3.3 × 105 CFU (95% confidence interval, 0.8 × 105 to 14 × 105 CFU).
For 1,25-D3 versus medium alone.
For l-NMMA versus medium alone. NS, not significant.
For 1,25-D3 plus l-NMMA versus 1,25-D3 alone.
DISCUSSION
The HL-60hca cell line has several notable features. The NOS2 gene is expressed, and high levels of nitric oxide are generated following stimulation with LPS or 1,25-D3. This unusual degree of NO inducibility is of particular interest since the cells have a macrophage-like phenotype. Our data indicate that 1,25-D3 acts to inhibit the growth or viability of M. tuberculosis in HL-60hca cells through an NO-dependent mechanism.
It is puzzling that human macrophages generally fail to produce NO in response to stimuli such as LPS plus gamma interferon in vitro, considering the critical role of NO in defending mouse macrophages against a variety of intracellular pathogens. Since NOS2 expression has been demonstrated in pulmonary alveolar macrophages from humans with tuberculosis, it is conceivable that inducible NO synthesis in humans depends on specific processes of macrophage differentiation in vivo (27). HL-60 is a human promyelocytic cell line which can be induced to differentiate in various ways: for example, a monocyte/macrophage-like phenotype can be induced by vitamin D or gamma interferon, whereas neutrophil- or eosinophil-like phenotypes are induced by DMSO or alkaline media, respectively (11). The undifferentiated parent cell line is nonadherent, and the present investigation stems from our chance observation that a significant proportion of the cells spontaneously became adherent after several weeks in culture at 7.5% CO2, allowing us to select an adherent line that we have termed HL-60hca. The adherent properties of HL-60hca cells appear to depend on continual high CO2 concentration, but we cannot exclude the possibility that unknown factors have contributed to differentiation of this phenotype. Importantly, adherence is preserved after storage in liquid nitrogen. HL-60hca cells express CD14 and readily phagocytose latex particles, whereas they do not reduce NBT. They also ingest M. tuberculosis. Taking these findings together with the published literature on HL-60 differentiation pathways (2, 3, 11), we conclude that the HL-60hca line is essentially macrophage-like.
A striking feature of HL-60hca cells is that LPS alone is sufficient to induce NO production. This contrasts with the case for undifferentiated HL-60 cells, which require more complex combinations of stimuli to produce NO (20, 37, 40). Our data confirm that the parent HL-60 cell line can produce NO after stimulation with PMA and LPS in combination but not with either agent alone. Levels of RNI in the culture supernatant of LPS-stimulated HL-60hca cells can reach almost half the levels obtained from murine macrophages optimally stimulated with LPS plus gamma interferon (34).
Several lines of evidence indicate that the LPS-induced NO response in HL-60hca cells is due to activity of an NOS, which we identify as NOS2. Diaphorase activity is present in LPS-stimulated but not in unstimulated cells, and both this and the inducible rise in RNI are inhibited by l-NMMA. Following LPS stimulation, there is a marked rise in the rate of formation of [14C]citrulline from [14C]arginine, whose time course is consistent with the rate of accumulation of RNI in the culture medium. RT-PCR of LPS-stimulated cells yields a product, confirmed as human NOS2 by sequencing, which is absent in the unstimulated state. Northern analysis confirms that levels of NOS2 mRNA rise appreciably after LPS stimulation. Taking these data together, we can exclude the possibility that the accumulation of RNI is simply due to alternate mechanisms, as has been recently proposed for other experimental systems involving cultured human macrophages (38, 39).
Our time course experiments with HL-60hca cells suggest that the relationship between NOS2 mRNA expression and the rate of NO production is complex. Both Northern and RT-PCR analyses show that levels of NOS2 mRNA rise markedly within 12 h and are maintained for at least 14 days after stimulation with LPS. The daily rate of conversion of arginine to citrulline rises significantly during the 2nd day, reaches a peak during the 5th and 6th days, and declines to resting levels on the 10th day after stimulation. Consistent with the latter observation, the amount of RNI in the culture medium rises progressively for 7 or 8 days and thereafter remains more or less constant. That is, high levels of NOS2 mRNA are accompanied by increasing rates of NO generation in the first few days, but after day 6 the rate of NO generation declines while NOS2 mRNA levels remain high. The decline does not appear to be due to nutrient depletion, since we replaced 10% of the culture supernatant with fresh medium every 24 h, and a similar time course was observed in experiments where the medium was not replaced (data not shown). In other experimental contexts it has been found that NO generation can remain low despite high levels of NOS2 mRNA and NOS2 protein (31), and there is growing evidence of mechanisms that can modulate the functional activity of NO synthases (17, 19). Thus, it is interesting to speculate that the eventual decline in rates of NO generation in LPS-induced HL-60hca cells might be caused by autoregulatory processes on NOS2 gene expression (1, 10, 26, 29, 32), and further investigation of this phenomenon is warranted.
It is well known that vitamin D has potent immunomodulatory properties (6), but little attention has been paid to the possibility that one of its important immunological functions may be to enhance expression of the NOS2 gene, despite a previous report that undifferentiated HL-60 cells produce NO when stimulated with a combination of 1,25-D3 plus prostaglandin E1 (20). We find that 1,25-D3 stimulates HL-60hca cells to express NOS2 mRNA and generate NO, whereas cholecalciferol and ergocalciferol do not. The ability of 1,25-D3 to stimulate a detectable NO output at concentrations as low as 1 nM suggests that the observed effects are physiologically plausible. The concentration of 1,25-D3 in normal human serum is around 0.1 nM, but that of its immediate precursor 25-hydoxyvitamin D3 is around 100 nM (12). A critical point is that 25-hydroxyvitamin D3 is converted to 1,25-D3 within the macrophage, and the rate of conversion increases markedly within pulmonary alveolar macrophages and human monocytes following stimulation with gamma interferon (21, 36).
The last observation may be highly relevant to the role of NO in the host defense against human tuberculosis. Although gene knockout experiments with mice have formally identified the NOS2 gene as a potentially important component of the host defense against tuberculosis (24), whether human macrophages can produce NO at antimicrobial concentrations remains controversial (15). NOS2 protein is expressed in pulmonary alveolar macrophages from tuberculous patients (27), raising the possibility that the natural NO response in human tuberculosis depends on factors that have not been adequately explored in vitro. Several lines of evidence point to vitamin D as one of the missing factors. There is a substantial body of circumstantial clinical evidence that vitamin D is protective against human tuberculosis (4, 25, 35), and we have recently found that polymorphisms in the vitamin D receptor gene are associated with susceptibility to pulmonary tuberculosis in an African population (1a). 1,25-D3 suppresses the growth of M. tuberculosis in human monocyte-derived macrophages (13, 36). Taking these observations together with the present data, we can speculate that 1,25-D3 acts to control tuberculosis in human macrophages by an NO-dependent mechanism.
Our observations on the growth of M. tuberculosis in HL-60hca cells support this proposition. The number of CFU recovered from HL-60hca cells 6 days after infection was significantly reduced as a result of adding 20 nM 1,25-D3 to the culture medium, and the level of RNI generation was increased. Both of these effects of 1,25-D3 were reversed by the addition of l-NMMA. However, l-NMMA had no effect on the recovery of M. tuberculosis or RNI generation compared to control cultures without 1,25-D3. Taken together, these results indicate that ingestion of M. tuberculosis does not directly stimulate HL-60hca cells to produce NO but that cells stimulated with 1,25-D3 inhibit M. tuberculosis via a mechanism that involves NO production.
Several important questions arise from these observations. Are there specific differentiation processes and costimulatory factors that permit 1,25-D3 to induce NOS2 gene expression in natural human macrophages? Is gamma interferon the major stimulus for intracellular generation of 1,25-D3, and thereby NO, in the pulmonary alveolar macrophages of humans with tuberculosis, or are there other important immune stimuli? How does NO suppress the intracellular growth of M. tuberculosis; i.e., does it act directly on the microbe, affecting its survival or its ability to replicate, or is the suppression an indirect consequence of metabolic changes in the host cell that are caused by NO? The HL-60hca cell line provides a tool for exploring these issues and the general question of how the human NOS2 gene is regulated.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust and The Medical Research Council.
We thank Andrew Skinner for electron microscopy, the Public Health Laboratory Service of the John Radcliffe Hospital for category 3 facilities, and Warrick Britton, Mycobacterial Research Group, Centenary Institute of Cancer Medicine and Cell Biology, Sydney, Australia, for helpful discussion and advice.
The first two authors contributed equally to this work.
REFERENCES
- 1.Assreuy J, Cunha F Q, Liew F Y, Moncada S. Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol. 1993;108:833–837. doi: 10.1111/j.1476-5381.1993.tb12886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Bellamy, R., A. V. S. Hill, et al. Unpublished data.
- 2.Bober L A, Waters T A, Pugliese Sivo C C, Sullivan L M, Narula S K, Grace M J. IL-4 induces neutrophilic maturation of HL-60 cells and activation of human peripheral blood neutrophils. Clin Exp Immunol. 1995;99:129–136. doi: 10.1111/j.1365-2249.1995.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brackman D, Lund Johansen F, Aarskog D. Expression of leukocyte differentiation antigens during the differentiation of HL-60 cells induced by 1,25-dihydroxyvitamin D3: comparison with the maturation of normal monocytic and granulocytic bone marrow cells. J Leukoc Biol. 1995;58:547–555. doi: 10.1002/jlb.58.5.547. [DOI] [PubMed] [Google Scholar]
- 4.Brincourt J. Le calcifél a-t-il une action liquéfiante sur le caséum. Le poumon et la coeur. 1967;23:841–851. [PubMed] [Google Scholar]
- 5.Bukrinsky M I, Nottet H S, Schmidtmayerova H, Dubrovsky L, Flanagan C R, Mullins M E, Lipton S A, Gendelman H E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casteels K, Bouillon R, Waer M, Mathieu C. Immunomodulatory effects of 1,25-dihydroxyvitamin D3. Curr Opin Nephrol Hyperten. 1995;4:313–318. doi: 10.1097/00041552-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Chan J, Tanaka K, Carroll D, Flynn J, Bloom B R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chenais B, Yapo A, Leppoivre M, Tenu J P. High performance liquid chromatographic analysis of the unusual pathway of oxidation of l-arginine to citrulline and nitric oxide in mammalian cells. J Chromatogr. 1991;539:433–441. doi: 10.1016/s0021-9673(01)83952-2. [DOI] [PubMed] [Google Scholar]
- 9.Clark I A, Rockett K A. Nitric oxide and parasitic disease. In: Dawer B, editor. Advances in parasitology. London, United Kingdom: Academic Press; 1996. pp. 1–56. [DOI] [PubMed] [Google Scholar]
- 10.Colasanti M, Persichini T, Menegazzi M, Mariotto S, Giordano E, Caldarera C M, Sogos V, Lauro G M, Suzuki H. Induction of nitric oxide synthase mRNA expression. Suppression by exogenous nitric oxide. J Biol Chem. 1995;270:26731–26733. doi: 10.1074/jbc.270.45.26731. [DOI] [PubMed] [Google Scholar]
- 11.Collins S J. The HL-60 promyelocytic cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- 12.Combs G F. The vitamins: fundamental aspects in nutrition and health. London, United Kingdom: Academic Press; 1992. pp. 151–177. [Google Scholar]
- 13.Crowle A J, Ross E J, May M H. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987;55:2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Maria R, Cifone M G, Trotta R, Rippo M R, Festuccia C, Santoni A, Testi R. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med. 1994;180:1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 16.Green S J, Scheller L F, Marletta M A, Seguin M C, Klotz F W, Slayter M, Nelson B J, Nacy C A. Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol Lett. 1994;43:87–94. doi: 10.1016/0165-2478(94)00158-8. [DOI] [PubMed] [Google Scholar]
- 17.Griffith O W, Gross S S. Inhibitors of nitric oxide inhibitors. In: Feelisch M, Stamler J S, editors. Methods in nitric oxide research. Chichester, United Kingdom: John Wiley and Sons; 1996. pp. 187–208. [Google Scholar]
- 18.Hon W M, Chhatwal V J S, Khoo H E, Moochhala S M. Histochemical method for detecting nitric oxide synthase activity in cell cultures. Biotechnic Histochem. 1997;72:29–32. doi: 10.3109/10520299709082208. [DOI] [PubMed] [Google Scholar]
- 19.Jaffrey S R, Snyder S H. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- 20.Kawase T, Ogata S, Orikasa M, Burns D M. 1,25-Dihydroxyvitamin D3 promotes prostaglandin E1-induced differentiation of HL-60 cells. Calcif Tissue Int. 1995;57:359–66. doi: 10.1007/BF00302071. [DOI] [PubMed] [Google Scholar]
- 21.Koeffler H P, Reichel H, Bishop J E, Norman A W. γ-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun. 1985;127:596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- 22.Linn S C, Morelli P J, Edry I, Cottongim S E, Szabo C, Salzman A L. Transcriptional regulation of human inducible nitric oxide synthase gene in an intestinal epithelial cell line. Am J Physiol. 1997;272:G1499–G1508. doi: 10.1152/ajpgi.1997.272.6.G1499. [DOI] [PubMed] [Google Scholar]
- 23.Macmicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 24.Macmicking J D, North R J, Lacourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macrae D. Calciferol treatment in lupus vulgaris. Br J Dermatol. 1947;59:333–338. doi: 10.1111/j.1365-2133.1947.tb10871.x. [DOI] [PubMed] [Google Scholar]
- 26.Matthews J R, Botting C H, Panico M, Morris H R, Hay R T. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson S, Bonecini Almeida M D G, Lapa e Silva J R, Nathan C, Xie Q W, Mumford R, Weidner J R, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho J L. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nozaki Y, Yoshinori H, Ichiyama S, Nakahima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun. 1997;65:3644–3647. doi: 10.1128/iai.65.9.3644-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S K, Lin H L, Murphy S. Nitric oxide regulates nitric oxide synthase-2 gene expression by inhibiting NF-kappa b binding to DNA. Biochem J. 1997;322:609–613. doi: 10.1042/bj3220609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patthy A, Bajusz S, Patthy L. Preparation and characterisation of NG-mono-, di-, and trimethylated arginines. Acta Biochim Biophys Acad Sci Hung. 1977;12:191–195. [PubMed] [Google Scholar]
- 31.Reiling N, Ulmer A J, Duchrow M, Ernst M, Flad H D, Hauschildt S. Nitric oxide synthase: mRNA expression of different isoforms in human monocytes/macrophages. Eur J Immunol. 1994;24:1941–1944. doi: 10.1002/eji.1830240836. [DOI] [PubMed] [Google Scholar]
- 32.Rengasamy A, Johns R A. Regulation of nitric oxide synthase by nitric oxide. Mol Pharmacol. 1993;44:124–128. [PubMed] [Google Scholar]
- 33.Rhoades E R, Orme I M. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rockett K A, Kwiatkowski D, Bate C A W, Awburn M M, Rockett E J, Clark I A. In vitro induction of nitric oxide by an extract of plasmodium falciparum. J Infect. 1996;32:187–196. doi: 10.1016/s0163-4453(96)80018-1. [DOI] [PubMed] [Google Scholar]
- 35.Rook G A. The role of vitamin D in tuberculosis. Am Rev Respir Dis. 1988;138:768–770. doi: 10.1164/ajrccm/138.4.768. [DOI] [PubMed] [Google Scholar]
- 36.Rook G A, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt H H, Seifert R, Bohme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor and leukotriene B4. FEBS Lett. 1989;244:357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- 38.Schneemann M, Schoedon G, Linscheid P, Walter R, Blau N, Schaffner A. Nitrite generation in interleukin-4-treated human macrophage cultures does not involve the nitric oxide synthase pathway. J Infect Dis. 1997;175:130–135. doi: 10.1093/infdis/175.1.130. [DOI] [PubMed] [Google Scholar]
- 39.Singh R, Pervin S, Rogers N E, Ignarro L J, Chaudhuri G. Evidence for the presence of an unusual nitric oxide- and citrulline-producing enzyme in rat kidney. Biochem Biophys Res Commun. 1997;232:672–677. doi: 10.1006/bbrc.1997.6354. [DOI] [PubMed] [Google Scholar]
- 40.Tsuda H, Hasegawa M. Malignant transformation of mouse BALB/c3T3 cells induced by NaNO2. Carcinogenesis. 1990;11:595–597. doi: 10.1093/carcin/11.4.595. [DOI] [PubMed] [Google Scholar]
- 41.Vouldoukis I, Riverosmoreno V, Dugas B, Ouaaz F, Becherel P, Debre P, Moncada S, Mossalayi M D. The killing of leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the fc epsilon RII/CD23 surface antigen. Proc Natl Acad Sci USA. 1995;92:7804–7808. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]