Abstract
Maternal experience can promote a long-lasting increase in maternal motivation. This maintenance of caregiving behaviors, rather than avoidant or agnostic responses towards young is advantageous for the survival of subsequent offspring. We have previously reported that maternal motivation is associated with differential immediate early gene expression in central motivation circuits and aversion circuits. Here we ask how these circuits come to differentially respond to infant cues. We used Targeted Recombination in Active Populations (TRAP) to identify cells that respond to pups in maternally hesitant TRAP2;Ai14 virgin female mice. Following an initial 60 min exposure to foster pups, virgin TRAP2;Ai14 mice were injected with 4-hydroxytamoxifen to induce recombination in c-Fos expressing cells and subsequent permanent expression of a red fluorescent reporter. We then examined whether the same cells that encode pup cues are reactivated during maternal memory retrieval two weeks later using cFos immunohistochemistry. Whereas initial pup exposure induced cFos activation exclusively in the medial preoptic area (MPOA), following repeated experience, cFos expression was significantly higher than baseline in multiple regions of maternal and central aversion circuits (e.g., ventral bed nucleus of the stria terminalis, nucleus accumbens, basolateral amygdala, prefrontal cortex, medial amygdala, and ventromedial nucleus of the hypothalamus). Further, cells in many of these sites were significantly reactivated during maternal memory retrieval. These data suggest that cells across both maternal motivation and central aversion circuits are stably responsive to pups and thus may form the cellular representation of maternal memory.
Keywords: maternal experience, Targeted Recombination in Active Populations, FosTRAP2, engram, maternal memory
Introduction
At birth, female mammals are attracted to infant cues and motivated to protect and care for their young. This maternal motivation represents a significant divergence from avoidant or agnostic responses toward young, which tend to occur by default or under stressful conditions depending on the species. Importantly, maternal motivation can persist long-term to prevent infant avoidance and promote survival of subsequent offspring. Decades of research in laboratory rats and mice support the idea that maternal experience produces long-term changes in how pups are perceived and responded to in the future, which is referred to as maternal memory (Bridges, 1978, 1975; Cohen and Bridges, 1981; Ehret and Buckenmaier, 1994; Orpen and Fleming, 1987; Stolzenberg et al., 2014).
In laboratory rats and mice, maternal memory functions to prevent avoidant or agnostic responses to pups and promote caregiving behavior. For example, female rats with as little as 2 hours of postpartum pup experience care for foster pups after a 10-day separation from their infants post-birth, whereas inexperienced dams avoid pups as a result of separation from their litters (Fleming and Sarker, 1990). Maternal memory drives maternal motivation in laboratory mice as well. Given that many laboratory strains of female mice are spontaneously attracted to pups, experience-induced changes in motivation can be investigated in virgins in the absence of hormone exposure. For example, virgin C57BL/6J female mice with as little as 2 hours of pup experience on 4 consecutive days are motivated to care for pups in a challenging environment, whereas inexperienced females avoid or ignore pups (Mayer et al., 2019; Stolzenberg et al., 2014, 2012; Stolzenberg and Rissman, 2011). Thus, similar to other learning experiences, maternal experiences are believed to be consolidated into a maternal memory that allows for care to be sustained well past the initial encoding event.
Our laboratory has investigated maternal memory in virgin C57BL/6J mice. Based on evidence that maternal memory functions to prevent infant avoidance and sustain infant care, we hypothesize that maternal experiences alter the way central avoidance and motivation circuits respond to infant stimuli. This hypothesis is based on seminal experiments conducted in female rats that used cFos immunohistochemistry as an indirect marker of neuronal activity to delineate neural circuits that respond to pup cues in both maternal and non-maternal rats. Subsequent work established causal evidence for two competing neural circuits that regulate motivated and avoidant behavioral responses toward infants. The activity of these two competing circuits, and thus the behavioral response selected is likely coordinated by the medial preoptic area (MPOA) of the anterior hypothalamus through its projections to these systems (Stolzenberg and Mayer, 2019). The MPOA is considered the central neural site for processing pup-related cues and coordinating care for infants, as disruption of MPOA activity interferes with both the onset and maintenance of caregiving behaviors (Arrati et al., 2006; Jacobson et al., 1980; Kohl et al., 2018; Numan, 1974, p. 197; Numan et al., 1988, 1977; Pereira and Morrell, 2009). In mice, the central part of the MPOA (cMPOA) may not only be critical for regulating care, but may also actively prevent infanticide (Kohl et al., 2018; Tsuneoka et al., 2013).
Abundant evidence indicates that maternal motivation is regulated through MPOA activation of the mesolimbic dopamine system. MPOA neurons that project to the ventral tegmental area (VTA) function to disinhibit the release of dopamine in the nucleus accumbens (NA) (Fang et al., 2018). The NA has been identified as a critical regulator of maternal memory in rats (d’Cunha et al., 2011; Lee et al., 1999; Li and Fleming, 2003; Parada et al., 2008) and receives pup-related inputs from the basolateral amygdala (BLA) and prefrontal cortex (PFC) to initiate maternally motivated responses (Fleming and Korsmit, 1996; Mattson and Morrell, 2005; Numan et al., 2010; Vertes, 2004). Further, cells within these nodes of the central motivation system respond to infant cues with the expression of cFos in maternal rats and mice (Alsina-Llanes and Olazábal, 2020; Caba et al., 2019; Lonstein et al., 1997; Matsushita et al., 2015; Mayer et al., 2019). In contrast, infant avoidance is associated with cFos expression in several nodes of a central aversion system. For example, non-maternal rats display greater cFos expression the medial amygdala (meA), dorsal bed nucleus of the stria terminalis (dBNST), anterior hypothalamic nucleus (AHN) and ventromedial nucleus of the hypothalamus (VMN) (Alsina-Llanes and Olazábal, 2020; Sheehan et al., 2000). Similarly, C57BL/6J mice that avoid or ignore pups in a novel environment show greater expression of the cFos transcript in AHN/VMN (Mayer et al., 2019). Interference with this central aversion system prevents pup avoidance in female rats (Bridges et al., 1999; Sheehan et al., 2001). The MPOA likely coordinates suppression of avoidance behavior through its projections to the AHN/VMN, although causal evidence for this interaction is lacking (Stolzenberg and Mayer, 2019).
Based on evidence that maternal memory functions to prevent infant avoidance and sustain infant care, we hypothesized that experience alters how neural systems that regulate pup avoidance and maternal motivation respond to pup stimuli. Specifically, we predict that experience should prevent pup cues from activating nodes of the central aversion pathway. Instead, pup interaction should selectively strengthen the connections between pup-responsive cells in the MPOA and nodes within a central motivation system (VTA, NA, BLA and PFC). We hypothesize this neuronal ensemble forms the cellular representation of maternal memory or the maternal engram (Josselyn et al., 2015).
To date this hypothesis has been difficult to test for several reasons. First, experienced and inexperienced females differ not only in their previous exposure to pups, but also their behavioral response to them. Thus, the extent to which pup-induced cFos activation reflects the impact of experience (altered perception of pups) has primarily been investigated in the absence of direct pup interaction. For example, Fleming and Korsmit (1996) report that pup cues (olfactory, visual, auditory) induce cFos to a greater extent in the MPOA and BLA of experienced versus inexperienced rats (Fleming and Korsmit, 1996). Similarly, auditory stimuli that mimic pup calls induce greater cFos activation in the MPOA and adjoining ventral bed nucleus of the stria terminalis (vBNST) of experienced mice compared with inexperienced females (Geissler et al., 2013). However, it is unclear whether the processing of pup associated cues would be consistent with an experience-dependent cFos response to direct pup exposure. A second challenge in elucidating how maternal experience is encoded within maternal neural circuits is related to the limitations of the cFos immunostaining technique. Because the protein is labeled in post-mortem tissue, inexperienced mice are compared to a separate group of experienced mice. This comparison does not test the hypothesis that cells that initially encode maternal experiences are reactivated during maternal memory retrieval. This issue is important as we have hypothesized that experience-dependent transcriptional changes within pup-responsive cells are likely driving long-term changes in behavior and activity in the MPOA and central motivation system. The present study was designed using a novel model to test these important and previously unanswered questions.
In the present study we examined the impact of maternal experience on pup-induced cFos activation in motivation and aversion circuits across time using a within subject design. We took advantage of the fact that behavioral responses to pups only subtly vary between inexperienced and experienced C57BL/6J mice in a familiar home cage environment. For example, naïve virgin C57BL/6J females are spontaneously attracted to pups placed in their home cage. Whereas caregiving behaviors can be quite disorganized and variable during the initial experience with infants, the amount of time females spend in tactile contact with infants does not vary as a function of experience (Stolzenberg and Rissman, 2011). To determine whether cells that encode pup cues initially are reactivated during maternal memory retrieval, we used a mouse model in which cFos expression induces a permanent fluorescent reporter via Targeted Recombination in Active Populations (TRAP) (DeNardo and Luo, 2017). Colocalization analyses were performed to determine the overlap in cellular activity as female mice transition from pup naïve to maternally experienced.
Methods and Materials
Subjects
All mice used in this study were obtained from our breeding colony and all procedures were in compliance with the University of California, Davis Institutional Animal Care and Use Committee. TRAP2;Ai14 mice were generated by crossing homozygous FosTRAP2 (Fostm2.1(icre/ERT2)Luo/J; product number 030323) and Ai14 (Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; product number 007914) mice, which were originally obtained from Jackson Laboratories. Upon arrival, FosTRAP2 mice were backcrossed 5 times, whereas Ai14 mice were on a C57BL/6J background upon arrival. FosTRAP2 and Ai14 mice were crossed to produce TRAP2;Ai14 mice. Genotyping for the FosTRAP2 and Ai14 alleles was performed using Cre primers (Fwd: CCTTGCAAAAGTATTACATCACG, Rev: GAACCTTCGAGGGAAGACG) and Ai14 primers (Fwd: CTGTTCCTGTACGGCATGG, Rev: GGCATTAAAGCAGCGTATCC). All nulliparous female mice were on a C57BL/6J background, 60+ days of age, and naïve to pups at the start of the experiment. They were single housed for at least 48 hours prior to the start of the experiment and were handled one day prior to testing. A separate group of C57BL/6J mice served as foster dams that provided stimulus pups. Mice were housed on a 12-h reverse light cycle and given food and water ad libitum. Behavioral testing began 30 minutes into the dark phase of the light/dark cycle under dim red light.
4-hydroxytamoxifen preparation
4-OHT (Sigma-Aldrich H6278) was dissolved at 25mg/ml in warmed (37°C) ethanol by shaking on a vortex for 10 minutes (Chevalier et al., 2013). The dissolved 4-OHT was warmed to 60°C for 10 minutes and was diluted with warmed Kolliphor oil (Sigma-Aldrich C5135) to make a 12.5mg/ml stock solution. Aliquots of stock solution were either used immediately or stored at −20°C for several weeks until used. Just before use, the stock solution was rapidly thawed at 37°C and warm sterile phosphate buffer saline (PBS) was added to make a 10mg/ml concentration. Stock solutions of oil were made up following the same protocol, except without the addition of 4-OHT.
TRAP2;Ai14 model validation
TRAP2;Ai14 mice possess a 4- hydroxytamoxifen (4-OHT)-inducible Cre recombinase sequence fused to a mutated estrogen hormone binding domain (iCreERT2) that lies downstream from a cFos coding sequence and a floxed tdTomato reporter (Fig. 1A). The insertion of iCreERT2 downstream from the cFos coding sequence prevents disruption of endogenous cFos expression (DeNardo et al., 2019). cFos expression drives iCreERT2; however, in the absence of 4-OHT, iCreER is sequestered to the cytoplasm (Fig. 1B). In the presence of 4-OHT, iCreERT2 enters the nucleus and recombination excises a LoxP-flanked stop signal upstream from a tdTomato reporter. TRAP occurs within a 6-hour window around the time 4-OHT is present and can be identified by the presence of a stable tdTomato signal (DeNardo et al., 2019). Although TRAP2;Ai14 mice have been used to investigate neural circuit reactivation in response to homeostatic and social stimuli as well as during hippocampal-dependent memory consolidation (Allen et al., 2017; Erwin et al., 2020; Shang et al., 2019; Tasaka et al., 2018), to our knowledge we are the first to use TRAP2;Ai14 mice to examine reactivation of cells in the maternal neural circuits. Thus, we first determined whether the pup-responsive cells in the MPOA could be selectively TRAPed by 4-OHT administration. To account for non-specific TRAP induction, a separate group of TRAP2;Ai14 mice were exposed to a mock pup exposure, which consisted of the experimenter briefly placing their hand in the cage to mimic the presentation and removal of pups. Following 60 minutes of pup (Pup-TRAP, n=4) or mock (Mock-TRAP, n=4) exposure in the home cage, pups/no pups were removed from the cages of pup-naïve TRAP2;Ai14 virgin female mice and mice were immediately injected with 4-OHT. Additionally, to ensure that reporter expression was driven by 4-OHT, a second control group of TRAP2;Ai14 mice was injected with oil following 60 minutes of pup exposure (Oil, N=3). Further, because 4-OHT has the potential to antagonize or agonize endogenous estrogen receptors (Gallo and Kaufman, 1997; Shiau et al., 1998), we wanted to verify that 4-OHT injection would not interfere with experience-dependent changes in maternal care. Thus, TRAP2;Ai14 virgin female mice were tested for maternal responsiveness one week following 4-OHT (N=9) or oil (N=3) injection using our standard experience paradigm described below (Fig. 2).
Figure 1. TRAP2;Ai14 Model.
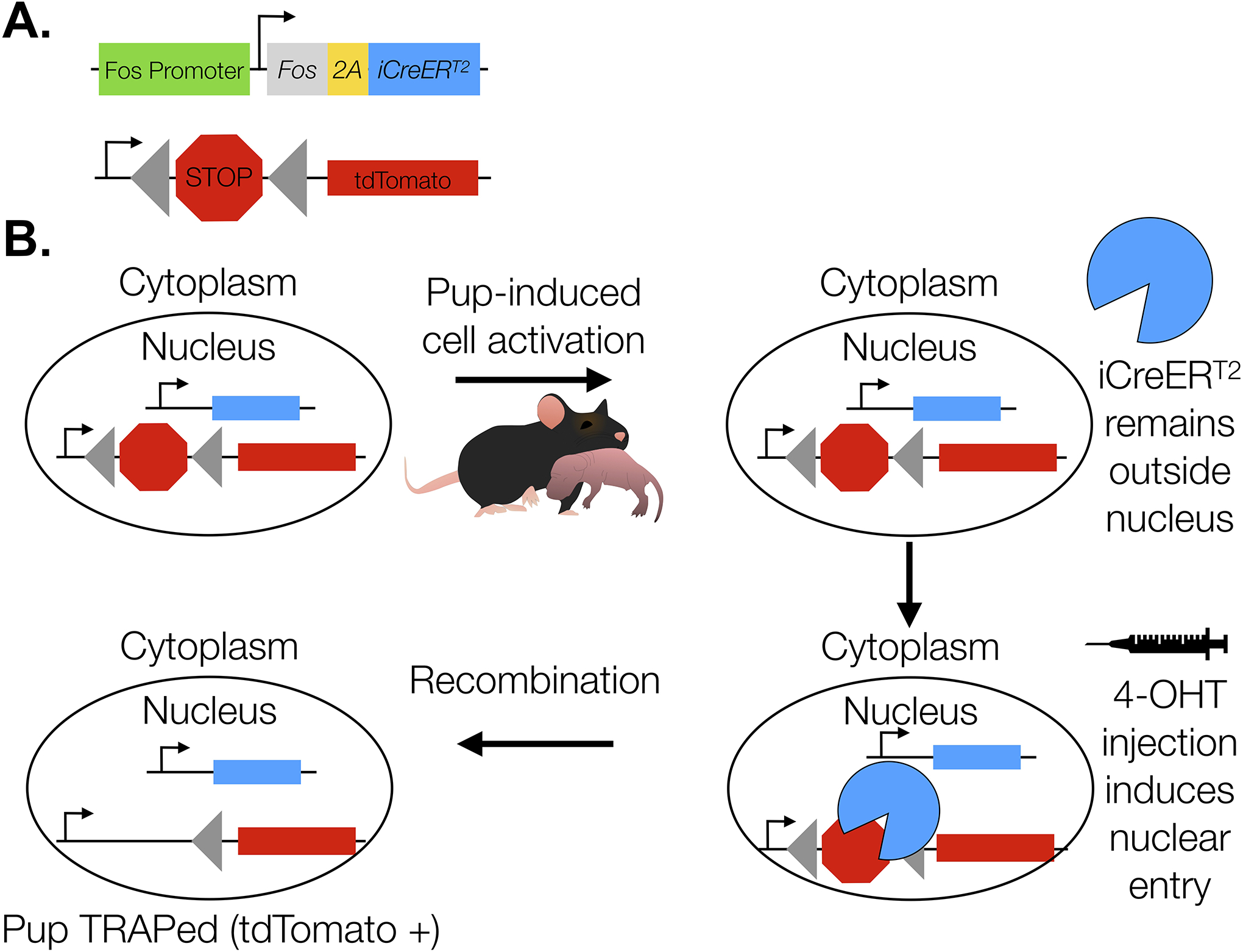
Construct design (A). Neurons that are activated by pups during the 4-OHT exposure period undergo Cre/loxP-mediated recombination as a result of iCreERT2 nuclear entry (B). Recombination induces stable tdTomato expression. In the absence of 4-OHT, iCreERT2 remains in the cytoplasm and recombination does not occur.
Figure 2. Experimental Timeline.
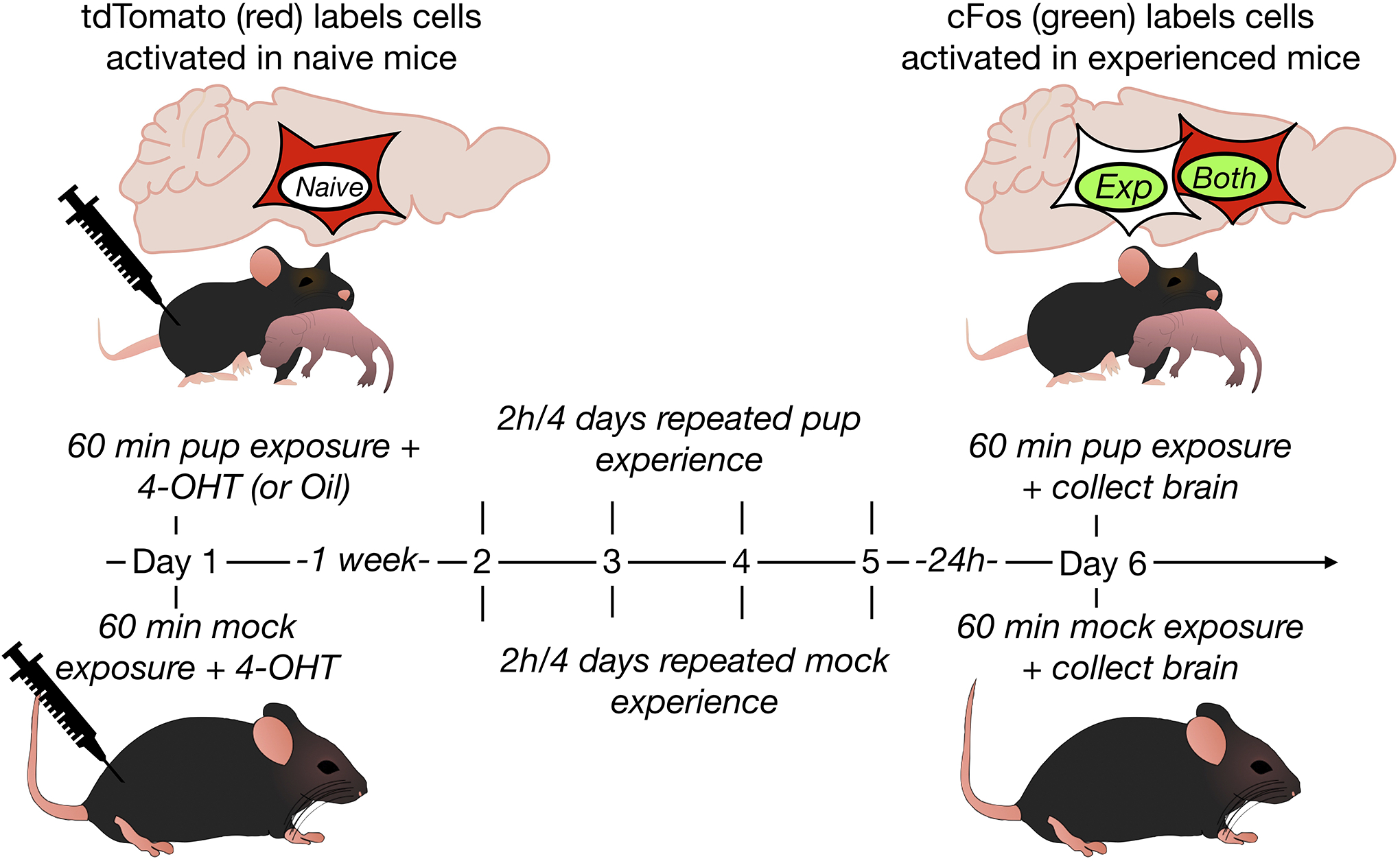
Cells activated during an initial pup exposure were TRAPed following 4-OHT injection. A direct cFos assay labeled cells activated after repeated experience. Oil injected virgin mice were included for the model validation experiment, otherwise virgins with mock exposure served as controls.
TRAP induction in naïve mice
To investigate the extent to which cells that are activated by spontaneous maternal care are reactivated in maternally experienced virgin mice, we injected naïve females with 4-OHT following a brief exposure to pups to TRAP cells initially activated in response to pups. Females were then euthanized following repeated experience (2hours/4days) with pups and a direct cFos assay was used to examine experience-dependent cell activation in response to pups. TRAP2;Ai14 mice were randomly assigned to either Pup-TRAP (N=9) or Mock-TRAP (N=6) groups. During the dark phase of the light/dark cycle, 3 stimulus pups (0–7 days old, mixed sex) were scattered in the home cage of naïve female mice assigned to the Pup-TRAP group. Latency to sniff, retrieve the first pup back to the nest, retrieve all 3 stimulus pups back to the nest, and lick and hover over pups in the nest were recorded during the first 15 minutes of the test. Throughout the 60-minute test, the frequencies of licking, crouching over pups in a nursing posture, and nestbuilding were recorded every 3 minutes. Pups were removed at the end of the 60-minute test period and returned to a lactating dam. Immediately upon pup removal, 4-OHT was injected intraperitoneally at a dose of 50mg/kg (Allen et al., 2017). At the end of the 60-minute control exposure, mice were immediately injected with 4-OHT.
Experience Paradigm
At least one week following TRAP induction (Allen et al., 2017), Pup-TRAP mice underwent a 4-day maternal experience paradigm. During the dark phase of the light/dark cycle, Pup-TRAP mice were presented with 3 stimulus pups (0–7 days old, mixed sex) for 2 hours/day. Maternal behavior observations were recorded (as described above) for the first 30 minutes of the first 2 days of repeated experience. Mock-TRAP mice were given mock exposure (as described above) for 2 hours/4 consecutive days.
cFos immunohistochemistry in experienced mice
Twenty-four hours after the last pup exposure, Pup-TRAP mice were exposed to 3 stimulus pups (0–7 days old, mixed sex) for 60 minutes. Maternal behavior was recorded for 60 minutes as described above. Mock-TRAP mice were undisturbed in their home cage with the exception of two brief disruptions by the experimenter’s hand to simulate the presentation and removal of pups. Immediately following pup/mock removal, all females were transcardially perfused with 4% paraformaldehyde and brains were collected. Brains were post-fixed in 4% paraformaldehyde for 18–24 hours and were soaked in 30% sucrose until the brain sank before being frozen in dry ice. Free floating 40μm coronal sections were prepared using a cryostat and were blocked for 1 hour in 10% normal goat serum in PBS, followed by an overnight incubation at room temperature in a solution containing PBS, 2% normal goat serum, and anti-cFos rabbit primary antibody (SySy 226 003, 1:750). After several washes in PBS, tissue was incubated in 0.5% triton, 2% normal goat serum, and Alexa Fluor 488 goat anti-rabbit (Fisher A11008, 1:500). Stained sections were rinsed in PBS followed by a wash in 0.1% triton, and were then mounted onto slides and coverslipped with DAPI (Vector H1200). Images were taken at 10X and 4X magnification using a Zeiss Axio Imager microscope. tdTomato and cFos positive cells were manually counted in ImageJ. DAPI was counted in ImageJ using an automated system. Anatomical borders of brain regions were determined based on the Paxinos and Franklin atlas. Two sections of the cMPOA (Bregma 0.13 to 0.01), three sections of the vBNST (Bregma 0.13 to −0.11), four sections of the VTA (Bregma −2.79 to −3.15), three sections of the NAc (Bregma 1.33 to 1.09), three sections of the NAs (Bregma 1.33 to 1.09), four sections of the BLA (Bregma −1.23 to −1.55), four sections of the PFC (Bregma 1.97 to 1.69), four sections of the MeA (Bregma −1.23 to −1.55), five sections of the dBNST (Bregma 0.25 to −0.23), four sections of the AHN (Bregma −0.71 to −1.07), and four sections of the VMNvl (Bregma −1.43 to −1.79) were quantified. For all cell quantification analyses, the percentage of DAPI-labeled cells containing tdTomato, cFos, or both was calculated for each image and averaged for each region.
Statistical Analyses
In validation experiments, the latencies to retrieve the first and last pup, as well as the percentage of observations nestbuilding, sniffing/licking, in a nursing posture (hover or crouch) or in physical contact with pups in Pup-TRAP and Oil-TRAP mice were analyzed with two-way mixed ANOVAs (group × day) with a repeated measure on the second factor. Significant main effects were followed up with Sidak post-hoc analyses to compare test day 1 with test day 6. The effects of 4-OHT treatment on TRAP induction were analyzed by a one-way between subjects ANOVA followed by a Sidak post-hoc test.
To investigate maternal experience-induced behavioral changes in TRAP2;Ai14 mice, the latencies to retrieve the first and last pup, as well as the percentage of observations nestbuilding, sniffing/licking, in a nursing posture (hover or crouch) or in physical contact with pups, were analyzed by one-way within subject ANOVAs, with test day as the repeated measure. Significant main effects were followed up with Sidak post-hoc analyses to compare test day 1 with test day 6.
All tdTomato and cFos positive cells were analyzed as a percentage of DAPI cells: (tdTomato cells / total number of DAPI cells) * 100% or (cFos cells / total number of DAPI cells) * 100%. Multiple independent samples t tests were used to analyze differences in the percentage of cells expressing tdTomato or cFos between mock and pup exposed mice for each brain region. The Benjamini, Krieger, and Yekutieli false discovery rate approach (FDR=0.1) was used to correct for these multiple comparisons (DeNardo et al., 2019).
Reactivation was analyzed as colocalization of tdTomato and cFos labels above chance levels of colocalization in pup and mock exposed mice. Chance colocalization was calculated as: (total number of tdTomato cells / DAPI) * (total number of cFos cells / DAPI) * 100% and subtracted from the quantified colocalization for each brain region (Reijmers et al., 2007; Tayler et al., 2013). Multiple independent samples t tests were used to compare reactivation in pup or mock exposed mice per brain region with the Benjamini, Krieger, and Yekutieli false discovery rate approach (FDR=0.1) correction (DeNardo et al., 2019). We were also interested in whether the level of reactivation (fold change to mock) differed across brain regions that showed significant reactivation to pup versus mock exposure. Although the regions we examined differed dramatically in total cell number, because we normalized all labels to DAPI expression in each region, we were able to make this comparison. A one-way within subject ANOVA was used to compare brain regions in which significant reactivation occurred, followed by a Sidak post-hoc test to compare MPOA reactivation with other reactivated regions. All statistical tests were two tailed. All data were analyzed using GraphPad Prism 9 software (GraphPad, Inc., La Jolla, CA). Effect sizes (eta squared and Cohen’s d) were calculated using Excel. Finally, we used multiple regression analyses to determine whether cellular activation or reactivation were predictive of experience-dependent changes in maternal care. For all analyses, differences were considered statistically significant at p< 0.05.
Results
Validation of the TRAP2;Ai14 model
The present series of experiments represent the first investigation of maternal experience-dependent alterations in maternal neural circuits. Thus, we began by verifying that 4-OHT injection would not interfere with experience-dependent changes in maternal care. We found no effect of 4-OHT treatment on any maternal response measured. All mice, regardless of group or test day, retrieved pups presented (data not shown). A two-way mixed ANOVA (group × day) with a repeated measure on the second factor revealed a significant main effect of day on latency to initiate pup retrieval [F(3,30)=10.63, p<0.0001, η2=0.372] and complete pup retrieval [F(3,30)=13.55, p<0.0001, η2=0.298]. Post hoc analyses showed that all mice, regardless of 4-OHT exposure, were significantly faster to initiate pup retrieval (p<0.0001, d=3.87) and complete pup retrieval (p<0.0001, d=14.27) on the last compared to the first day of testing (Fig. 3A–B). We also detected a significant main effect of day on the percentage of time mice spent in nursing postures over the pups [F(3,30)=4.622, p=0.009, η2=0.176] (Fig. 3C). However, a post-hoc comparison of first and last test days did not reach statistical significance (p=0.112, d= −5.31). Similarly, there was a significant main effect of day on the percentage of time mice spent nestbuilding [F(3,30)=9.0, p=0.0002, η2=0.352, Fig. 3D) and the percentage of time spent sniffing/licking [F(3,30)=6.338, p=0.0019, η2=0.263, Fig. 3E], however post-hoc comparison of the first and last days did not reach statistical significance (p=0.8645, d=1.19; p=0.468, d=3.62, respectively). There were also no significant differences in total percent of observations spent in tactile contact with pups across test days (p=0.2243, η2=0.076) or treatment group (p=0.9158, η2=0.000) (Fig. 3F).
Figure 3. TRAP2;Ai14 Model Validation.
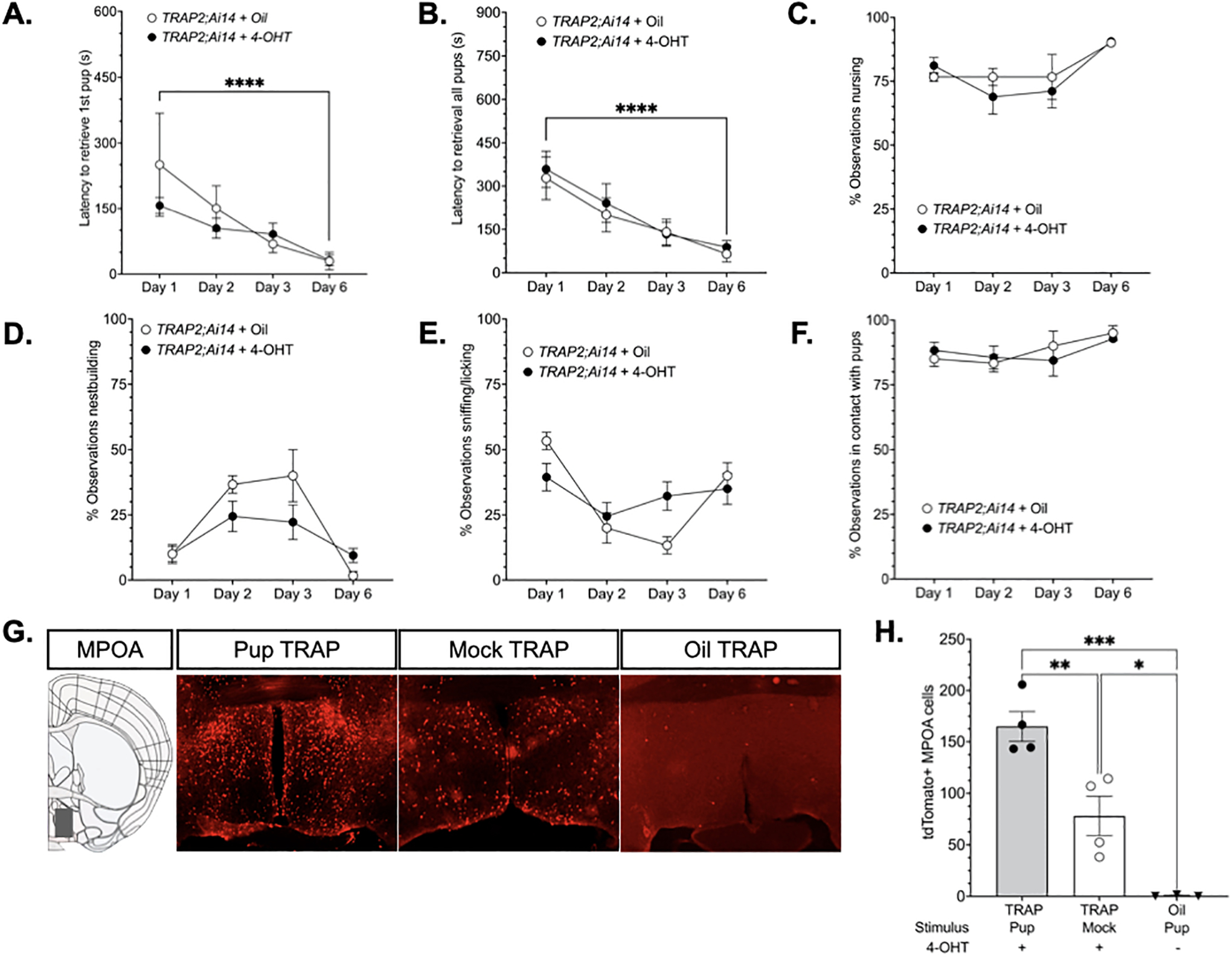
The results of two validation experiments are illustrated. The first experiment tested whether 4-OHT injection (N=9) following an initial pup exposure produced alterations in caregiving behavior relative oil-injected (N= 3) mice on test days 2–6. 4-OHT and oil-treated groups did not significantly differ on any measure. Latencies initiate (A) and complete pup retrieval (B) declined from day 1 to day 6 (p<0.0001, d=3.87; p<0.0001, d=14.27, respectively). Time spent in nursing postures (C) and nestbuilding (D) varied by day, however the differences between day 1 and day 6 did not reach statistical significance (p=0.112, d= −5.31; p=0.8645, d=1.19, respectively). The percentage of observations sniffing/licking (E) or in physical contact with pups (F) did not significantly vary by group or day. The second validation experiment tested whether 4-OHT injection sufficiently TRAPed cells activated by pup exposure in the medial preoptic area. Representative photomicrographs of tdTomato expression in the MPOA (G). Quantification of tdTomato expression in 4-OHT treated mice that were given mock (N=4) or pup exposure (N=4) and oil-treated mice (N=3) exposed to pups (H). ****Significant effect of time, Day 1 v. Day 6, p<0.0001;***Significant main effect of group on tdTomato expression, p<0.0003; ***pup TRAP v. Oil TRAP, p<0.0003;**pup TRAP v mock TRAP, p<0.0093; *mock TRAP v oil TRAP, p<0.0265. All p values adjusted for multiple comparisons.
Second, we wanted to test whether pup-responsive cells in the MPOA could be selectively TRAPed by 4-OHT administration. A one-way ANOVA revealed a significant main effect of group [F(2,8)=27.05, p=0.0003, η2=0.871], and post hoc analyses showed that pup exposed mice (N = 4) exhibited greater tdTomato expression than mock exposed control mice (N = 4, p=0.0093, d=2.95). Further, in the absence of 4-OHT administration tdTomato labeling is absent. Thus, endogenous estradiol does not appear to be capable of driving recombination. For example, TRAP2;Ai14 mice treated with vehicle (oil) (N = 3) injections had significantly less tdTomato expression compared to both groups of 4-OHT-treated mice (Oil vs Pup, p=0.0003, d=8.59; Oil vs Mock, p=0.0265, d= −3.09) (Fig. 3E–F).
Maternal experience-induced behavioral changes in TRAP2;Ai14 mice
Maternal behaviors were quantified in pup exposed TRAP2;Ai14 virgins on exposure days 1, 2, 3, and 6. Nearly all mice retrieved all pups presented on each day (Fig. 4A). A one-way repeated measures ANOVA revealed a significant main effect of day on latency to retrieve the first pup [F(1.371, 10.97)=3.407, p=0.0832, η2=0.408]. The effect of day on latency to retrieve the last pup was marginally significant [F(2.142,17.14)=6.78, p=0.057, η2=0.261). Post-hoc analyses showed that mice were significantly faster to initiate (p=0.0079, d=1.62) and complete (p=0.0046, d=1.61) pup retrieval on the last compared to the first day of testing (Fig. 4B–C). TRAP2;Ai14 mice spent more time in nursing postures [F(2.307, 18.46)=7.591, p=0.029, η2=0.295, Fig. 4D] and sniffing/licking [F(2.250,18)=15.03, p<0.0001, η2=0.423, Fig. 4E] on the last test day compared to the first (p=0.0132, d= −1.55; p=0.012, d= −2.04, respectively). Nestbuilding behavior also significantly varied by day [F(1.943,15.54)=4.159, p=0.0367, η2=0.233, Fig. 4F). However, there were no significant differences between test days 1 and 6 (p=0.1346, d= 0.76). There was a marginally significant difference in the amount of time spent in tactile contact with pups across test day [F(1.983,15.87)=3.45, p=0.0570, η2=0.184, Fig. 4G), however no statistically significant difference was detected between test days 1 and 6 (p=0.112, d= −0.90).
Figure 4. Maternal experience-dependent changes in behavior.
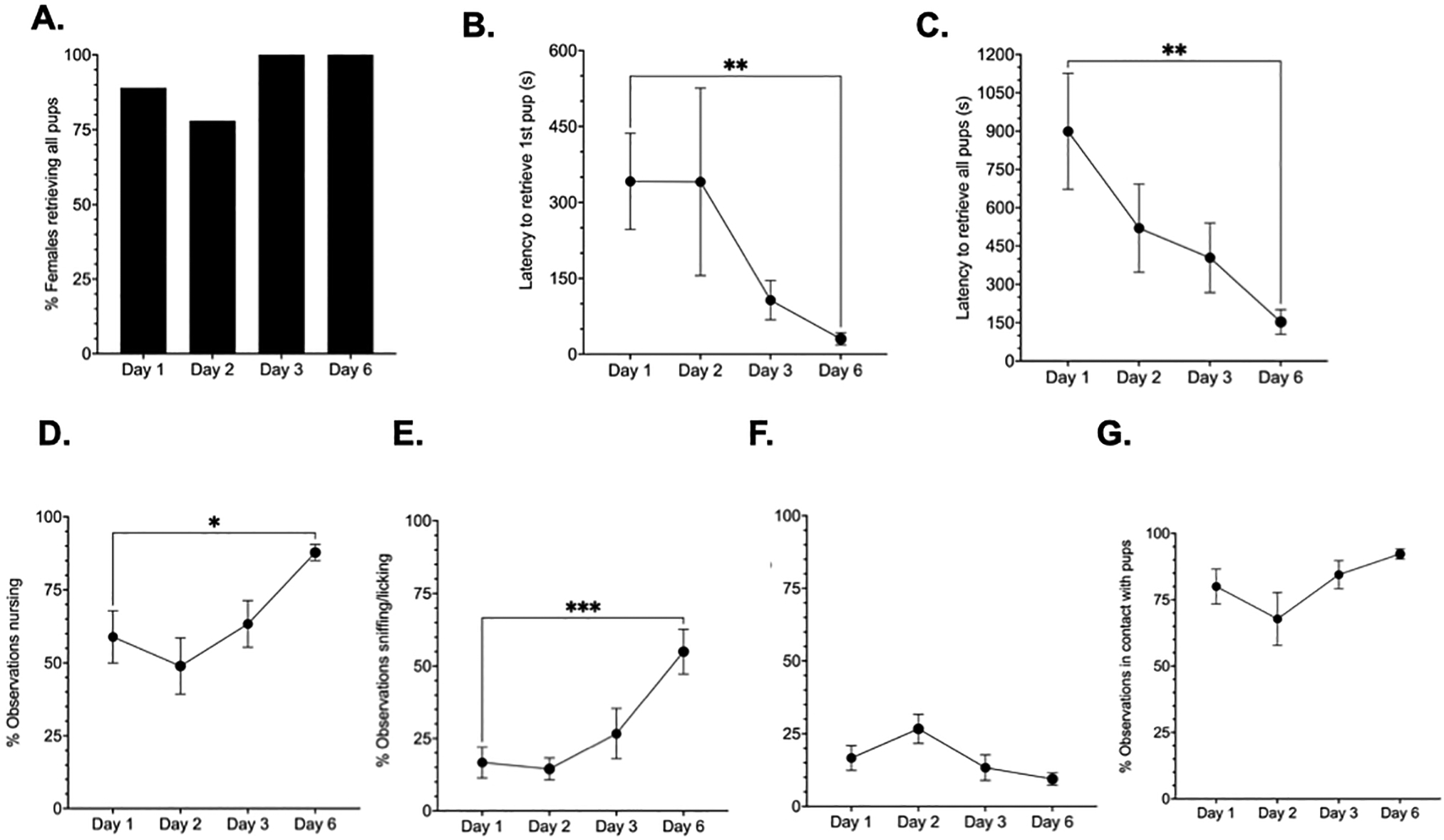
A second independent group of TRAP2;Ai14 virgin female mice (N=9) were used to investigate experience-induced alterations in maternal circuit activation. Experienced-induced changes in multiple aspects of behavior were quantified on each test day following foster pup presentation. Nearly all TRAP2;Ai14 mice retrieved pups on each test day (A). Repeated maternal experience reduced the latency to initiate and complete pup retrieval in TRAP2;Ai14 virgin mice (B-C). Repeated experience also increased time spent in nursing postures and sniffing/licking behavior (D-E), but had no effect on the nestbuilding (F) or total time spent in tactile contact with pups (G). *Day 1 versus Day 6, p< 0.05; **Day 1 vs Day 6, p<0.005; ***Day1 vs Day 6, p<0.0001
tdTomato activation in response to initial pup or mock exposure
Tdtomato expression was used to label cells activated by an initial pup or mock experience. An initial pup exposure induced significantly higher tdTomato expression relative to mock in the cMPOA [t(12)=4.738, p=0.000482, d= −2.76]. No differences were found in the vBNST (p=0.115, d= −0.96), VTA (p=0.867, d=0.10), NAc (p=0.509, d=0.38), NAs (p=0.542, d=0.35), BLA (p=0.132, d= −0.91), PFC (p=0.475, d=0.42), meA (p=0.659, d=0.26), dBNST (p=0.4113, d=0.48), AHN (p=0.189, d=0.81), and VMNvl (p=0.54, d= −0.31) (Fig. 5A).
Figure 5. Maternal neural circuits respond to pups following maternal experience.
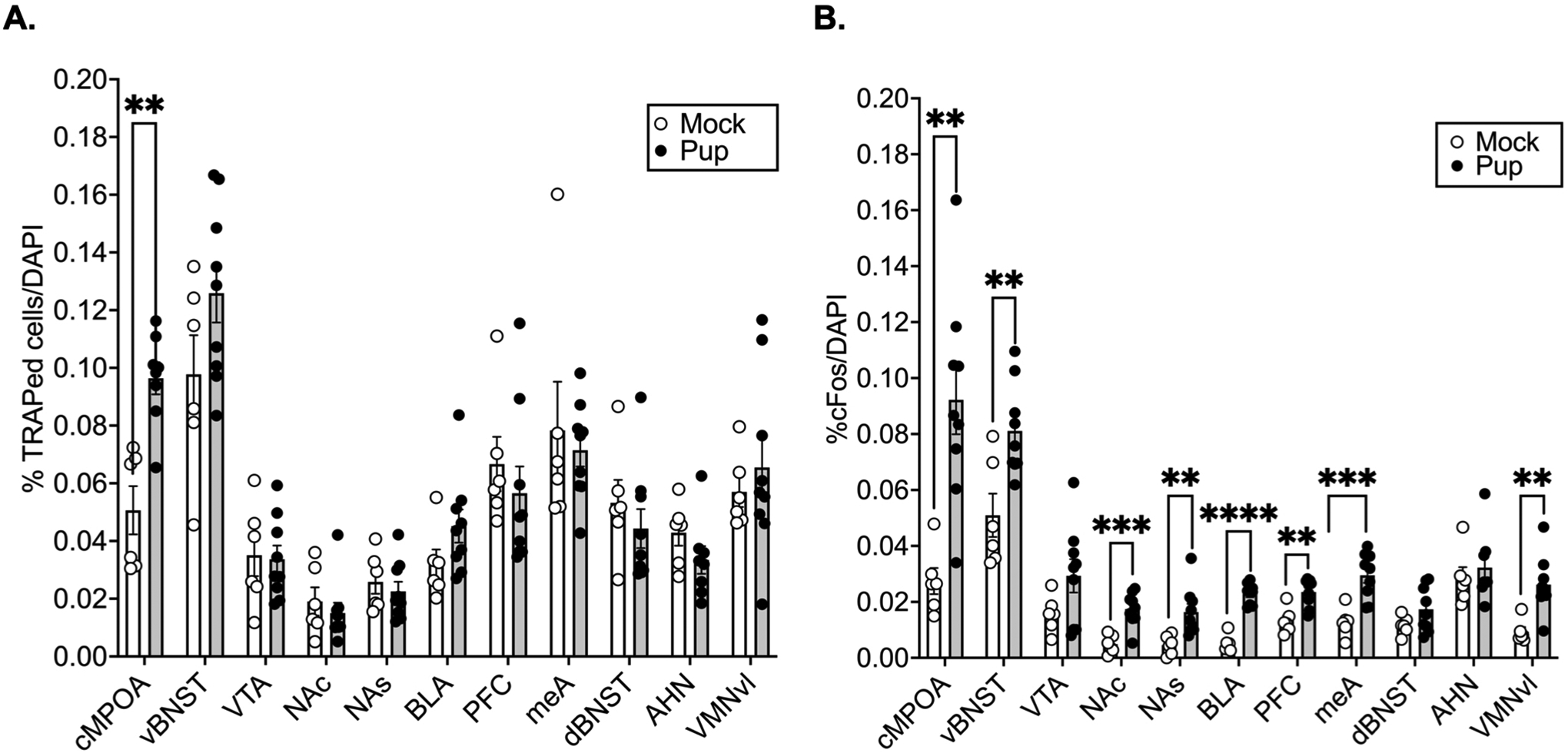
Percentage of DAPI cells that were TRAPed by pup (N=9) versus mock (N=6) exposure (A). Percentage of cFos positive cells/DAPI (B). Multiple independent samples t tests per brain region adjusted for multiple comparisons with the Benjamini, Krieger, and Yekutieli false discovery rate approach (FDR=0.1). ****p<0.0001, ***p<0.001, **p<0.01.
cFos expression in response to pup or mock exposure following repeated experience
Following repeated experience, pups induced significantly more cFos expression relative to mock in several regions in the central motivation circuit, such as the cMPOA [t(13)=4.124, p=0.001, d= −2.33], vBNST [t(13=3.289, p=0.006, d= −1.86], NAc [t(13)=4.656, p=0.0004, d = −2.64], NAs [t(13)=3.159, p=0.0075, d= −1.79], BLA [t(13)=9.835, p<0.000001, d= −5.57], PFC [t(13)=4.075, p=0.001, d= −2.31], as well as some regions in the central avoidance circuit, such as the meA [t(13)=4.478, p=0.000622, d= −2.54] and VMNvl [t(13)=3.889, p=0.002, d= −2.20]. However, no significant experience-dependent increases in cFos expression were found in the VTA (p=0.099, d= −1.00), dBNST (p=0.123, d= −0.93), or AHN (p=0.543, −0.36) (Fig. 5B).
Experience-induced reactivation of TRAPed neurons in response to pup or mock exposure
Whereas pup TRAPed cells were exclusively identified in the cMPOA, pup exposure induced cFos expression through maternal motivation and aversion circuits. To control for these differences in expression, we compared reactivation through motivation and aversion circuits by subtracting colocalization expected by chance from the percentage of colocalized cells for each subject. Student’s t tests revealed significant reactivation in the cMPOA [t(13)=5.547, p=0.000094, d= −3.14] and vBNST [t(13)=5.315, p=0.000140, d= −3.01] (Fig 7B). Several other regions of both motivation and aversion circuits were significantly reactivated by pups relative to mock exposure (Fig 7C): BLA [t(13)=4.145, p=0.00115, d= −2.35], PFC [t(13)=2.908, p=0.0122, d= −1.65] and VMNvl [t(13)=2.513, p=0.0259, d= −1.42). No significant experience-induced reactivation was seen in the VTA (p=0.656, d= −0.26), NAc (p=0.254, d= −0.68), NAs (p=0.144, d= −0.88), AHN (p=0.161, d=0.91), dBNST (p=0.858), or MeA (p=0.163, d= −0.84) (Fig 7C).
Figure 7. Experience-dependent cell reactivation in maternal neural circuits.
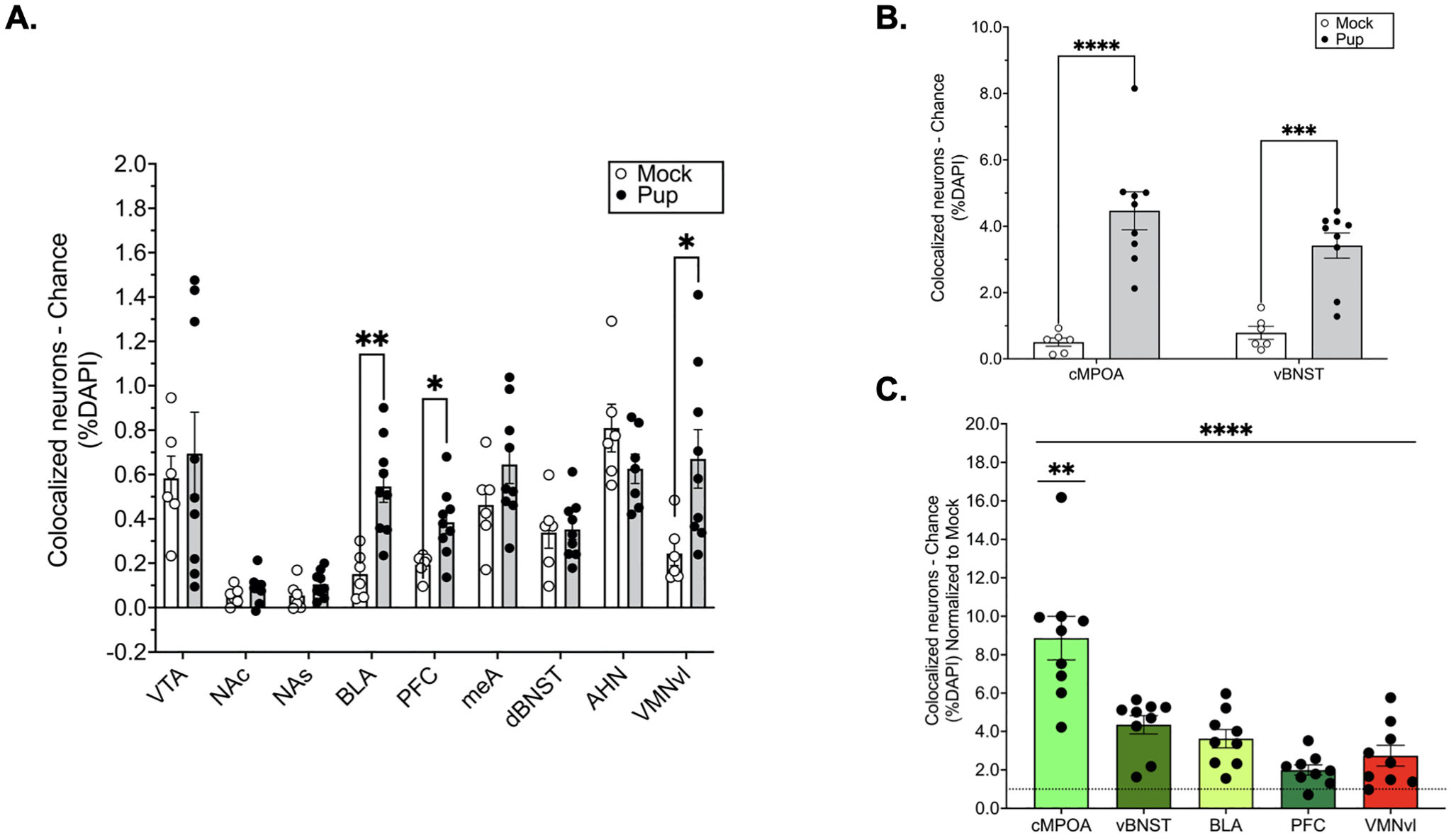
The percentage of DAPI cells that expressed both tdTomato and cFos in response to mock (N=6) or pup exposure (N=9) across regions in the central motivation and avoidance circuits (A) and in the cMPOA and vBNST (B). Relative differences in reactivation across regions (C). Each region normalized to mock control (dotted line). Multiple t tests (per region) adjusted for multiple comparisons with the Benjamini, Krieger, and Yekutieli false discovery rate approach (FDR=0.1).****p<0.0001, ***p<0.001, **p<0.01.
Relative levels of reactivation across regions
Relative differences in reactivation were compared across regions that showed significant pup induced reactivation relative to mock exposure (cMPOA, vBNST, BLA, PFC and VMNvl). A one-way within subjects ANOVA revealed a main effect of region [F(1.860, 14.88)=19.32, p<0.0001, η2=0.633]. Post hoc analyses showed that the cMPOA had the greatest number of reactivated cells compared to the vBNST (p=0.0082, d=1.84), BLA (p=0.0053, d=2.13), PFC (p=0.0012, d=2.95) and VMNvl (p=0.0086, d=2.44) (Fig. 7D).
Multiple regression analyses
Multiple Regression Analyses were used to evaluate whether cellular activation and reactivation patterns were predictive for experience-dependent changes in maternal care. Specifically, we investigated the impact of cellular activation and reactivation in regions of the maternal motivation or central aversion circuits on maternal responsiveness on test day 6. Variation in nursing behavior was found to be significantly predicted by cFos activation in the cMPOA, VTA, NAc, NAs, BLA and PFC. For this analysis, Multiple R value was 0.999 and the six variables taken together were responsible for 99% of the variance in nursing behavior on test day 6 (Table 1). In contrast, cFos expression in the central aversion circuit did not significantly predict variation in nursing. Cellular reactivation patterns did not significantly predict any of variations in experience-dependent maternal care observed on day 6.
Table 1:
cFos expression in the maternal motivation circuit predicts variation in nursing behavior on test day 6.
| % Nursing on Day 6 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Brain regions | Statistics | |||||||
| cMPOA | vBNST | VTA | NAc | NAs | BLA | PFC | ||
| x | x | x | x | x | x | x | F (7, 1) = 1673; p= 0.0188 | |
| x | F (1, 1) = 1258; p= 0.0179 | |||||||
| x | F (1, 1) = 61.17; p= 0.0810 | |||||||
| x | F (1, 1) = 196.7; p= 0.0453 | |||||||
| x | F (1, 1) = 339.6; p= 0.0345 | |||||||
| x | F (1, 1) = 164.2; p= 0.0496 | |||||||
| x | F (1, 1) = 489.5; p= 0.0288 | |||||||
| x | F (1, 1) = 1172; p= 0.0186 | |||||||
| meA | dBNST | AHN | VMNvl | |||||
| x | x | x | x | F (4, 3) = 1.736; p= 0.3393 | ||||
| x | F (1, 3) = 0.0254; p= 0.8839 | |||||||
| x | F (1, 3) = 1.534; p= 0.3035 | |||||||
| x | F (1, 3) = 0.1943; p= 0.6892 | |||||||
| x | F (1, 3) = 1.370; p= 0.3264 | |||||||
“X” indicates the brain region in the column heading was included in the multiple regression analysis. Abbreviations: AHN, anterior hypothalamic nucleaus; BLA, basolateral amygdala; cMPOA, central medial preoptic area; dBNST, dorsal bed nucleus of the stria terminalis; meA, medial amygdala; NAc, nucleus accumbens core; NAs, nucleus accumbens shell; PFC, prefrontal cortex; vBNST, ventral bed nucleus of the stria terminalis; VMNvl, ventro lateral part of the ventromedial nucleus of the hypothalamus; VTA, ventral tegmental area.
Significant effects indicated in bold.
Discussion
We have previously reported that IEG expression throughout central motivation and aversion circuits is consistent with motivated caregiving versus indifferent or avoidant responses toward pups, respectively (Mayer et al., 2019). Here we ask how these circuits came to differentially respond to infant cues. We have hypothesized that repeated experience with pups enhances the cellular and molecular response to pups in regions of the central motivation system and prevents a pup-induced activation of a central aversion system. As the MPOA is sensitive to pup cues by default, and sends projections to the VTA and the VMNvl (Fang et al., 2018; Kohl et al., 2018; Numan and Numan, 1997), we have also hypothesized that MPOA neurons gate access of these systems to pup stimuli such that the mesolimbic dopamine system is more responsive to pups whereas pup-induced activation of the VMNvl is suppressed. The results of the present study largely support this hypothesis. First, MPOA cells are highly responsive to pups by default whereas central motivation and avoidance circuits are not. Second, several regions of the central motivation circuit became responsive to pups following repeated experience (vBNST, NAc/s, BLA, PFC). Further, cFos expression throughout the entire motivation circuit (cMPOA, vBNST, VTA, NAc/s, BLA and PFC) accounted for 99% of the variance in the frequency of nursing responses in experienced mice. Whereas cFos activation in the avoidance circuit was not predictive of any aspect of maternal care. Third, multiple regions in this pathway (vBNST, BLA, PFC) were more likely to become reactivated in response to pup compared with mock exposure, which indicates that the sub-threshold number of cells initially responsive to pup cues are stably responsive in experienced C57BL/6J mice. Finally, in support of the idea that cMPOA coordinates experience-dependent changes in circuit activation, cells in the cMPOA were significantly more likely to be reactivated than any other region. To our surprise, several regions of the central aversion circuit (meA, VMNvl) also became responsive to pups following repeated maternal experience. Further, the fact that VMNvl cells were more likely to become reactivated in response to pup compared with mock exposure indicates that cells in this region may also be part of the cellular representation of maternal memory. Together the results presented here are the first evidence for a maternal engram.
Although the present data are the first to examine maternal experience-dependent alterations in pup-induced circuit activation in C57BL/6J mice, our findings are consistent with the literature examining maternal and social memory consolidation. BLA and PFC process pup-associated information in rats and, in turn, route this information to the mesolimbic dopamine system (Numan et al., 2010; Petrovich et al., 1996; Vertes, 2004). Maternally experienced rats show a significantly greater cFos response to pups and pup-associated cues in the MPOA and BLA than inexperienced rats (Fleming and Korsmit, 1996). Recently PFC neurons have been linked to social memory in male C57BL/6J mice. For example, ablation of TRAPed PFC cells abolishes social recognition, whereas activation of TRAPed PFC cells can restore recognition of a forgotten mouse (Xing et al., 2021). Finally, the NAs has been causally linked to maternal memory formation as lesions of the shell, but not core, prevent maternal memory formation in rats (Li and Fleming, 2003). Thus, we were surprised to find that NA neurons were not significantly reactivated. However, our finding that repeated experience results in newly pup-responsive NA cells suggests that NA cells activated in experienced mice could become reactivated upon remote maternal memory activation. In support of this idea, DeNardo and colleagues (2019) recently reported increased reactivation of prelimbic cells TRAPed at increasing time points post-fear conditioning acquisition (DeNardo et al., 2019). This example of dynamic ensemble activation has also been identified as critical for memory formation.
Although our lab has previously shown that motivated caregiving behavior in a novel context is linked to a significant increase in cFos transcript in the VTA relative to avoidant behavior (Mayer et al., 2019), in the present study we find no evidence for TRAP or cFos expression in VTA cells following pup exposure. One possibility is that the VTA cells only respond to pups in stressful contexts, which might rely on VTA activation for maternal responsiveness. In support of this idea, Fang and colleagues (2018) have shown that optogenetic stimulation of MPOA neurons that project to the VTA induce pup retrieval in a novel context in pup naïve C57BL/6J mice (Fang et al., 2018). In the present study, we assessed experience-dependent changes in maternal circuit activation within a familiar, home cage context. Thus, it is possible that VTA cells are only activated in challenging environments that require a strong motivational drive to respond to infants. The extent to which VTA cells recruited under challenging conditions are reactivated by pup cues during maternal motivation will need to be addressed by future experiments. Here, we find no evidence for a stable representation of pup cues in VTA cells. However, given that the VTA is a site with a complex micro-circuitry, it is certainly possible that maternal experience-induced plasticity within the VTA involves a dynamic (rather than stable) neural ensemble. For example, optogenetic activation of the MPOA-to-VTA pathway that induces maternal motivation in naïve C57BL/6J mice involves the inhibition of GABAergic interneurons, which results in the disinhibition of VTA dopamine neurons (Fang et al., 2018). Repeated experience with pups could ultimately function to directly alter the responsiveness of dopaminergic cells to pup stimuli. For example, while the MPOA may initially inhibit interneurons, perhaps an experience-dependent shift allows for dopaminergic cells to become responsive to pups under maternal challenge in the absence of disinhibition. If this were the case, we would not expect significant reactivation of VTA cells because the pattern of VTA cells that respond to pups would change over time.
In contrast to our hypothesis that maternal experience biases pup-induced neural activation to a central motivation rather than avoidance pathway, we identified significant experience-dependent cFos activation in two regions of the central avoidance circuit (MeA and VMNvl). Further, cells in the VMNvl were more likely to be reactivated following maternal experience. The VMNvl has been highly implicated in aggressive responses, as stimulation of this subregion has been shown to evoke aggression in male mice towards females, inanimate objects, and males (Lin et al., 2011). Unlike males, female mice are not aggressive towards infants; however more recent findings have indicated that the VMNvl may play a role in mediating aggression towards potentially harmful intruders. For instance, Hashikawa and colleagues (2017) have discovered a population of estrogen receptor-expressing cells in the VMNvl that is highly active in lactating mice during aggressive responses towards males, and chemogenetic inhibition of these cells reduces maternal aggression in lactating mice (Hashikawa et al., 2017). Thus, because female mice are naturally protective of infants, one possibility is that cells within the VMNvl are primed early on to respond to potential threats towards offspring.
Alternatively, one limitation of using cFos as an indirect marker of neural activity as it is used in the current experiment is that although it is commonly assumed that cFos expression is correlated with neuron activity, this is not always the case, as immediate early genes are not always expressed in response to depolarization (Hoffman and Lyo, 2002). An extracellular signal may actually lead to the depression of the cFos-expressing neuron. Considering the MPOA has inhibitory projections to the VMNvl (Fang et al., 2018; Kohl et al., 2018; Numan and Numan, 1997) and central MPOA lesions induce infanticide in female mice (Tsuneoka et al., 2013), it is likely that cells expressing cFos in the VMNvl, at least in female mice, are always being inhibited by the MPOA. Thus, the cFos being expressed at both time points may represent the inhibition, rather than the activation, of cells in the VMNvl in response to pups.
The MeA has also long been implicated in the suppression of maternal behavior because of work showing more pup-induced cFos expression in this region in non-parental compared to parental female rats and male mice (Sheehan et al., 2000; Tachikawa et al., 2013). Further, disconnection between the MeA and AHN/VMN has been shown to facilitate the onset of care in non-maternal rats (Sheehan et al., 2001). In spite of this previous work, here we find an experience-dependent increase in cFos expression in this region. One possibility for this finding is that although a subpopulation of cells that routes pup-related information to the central aversion circuit is initially activated in animals who by default show aversion towards infants, this same subpopulation never becomes activated in female mice, who naturally do not show aversive responses towards infants. However, following maternal experience, a separate subpopulation of cells involved in routing sensory information to the central motivation, rather than the avoidance, circuit may be recruited. In support of this idea, mice, in contrast to rats, actually do require olfaction to be intact in order to display maternal care (Gandelman et al., 1971; Sato et al., 2010). Thus, the only way in which central motivation circuits may gain access to pup olfactory cues is through the MeA. Further, optogenetic inhibition of GABAergic neurons in the MeA results in deficits in maternal care, such as a decrease in pup grooming, in virgin female C57BL/6J mice (Chen et al., 2019), suggesting that different subsets of cells in the MeA may be involved in regulating caregiving and defensive behaviors. The establishment of a maternal memory, then, may require a reorganization in cellular activity within the MeA that allows for pup cues to be continuously routed to the central motivation circuit to promote care.
Interestingly, of all regions in which cells were significantly more likely to be reactivated to pup exposure, the MPOA showed the greatest level of experience-dependent reactivation. These results are in agreement with our hypothesis that the MPOA may be the primary site where maternal memories are stored, and a few other pieces of evidence lend further support for this idea. For example, caregiving behavior never becomes independent from MPOA activity, as disruption of the MPOA interferes with both the onset and maintenance of caregiving behaviors (Arrati et al., 2006; Jacobson et al., 1980; Kohl et al., 2018; Numan, 1974; Numan et al., 1988, 1977; Pereira and Morrell, 2009; Tsuneoka et al., 2013). Second, the fact that cMPOA shows the greatest reactivation to pups is consistent with other work showing cells with similar transcriptional profiles express cFos in response to pups in virgin, postpartum and male mice (Moffitt et al., 2018).
The TRAP2;Ai14 model offers a unique opportunity to identify experience-induced alterations in maternal neural circuit activation. Although the TRAP2;Ai14 mice have been used to study plasticity in the auditory cortex in response to pup vocalizations, the data presented here are the first to use TRAP2;Ai14 mice to study plasticity in reproductive behavior (Tasaka et al., 2018). The TRAP2;Ai14 model relies on administration of a selective estrogen receptor modulator, which could potentially interfere with reproductive behavior. Further, the extent to which regions of the brain that contain cells that are highly sensitive to endogenous estradiol could be selectively TRAPed by 4-OHT (rather than endogenous estradiol) was unclear. Our results indicate that recombination in TRAP2;Ai14 mice is selectively driven by a mutated estrogen receptor, as we found almost no TRAPed cells in the absence of 4-OHT. Further, although 4-OHT is a known estrogen receptor modulator (Gallo and Kaufman, 1997) and has the potential to interfere with maternal behavior, we did not see any significant deficits or enhancements in maternal behavior relating to 4-OHT administration. For instance, all mice, regardless of treatment, showed improved behavior over time, which is consistent with what we have previously reported in virgin and ovariectomized mice (Stolzenberg and Rissman, 2011). Our finding that behavioral response to pups is largely variable during the initial pup exposure is also consistent with our previous findings. Importantly, the variability recorded on test day 1 is not related to 4-OHT injection because administration occurred after pups were removed from the cage. Further, because the second pup test day actually occurred 5 days following 4-OHT administration, 4-OHT would no longer be present in the system (Wayne et al., 2019).
Although the TRAP2; Ai14 model is useful, it also has a few limitations. First, although we have contrasted the pattern of tdTomato expression in response to one-hour of pup exposure in naïve mice with the pattern of cFos expression in response to one-hour of pup exposure in experienced mice, there are some important differences between these two signals. Whereas cFos antibody labels cells that express cFos protein within one hour of pup exposure, tdTomato represents cFos driven recombination over a larger (<6h) window (DeNardo et al., 2019). Further, because recombination is driven by 4-OHT injection, all naïve mice (mock and pup) were exposed to subcutaneous injection and handling stress that could have impacted cFos activity.
In conclusion, our current findings demonstrate that the same cells in the MPOA and central approach circuit that are initially responsive during spontaneous maternal care are reactivated as a consequence of experience. We do not find this same experience-dependent increase in reactivation in the majority of regions that were examined in the central avoidance circuit, with the exception of the VMNvl. We have previously hypothesized that experience-dependent transcriptional changes within these regions are likely occurring to promote the long-term maintenance of care. Although the molecular mechanisms that underlie maternal memory consolidation largely remain unknown, one possibility is that pup-induced gene expression alters the phenotype of maternally relevant neurons so that these neurons become permanently more responsive to infants, an idea that is referred to as transcriptional memory (D’Urso and Brickner, 2017). The present results, which suggest that cells initially encoding pup cues in naïve mice are reactivated following maternal experience in both motivation and aversion neural nodes, is consistent with the idea that transcriptional changes may not only be occurring in the central motivation circuit, but also in the central aversive circuit to possibly silence cells that would typically promote aversion towards infants. Future work will be necessary to investigate how repeated experience changes the transcriptional profile within pup-responsive cells in regions across the central approach and avoidance circuits.
Figure 6. Representative photomicrographs.
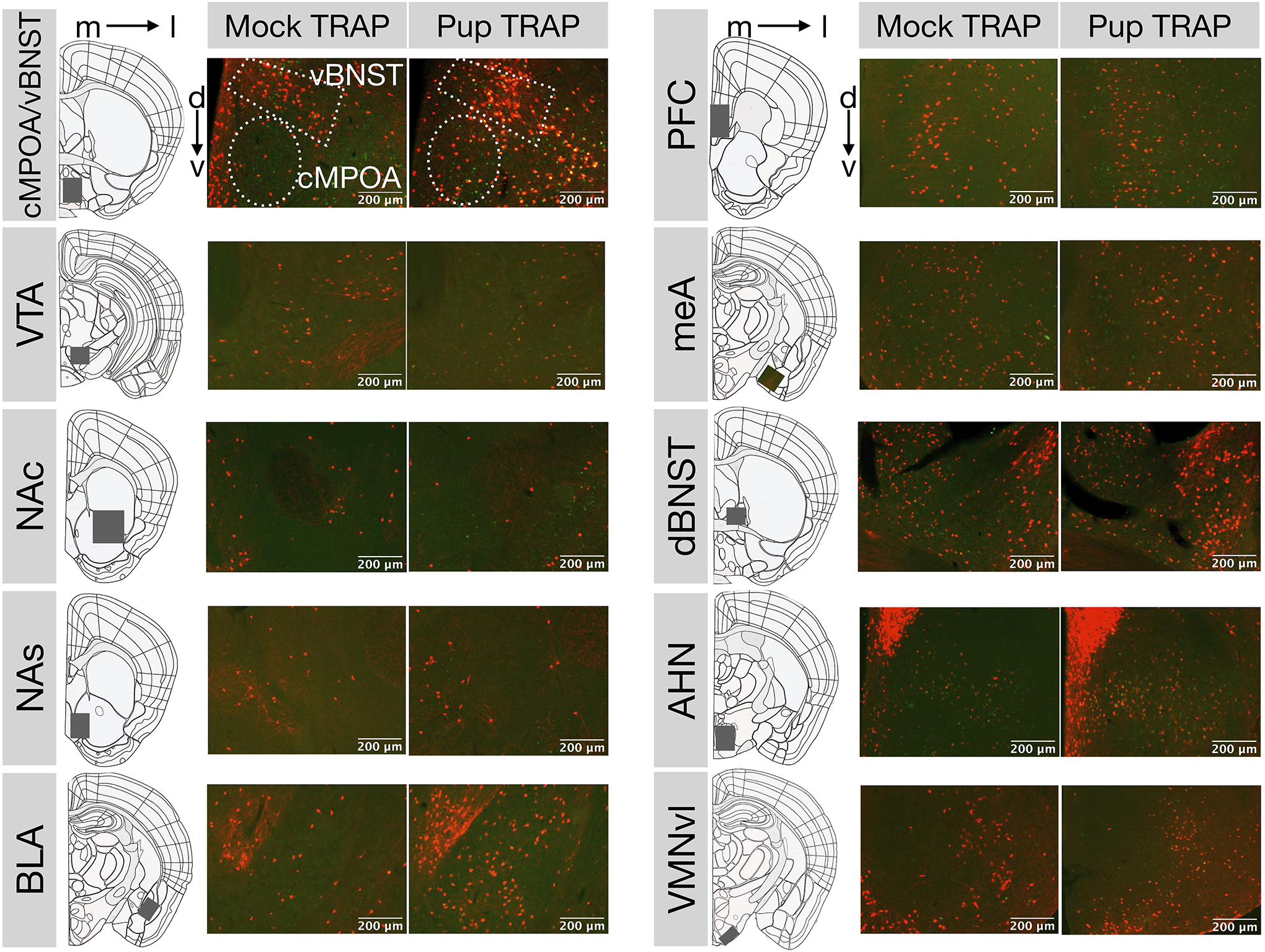
Colocalized expression of tdTomato (red) and cFos (green) in coronal sections of 4-OHT-injected females that received either mock (N=6) or pup (N=9) experience. (m: medial, l: lateral, d: dorsal, v: ventral).
Funding:
This work was supported by the National Institute of Child Health and Human Development [R01HD087709-01A1].
References
- Allen WE, DeNardo LA, Chen MZ, Liu CD, Loh KM, Fenno LE, Ramakrishnan C, Deisseroth K, Luo L, 2017. Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357, 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina-Llanes M, Olazábal DE, 2020. Prefrontal cortex is associated with the rapid onset of parental behavior in inexperienced adult mice (C57BL/6). Behavioural brain research 385, 112556. [DOI] [PubMed] [Google Scholar]
- Arrati PG, Carmona C, Dominguez G, Beyer C, Rosenblatt JS, 2006. GABA receptor agonists in the medial preoptic area and maternal behavior in lactating rats. Physiology & behavior 87, 51–65. [DOI] [PubMed] [Google Scholar]
- Bridges RS, 1978. Retention of rapid onset of maternal behavior during pregnancy in primiparous rats. Behavioral Biology 24, 113–117. [DOI] [PubMed] [Google Scholar]
- Bridges RS, 1975. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiology & Behavior 14, 245–249. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Mann PE, Coppeta JS, 1999. Hypothalamic involvement in the regulation of maternal behaviour in the rat: inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. Journal of neuroendocrinology. [DOI] [PubMed] [Google Scholar]
- Caba M, Melo AI, Fleming A, Meza E, 2019. Maternal care activates the ventral tegmental area but not dopaminergic cells in the rat. Journal of neuroendocrinology 31, e12713. [DOI] [PubMed] [Google Scholar]
- Chen PB, Hu RK, Wu YE, Pan L, Huang S, Micevych PE, Hong W, 2019. Sexually dimorphic control of parenting behavior by the medial amygdala. Cell 176, 1206–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier C, Nicolas J-F, Petit A-C, 2013. Preparation and delivery of 4-hydroxy-tamoxifen for clonal and polyclonal labeling of cells of the surface ectoderm, skin, and hair follicle, in: Epidermal Cells. Springer, pp. 239–245. [DOI] [PubMed] [Google Scholar]
- Cohen J, Bridges RS, 1981. Retention of maternal behavior in nulliparous and primiparous rats: Effects of duration of previous maternal experience. Journal of Comparative and Physiological Psychology 95, 450. [Google Scholar]
- d’Cunha TM, King SJ, Fleming AS, Lévy F, 2011. Oxytocin receptors in the nucleus accumbens shell are involved in the consolidation of maternal memory in postpartum rats. Hormones and behavior 59, 14–21. [DOI] [PubMed] [Google Scholar]
- DeNardo L, Luo L, 2017. Genetic strategies to access activated neurons. Current opinion in neurobiology 45, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo LA, Liu CD, Allen WE, Adams EL, Friedmann D, Fu L, Guenthner CJ, Tessier-Lavigne M, Luo L, 2019. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nature neuroscience 22, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Urso A, Brickner JH, 2017. Epigenetic transcriptional memory. Current genetics 63, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Buckenmaier J, 1994. Estrogen-receptor occurrence in the female mouse brain: effects of maternal experience, ovariectomy, estrogen and anosmia. Journal of Physiology-Paris 88, 315–329. [DOI] [PubMed] [Google Scholar]
- Erwin SR, Sun W, Copeland M, Lindo S, Spruston N, Cembrowski MS, 2020. A sparse, spatially biased subtype of mature granule cell dominates recruitment in hippocampal-associated behaviors. Cell reports 31, 107551. [DOI] [PubMed] [Google Scholar]
- Fang Y-Y, Yamaguchi T, Song SC, Tritsch NX, Lin D, 2018. A hypothalamic midbrain pathway essential for driving maternal behaviors. Neuron 98, 192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, 1996. Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behavioral neuroscience 110, 567. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Sarker J, 1990. Experience-hormone interactions and maternal behavior in rats. Physiology & behavior 47, 1165–1173. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Kaufman D, 1997. Antagonistic and agonistic effects of tamoxifen: significance in human cancer., in: Seminars in Oncology. pp. S1–71. [PubMed] [Google Scholar]
- Gandelman R, Zarrow MX, Denenberg VH, Myers M, 1971. Olfactory bulb removal eliminates maternal behavior in mouse. Science. [DOI] [PubMed] [Google Scholar]
- Geissler DB, Schmidt HS, Ehret G, 2013. Limbic brain activation for maternal acoustic perception and responding is different in mothers and virgin female mice. Journal of Physiology-Paris 107, 62–71. [DOI] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Piper WT, Lee H, Rudy B, Lin D, 2017. Esr1+ cells in the ventromedial hypothalamus control female aggression. Nature neuroscience 20, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D, 2002. Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out’? Journal of neuroendocrinology 14, 259–268. [DOI] [PubMed] [Google Scholar]
- Jacobson CD, Terkel J, Gorski RA, Sawyer CH, 1980. Effects of small medial preoptic area lesions on maternal behavior: Retreiving and nest building in the rat. Brain research 194, 471–478. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Köhler S, Frankland PW, 2015. Finding the engram. Nature Reviews Neuroscience 16, 521–534. [DOI] [PubMed] [Google Scholar]
- Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, Miyamishi K, Zweifel LS, Luo L, Uchida N, 2018. Functional circuit architecture underlying parental behaviour. Nature 556, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Li M, Watchus J, Fleming AS, 1999. Neuroanatomical basis of maternal memory in postpartum rats: selective role for the nucleus accumbens. Behavioral neuroscience 113, 523. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS, 2003. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behavioral neuroscience 117, 426. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ, 2011. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM, 1997. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience 82, 267–281. [DOI] [PubMed] [Google Scholar]
- Matsushita N, Muroi Y, Kinoshita K, Ishii T, 2015. Comparison of c-Fos expression in brain regions involved in maternal behavior of virgin and lactating female mice. Neuroscience letters 590, 166–171. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI, 2005. Preference for cocaine-versus pup-associated cues differentially activates neurons expressing either Fos or cocaine-and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience 135, 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer HS, Helton J, Torres LY, Cortina I, Brown WM, Stolzenberg DS, 2019. Histone deacetylase inhibitor treatment induces postpartum-like maternal behavior and immediate early gene expression in the maternal neural pathway in virgin mice. Hormones and behavior 108, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, Rubinstein ND, Hao J, Regev A, Dulac C, 2018. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, 1974. Medial preoptic area and maternal behavior in the female rat. Journal of comparative and physiological psychology 87, 746. [DOI] [PubMed] [Google Scholar]
- Numan M, Bress JA, Ranker LR, Gary AJ, DeNicola AL, Bettis JK, Knapp SE, 2010. The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats. Behavioural brain research 214, 368–376. [DOI] [PubMed] [Google Scholar]
- Numan M, Corodimas KP, Numan MJ, Factor EM, Piers WD, 1988. Axon-sparing lesions of the preoptic region and substantia innominata disrupt maternal behavior in rats. Behavioral neuroscience 102, 381. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, 1997. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. Journal of neuroendocrinology 9, 369–384. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR, 1977. Medial preoptic area and onset of maternal behavior in the rat. Journal of comparative and physiological psychology 91, 146. [DOI] [PubMed] [Google Scholar]
- Orpen BG, Fleming AS, 1987. Experience with pups sustains maternal responding in postpartum rats. Physiology & Behavior 40, 47–54. [DOI] [PubMed] [Google Scholar]
- Parada M, King S, Li M, Fleming AS, 2008. The roles of accumbal dopamine D1 and D2 receptors in maternal memory in rats. Behavioral neuroscience 122, 368. [DOI] [PubMed] [Google Scholar]
- Pereira M, Morrell JI, 2009. The changing role of the medial preoptic area in the regulation of maternal behavior across the postpartum period: facilitation followed by inhibition. Behavioural brain research 205, 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW, 1996. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. Journal of Comparative Neurology 374, 387–420. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M, 2007. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Sato A, Nakagawasai O, Tan-No K, Onogi H, Niijima F, Tadano T, 2010. Influence of olfactory bulbectomy on maternal behavior and dopaminergic function in nucleus accumbens in mice. Behavioural brain research 215, 141–145. [DOI] [PubMed] [Google Scholar]
- Shang C, Liu A, Li D, Xie Z, Chen Z, Huang M, Li Y, Wang Y, Shen WL, Cao P, 2019. A subcortical excitatory circuit for sensory-triggered predatory hunting in mice. Nature neuroscience 22, 909–920. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan MJ, Numan M, 2001. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience 106, 341–356. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Cirrito J, Numan MJ, Numan M, 2000. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behavioral neuroscience 114, 337. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL, 1998. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95, 927–937. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Mayer HS, 2019. Experience-dependent mechanisms in the regulation of parental care. Frontiers in neuroendocrinology 54, 100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Rissman EF, 2011. Oestrogen-independent, experience-induced maternal behaviour in female mice. Journal of neuroendocrinology 23, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Stevens JS, Rissman EF, 2014. Histone deacetylase inhibition induces long-lasting changes in maternal behavior and gene expression in female mice. Endocrinology 155, 3674–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Stevens JS, Rissman EF, 2012. Experience-facilitated improvements in pup retrieval; evidence for an epigenetic effect. Hormones and behavior 62, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa KS, Yoshihara Y, Kuroda KO, 2013. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. Journal of Neuroscience 33, 5120–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka G, Guenthner CJ, Shalev A, Gilday O, Luo L, Mizrahi A, 2018. Genetic tagging of active neurons in auditory cortex reveals maternal plasticity of coding ultrasonic vocalizations. Nature communications 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ, 2013. Reactivation of neural ensembles during the retrieval of recent and remote memory. Current Biology 23, 99–106. [DOI] [PubMed] [Google Scholar]
- Tsuneoka Y, Maruyama T, Yoshida S, Nishimori K, Kato T, Numan M, Kuroda KO, 2013. Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. Journal of Comparative Neurology 521, 1633–1663. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Wayne NL, Duarte-Vogel S, Kraemer S, Rager JD, 2019. Exposure assessment of laboratory workers to hazardous waste from mice treated with tamoxifen and bromodeoxyuridine. Journal of Chemical Health & Safety 26, 2–8. [Google Scholar]
- Xing B, Mack NR, Guo K-M, Zhang Y-X, Ramirez B, Yang S-S, Lin L, Wang DV, Li Y-C, Gao W-J, 2021. A subpopulation of prefrontal cortical neurons is required for social memory. Biological Psychiatry 89, 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]


