Abstract
Background
Surveillance of malaria vectors is crucial for assessing the transmission risk and impact of control measures. Human landing catches (HLC) directly estimate the biting rates but raise ethical concerns due to the exposure of volunteers to mosquito-borne pathogens. A common alternative is the CDC-light trap, which is effective for catching host-seeking mosquitoes indoors but not outdoors. New, exposure-free methods are needed for sampling mosquitoes indoors and outdoors in ways that reflect their natural risk profiles. The aim of this study was therefore to evaluate the efficacy of the miniaturized double net trap (DN-Mini) for sampling host-seeking mosquitoes in south-eastern Tanzania, where malaria transmission is dominated by Anopheles funestus.
Methods
Adult mosquitoes were collected from 222 randomly selected houses across three villages (74 per village) in Ulanga district, south-eastern Tanzania, using the DN-Mini traps, CDC-Light traps, and Prokopack aspirators. First, we compared CDC-light and DN-Mini traps for collecting indoor host-seeking mosquitoes, while Prokopack aspirators were used for indoor-resting mosquitoes. Second, we deployed the DN-Mini and Prokopack aspirators to collect host-seeking and resting mosquitoes indoors and outdoors. Generalized linear mixed models (GLMM) with a negative binomial distribution were used to compare the effectiveness of the traps for catching different mosquito species.
Results
The DN-Mini was 1.53 times more efficient in collecting An. funestus indoors (RR = 1.53, 95% CI: 1.190–1.98) compared to the CDC-Light trap. However, for Anopheles arabiensis, the DN-Mini caught only 0.32 times as many mosquitoes indoors as the CDC-Light traps (RR = 0.32, 95% CI: 0.183–0.567). Both An. funestus and An. arabiensis were found to be more abundant indoors than outdoors when collected using the DN-Mini trap. Similarly, the Prokopack aspirator was greater indoors than outdoors for both An. funestus and An. arabiensis.
Conclusion
The DN-Mini outperformed the CDC-light trap in sampling the dominant malaria vector, An. funestus species, but was less effective in capturing An. arabiensis, and for both vector species, the biting risk was greater indoors than outdoors when measured using the DN-Mini trap. These findings highlight the importance of selecting appropriate trapping methods based on mosquito species and behaviors.
Introduction
Entomological indicators of malaria are key metrics used to assess the burden of malaria transmission and evaluate the effectiveness of vector control interventions. Furthermore, the development and implementation of effective interventions require in-depth knowledge of the local vector species on their vector species composition, distribution, abundance, feeding behaviors, host preference, parity status, human biting rates and pathogen infection rates [1]. To generating such knowledge requires reliable and standardized efficient mosquito sampling techniques for mosquitoes in their different physiological states, e.g., host-seeking and resting populations. Unfortunately, the standardized methods/tools for estimating these important parameters has been a major impediment [2, 3] especially in settings where substantial malaria transmission occurs outdoors [4].
Human Landing Catches (HLC), where volunteers expose their legs and catch mosquitoes landing on them, is the most direct method for measuring human biting rates and is constantly used as a reference when evaluating other sampling techniques [5]. One advantage of using HLC is that the mosquitoes are caught in the act of attacking the human host [1, 6]. The collected mosquitoes are considered representative of the natural human biting rates and are used to understand pathogen transmission risk. On the other hand, the main weaknesses of HLC include high costs, laboriousness, and the potential exposure of the volunteers to mosquito bites and mosquito-borne diseases (e.g., dengue fever, Zika, malaria, chikungunya, and others). While other studies suggest that proper use of HLC may reduce the risks of pathogen transmission to volunteers [7, 8]. Therefore, there is a strong need for alternative mosquito sampling methods that enable entomological surveillance without exposing human volunteers to mosquito bites and the risks of pathogen transmission. Various alternative methods for collecting mosquitoes have been developed [6, 9, 10], but CDC light traps have gained the most popularity due to their reliability, portability, cost-effectiveness, and scalability [11–14]. While CDC light traps are commonly used indoors alongside human-occupied bed nets and are considered more sensitive [6], their effectiveness diminishes significantly when used outdoors [15, 16].
In response to the challenges posed by traditional mosquito sampling methods, the miniaturized double net trap (DN-Mini) was introduced in 2019 as an exposure-free alternative for capturing host-seeking mosquitoes both indoors and outdoors (Fig 1). This system is a variant of the traditional double net trap designs [1, 2], but allows for direct assessment of human biting risk without exposing the volunteers. The DN-Mini has been widely tested agais in various locations, including Zanzibar [17] and mainland Tanzania [3, 18, 19] as an ethical alternative to HLC. It is becoming a common alternative method when HLC is not acceptable due to ethical reasons [2, 5, 20]. However, limited data exist on its direct evaluation as a sampling tool, except for one study in rural Tanzania that examined its use for assessing indoor-outdoor biting preferences and physiological ages of malaria vectors [3].
Fig 1. Showing a miniaturized double net trap (DN-Mini trap).
The overall aim of this study was to evaluate the performance of DN-Mini traps as a sampling and surveillance tool for malaria vectors from indoors and outdoors of houses in southeastern Tanzania. The specific aims of this study include: 1) to compare the efficacy of the DN-Mini traps with that of the CDC-Light traps in capturing mosquitoes from inside houses (indoors); 2) to assess the efficacy of DN-Mini traps in sampling mosquitoes from both indoors and outdoors of houses; 3) to compare catches of resting mosquitoes in prokopack aspirators from indoors and outdoors of houses with DN-Mini traps and CDC-Light traps.
Methods
Study area
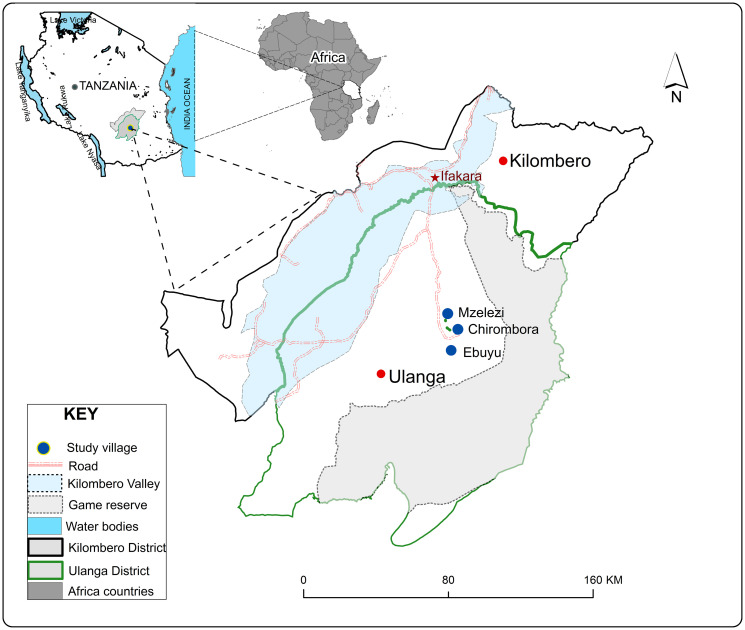
The study was conducted in Ulanga district, Morogoro region, southeastern Tanzania (Fig 2). The three villages involved were Mzelezi (-8.898 S, 36.735 E), Chirombola (-8.926 S, 36.753 E), and Ebuyu (-8.979 S, 36.760 E), all situated 500m above sea level. The area experiences an annual rainfall between 1200 and 1800mm, with a hot and humid season (December to May), followed by a cool-dry period (June–July), and a hot dry season (August–November). The main economic activities in the area are maize and rice farming. Furthermore, Anopheles arabiensis and An. funestus stands as the principal malaria vector in the study area. The prevalent housing structures typically include clay brick walls, open eaves, and open windows. Malaria transmission is moderate to high and mainly mediated by An. funestus. [11, 21].
Fig 2. Map of the study area, showing study villages.
The miniaturized double net (DN-Mini) trap
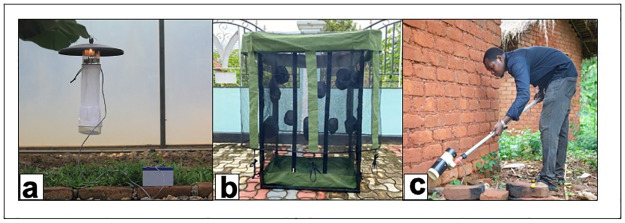
The DN-Mini Trap is an exposure-free mosquito sampling tool designed to capture mosquitoes indoors and outdoors without interfering with their natural behavior (Fig 3). It is based on the original bed net system [1], also used in Lao PDR by Tangena et al. [2]. The trap comprises a fiberglass-netted cage with canvas-reinforced corners and a Polyvinyl Chloride (PVC) base, where a human volunteer sits as bait for mosquitoes. Multiple protective sleeves on the inner wall allow volunteers to safely retrieve captured mosquitoes using mouth aspirators. Mosquitoes enter the trap through the gap between the two nets and are collected at regular intervals (Fig 3). The trap’s dimensions are 60 in cm width, 100 cm in length, and 180 cm in height [3, 22]. It is made of UV-resistant netting in an iron-framed cage. The current version costs approximately 100 USD per unit per year and is locally available.
Fig 3.
Illustrations of Mosquito Traps A) Standard CDC-LT (Model 512; John W. Hock Company, Gainesville, FL) B) A miniaturized double net trap (DN-Mini). C) Prokopack aspirator (John W. Hock Company model 1412).
The trap can be easily assembled and dismantled, requiring less than five minutes for setup and takedown. It is low-maintenance, with minimal repairs needed in case of damage during data collection. To ensure its longevity, the trap should be stored away from direct sunlight to protect the netting fibers. Handling should pay particular attention when entering or exiting the trap, as damage typically occurs when the chair inside the trap rubs tightly on the walls, affecting the edges of the nets fitted to iron poles.
CDC-light trap
The Centers for Disease Control and Prevention (CDC) light traps (John W. Hock Ltd, Gainesville, FL., USA) (Fig 3) are portable devices comprising a light source, a fan, and a collection container. Mosquitoes are attracted to the light and drawn into the trap by the fan. While commonly used for collecting indoor mosquitoes [16, 23, 24], they can also be applied outdoors alone [5] or when combined with other traps. It is one of the most common sampling tools for Anopheles mosquitoes in Africa today.
Prokopack aspirator
The Prokopack aspirator (John W. Hock Company model 1412) [25] is a portable and user-friendly trap designed for sampling resting mosquitoes indoors and outdoors. It has a cylindrical shape housing a fan powered by 12-volt batteries. The fan pulls and sucks mosquitoes into the wire mesh surface, where they are collected until the fan is turned off and then packed into a collection bag or paper cup. This trap is designed for collecting both indoor and outdoor resting mosquitoes (Fig 3).
Study design
This cross-sectional entomological survey was conducted in three purposefully selected villages: Mzelezi, Chirombora, and Ebuyu, in Ulanga district, southeastern Tanzania (Fig 2). These villages are approximately 70km from Ifakara town, where the Ifakara Health Institute offices are located. We sampled mosquitoes in a total of 222 households that were systematically randomly selected in three villages, each with 74 houses (74 houses/villages x 3 villages = 222 houses). Among these 74 households per village, 36 houses were randomly assigned to DN-Mini traps for random mosquito collections as random households, and 1 house was assigned as a sentinel household; the other 36 houses were assigned to CDC-Traps for random mosquito collections (random households), and 1 house was assigned as a sentinel household. The mosquito catches from random households enable us to control for the spatial variations to compare the efficacy of CDC traps and DN-Mini traps in collecting host-seeking malaria vectors from inside houses (indoors), and those from sentinel households monitor the variations in catches and vector behavior with time and space (temporal and spatial variations) of mosquitoes collected through CDC traps, DN-Mini traps, and Prokopack aspirators from inside (indoors) and outside (outdoors) houses. Each of these households was 30 meters from one another to avoid competition between traps. Mosquito collections were conducted for three consecutive days, with a one-day interval between each collection day, resulting in three days of collections within a single week. A total of three households were covered each day of mosquito collection, including one sentinel and two random households. Each time of collection of host-seeking mosquitoes from random households, mosquitoes were collected from two houses per day and moved to the other two different houses until 36 houses were covered for DN-Mini traps and those for CDC-Traps (2 houses per day x 18 days = 36 houses for each trap design). Additionally, the collection of host-seeking mosquitoes from sentinel households using the DN-Mini Trap from inside (indoors) and outside (outdoor) houses was performed repeatedly from the same house once per week for 16 weeks (1 day per week x 16 weeks = 16 days/nights). The resting mosquitoes were also collected from sentinel households using Prokopack aspirators once per week for 16 weeks from inside and outside the house (1 day per week x 16 weeks = 16 days). The GPS coordinates (latitudes and longitudes) of random and sentinel households were collected using a Garmin eTrex 20GPS device.
Mosquito collection
During the high malaria transmission period (March to July) in 2022, adult mosquitoes were collected monthly in each village by eight trained volunteers (Fig 2).
Comparison of the DN-Mini trap against CDC light trap
Host-seeking mosquitoes were collected indoors for three days each week for different 74 houses, 37 households for the DN-Mini trap and CDC light traps (1 sentinel and 36 random households). The CDC light trap was placed in the sleeping room about 1.5 meters from the floor surface, close to the feet of household member sleeping under treated bed net [26]. The DN-Mini trap was positioned in the sitting room, and was occupied by an adult volunteer, who also retrieved mosquitoes periodically as previously described [3]. Both traps CDC-Light trap and DN-Mini trap were set at 18:00 hrs and mosquitoes retrieved at 6:00 hrs the following morning.
Mosquito composition between indoors and outdoors biting
Two pairs of human volunteers in a DN-Mini trap were used for the collection of indoor and outdoor mosquito compositions. One DN-Mini trap was placed indoors in the sitting room and another outdoors, five meters away from the house. In these surveys, the DN-Mini traps were used to collect mosquitoes every one-hour interval between 18:00 hrs. and 6:00 hrs. the following morning. Mosquitoes collection was done once a week for four months at the sentinel houses (1 sentinel house/village x 3 villages = 3 houses/day).
Composition of resting mosquitoes
Resting mosquitoes were collected indoors from ceilings and walls and outdoors around five meters from the sampled house from flowerpots, plants, walls, and general outdoor surfaces using the Prokopack aspirator. Resting mosquitoes were collected indoors and outdoors to assess the composition of mosquitoes and whether the species composition captured by host-seeking mosquitoes matches the composition resting indoors. The same person who collected mosquitoes from the DN-Mini traps was assigned to perform the Prokopack aspirators once a week, between 6:00 a.m. and 7:30 a.m., for the duration of four months at the sentinel houses to which the DN-Mini trap had been assigned.
Mosquito identification and parasite detection
All the collected mosquitoes were killed each morning using petroleum or alcohol fumes, then sorted by taxa and sex using the keys for morphological mosquito identification [27]. The abdominal status of each female Anopheles mosquitoes was recorded as blood fed, unfed, partially fed or semi-gravid or gravid. Female Anopheles mosquitoes were packaged individually or in pools in microcentrifuge (Eppendorf®) tubes filled with silica gel as a preservative. Each tube was provided with a unique identification number and packed in the storage boxes labeled with village name, house number, trap position, mosquito species name and date of collection; then submitted for further laboratory analysis. Additionally, a sub-sample of female malaria vectors, An. gambiae s.l were examined by multiplex polymerase chain reaction (PCR) for species identification to confirm and distinguish the sibling species [28]. A sub sample of An. funestus s.l. were also examined by PCR, using a technique adapted from Koekemoer at al [29] and Cohuet at al [30], to identify sibling species in An. funestus group. Furthermore, the head and thorax of Anopheles mosquitoes were separated from abdomen and tested for the presence of Plasmodium falciparum circum-sporozoite protein (Pf CSP) using the enzyme-linked immunosorbent assay (ELISA) method [29].
Data analysis
Data were analyzed using R open-source statistical software version 4.2.1 [31]. Descriptive statistics were used to summarize the data using frequencies, means and proportions. Generalized linear mixed models (GLMM) following negative binomial distribution was implemented using lme4 package [31], to assess the relationship between number of mosquitoes collected and different trap types. The number of mosquitoes collected were modeled as a response variable while mosquito traps and villages were added as fixed factors. To take into account of the variations in the mosquito catches, the different days, household ID and collection date were included as random factors. Each mosquito species was analyzed separately, in this model, our focus was solely on An. arabiensis, An. funestus and Culex mosquitoes. These particular species were selected due to their substantial collection numbers, while vector species with fewer numbers or catches were excluded from the analysis. The relative rates of mosquito catches were obtained with their associated 95% confidence intervals and considered significant when p-values were less than or equal to the 5% significance level. The models were also used to generate the estimated marginal means (EMM) for catches in each trap using ggeffects package [32]. Graphs were plotted using ggplot2 [33] package.
Ethical clearance and informed consent
The research was conducted following the principles of the Declaration of Helsinki. Ethical approval was obtained from the Muhimbili University of Health and Allied Sciences Review Board (MUHAS-REC-12-2021-910), and permission to publish the work was granted by NIMR (Ref: NIMR/HQ/P.12VOL.XXXVI/27). Approval to conduct the study was obtained from the district medical officer of Ulanga district and the local government leadership in the selected villages. Before commencing the study, meetings were held with local government leaders to explain the study’s aim and procedures. Verbal and written informed consent was obtained from individual house occupants and human volunteers involved in mosquito collection. Participants were fully informed of potential benefits and risks, and their voluntary participation was assured. Participants were also informed of their right to withdraw from the study at any time without consequences. Confidentiality was maintained to ensure the anonymity of participants.
Results
Mosquito species composition and abundance
Overall, a total of 19,841 female mosquitoes were collected indoors and outdoors between March and July 2022 using CDC-light traps, DN-Mini traps, and Prokopack aspirators. About equal numbers of mosquitoes were collected between CDC light traps (45.4%, n = 9,008) and DN-Mini traps (44.0%, n = 8732), and 10.6% (n = 2101) were collected through prokopack aspirators. The majority of mosquitoes collected through the DN-Mini trap were collected indoors (89.9%, n = 7851), and 10.1% (n = 881) were collected outdoors (Table 1). An. funestus accounted for 6.1% (n = 1219), An. gambiae ss accounted for 1.6% (n = 313), and other anopheles species accounted for 0.1% (n = 10) (Table 1). A majority of the mosquitoes collected were of the culicine group, of which Culex species accounted for 92.1% (n = 18268), Mansonia species accounted for 0.1% (n = 29), and Aedes species accounted for 0.0% (n = 2) of all mosquitoes.
Table 1. Total number of mosquitoes species collected by the different traps.
| Trap types | Vector species | Number collected (%) |
|---|---|---|
| CDC-Light trap indoors | An. funestus s.l. | 399 (4.4) |
| An. gambiae s.l. | 202 (2.2) | |
| An. coustani spp. | 4 (0.0) | |
| Culex spp. | 8396 (93.2) | |
| Mansonia spp. | 7 (0.1) | |
| Aedes spp. | 0 (0.0) | |
| DN-Mini trap indoors | An. funestus s.l. | 704 (9.0) |
| An. gambiae s.l. | 67 (0.9) | |
| An.coustani spp. | 6 (0.1) | |
| Culex spp. | 7055 (89.8) | |
| Mansonia spp. | 17 (0.2) | |
| Aedes spp. | 2 (0.0) | |
| DN-Mini trap outdoors | An. funestus s.l. | 56 (6.4) |
| An. gambiae s.l. | 9 (1.0) | |
| An.coustani spp. | 0 (0.0) | |
| Culex spp. | 812 (92.1) | |
| Mansonia spp. | 4 (0.5) | |
| Aedes spp. | 0 (0.0) | |
| Prokopack aspirator indoor | An. funestus s.l. | 54 (3.1) |
| An. gambiae s.l. | 26 (1.5) | |
| An.coustani spp. | 0 (0.0) | |
| Culex spp. | 1656 (95.3) | |
| Mansonia spp. | 1 (0.1) | |
| Aedes spp. | 0 (0.0) | |
| Prokopack aspirator outdoor | An. funestus s.l. | 6 (1.6) |
| An. gambiae s.l. | 9 (2.5) | |
| An.coustani spp. | 0 (0.0) | |
| Culex spp. | 349 (95.9) | |
| Mansonia spp. | 0 (0.0) | |
| Aedes spp. | 0 (0.0) |
Mosquito species composition
A total of 487 female malaria vectors were submitted to the molecular laboratory for species identification, of which 419 were An. funestus s.l. and 68 were An. gambiae s.l. The overall amplification rate for the An. funestus group was 87.8% (368/419), of which 99.7% (367/368) were confirmed as An. funestus and 0.3% (1/368) were confirmed as Anopheles rivulorum. On the other hand, the overall amplification rate for the sub-sample of An. gambiae complex was 94.1% (64/68), of which 93.7% (60/64) were confirmed as Anopheles arabiensis and 6.6% (4/64) were confirmed as Anopheles gambiae s.s.
Detection of sporozoite rate infection in malaria vectors
Out of 487 Anopheles mosquitoes submitted to the molecular laboratory for ELISA, 14 Anopheles were confirmed to be infected with Plasmodium falciparum sporozoites. Of these infected mosquitoes, 92.8% (n = 13) were An. funestus s.s., and only one mosquito, 7.2% (n = 1), was An. arabiensis.
Performance of CDC- light trap and DN-Mini trap for indoor mosquito collection
Comparing the performance of the two traps indoors, the DN-Mini trap outperformed the CDC light trap in capturing An. funestus (RR: 1.536, p = 0.001 Table 2). On the other hand, CDC light traps outperformed DN-Mini traps in capturing An. gambiae (RR: 0.305, p<0.001 Table 2) and Culex species (RR: 0.710, p<0.001) (Table 2).
Table 2. Comparison of performance of the indoor host-seeking traps.
| Species | Traps type | Number collected (%) | RR (95% CI) | P-value |
|---|---|---|---|---|
| An. arabiensis | CDC-Light | 202 (75.1) | 1 | |
| DN-Mini | 67 (24.9) | 0.305 (0.178–0.522) | <0.001 | |
| An. funestus | CDC-Light | 399 (36.2) | 1 | |
| DN-Mini | 704 (63.8) | 1.536 (1.204–1.958) | 0.001 | |
| Culex species | CDC-Light | 8396 (54.3) | 1 | |
| DN-Mini | 7055 (45.7) | 0.710 (0.610–0.828) | <0.001 |
Composition of indoor and outdoor mosquitoes by using DN-Mini trap
Overall total of mosquito composition: 78.5% (n = 204) were An. funestus collected indoors while 21.5% (n = 56) were collected outdoors; 66.6% (n = 18) were An. gambiae collected indoors while 33.3% (n = 9) were collected outdoor; other anopheline 100% (n = 2) were collected indoors; for the non-malaria vector, about 74.1% (n = 2320) were Culex collected indoors while 25.9% (n = 812) were collected outdoors; Mansonia were 73.3% (n = 11) were collected indoors, while 26.7% (n = 4) were collected outdoor; and 100% (n = 2) ware Aedes mosquitoes.
Resting mosquito composition by using prokopack aspirator
Generally, there was no significant difference between An. funestus mosquitoes collected indoors and outdoors (RR: 0.351, p = 0.210), but more outdoor collections were made for An. arabiensis (RR: 1.093, p = 0.936) and Culex species (RR: 0.665, p = 0.194) (Table 3). Comparing resting mosquitoes collected in houses with CDC-light traps and those with DN-Mini traps, for indoor collections, there was no significant difference between all major mosquitoes collected indoors by prokopack aspirators in the house with CDC-light trap and DN-Mini rap (RR: 1.434, p = 0.597), An. funestus, An. gambiae (RR: 1.434, p = 0.545), and Culex species (RR: 0.677, p = 0.143) (Table 4).
Table 3. Resting composition of indoor and outdoor mosquitoes by using prokopack aspirators (PP).
| Species | Traps position | RR | (95% CI) | P-value |
|---|---|---|---|---|
| An. gambiae | Indoor | 1 | ||
| Outdoor | 1.093 | 0.124–9.624 | 0.936 | |
| An. funestus | Indoor | 1 | ||
| Outdoor | 0.351 | 0.068–1.806 | 0.210 | |
| Culex species | Indoor | 1 | ||
| Outdoor | 0.665 | 0.359–1.231 | 0.194 |
Table 4. Resting composition of indoor mosquitoes collected by prokopack aspirator (PP) in the house of CDC-Light trap and DN-Mini trap.
| Vector Species | Trap types | RR | (95% CI) | P-value |
|---|---|---|---|---|
| An. gambiae | PP-CDC-Light | 1 | ||
| PP-DN-Mini | 0.565 | 0.089–3.599 | 0.545 | |
| An. funestus | PP-CDC-Light | 1 | ||
| PP-DN-Mini | 1.434 | 0.377–5.448 | 0.597 | |
| Culex species | PP-CDC-Light | 1 | ||
| PP-DN-Mini | 0.677 | 0.401–1.142 | 0.143 |
Comparison of feeding status between mosquitoes collected by CDC light trap and DNM trap
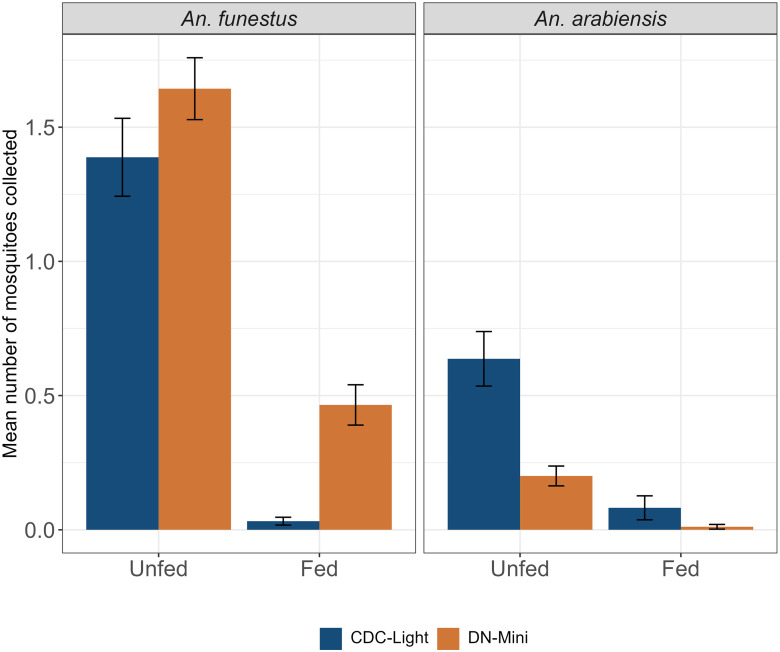
A total of 157 An. funestus were identified as blood-fed. Of these, 5.7% (n = 9) were collected through the CDC light traps, and 94.3% (n = 148) were collected through the DN-Mini trap. Of the 27 blood-fed An. gambiae sl, 85.2% (n = 23) were collected by a CDC-light trap, and 14.8% (n = 4) were collected by a DN-Mini trap (Fig 4).
Fig 4. Feeding status of dominant malaria vectors caught by the CDC-light trap and the DN-Mini trap.
Discussion
In our study, the two candidate traps for the collection of host-seeking African malaria vectors, including the DN-Mini and CDC-light traps, were compared in indoor settings. And the DN Mini was also used to compare indoor and outdoor catches. The results show that the DN-Mini trap collects about twice as many An. funestus compared to the CDC-light trap indoors. This indicates that the DN-mini trap, which is baited by a sitting volunteer, can be especially efficient for sampling An. funestus mosquitoes, though it also catches other vector species. In this area, where An. funestus now dominates malaria transmission, it appears that the DN-Mini trap can be especially useful for sampling malaria vectors. On the other hand, the CDC light traps, which depend mostly on odor from people sleeping under the bed net to attract host-seeking mosquitoes, were superior in capturing An. arabiensis mosquitoes, which also contribute to malaria transmission in the region and can be especially important for outdoor transmission [14, 34]. The CDC-light trap has previously been reported to attract nearly as many Anopheles mosquitoes as the HLC and, in some cases, even equivalent numbers [12, 13]. The only limitations of this trap is that while it is effective for indoor collections, it is not always sufficient for outdoor collections [2]. Other studies suggest the use of CDC-light outdoor to be embedded into the other trap as an additional trap (Limwagu et al., unpublished).
The current study found that An. funestus is not only the most efficient malaria vector than An. arabiensis, but it is also now becoming the most abundant vector species of Anopheline mosquitoes in this study area. For example, the number of host-seeking An. funestus s.s. was 2–10 times higher than that of host-seeking An. arabiensis in indoors, but it was 6 times higher than that of host seeking An. arabiensis outdoors (Tables 1 and 2). These findings are consistent with previous studies in the Kilombero valley, which have reported Anopheles funestus to be responsible for over 85% of malaria transmission [35]. Our analysis of Plasmodium sporozoite infections also suggests that nearly all the infected mosquitoes were An. funestus and only one was An. arabiensis, thus suggesting that An. funestus dominates in both densities and malaria infection rates.
Furthermore, An. funestus could be more efficient at transmitting malaria and may be more resistant to primary control measures such as long-lasting insecticide-treated nets (LLINs) and indoor residual spray (IRS) in the study area. These interventions primarily target indoor mosquitoes, but An. funestus, which normally bite and rest indoors, are not responsive to them. Additionally, the study also found that there were higher numbers of An. funestus and An. arabiensis mosquitoes indoors compared to outdoors (Table 2). This is inconsistent with the findings of a study by Limwagu et al. [3] in the evaluation of DN-Mini traps, where An. funestus were dominant indoors while An. arabiensis were dominant outdoors. Moreover, using Prokopack aspirators, the density of An. funestus was higher indoors compared to outdoors, while for An. arabiensis, the majority was found outdoors compared to indoors.
The study also found that the DN-Mini trap is effective for catching mosquitoes that have recently had a blood meal, as it caught more fed mosquitoes than the CDC-light trap (Fig 4). This indicates that the mosquitoes caught by the DN-Mini trap are likely coming from a different host that had obtained a partially blood meal and are being caught by the trap while attempting to obtain a second blood meal. This could signify that the DN-Mini trap can sample host-seeking mosquitoes in the process of seeking a first or second blood meal. Hence, the HLC is a usual tool for understanding the transmission dynamic as well as host preference of mosquitoes, and such details cannot be observed from the HLC since mosquitoes collected might also feed on the HLC collector. Multiple blood feeding (taking more than one blood meal per gonotrophic cycle) was found to be the most important information when calculating human biting rate [36], such data that can easily be obtained from the DN-Mini trap. Though unlikely, the blood-fed mosquitoes may have been from the trap handlers or volunteers inside the traps. This might suggest that while the DN-Mini is designed to be exposure-free, the volunteers sitting inside the trap and retrieving the mosquitoes periodically still need to be well trained to avoid mosquito bites.
Overall, the findings taken together suggest that the DN-Mini trap can be effective for sampling malaria vectors inside houses and might, in certain cases, be even more efficient than the CDC light traps. The study also suggests that the DN Mini can be efficient for comparing indoor-outdoor biting patterns for the malaria vector species. This study therefore proves that it can be widely used for surveillance of malaria vectors across the country.
In regard to the composition of mosquitoes collected indoors and outdoors by DN-Mini trap, it was revealed that, among the anopheline mosquitoes, both An. funestus and An. arabiensis were found to be dominant, while among the culicine group, Culex mosquitoes were the most abundant. These results, however, appear to be inconsistent with the findings of a previous study conducted by Limwagu et al. 2019 [3]. In their study, they reported a different distribution pattern of An. funestus and An. arabiensis between indoor and outdoor collections. Limwagu et al. found that An. funestus was more prevalent indoors than outdoors. Additionally, our study revealed that Culex species were more dominant indoors compared to outdoors.
In resting mosquito composition by Prokopack aspirator, we found that the majority of anopheline mosquitoes were An. funestus and An. arabiensis, while Culex species were more abundant in the culicine group. In regards to indoor and outdoor locations, there were more An. funestus indoors, while An. arabiensis were more outdoors. Contrary to the results reported by Kreppel et al. 2020 [35], our study revealed that An. funestus found resting outdoors, while An. arabiensis exhibited a preference for outdoor resting sites.
The study conducted by Mmbando et al. 2020 [36] suggests that when employing the resting bucket (RBu), similar results are likely to be obtained, with the majority of outdoor mosquitoes being culicine and a proportion belonging to anopheline mosquitoes. It’s worth noting that the resting bucket is predominantly favored for outdoor use.
This study has certain limitations. First, the performance of the DN-Mini trap is influenced by environmental factors such as wind speed and rainfall. These factors might influence mosquito behavior and compromise trap performance, particularly when the traps are deployed outdoors. Additionally, there is a limitation related to community acceptance. When using the DN-Mini trap for indoor collection, we normally position it in living rooms. However, in some households, the spaces also serve as storage areas for harvested food items, such as bags of rice.
Conclusion
In conclusion, the study demonstrates that the DN-Mini trap exhibits higher efficacy, being 53% more efficient in collecting An. funestus species indoors compared to the CDC-Light trap. However, for An. arabiensis, the DN-Mini trap’s efficiency is reduced, being 68% less effective indoors than the CDC-Light trap. The results also indicate that both An. funestus and An. arabiensis species are more abundant indoors than outdoors when collected using the DN-Mini trap. These findings highlight the importance of selecting appropriate trapping methods based on mosquito species and their behavioral patterns. The findings also emphasize the potential of the DN-Mini trap for malaria vector surveillance and compare indoor and outdoor biting profiles of different vector species. The DN-Mini can be particularly useful in areas such as the southeastern Tanzanian villages where An. funestus dominates transmission.
Acknowledgments
We express our sincere gratitude to the village leaders and communities of Ulanga district for their cooperation and permission to conduct this study. We are thankful to all the volunteers involved in the data collection. Special thanks to our project administrator, Rukiya Mohamed, for assisting with the logistics, Dr. Marceline Finda and Dr. Halfan Ngowo for reviewing the manuscript, and Najat Kahamba and Mohamed Jumanne for their valuable contributions during fieldwork. We also acknowledge the efforts of Said Abbasi and Francis Tumbo in processing laboratory samples.
Data Availability
Relevant data are within the paper.
Funding Statement
his study was funded by the Bill and Melinda Gates Foundation, awarded through the Pan-African Mosquito Control Association (PAMCA) (Grant No. OPP 1214408, Ifakara Health Institute).
References
- 1.WHO. Manual on practical entomology in malaria. Part II. Methods and techniques. Man. Pract. Entomol. malaria. Part II. Methods Tech. 1975. p. 6+191 pp.
- 2.Tangena JAA, Thammavong P, Hiscox A, Lindsay SW, Brey PT. The human-baited double net trap: An alternative to human landing catches for collecting outdoor biting mosquitoes in Lao PDR. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0138735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limwagu AJ, Kaindoa EW, Ngowo HS, Hape E, Finda M, Mkandawile G, et al. Using a miniaturized double-net trap (DN-Mini) to assess relationships between indoor–outdoor biting preferences and physiological ages of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Malar J. 2019;18:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF, et al. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degefa T, Yewhalaw D, Zhou G, Atieli H, Githeko AK. Evaluation of human—baited double net trap and human—odour—baited CDC light trap for outdoor host—seeking malaria vector surveillance in Kenya and Ethiopia. Malar J. 2020;1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mboera LEG. Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rate. Tanzan Health Res Bull. 2005;7:117–24. doi: 10.4314/thrb.v7i3.14248 [DOI] [PubMed] [Google Scholar]
- 7.Wotodjo AN, Trape J-F, Richard V, Doucouré S, Diagne N, Tall A, et al. No Difference in the Incidence of Malaria in Human-Landing Mosquito Catch Collectors and Non-Collectors in a Senegalese Village with Endemic Malaria. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, et al. Incidence of Malaria among Mosquito Collectors Conducting Human Landing Catches in Western Kenya. 2013;88:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maliti D V, Govella NJ, Killeen GF, Mirzai N, Johnson PCD, Kreppel K, et al. Development and evaluation of mosquito-electrocuting traps as alternatives to the human landing catch technique for sampling host-seeking malaria vectors. Malar J. 2015;14:502. doi: 10.1186/s12936-015-1025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govella NJ, Chaki PP, Geissbuhler Y, Kannady K, Okumu F, Charlwood JD, et al. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J. 2009;8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS One. 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namango IH, Marshall C, Saddler A, Ross A, Kaftan D, Tenywa F, et al. The Centres for Disease Control light trap (CDC—LT) and the human decoy trap (HDT) compared to the human landing catch (HLC) for measuring Anopheles biting in rural Tanzania. Malar J. 2022;1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finda MF, Limwagu AJ, Ngowo HS, Matowo NS, Swai JK, Kaindoa E, et al. Dramatic decreases of malaria transmission intensities in Ifakara, south-eastern Tanzania since early 2000s. Malar J. 2018;17:362. doi: 10.1186/s12936-018-2511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okumu FO, Kotas ME, Kihonda J, Mathenge E, Killeen GF, Moore SJ. Comparative Evaluation of Methods Used for Sampling Malaria Vectors in the Kilombero Valley, South Eastern Tanzania. Open Trop Med J. 2008;1:51–5. [Google Scholar]
- 15.Fornadel CM, Norris LC, Norris DE. Centers for Disease Control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am J Trop Med Hyg. 2010;83:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis JR, HALL T, Chee EM, Majala A, Minjas J, Shiff CJ. Comparison of sampling anopheline mosquitoes by light-trap and human-bait collections indoors at Bagamoyo, Tanzania. Med Vete Entomolo. 1995;9:249–55. doi: 10.1111/j.1365-2915.1995.tb00130.x [DOI] [PubMed] [Google Scholar]
- 17.Polovodova VP.Age Changes in Ovaries of Anopheles and Methods of determination of age composition in mosquito populations.Med Parazit (Mosk), 1941. [Google Scholar]
- 18.Mmbando AS, Ngowo H, Limwagu A, Kilalangongono M, Kifungo K, Okumu FO. Eave ribbons treated with the spatial repellent, transfluthrin, can effectively protect against indoor-biting and outdoor-biting malaria mosquitoes. Malar J. 2018;17:368. doi: 10.1186/s12936-018-2520-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS One. 2019;14:e0217414. doi: 10.1371/journal.pone.0217414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Q, Wang F, Lv X, Cao H, Zhou J, Su F, et al. Comparison of the human-baited double net trap with the human landing catch for Aedes albopictus monitoring in Shanghai, China. Parasit Vectors. 2018;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mapua SA, Hape EE, Kihonda J, Bwanary H, Kifungo K, Kilalangongono M, et al. Persistently high proportions of plasmodium-infected Anopheles funestus mosquitoes in two villages in the Kilombero valley, South-Eastern Tanzania. Parasite Epidemiol Control. 2022;18:e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musiba RM, Tarimo BB, Monroe A, Msaky D, Ngowo H, Mihayo K, et al. Outdoor biting and pyrethroid resistance as potential drivers of persistent malaria transmission in Zanzibar. Malar J. 2022;21:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiscox A, Otieno B, Kibet A, Mweresa CK, Omusula P, Geier M, et al. Development and optimization of the Suna trap as a tool for mosquito monitoring and control. Malar J. 2014;13:257. doi: 10.1186/1475-2875-13-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurent BS, Zio M, Sumardi S, Sudibyo H, Suwito S, Fitri S, et al. Rapid comparative evaluation of anopheline sampling methods in three localities in Indonesia. Am J Trop Med Hyg. 2012;Conference:368–9. [Google Scholar]
- 25.Rico P. Improved Prokopack Aspirator—Model 1419. Expert Insect Sampl. 2012;P2. [Google Scholar]
- 26.Mboera LE, Knols BG, Kihonda J, Braks MA. Short report: Influence of centers for disease control light trap position, relative to a human-baited bed net, on catches of Anopheles gambiae and Culex quinquefasciatus in Tanzania. Am J Trop Med Hyg. 1998;59:595–6. [DOI] [PubMed] [Google Scholar]
- 27.Gillies MT, Coetzee M. A Supplement to the Anophelinae of the South of the Sahara (Afrotropical Region). Publ. South African Inst. Med. Res. 1987. p. 1–143. [Google Scholar]
- 28.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9. [DOI] [PubMed] [Google Scholar]
- 29.Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–11. [DOI] [PubMed] [Google Scholar]
- 30.Cohuet A, Simard F, Toto J, Kengne P, Coetzee M. Species Identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for new a new species. 2003;69:200–5. [PubMed] [Google Scholar]
- 31.R Development Core Team R. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2011.
- 32.Daniel L. Package ‘ ggeffects.’ 2018;1–21.
- 33.Ginestet C. ggplot2: Elegant Graphics for Data Analysis. J R Stat Soc Ser A Stat Soc. 2011; [Google Scholar]
- 34.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south–eastern Tanzania. Brooke B, editor. PLoS One. 2017;12:e0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreppel KS, Viana M, Main BJ, Johnson PCD, Govella NJ, Lee Y, et al. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci Rep. 2020;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mmbando AS, Kaindoa EW, Ngowo HS, Swai JK, Matowo NS, Kilalangongono M, et al. Fine-scale distribution of malaria mosquitoes biting or resting outside human dwellings in three low-altitude Tanzanian villages. PLoS One. 2021;16:1–19. doi: 10.1371/journal.pone.0245750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data are within the paper.