Abstract
Neospora caninum is an apicomplexan parasite that is closely related to Toxoplasma gondii and has been found to be associated with neurological disorders in dogs and congenital infections and abortions in cattle. We have identified two surface proteins of 29 and 35 kDa (designated Ncp29 and Ncp35, respectively) from N. caninum tachyzoites that are the predominant antigens recognized by antisera from Neospora-infected animals. Monoclonal antibodies against Ncp29 and Ncp35 were used to analyze several independent and diverse N. caninum isolates; both antigens were recognized in all isolates, suggesting that they are well conserved. Localization studies and surface labeling with biotin demonstrated that Ncp29 and Ncp35 are membrane associated and displayed on the surface of the parasite. After treatment with phosphatidylinositol-specific phospholipase C, parasite lysates were analyzed with antibodies against the cross-reacting determinant of glycosylphosphatidylinositol anchors. Approximately six glycolipid-anchored surface proteins were identified, with the two most prominent corresponding to Ncp29 and Ncp35. Sequence comparisons of Ncp29 and Ncp35 with GenBank indicated that they are most similar to the T. gondii surface antigen 1 (SAG1) and surface antigen 1-related sequence 2 (SRS2), respectively. Consequently, Ncp29 has been designated NcSAG1 and Ncp35 has been designated NcSRS2. Both NcSAG1 and NcSRS2 contain a tandemly duplicated motif and 12 absolutely conserved cysteines which are also found in all of the SAG and SRS proteins of T. gondii. Maintenance of these motifs and the 12 cysteine residues suggests that these surface antigens share a similar secondary and tertiary structure that is presumably important for a conserved function that these antigens serve during infection.
Neospora caninum is an apicomplexan parasite that was originally identified as an etiologic agent of neurological disease in dogs and has subsequently been found to be associated with abortions in dairy cattle. N. caninum is an obligate intracellular pathogen that appears to be capable of utilizing a fairly wide range of animals as intermediate hosts (17) and has the ability to parasitize many types of nucleated cells (44). Tachyzoites of N. caninum contain the characteristic organelles (i.e., dense granules, rhoptries, micronemes, and an apical complex, etc.) found in other coccidian parasites and are virtually indistinguishable by light microscopy from Toxoplasma gondii tachyzoites (14). Both tachyzoites and cyst-forming bradyzoites have been characterized in the asexual phase of the N. caninum life cycle, but a definitive host that supports a sexual phase and the formation of oocysts has yet to be identified. Although N. caninum is assumed to form oocysts since herbivorous animals become infected, the absence of a well-defined life cycle has made it difficult to formulate good control measures to prevent infection.
Proteins displayed on the surfaces of intracellular pathogens are believed to play critical roles in infection. Since they represent the initial interaction with the host cell and components of the host immune system, these proteins may collectively serve a number of important functions, including adhesion to the host cell membrane and protection of the parasite from microbicidal immune responses. In T. gondii, the tachyzoite surface is covered by glycosylphospatidylinositol (GPI)-anchored antigens ranging in approximate size from 22 to 43 kDa (6). Surface antigen 1 (SAG1) is the immunodominant surface antigen of tachyzoites, and several recent studies, including the generation of >8,000 expressed sequence tags (1), have revealed that a number of the T. gondii surface proteins are structurally related to SAG1 (12, 22, 36). With the exception of SAG3, which had previously been characterized, these SAG1-like antigens have been designated SAG1-related sequence 1 (SRS1) to SRS4. Several of the T. gondii SAGs elicit strong antibody responses in infected animals, including humans, and are therefore attractive as diagnostic and/or vaccine antigens (8, 35, 40, 42). Definitive functions for these surface proteins have not been determined, but there is indirect evidence that at least one of these antigens, SAG1, is involved in the attachment of the parasite to host cells (20, 37).
N. caninum has only recently been recognized as a separate species, so much of its biology remains unknown. Consequently, molecular characterizations of N. caninum proteins are needed to improve our basic understanding of this parasite and to provide reagents that will be valuable for diagnosis as well as future studies on neosporosis. Here, we describe two immunodominant surface proteins of N. caninum tachyzoites that show striking similarity to the surface antigens of T. gondii tachyzoites.
MATERIALS AND METHODS
Parasite culture.
Strains of N. caninum were propagated as tachyzoites by serial passage in human foreskin fibroblast host cells maintained in Dulbecco’s modified Eagle medium supplemented with 10% horse serum, 2 mM glutamine, and 20 μg of gentamicin (Gibco BRL, Gaithersburg, Md.) per ml. All cultures were tested (Rapid Detection System; Gen-Probe, Inc., San Diego, Calif.) and determined to be free of mycoplasma contamination.
Monoclonal antibody (MAb) and polyclonal antiserum production.
Nc-1 strain tachyzoites were disrupted by hypotonic lysis, three freeze-thaw cycles, and Dounce homogenization. The sample was centrifuged at 13,600 × g to remove cytoskeletal components and cell debris, and the supernatant was centrifuged at 128,000 × g to obtain a pellet enriched for membrane-associated antigens. The pellet of membrane antigens was frozen at −20°C until injected into mice. Mice were immunized with the membrane antigen fraction, and stable hybridomas were generated from spleen cells of reactive mice, using standard methodology (21). For production of ascites fluid, approximately 106 hybridoma cells were administered to BALB/c mice by intraperitoneal injection, and ascites fluid was collected from the peritoneum (21).
To produce antisera from infected animals, female CD-1 outbred mice were infected by intraperitoneal injection with approximately 107 Nc-1 strain tachyzoites. After 30 days, mice were sacrificed and antisera were collected. Two dogs were infected by subcutaneous injection with 5 × 106 Nc-1 strain tachyzoites, followed by two subcutaneous boosts at about 1-week intervals. One dog was similarly infected and boosted with the Nc-2 strain of N. caninum. The dogs were sacrificed on day 25, and antisera were collected.
Western blot analysis.
Parasites were lysed in sodium dodecyl sulfate sample buffer supplemented with protease inhibitors trans-epoxysuccinyl-l-leucycloamido(4-guanidino)butane (E64), leupeptin, (4-amidinophenyl)methanesulfonyl fluoride hydrochloride (APMSF), and tosyl-lysine chloromethyl ketone (TLCK) (Sigma, St. Louis, Mo.), and the lysates were separated on 10% polyacrylamide gels (32). Proteins were transferred to nitrocellulose membranes by semidry electrophoretic transfer in Tris-glycine buffer (pH 8.3). Membranes were blocked with phosphate-buffered saline (PBS) containing nonfat dry milk and 5% goat serum and then incubated for 1 h with primary antibody. After washing, membranes were incubated with horseradish peroxidase (HRP)-conjugated immunoglobulin G secondary antibody (Jackson Immunoresearch Labs, West Grove, Pa.) at a 1:10,000 dilution. After washing, blots were processed for chemiluminescence by using Supersignal substrate (Pierce Chemical Company, Rockford, Ill.) and exposed to film.
Biotinylation of surface proteins and precipitation with MAbs or immobilized streptavidin.
Freshly harvested tachyzoites (approximately 5 × 107) were resuspended in cold PBS (pH 7.4) at approximately 2.5 × 107/ml. Sulfo–N-hydroxysuccinimide–biotin (50 mg/ml in dimethyl sulfoxide; Pierce) was added to a concentration of 0.5 mg/ml and incubated at room temperature for 30 min. The labeled parasites were washed twice with 5 ml of PBS and frozen at −20°C.
The labeled parasite pellet was lysed in 1 ml of radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.5], 1% Triton X-100, 0.5% sodium deoxycholate, 0.2% sodium dodecyl sulfate, 100 mM NaCl, 5 mM EDTA) supplemented with RNase, DNase, and protease inhibitors E64, APMSF, leupeptin, and TLCK, and the sample was centrifuged at 16,000 × g to remove the insoluble fraction. The soluble proteins were incubated with MAb 6C11 or 5H5 and precipitated with protein G-agarose (Sigma). The immunoprecipitated proteins were analyzed by Western blotting, as described above, using avidin-HRP conjugate (Bio-Rad Laboratories, Hercules, Calif.) and chemiluminescence to reveal biotin-labeled proteins. Alternatively, the biotin-labeled proteins were precipitated with streptavidin-agarose (Sigma) and analyzed by Western blotting, as described above, using the MAb 6C11 or 5H5.
Immunoelectron microscopy (immuno-EM).
Extracellular N. caninum tachyzoites were fixed for 1 h at 4°C with 2.5% formalin–0.5% glutaraldehyde in Tris-buffered NaCl, pH 7.2. Samples were then embedded in 10% gelatin, infiltrated with sucrose-polyvinylpyrolidine, and frozen in liquid N2. Ultrathin cryosections were blocked in 0.12 mM glycine and PBS–10% fetal bovine serum (FBS) and then incubated in primary antibodies diluted in PBS–1% FBS followed by secondary antibodies (goat antirabbit coupled to 18-nm-diameter colloidal gold [Jackson Immunoresearch Labs]) diluted in PBS–1% FBS. Sections were counterstained with 0.15 M oxalic acid–2% uranyl acetate, stained with a 1:12 mixture of 2% methylcellulose and 4% uranyl acetate, and examined with a Zeiss EM902 microscope.
Detection of glycolipid anchors.
Nc-1 strain tachyzoites of N. caninum or RH strain tachyzoites of T. gondii were lysed in 70 mM triethanolamine buffer (pH 7.5) with 0.16% Triton X-100 at a concentration of about 5 × 105 cells/μl. A 25-μl aliquot was incubated with or without 0.1 U of phosphatidylinositol-specific phospholipase C (Sigma) at 37°C for 2.5 h. The samples were analyzed by Western blotting with antibodies against the cross-reacting determinant (CRD) of GPI anchors (provided by J. Bangs, University of Wisconsin).
Protein purification and peptide sequencing.
To obtain antigen for peptide sequence analysis, proteins were immunopurified by precipitation with MAb 6C11 or 4H7. The precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane by semidry electroblotting. The membrane was stained with Coomassie brilliant blue, and the protein band was excised from the membrane and submitted for Edman degradation peptide sequence analysis (Midwest Analytical, Inc., St. Louis, Mo.).
Amplification and sequence analysis of Ncp35 cDNA.
Forward- and reverse-orientation degenerate oligonucleotides were designed from the amino acid sequence obtained by peptide sequencing. These oligonucleotides were paired with either the T3 or T7 oligonucleotide from the Uni-Zap lambda vector and used in PCR amplifications with an Nc-1 strain N. caninum cDNA library (4) as the template. The resulting amplification products were cloned into the pCR 2.1 plasmid vector (Invitrogen, Carlsbad, Calif.), and their nucleotide sequences were determined by PRISM dye terminator cycle sequencing (ABI, Foster City, Calif.). Specific primers based on the sequence obtained from these clones were used to amplify the full-length p35 cDNA with KlenTaq-LA polymerase (5) and the Nc-1 cDNA library as the template. The amplification product was cloned into the pCR 2.1 vector, and the complete double-strand sequence was obtained from the full-length clone, as described above.
Computer sequence analyses were conducted with Genetics Computer Group programs (13) and programs available on the National Center for Biotechnology Information web site (http://www2.ncbi.nlm.nih.gov).
Nucleotide sequence accession number.
The sequence of the Ncp35 gene has been assigned GenBank accession number AF061249.
RESULTS
Immunogenicities of Ncp29 and Ncp35.
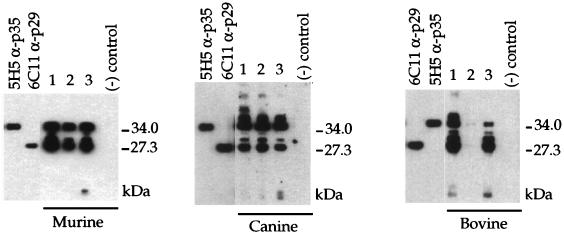
To identify the immunodominant antigens of N. caninum, we screened antisera from mice, canines, and bovines that were either experimentally or naturally infected with Neospora. Western blot analyses of N. caninum lysates with these sera revealed between four and seven highly immunogenic antigens, with two antigens at approximately 29 and 35 kDa recognized as immunodominant by all reactive antisera (one bovine serum did not react) (Fig. 1). The reactivity of these antisera is not limited to these few highly immunogenic antigens, since longer exposures and/or lower dilutions of antibodies (primary and secondary) revealed numerous additional antigens (data not shown). Nonetheless, the 29- and 35-kDa antigens appear to be immunodominant in a variety of different hosts.
FIG. 1.
The Ncp29 and Ncp35 proteins were recognized as immunodominant antigens by antisera from a wide range of infected hosts. Sera from three experimentally infected mice, three experimentally infected dogs, or three naturally infected cows were tested by Western blotting against the total parasite antigen of the Nc-1 strain of N. caninum. The two most immunogenic antigens comigrated with the Ncp29 antigen, recognized by MAb 6C11 (α-p29), and the Ncp35 antigen, recognized by MAb 5H5 (α-p35). Sera from noninfected animals did not react with parasite antigen.
Independently, we generated a collection of hybridomas against a membrane fraction of Nc-1 strain N. caninum tachyzoites and tested these by Western blotting against total parasite lysate. This analysis indicated that the 29-kDa antigen (designated Ncp29) was recognized by MAb 6C11 and that the 35-kDa antigen (designated Ncp35) was recognized by MAbs 5H5 and 4H7 (Fig. 1 and data not shown). Based on the immunodominance of the Ncp29 and Ncp35 antigens, these MAbs were selected for further study.
Presence of Ncp29 and Ncp35 in different isolates of N. caninum.
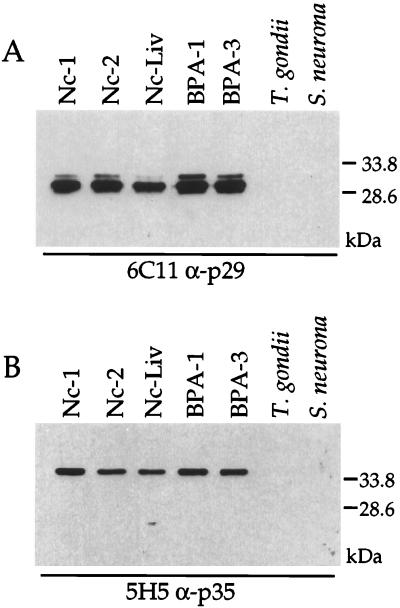
The 6C11 and 5H5 MAbs were tested against different strains of N. caninum to determine if the Ncp29 and Ncp35 antigens are conserved or unique to the Nc-1 strain. As shown in Fig. 2A, Ncp29 Western blot analysis with 6C11 indicated that Ncp29 was present in three independent isolates from infected dogs (Nc-1, Nc-2, and Nc-Liv) and in two independent isolates from infected cattle (BPA-1 and BPA-3). A second band that migrates slightly slower than Ncp29 was observed in all isolates, and this doublet was often seen when an abundance of protein was loaded on the gel (see also Fig. 3 and 5). Whether this second band is an alternative form of Ncp29 or a cross-reactive antigen is unknown. No cross-reaction of the 6C11 MAb with T. gondii or Sarcocystis was seen (Fig. 2A). Similarly, Western blot analysis with the 5H5 MAb indicated that Ncp35 was present in all N. caninum isolates tested, and no cross-reaction with T. gondii or S. neurona was observed (Fig. 2B). Western blot analysis with antisera against T. gondii and S. neurona confirmed that antigen was present in these parasite lysates (data not shown). These results indicate that Ncp29 and Ncp35 are conserved among N. caninum isolates but that the epitopes recognized by the 6C11 and 5H5 MAbs are not present in the two closely related parasites T. gondii and S. neurona.
FIG. 2.
The Ncp29 and Ncp35 surface antigens were recognized in different strains of N. caninum. The anti-Ncp29 (α-p29) MAb 6C11 (A) and the MAb 5H5 (α-p35) (B) reacted by Western blotting to parasite lysates of three independent N. caninum isolates from dogs (Nc-1, Nc-2, and Nc-LIV) and two independent N. caninum isolates from cattle (BPA-1 and BPA-3). These MAbs did not react to parasite lysates of T. gondii or S. neurona.
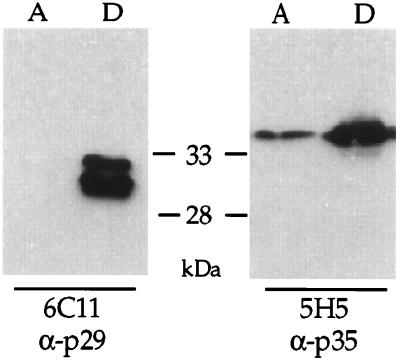
FIG. 3.
Triton X-114 partitioning assays indicated that Ncp29 and Ncp35 are membrane associated. Parasite lysates were separated with Triton X-114 into an aqueous phase (lanes A) that contains soluble proteins and a detergent phase (lanes D) that contains membrane-associated proteins. Western blot analysis with MAb 6C11 (α-p29) indicated that Ncp29 partitioned exclusively with the detergent phase. Western blot analysis with MAb 5H5 (α-p35) indicated that Ncp35 partitioned primarily with the detergent phase, although a minor portion was found in the aqueous phase.
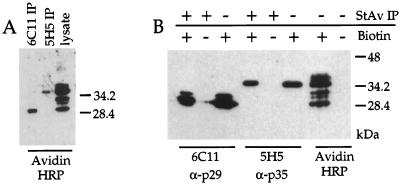
FIG. 5.
Approximately six dominant surface proteins, including Ncp29 and Ncp35, were revealed by surface biotinylation of N. caninum tachyzoites and Western blot detection with avidin-HRP conjugate. (A) Ncp29 (6C11) and Ncp35 (5H5) that were immunoprecipitated (IP) from labeled parasites were biotinylated, as determined by Western blotting with avidin-HRP. (B) Similarly, MAb 6C11 (α-p29) detected Ncp29 and MAb 5H5 (α-p35) detected Ncp35 in the biotinylated protein fraction precipitated with streptavidin-agarose (StAv) but not in the protein fraction from nonbiotinylated parasites, thus indicating that the streptavidin precipitation was specific for biotin-labeled proteins. Avidin-HRP detection was specific for biotinylated proteins, as revealed by Western blot analysis of nonbiotinylated parasites.
Demonstration of Ncp29 and Ncp35 membrane association and parasite surface localization.
MAbs 6C11 and 5H5 were generated against a membrane-enriched fraction of proteins, suggesting that they recognize membrane antigens. To determine if Ncp29 and Ncp35 are membrane associated, we performed Triton X-114 phase partitioning on tachyzoites, which separates proteins based on their amphiphilicity (7). As shown in Fig. 3, both Ncp29 and Ncp35 preferentially partitioned into the detergent phase, suggesting that these proteins are associated with membranes in N. caninum tachyzoites. A minor proportion of Ncp35 was also observed in the aqueous phase (Fig. 3).
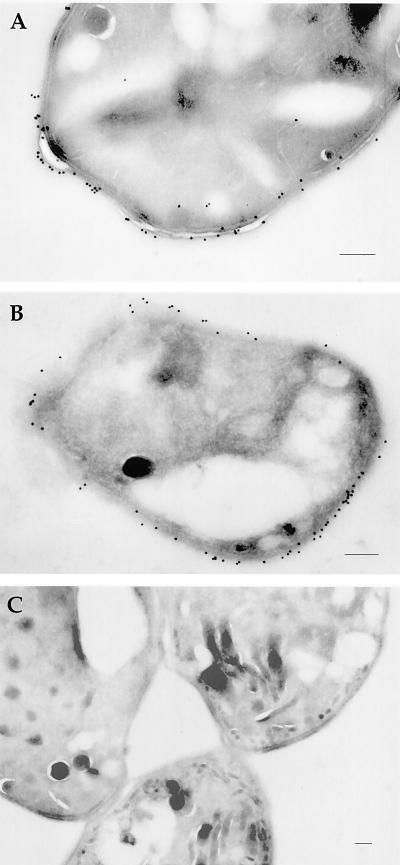
We also localized Ncp29 and Ncp35 in extracellular N. caninum tachyzoites by immuno-EM to determine if the antigens were associated with intracellular membranes (e.g., nuclear envelope or organelle membranes, etc.) or the parasite plasma membrane. The 6C11 MAb against Ncp29 (Fig. 4A) and the 5H5 MAb against Ncp35 (Fig. 4B) primarily labeled the tachyzoite plasma membrane, although gold labeling was occasionally seen in the cytoplasm, where it was associated with small vesicular structures (Fig. 4A). The antibodies did not label rhoptries, dense granules, or micronemes. As a negative control, we examined sections stained with MAb DG52, which recognizes the immunodominant T. gondii surface antigen SAG1. No cross-reaction to Nc-1 was observed (Fig. 4C).
FIG. 4.
Immuno-EM localization of Ncp29 and Ncp35 in extracellular tachyzoites of N. caninum. Both Ncp29 (MAb 6C11 (A) and Ncp35 (MAb 5H5) (B) were localized to the parasite cell membrane but were not found in micronemes, dense granules, or rhoptries. Occasional gold particles were found in the cytoplasm within small vesicles. No labeling of the parasites was observed with MAb DG52 against T. gondii SAG1 (C). Bars, approximately 0.2 μm.
Immuno-EM localization indicated that Ncp29 and Ncp35 are associated with the tachyzoite plasma membrane but did not distinguish whether the antigens are displayed on the cytoplasmic face or the extracellular surface of the parasite membrane. To determine if Ncp29 and Ncp35 were surface antigens and to assess the relative abundance of proteins on the cell surface, we labeled extracellular tachyzoites with sulfo–N-hydroxysuccinimide–biotin, which does not permeate membranes and selectively labels surface proteins. When surface-biotinylated parasites were analyzed by Western blotting with avidin-HRP to detect surface biotinylated proteins, approximately six dominant biotin-labeled proteins were revealed, including molecules that migrated at 29 and 35 kDa (Fig. 5A, lane 3). These prominent bands corresponded to Ncp29 and Ncp35 antigens, as confirmed by immunoprecipitation with MAbs 6C11 and 5H5 and detection with avidin-HRP (Fig. 5A, lanes 1 and 2). Immobilized streptavidin was also used to specifically precipitate the biotinylated proteins from the parasite lysate, and Western blot analysis with MAbs 6C11 and 5H5 revealed that Ncp29 and Ncp35 were in the fraction of biotinylated proteins (Fig. 5B, lanes 1 and 4). These data indicate that Ncp29 and Ncp35 are the two most abundant of approximately six distinct proteins that are displayed on the surface of N. caninum tachyzoites.
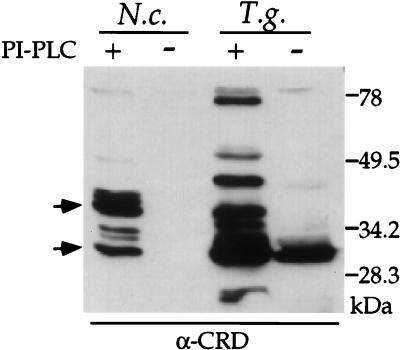
In many parasitic protozoa, including T. gondii, the dominant surface proteins are tethered to the plasma membrane by a glycolipid anchor (39, 47). To determine if Ncp29 and Ncp35 were similarly anchored, we utilized anti-CRD antibodies that recognize a carbohydrate epitope on the GPI anchor after cleavage with phosphatidylinositol-specific phospholipase C (41). Approximately six glycolipid-anchored proteins in N. caninum tachyzoites were revealed by the anti-CRD antibodies (Fig. 6). This profile matched the profile of biotin-labeled surface proteins (Fig. 5), and the two most prominent antigens revealed by both analyses comigrate with Ncp29 and Ncp35. Collectively, these data indicate that the surface of N. caninum tachyzoites is composed of approximately six dominant GPI-anchored proteins that include the Ncp29 and Ncp35 antigens.
FIG. 6.
Ncp29 and Ncp35 were detected by anti-CRD (α-CRD) after phosphatidylinositol-specific phospholipase (PI-PLC) treatment, indicating they contain a glycolipid anchor. Nc-1 strain N. caninum tachyzoites (N.c.) or RH strain T. gondii tachyzoites (T.g.) were lysed, treated with PI-PLC, and analyzed by Western blotting with antibodies against the CRD of GPI anchors. Approximately six GPI-anchored proteins were revealed in PI-PLC-treated N. caninum, two of which correspond to Ncp29 and Ncp35 (arrows). Anti-CRD antibodies did not react with parasites that had not been PI-PLC treated.
Identification of the Ncp29 and Ncp35 genes.
Since the MAbs against both Ncp29 and Ncp35 do not react with the antigens under reducing conditions, they would likely be ineffective for immunoscreening of cDNA expression libraries in Escherichia coli. The Ncp29 and Ncp35 proteins were therefore purified by immunoprecipitation and sodium dodecyl sulfate polyacrylamide gel electrophoresis, and the protein bands were transferred to polyvinylidene difluoride membranes and subjected to Edman degradation for peptide sequencing. An N-terminal sequence could not be obtained from Ncp29, but analysis of a peptide fragment generated by CNBr cleavage yielded an internal amino acid sequence of PPRASKLVNGV. With the exception of a S (serine) rather than a K (lysine) at residue 6, a BLAST search of GenBank (2) revealed that this peptide matches that encoded by the sequence under accession no. AJ005664, i.e., a 36-kDa surface protein of N. caninum (25).
Analysis of the Ncp35 N terminus yielded the amino acid sequence APFKSENEKFT. Degenerate oligonucleotides based on the sequence ENEKFT were utilized with the T3 or T7 vector primers to separately amplify the 5′ and 3′ ends of the Ncp35 gene from the Nc-1 cDNA library. The resulting PCR fragments were cloned and sequenced, and BLAST searches (2) revealed that the predicted products of several clones were significantly similar to T. gondii SAG proteins. The 5′ portion of the putative Ncp35 gene encoded the amino acid sequence APFKS at the immediate flank of the degenerate primer site, matching the N-terminal peptide sequence obtained from purified Ncp35 protein. To confirm that the two gene fragments represent a single continuous transcript, specific amplification primers were selected from the fragment sequences at the predicted 5′ and 3′ ends of the putative Ncp35 gene. These primers successfully amplified a DNA fragment of the correct size from both genomic DNA and the Nc-1 cDNA library. These data collectively indicate that the amplified and cloned fragments correctly represent the gene encoding the Ncp35 antigen.
The full-length amplified gene for Ncp35 was cloned, and double-strand sequence analysis revealed an open reading frame of 1,203 nucleotides that encodes a 401-amino-acid protein. Based on the N-terminal peptide sequence of purified Ncp35, the protein contains a 53-amino-acid signal peptide that is cleaved from the mature protein; despite its unusual length, this matches the computer-predicted site for signal peptide cleavage (Expasy). The predicted protein sequence also has a hydrophobic C-terminal tail, consistent with Ncp35 being a GPI-anchored protein, as shown above (Fig. 6). The most likely site for addition of the glycolipid anchor is 25 amino acids from the termination codon (31); this carboxyl terminus is presumably cleaved during the anchor addition process. Removal of the N-terminal signal peptide and the C-terminal peptide during GPI anchor addition would result in a mature protein of 323 amino acids that has a predicted molecular mass of 34.1 kDa.
A BLAST search with the partial Ncp35 clones indicated homology to T. gondii SAG proteins. Computer-generated alignments (Bestfit) (13) of the predicted full-length amino acid sequence of Ncp35 with proteins of the T. gondii SAG-SRS family indicated that Ncp35 is most similar to T. gondii SRS2 (36) (Fig. 7). Of interest, Ncp35 contains a tandemly duplicated motif and, more significantly, 12 conserved cysteine residues that have been described for the SAG and SRS proteins (12, 22, 36) (Fig. 7). A BLAST search with the GenBank sequence (AJ005664) that matches the peptide sequence obtained for Ncp29 indicated that this protein was also homologous to T. gondii SAG and SRS antigens. Computer-generated alignments (Bestfit) indicated that Ncp29 is most similar to SAG1 and also contains the duplicated motif and 12 conserved cysteines (data not shown).
FIG. 7.
Amino acid sequence alignment of Ncp35 with the SRS2 protein from T. gondii, showing the duplicated motif (shaded) and the 12 conserved cysteine residues (boxed). The putative signal peptide is underlined.
DISCUSSION
The Ncp29 and Ncp35 antigens were consistently recognized as immunodominant by antisera from Neospora-infected animals and were identified in five independent and diverse isolates of N. caninum. Analyses of these two antigens indicated that they are membrane associated and displayed as GPI-anchored proteins on the surface of tachyzoites, reminiscent of the SAG proteins in T. gondii. Comparison of the Ncp29 and Ncp35 genes with database sequences indicated that they are homologs of the SAG-SRS family of T. gondii surface antigens; Ncp29 is most similar to T. gondii SAG1, and Ncp35 is most similar to SRS2. Significantly, there are 12 conserved cysteine residues and a tandemly duplicated motif in Ncp29 and Ncp35 that have also been described for the SAG and SRS proteins (12, 22, 36), suggesting similar secondary and tertiary conformations that are likely important for the function of these surface antigens.
Methods for identifying N. caninum and Neospora-infected animals have typically utilized antigens and antibodies based on total parasite protein, thereby increasing the risk of false-positives due to cross-reaction with other closely related parasites (e.g., T. gondii) (27). The development of reliable reagents for N. caninum identification and diagnosis of neosporosis depends on the characterization of Neospora-specific antigens and antibodies. Antisera from Neospora-infected animals reacted strongly with Ncp29 and Ncp35 (Fig. 1), and the MAbs recognized both antigens in five independent N. caninum isolates (Fig. 2). These data indicate that Ncp29 and Ncp35 are highly immunogenic and well conserved; consequently, these should be ideal reagents for epidemiological studies and diagnostic purposes.
The data presented here quite clearly indicate that Ncp29 and Ncp35 are surface antigens with approximate molecular masses of 29 and 35 kDa, respectively. Immuno-EM localization indicated that both antigens are almost exclusively associated with the parasite plasma membrane (Fig. 4), where they appear to be tethered via GPI anchors (Fig. 6). Two immunogenic antigens designated Nc-p36 and Nc-p43 (corresponding to their apparent molecular masses of 36 and 43 kDa) have been previously described as surface proteins from N. caninum (23, 25, 26). These previous studies suggested that Nc-p36 could be found in dense granules, and Nc-p43 was also in dense granules as well as rhoptries, as determined by immuno-EM localization (23, 25). Comparison of the peptide sequence we obtained for Ncp29 with a nucleotide sequence described for Nc-p36 (GenBank accession no. AJ005664) suggested that these are the same protein, while our Ncp35 gene sequence partially matches an incomplete cDNA clone described for Nc-p43 (24). Despite their likely genetic identities, there are a number of inconsistencies in the characteristics we have observed for these antigens versus those previously reported. The discrepancy in molecular masses is most likely due to the altered migration of the proteins under native (our study) versus reduced (25, 26) conditions, as has previously been described for the SAG proteins of T. gondii (9, 12). The molecular masses of 29 and 35 kDa observed for Ncp29 and Ncp35 under native conditions correspond well with the masses of 28.2 and 34.1 kDa that are predicted from the gene sequences for Ncp29 (AJ005664) and Ncp35 (described in this study). The reported dense-granule localization of Ncp29 (Nc-p36) (25) and dense-granule/rhoptry localization of Ncp35 (Nc-p43) (23) are also inconsistent with our data, which indicate that both antigens are almost exclusively associated with the surface membrane. The previous studies on immunolocalization of Nc-p36 and Nc-p43 relied on affinity-purified antibodies from polyclonal antisera (23, 25, 26); thus, it is likely that the observed dense-granule and rhoptry labeling for these proteins was due to contaminating antibodies in the affinity-purified fraction. In addition, such a localization pattern is unlikely, as there is a clear separation between proteins found in the distinct secretory organelles of T. gondii (10, 11, 18). Using highly specific MAbs, we find that both Ncp29 and Ncp35 are largely confined to the surface membrane of N. caninum, a location that is entirely consistent with their homologies to SAG proteins of T. gondii.
The generation of an extensive expressed sequence tag database for T. gondii (1) has revealed that the surface of this parasite is composed of a number of antigens (at least six) that exhibit moderate sequence identity to the major tachyzoite surface antigen SAG1 and have apparent structural similarity to one another (36). Computer-generated alignments indicate that Ncp35 has about 44% amino acid sequence identity with SRS2 of T. gondii (Fig. 7) and relatively moderate (23 to 29%) identity with other T. gondii SAG-SRS proteins. Similarly, the GenBank sequence (AJ005664) for Ncp29 is approximately 53% identical to that for SAG1 of T. gondii and has moderate identity to those of the other SAG and SRS proteins (data not shown). Based on these obvious homologies, we propose that Ncp29 should be designated NcSAG1 and that Ncp35 should be designated NcSRS2, using nomenclature similar to that proposed for T. gondii (43).
Significantly, NcSAG1/Ncp29 and NcSRS2/Ncp35 contain an internally duplicated motif and the 12 absolutely conserved cysteine residues that have been identified in SAG1, SAG3, and the SRS antigens of T. gondii (12, 36) (Fig. 7). These cysteines are likely involved in intramolecular disulfide bonding, and their conservation may indicate that these proteins all have a similar folding pattern. Although the function of these surface antigens is unknown, their conserved secondary and tertiary structures are presumably important in both N. caninum and T. gondii.
Since they are displayed on the cell surface, NcSAG1/Ncp29 and NcSRS2/Ncp35 of N. caninum tachyzoites and the SAG and SRS antigens of T. gondii are likely involved in the parasite’s initial interactions with the host cell surface and the components of the host immune response. Consequently, a number of different potential functions can be suggested for these proteins. The SAG and SRS proteins of T. gondii may represent a set of diverse adhesins that have evolved to allow these parasites to infect the extensive variety of hosts and cell types that they encounter, and a similar scenario can be proposed for NcSAG1/Ncp29 and NcSRS2/Ncp35 in N. caninum. Studies on the T. gondii SAG1 (20, 38) and SAG3 (46) proteins and N. caninum NcSRS2/Ncp35 (Nc-p43) (23) have demonstrated that some antibodies against these antigens can reduce invasion. However, the specific binding to any one host cell receptor molecule has not been demonstrated for any of these SAG proteins. Nonetheless, they may provide low-affinity interactions during the initial contact with the host cell.
An alternative, but not mutually exclusive, hypothesis is that these surface antigens have evolved to modulate the immune response in the infected host. Support for this mechanism is provided by the extreme immunogenicity of these antigens. Protection against T. gondii infection is provided by a T-cell-dependent response that leads to a vigorous Th1 cytokine profile (28). It has been shown that the simultaneous presence of allelic variants of parasite epitopes reduce the cytotoxic-T-lymphocyte response during infection with Plasmodium falciparum (19). Thus, the concurrent expression of the related SAG and SRS proteins by T. gondii or their various homologs in N. caninum could serve a similar immunosuppressive function.
Vaccination of mice with N. caninum does not provide complete cross-protection against all strains of T. gondii (33). However, N. caninum-infected mice are protected against a lethal challenge with strain ME49 of T. gondii, and this process is mediated by CD8+ T cells (29). Interestingly, the cross-reactive T-cell epitopes are found in the molecular mass range of 15 to 50 kDa (29), which corresponds well to the sizes observed for the SAG and SRS proteins. Consequently, NcSAG1/Ncp29 and NcSRS2/Ncp35, and possibly additional related antigens, may contribute to the immunological cross-protection that has been observed between N. caninum and T. gondii.
Disease caused by N. caninum in several different animals, most notably canines (14, 15) and bovines (3, 16, 45), has been described. However, neosporosis in humans has not been described, and mice are quite resistant to high doses of N. caninum (27, 30, 34). Thus, N. caninum appears to be more limited than T. gondii in the range of animals in which it causes disease. It remains unknown whether the family of related surface antigens in N. caninum is equally diverse as it is in T. gondii. The profile of GPI-anchored proteins in Nc-1 is less complex than that in T. gondii RH (Fig. 6), which may indicate that there are fewer SAG and SRS antigens in Neospora. Assuming that the redundancy that has evolved in T. gondii is important for the function(s) of the SAG and SRS antigens, the apparent reduction in antigen complexity in N. caninum could be significant considering its decreased pathogenicity and narrower host range relative to T. gondii.
ACKNOWLEDGMENTS
We thank P. Conrad and S. Trees for providing strains of N. caninum, D. Granstrom for S. neurona antigen and antibodies, and J. Bangs for antibodies against CRD and advice on detection of GPI anchors. We also thank M. Levy for technical assistance.
This work was supported by a grant from the USDA National Research Initiative (97-35204-4770).
ADDENDUM IN PROOF
Since submission of this article, the dog has been identified as the definitive host of Neospora caninum (M. McAllister, J. P. Dubey, D. S. Lindsay, W. S. Jolley, R. A. Wills, and A. M. McGuire, Int. J. Parasitol. 28:1473–1479, 1998).
REFERENCES
- 1.Ajioka J A, Boothroyd J C, Brunk B P, Hehl A, Hillier L, Manger I D, Overton G C, Marra M, Roos D, Wan K L. Gene discovery by EST sequencing in Toxoplasma gondii reveals sequences restricted to the Apicomplexa. Genome Res. 1998;8:18–28. doi: 10.1101/gr.8.1.18. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M L, Blanchard P C, Barr B C, Dubey J P, Hoffman R L, Conrad P A. Neospora-like protozoan infection as a major cause of abortion in California dairy cattle. J Am Vet Med Assoc. 1991;198:241–244. [PubMed] [Google Scholar]
- 4.Asai, T., D. K. Howe, K. Nakajima, T. Nozaki, T. Takeuchi, and L. D. Sibley. Neospora caninum: tachyzoites express a potent type-I nucleoside triphosphate hydrolase, but lack nucleoside diphosphate hydrolase activity. Exp. Parasitol., in press. [DOI] [PubMed]
- 5.Barnes W M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc Natl Acad Sci USA. 1994;91:2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boothroyd J C, Hehl A, Knoll L J, Manger I D. The surface of Toxoplasma: more and less. Int J Parasitol. 1998;28:3–9. doi: 10.1016/s0020-7519(97)00182-3. [DOI] [PubMed] [Google Scholar]
- 7.Brusca J S, Radolf J D. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 1994;228:182–193. doi: 10.1016/0076-6879(94)28019-3. [DOI] [PubMed] [Google Scholar]
- 8.Bulow R, Boothroyd J C. Protection of mice from fatal Toxoplasma infection by immunization with p30 antigen in liposomes. J Immunol. 1991;147:3496–3500. [PubMed] [Google Scholar]
- 9.Burg J L, Perlman D, Kasper L H, Ware P L, Boothroyd J C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- 10.Carruthers V B, Sibley L D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 11.Cesbron-Delauw M F. Dense granule organelles of Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today. 1994;10:293–296. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 12.Cesbron-Delauw M F, Tomavo S, Beauchamps P, Fourmaux M P, Camus D, Capron A, Dubremetz J F. Similarities between the primary structures of two distinct major surface proteins of Toxoplasma gondii. J Biochem. 1994;269:16217–16222. [PubMed] [Google Scholar]
- 13.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey J P, Carpenter J L, Speer C A, Topper M J, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. 1988;192:1269–1285. [PubMed] [Google Scholar]
- 15.Dubey J P, Hattel A L, Lindsay D S, Topper M J. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc. 1988;193:1259–1263. [PubMed] [Google Scholar]
- 16.Dubey J P, Leathers C W, Lindsay D S. Neospora caninum-like protozoan associated with fatal myelitis in newborn calves. J Parasitol. 1989;75:146–148. [PubMed] [Google Scholar]
- 17.Dubey J P, Lindsay D S. Neosporosis. Parasitol Today. 1993;9:452–458. doi: 10.1016/0169-4758(93)90099-2. [DOI] [PubMed] [Google Scholar]
- 18.Dubremetz J F, Schwartzman J D. Subcellular organelles of Toxoplasma gondii and host cell invasion. Res Immunol. 1993;144:31–33. doi: 10.1016/s0923-2494(05)80093-8. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert S C, Plebanski M, Gupta S, Morris J, Cox M, Aidoo M, Kwiatkowski D, Greenwood B M, Whittle H C, Hill A V S. Association of malaria parasite population structure, HLA, and immunological antagonism. Science. 1998;279:1173–1177. doi: 10.1126/science.279.5354.1173. [DOI] [PubMed] [Google Scholar]
- 20.Grimwood J, Smith J E. Toxoplasma gondii: the role of a 30-kDa surface protein in host cell invasion. Exp Parasitol. 1992;74:106–111. doi: 10.1016/0014-4894(92)90144-y. [DOI] [PubMed] [Google Scholar]
- 21.Harlow E, Lane D, editors. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 22.Hehl A, Krieger T, Boothroyd J C. Identification and characterization of SRS1, a Toxoplasma gondii surface antigen upstream of and related to SAG1. Mol Biochem Parasitol. 1997;89:271–282. doi: 10.1016/s0166-6851(97)00126-6. [DOI] [PubMed] [Google Scholar]
- 23.Hemphill A. Subcellular localization and functional characterization of Nc-p43. Infect Immun. 1996;64:4279–4287. doi: 10.1128/iai.64.10.4279-4287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemphill A, Felleisen R, Connoly B, Gottstein B, Hentrich B, Muller N. Characterization of a cDNA-clone encoding Nc-p43, a major Neospora caninum tachyzoite surface protein. Parasitology. 1997;115:581–590. doi: 10.1017/s0031182097001650. [DOI] [PubMed] [Google Scholar]
- 25.Hemphill A, Fuchs N, Sonda S, Gottstein B, Hentrich B. Identification and partial characterization of a 36 kDa surface protein on Neospora caninum tachyzoites. Parasitology. 1997;115:371–380. doi: 10.1017/s0031182097001455. [DOI] [PubMed] [Google Scholar]
- 26.Hemphill A, Gottstein B. Identification of a major surface protein on Neospora caninum tachyzoites. Parasitol Res. 1996;82:497–504. doi: 10.1007/s004360050152. [DOI] [PubMed] [Google Scholar]
- 27.Howe D K, Sibley L D. Development of molecular genetics for Neospora caninum: a complementary system to Toxoplasma gondii. Methods Companion Methods Enzymol. 1997;13:123–133. doi: 10.1006/meth.1997.0505. [DOI] [PubMed] [Google Scholar]
- 28.Hunter C A, Suzuki Y, Subaste C S, Remington J S. Cells and cytokines in resistance to Toxoplasma gondii. In: Gross U, editor. Toxoplasma gondii. Heidelberg, Germany: Springer; 1996. pp. 113–125. [DOI] [PubMed] [Google Scholar]
- 29.Kasper L H, Khan I A. Antigen-specific T cells protect against lethal toxoplasmosis in mice infected with Neospora caninum. Infect Immun. 1998;66:1554–1560. doi: 10.1128/iai.66.4.1554-1560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan I A, Schwartzman J D, Fonseka S, Kasper L H. Neospora caninum: role for immune cytokines in host immunity. Exp Parasitol. 1997;85:24–34. doi: 10.1006/expr.1996.4110. [DOI] [PubMed] [Google Scholar]
- 31.Kodukula K, Gerber L D, Amthauer R, Brink L, Udenfried S. Biosynthesis of glycosylphosphatidylinositol (GPI)-anchored membrane proteins in intact cells: specific amino acid requirements adjacent to the site of cleavage and GPI attachment. J Cell Biol. 1993;120:657–664. doi: 10.1083/jcb.120.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay D S, Blagburn B L, Dubey J P. Infection of mice with Neospora caninum (Protozoa: Apicomplexa) does not protect against challenge with Toxoplasma gondii. Infect Immun. 1990;58:2699–2700. doi: 10.1128/iai.58.8.2699-2700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsay D S, Lenz S D, Cole R A, Dubey J P, Blagburn B L. Mouse model for central nervous system Neospora caninum infections. J Parasitol. 1995;81:313–315. [PubMed] [Google Scholar]
- 35.Lunden A, Parmley S F, Bengtsson K L, Araujo F G. Use of a recombinant antigen, SAG2, expressed as a glutathione-S-transferase fusion protein to immunize mice against Toxoplasma gondii. Parasitol Res. 1997;83:6–9. doi: 10.1007/s004360050198. [DOI] [PubMed] [Google Scholar]
- 36.Manger I, Hehl A B, Boothroyd J C. The surface of Toxoplasma tachyzoites is dominated by a family of glycosylphosphatidylinositol-anchored antigens related to SAG1. Infect Immun. 1998;66:2237–2244. doi: 10.1128/iai.66.5.2237-2244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mineo J R, Kasper L H. Attachment of Toxoplasma gondii to host cells involves major surface protein SAG-1 (P30) Exp Parasitol. 1994;79:11–20. doi: 10.1006/expr.1994.1054. [DOI] [PubMed] [Google Scholar]
- 38.Mineo J R, McLeod R, Mack D, Smith J, Khan I A, Ely K H, Kasper L H. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J Immunol. 1993;150:3951–3964. [PubMed] [Google Scholar]
- 39.Nagel S D, Boothroyd J C. The major surface antigen, p30, of Toxoplasma gondii is anchored by a glycolipid. J Biochem. 1989;264:5569–5574. [PubMed] [Google Scholar]
- 40.Potasman I, Araujo F G, Desmonts G, Remington J S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986;154:650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- 41.Shak S, Davitz M A, Wolinsky M L, Nussenzweig V, Turner M J, Gurnett A. Partial characterization of the cross-reacting determinant, a carbohydrate epitope shared by decay accelerating factor and the variant surface glycoprotein of the African Trypanosoma brucei. J Immunol. 1988;140:2046–2050. [PubMed] [Google Scholar]
- 42.Sharma S D, Mullenax J, Araujo F G, Ehrlich H A, Remington J S. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 43.Sibley L D, Pfefferkorn E R, Boothroyd J C. Proposal for a uniform genetic nomenclature in Toxoplasma gondii. Parasitol Today. 1991;7:327–328. doi: 10.1016/0169-4758(91)90210-f. [DOI] [PubMed] [Google Scholar]
- 44.Speer C A, Dubey J P. Ultrastructure of tachyzoites, bradyzoites, and tissue cysts of Neospora caninum. J Protozool. 1989;36:458–463. doi: 10.1111/j.1550-7408.1989.tb01081.x. [DOI] [PubMed] [Google Scholar]
- 45.Thilsted J P, Dubey J P. Neosporosis-like abortions in a herd of dairy cattle. J Vet Diag Invest. 1989;1:205–209. doi: 10.1177/104063878900100301. [DOI] [PubMed] [Google Scholar]
- 46.Tomavo S. The major surface antigens of Toxoplasma gondii: structures and functions. In: Gross U, editor. Toxoplasma gondii. Heidelberg, Germany: Springer; 1996. pp. 45–54. [DOI] [PubMed] [Google Scholar]
- 47.Tomavo S, Schwartz R T, Dubremetz J F. Evidence for glycosylphosphatidylinositol anchoring of Toxoplasma gondii major surface antigens. Mol Cell Biol. 1989;9:4576–4580. doi: 10.1128/mcb.9.10.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]