Abstract
Postnatal growth failure remains a significant problem for infants born prematurely, despite aggressive efforts to improve perinatal nutrition. Though often dysregulated in early life when children are born preterm, sodium (Na) homeostasis is vital to achieve optimal growth. We hypothesize that insufficient Na supply in this critical period contributes to growth restriction and programmed risks for cardiometabolic disease in later adulthood. Thus, we sought to ascertain the effects of prolonged versus early-life Na depletion on weight gain, body composition, food and water intake behaviors, and energy expenditure in C57BL/6J mice. In one study, mice were provided a low (0.04%)- or normal/high (0.30%)-Na diet between 3 and 18 wk of age. Na-restricted mice demonstrated delayed growth and elevated basal metabolic rate. In a second study, mice were provided 0.04% or 0.30% Na diet between 3 and 6 wk of age and then returned to standard (0.15%)-Na diet through the end of the study. Na-restricted mice exhibited growth delays that quickly caught up on return to standard diet. Between 6 and 18 wk of age, previously restricted mice exhibited sustained, programmed changes in feeding behaviors, reductions in total food intake, and increases in water intake and aerobic energy expenditure while maintaining normal body composition. Although having no effect in control mice, administration of the ganglionic blocker hexamethonium abolished the programmed increase in basal metabolic rate in previously restricted mice. Together these data indicate that early-life Na restriction can cause programmed changes in ingestive behaviors, autonomic function, and energy expenditure that persist well into adulthood.
Keywords: energy, feeding, metabolism, programming, sodium
INTRODUCTION
Premature birth, defined as birth before 37 wk of gestation, is a frequent complication of pregnancy occurring in about 1 in 10 pregnancies (1). Postnatal growth failure, defined as weight < 10th percentile at 36 wk of postmenstrual age, is a significant morbidity in very low-birth-weight (VLBW; <1,500 g at birth) infants (2). Recent efforts to promote growth and optimize nutritional support in this population have included earlier initiation of parenteral nutrition and increased caloric and protein administration (3). Despite these advances in nutritional practices, up to 50% of VLBW infants continue to experience postnatal growth failure and >25% experience severe postnatal growth failure (< 3rd percentile) (4). Strong associations exist between in-hospital growth failure and impaired short- and long-term neurodevelopment and, to a lesser extent, adult metabolic derangements (5–7). Thus, there remains a critical need to optimize nutritional supply to prematurely born infants, including both caloric and noncaloric components, to support growth and neurodevelopment in this critical period.
The need for adequate sodium (Na) intake and the maintenance of a Na-replete state to support optimal growth is apparent from studies in animals and humans. A century ago, Mitchell and Carmen (8) identified that young rats and chicks experienced greater weight gain and nitrogen retention when provided rations with added NaCl, concluding that deficiency of NaCl limited the utilization of energy and protein for growth. More recent studies in weanling rats have confirmed these findings, demonstrating that restricted Na intake early in life impairs weight and length gain, impairs bone growth, diminishes nitrogen retention, and decreases muscle protein synthesis and RNA concentrations, whereas Na supplementation to Na-depleted animals restores normal rates of weight and length growth and protein synthesis (9–11).
Premature infants are at high risk of Na depletion due to urine Na loss from immature renal tubular function along with the naturally low abundance of Na in breast milk (12–14). Additionally, we have postulated that prematurely born infants lack osmotically inactive Na storage pools from which Na can be mobilized for growth (12). Several relatively small clinical studies have demonstrated a beneficial effect of Na supplementation above that typically provided in the diet to optimize postnatal growth (15, 16). Despite long-standing knowledge of the relationship between early-life Na deficiency and growth failure, little is known regarding potential mechanisms involved in this relationship.
Mouse models have been used extensively for metabolic studies, allowing identification of mechanisms and pathways regulating energy balance and thermogenesis. We previously demonstrated in C57BL/6J mice that weanling animals of both sexes fed a 0.04% Na diet from postnatal day (P)21 to P42 exhibited pronounced reductions in weight gain compared with animals fed a normal (0.15%)- or high (0.30%)-Na diet (17). Consistent with previous work, we also found that decreased weight gain from Na depletion in mice was associated with decreased efficiency of food energy utilization. In the present study, we expanded on this work to ascertain the long-term, programmed effects of chronic versus early-life Na depletion on body mass and composition, food and water intake behaviors, and energy expenditure. We specifically chose to examine the effects of Na depletion after weaning at P21 to simultaneously minimize confounding effects of Na manipulation on renal development while more specifically permitting analysis of the effects of Na manipulation on behaviors and functions controlled by the brain. Mouse renal development is complete at this age (18), whereas human renal maturation is essentially complete around 37 wk of gestation (19). The development and maturation of mouse forebrain and hypothalamic structures involved in the coordinated control of ingestive behaviors and energy balance continue through 6 wk of age (20–22), whereas the development of substructures of the human hypothalamus continues years after birth (23). Thus, because the relative timelines of kidney versus brain development between species are complex, manipulating Na supply after nephrogenesis is complete in mice allows for the examination of the effects of Na on hypothalamic and forebrain maturation while avoiding confounding effects on kidney development. Ultimately, our findings demonstrate a robust effect of early-life Na restriction on long-term energy homeostasis mechanisms and thereby provide insight and rationale for enhanced monitoring of Na homeostasis during the early postnatal period.
MATERIALS AND METHODS
Ethical Approval
All animal use complied with the American Physiological Society’s “Guiding Principles in the Care and Use of Laboratory Animals” and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (24) and received prior approval from the Medical College of Wisconsin (MCW) Institutional Animal Care and Use Committee.
Animals
Timed-pregnant C57BL/6J dams were purchased from the Jackson Laboratories and arrived at MCW on gestational day 14.5. At the Jackson Laboratories, mice were maintained on LabDiet 5K52 (3.17 kcal/g metabolizable energy; 22% kcal from protein, 16% kcal from fat, and 62% kcal from carbohydrates; 0.26% Na, which is equivalent to 2.6 g Na/kg or 0.113 mEq Na/g). Upon arrival at MCW, dams were immediately switched to Envigo/Teklad 2920x, which is a soy protein-free (and thereby phytoestrogen free) purified diet (3.13 kcal/g metabolizable energy; 24% kcal from protein, 16% kcal from fat, and 60% kcal from carbohydrates; 0.15% Na, which is equivalent to 1.5 g Na/kg or 0.065 mEq Na/g). All animals were maintained on a 14:10-h light-dark cycle at standard room temperature (22–23°C) (25). Dams were allowed to deliver, and offspring were culled to six or seven per dam by P3 and then weaned at P21.
Dietary Interventions
At weaning on P21, offspring from each litter were individually housed and randomly and evenly distributed within sex to one dietary intervention protocol. All animals received ad libitum access to reverse osmosis-filtered, chlorinated water throughout the study protocols.
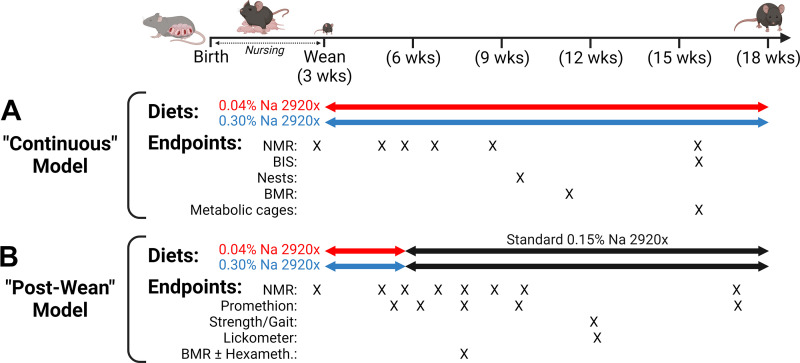
In one protocol designed to evaluate the effects of sustained Na restriction (referred to as the Continuous model; Fig. 1A), male C57BL/6J mice were randomized to receive a custom-modified version of the Teklad 2920x diet with a very low (trace) Na concentration (0.04% = 0.017 mEq Na/g, n = 8) beginning at weaning (at P21) and continuing throughout the study. Littermate male mice were assigned to receive a custom-modified version of the 2920x diet containing a normal/high concentration of Na (0.30% = 0.130 mEq Na/g, n = 7) from weaning to the end of the study.
Figure 1.
Experimental design. A: continuous model. Timeline of manipulations and measurements performed on mice that were maintained on customized trace (0.04%)- or normal/high (0.30%)-Na versions of Teklad 2920x soy-free diet. B: postwean model. Timeline of manipulations and measurements performed on mice that were treated with 0.04% or 0.30% Na diets from weaning [postnatal day (P)21] to 6 wk of age (P42), before return to standard (0.15% Na) 2920x diet. BIS, bioimpedance spectroscopy; BMR, basal metabolic rate; NMR, nuclear magnetic resonance. Figure created with BioRender.
In a second protocol designed to evaluate the programming effects of early-life Na restriction (referred to as the Postwean model; Fig. 1B), male (n = 8) and female (n = 8) C57BL/6J mice were assigned to receive the 0.04% Na version of 2920x from weaning (at P21) for 3 wk (to P42). Littermate male (n = 8) and female (n = 8) mice were assigned to receive the 0.30% Na version of 2920x from weaning until P42. At P42, both groups were then switched to the standard 2920x formulation (containing 0.15% Na = 0.065 mEq Na/g).
Body Composition
Animals were weighed to the nearest 0.01 g. Body composition was then determined by time-domain nuclear magnetic resonance (NMR; Bruker LF110) as previously described (26). Fat-free mass (FFM) was calculated as total body mass minus fat mass. Total body water (TBW) was estimated as 73.2% of FFM and total body hydration as TBW divided by total body mass. In a subset of animals, fluid compartmentalization in intracellular versus extracellular compartments was determined by NMR and bioimpedance spectroscopy as previously described (26, 27).
Basal Metabolic Rate
Basal metabolic rate (BMR) was determined with gas respirometry (a common form of indirect calorimetry), using a push-pull respirometry system previously described in detail (26). Briefly, animals were placed into a gastight chamber maintained at 28–30°C, with airflow through the chamber maintained at 400 mL/min and no food supply within the chamber. Rates of respirometric gas exchange [oxygen consumption (V̇o2) and carbon dioxide production (V̇co2)] were determined during sustained immobility (>20 min) between 4 and 6 h after the animal entered the chamber via measurements of O2 and CO2 concentrations in the effluent air stream (FMS3; Sable Systems International) and corrected for water vapor pressure. Heat production was then estimated with the modified Weir equation (28). As these determinations were performed in resting, fasted mice at thermoneutrality, these values reflect the BMR of the animal.
Metabolic Caging
Ingestive behaviors were assessed and urine and feces were quantitatively collected at 16 wk of age in mice subjected to the Continuous model of Na restriction by metabolic caging as previously described (25, 26). Animals were singly housed in Nalgene-style metabolic cages, in which mice were supplied ad libitum access to powdered chow and water. Urine and feces were used for subsequent analyses of electrolyte and caloric contents.
Electrolyte Analyses
Blood chemistry end points were determined with a handheld iSTAT analyzer with CHEM8+ cartridges (Abbott), as previously described (26). Na and potassium (K) content of urine was determined by flame atomic absorption spectrophotometry as previously described (26). Similarly, after ashing at 600°C and reconstitution in nitric acid (10% in deionized water), electrolyte contents of feces were also determined by flame atomic absorption spectrophotometry (26). Osmolality of urine was determined by the freezing-point depression method (26).
Bomb Calorimetry
Caloric density of food and fecal samples was determined by bomb calorimetry as previously described (26). Caloric densities were then used to determine calories absorbed by the animal versus those lost to stool. These values were then used to calculate digestive efficiency (i.e., fraction of calories absorbed vs. total ingested) and energy efficiency (i.e., weight gain per calorie absorbed).
Multiplexed Metabolic Phenotyping
Simultaneous, multiplexed metabolic phenotyping was carried out in mice subjected to the Postwean Na restriction model with a commercially available 16-chamber metabolic phenotyping system (Promethion; Sable Systems International) as previously described (26, 29). This system provides nearly continuous analyses of respiratory gas exchange, food and water intake, sleep and locomotion behaviors, and meal and drink patterning over four consecutive day and night cycles (∼96 total hours) as previously described (26, 29). Body composition was determined immediately before and after the 96-h session as described above. Food intake was determined by analyzing food consumption patterns during the final 48-h period from each 96-h session, to minimize any variance that might be introduced as the animals acclimate/reacclimate to the Promethion caging.
Energy expenditure (i.e., heat production) was estimated from V̇o2 and V̇co2 with the modified Weir equation (28). As V̇o2 and V̇co2 exchange rates are measured every 3 min in the Promethion apparatus used here, total daily heat production can be summed from the serial data points collected throughout the day. The system automatically estimates the resting metabolic rate (RMR) of the animal by noting the lowest 30-min average heat production rate throughout the 24-h cycle (noted in the software output as “QR_EE_30”). Energy expenditure due to locomotion can then be estimated by subtracting the RMR of the animal from its 24-h average heat production. Finally, because the system also calculates total distance traveled (“AllMeters”) and true locomotion (“PedMeters”) via photoelectric grid beam interruptions, the efficiency of energy expenditure during locomotion can be estimated by dividing the extra energy invested in locomotion per unit time by the distance traveled in that same period of time.
Lickometry
Ingestive behaviors were examined in mice subjected to the Postwean Na restriction model by lickometry as previously described (26). Briefly, mice were placed individually into mouse-sized metabolic cages that included a metal floor grid. Separate burettes filled with water or 1% sucrose solutions and fitted with metal sipper tube tips were provided, which were electrically isolated from the metal floor. Voltage fluctuations occurring between the sipper tube tips and the metal floor occurred when the animal’s tongue contacted the sipper tube tip, which were recorded by chart recorder (PowerLab; ADInstruments) and quantified to document licking behaviors during this two-bottle choice assay. Licking behavior was analyzed over 2 h of exposure to the assay.
Ganglionic Blockade
In one cohort of mice subjected to the Postwean model dietary intervention protocol, BMR was determined at 8 wk of age. Mice were removed from the chamber, received acute injection of the ganglionic blocking agent hexamethonium (30 mg/kg ip), and were then immediately returned to the testing chamber.
Statistics
Data are reported from individual animals where possible, and summary data are presented as means ± SE. Data were analyzed by one-sample t test, independent t test, or two-way ANOVA with or without repeated measures followed by the Šídák's multiple comparisons procedure or general linear modeling (GLM) as indicated in individual figures. Data were analyzed with GraphPad Prism v9.5.1 or SPSS v27. All comparisons were performed as two-tailed tests, and differences were considered significant with P < 0.05.
RESULTS
Chronic Na Depletion Restricts Growth through Increased Basal Metabolism
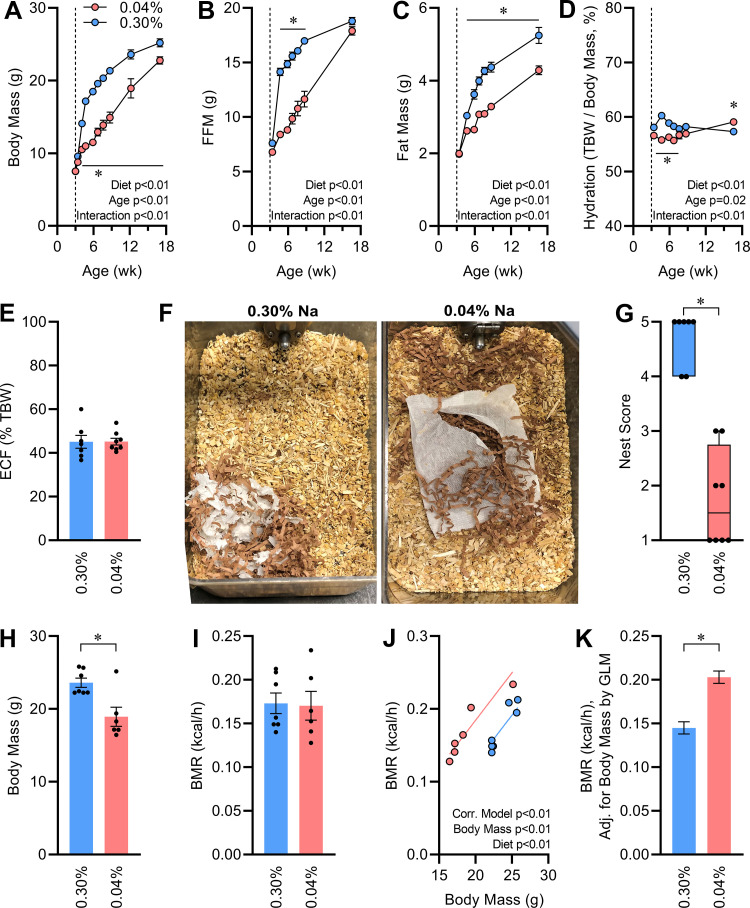
Chronic Na depletion led to impaired body mass gains through the duration of study (Fig. 2A). Differences in body composition evolved over time, with an initial large difference in fat-free mass (FFM) between groups that was ameliorated by 17 wk of age (Fig. 2B), whereas significant differences in fat mass persisted through the experiment (Fig. 2C). Total body hydration was initially reduced by chronic Na depletion, but this effect was reversed by the end of the study (Fig. 2D). At 16 wk of age, fluid compartmentalization was assessed by bioimpedance (27). At this age, the distribution of total body water between intracellular and extracellular compartments was similar in each diet group (Fig. 2E).
Figure 2.
Effects of continuous Na depletion on growth and metabolism. A: body mass vs. age. B: fat-free mass (FFM) vs. age. C: fat mass vs. age. D: total body hydration calculated as total body water (TBW) per body mass vs. age. E: extracellular fluid (ECF) volume, expressed as fraction of TBW, at 16 wk of age. F: representative photographs of nests within home cage, 24 h after cage change. G: semiquantification of nest building. H: body mass at 12 wk of age. I: basal metabolic rate (BMR) at 12 wk of age. J and K: BMR regression against body mass and BMR corrected for body mass (21.45 g) by general linear modeling (GLM). For A–H, 0.04% n = 8 vs. 0.30% n = 7, and for I–K, n = 6 vs. 7. For A–D, data analyzed by 2-way repeated-measures ANOVA, followed by Šídák's multiple comparisons test (*P < 0.05). For E, H, and I, data analyzed by 2-tailed independent t test. For all panels except G and K, summary data presented as means ± SE. For G, data analyzed by Mann-Whitney U test, and data are presented as median and 10–90%. For K, data analyzed by GLM, and data are presented as estimated marginal means ± SE.
At 10 wk of age, investigators noted a consistent and robust difference in nest building activity between groups. Na-depleted mice exhibited disorganized nest construction, whereas Na-replete mice constructed nests of more typical size and shape (Fig. 2F). Nests were photographed 24 h after regular cage changes, to enable semiquantification of nest building behavior via a 5-point scale using a previously reported protocol (30). Results indicated that Na-depleted mice consistently failed to develop an organized nest structure compared with Na-replete littermates (Fig. 2G). As nest construction is dependent on cognitive function but is also reflective of thermoregulatory behavior (31–33), we hypothesized that Na depletion may be associated with altered energy expenditure.
To examine energy expenditure, at 12 wk of age basal metabolic rate was assessed via gas respirometry in mice that were fasted and held at thermoneutral temperatures. At this age Na-depleted mice were significantly smaller than Na-replete mice (Fig. 2H), but aerobic heat production was indistinguishable (Fig. 2I). Therefore, correcting heat production (i.e., metabolic rate) for the covariate of body mass highlighted that heat production relative to body mass was grossly elevated in Na-depleted mice (Fig 2, J and K).
At 16 wk of age, energy and electrolyte turnovers were assessed with metabolic caging. The smaller Na-depleted mice exhibited a marginal increase in total food ingestion, and therefore food intake relative to body mass was increased (Table 1). No overall change in digestive efficiency was noted, and therefore the Na-depleted group absorbed more calories per day. Consistent with increased basal metabolism, Na-depleted animals exhibited a reduced energy efficiency (i.e., the ratio of mass gained per calorie absorbed, and thus an inverse metric of all forms of energy expenditure combined). Water intake was similar between groups, despite the difference in body sizes between groups. Total daily Na intake and Na elimination to urine and to feces were significantly suppressed in the Na-depleted group, resulting in a significant decrease in urine osmolality. Potassium (K) intake along with K elimination to urine and feces were all unchanged.
Table 1.
Metabolic cage study of Continuous Na-depleted mice at 16 wk of age
| End Point | 0.04% Na (n = 8) | 0.30% Na (n = 7) | P Value |
|---|---|---|---|
| Body mass, g | 22.18 ± 0.44 | 24.03 ± 0.52 | 0.02 |
| Fat-free mass, g | 17.90 ± 0.39 | 18.81 ± 0.33 | 0.11 |
| Fat mass, g | 4.28 ± 0.12 | 5.22 ± 0.22 | <0.01 |
| Fat, % total mass | 19.3 ± 0.5 | 21.7 ± 0.6 | <0.01 |
| Total body water, mL | 13.11 ± 0.29 | 13.77 ± 0.24 | 0.11 |
| Hydration, % total mass | 59.1 ± 0.4 | 57.3 ± 0.4 | <0.01 |
| Food consumed, kcal/day | 13.97 ± 0.35 | 12.52 ± 0.60 | 0.05 |
| Digestive efficiency, % | 84.8 ± 0.6 | 84.3 ± 0.5 | 0.48 |
| Calories absorbed, kcal/day | 11.85 ± 0.34 | 10.55 ± 0.50 | 0.04 |
| Energy efficiency, Δmg body mass/Σ kcal absorbed | 18.8 ± 0.8 | 24.9 ± 1.7 | <0.01 |
| Water intake, mL/day | 3.56 ± 0.26 | 3.31 ± 0.26 | 0.51 |
| Total Na intake, mEq/day | 0.053 ± 0.001 | 0.371 ± 0.018 | <0.01 |
| Urine Na concentration, mmol/L | 3.6 ± 1.1 | 294.5 ± 28.3 | <0.01 |
| Na eliminated to urine, mEq/day | 0.005 ± 0.001 | 0.344 ± 0.016 | <0.01 |
| Na eliminated to feces, mEq/day | 0.020 ± 0.003 | 0.108 ± 0.008 | <0.01 |
| Total K intake, mEq/day | 0.318 ± 0.008 | 0.291 ± 0.014 | 0.10 |
| Urine K concentration, mmol/L | 255.1 ± 22.2 | 254.2 ± 15.1 | 0.98 |
| K eliminated to urine, mEq/day | 0.402 ± 0.038 | 0.304 ± 0.023 | 0.06 |
| K eliminated to feces, mEq/day | 0.050 ± 0.009 | 0.035 ± 0.004 | 0.15 |
| Urine osmolality, mosmol/kgH2O | 2,278 ± 308 | 3,169 ± 233 | 0.04 |
| Total daily osmolytes to urine, mosmol/day | 3.35 ± 0.14 | 3.75 ± 0.21 | 0.12 |
| Urine volume, mL/day | 1.69 ± 0.26 | 1.22 ± 0.11 | 0.13 |
| Water in feces, g/day | 0.103 ± 0.006 | 0.089 ± 0.005 | 0.10 |
| Water in feces, % feces mass | 33.4 ± 0.6 | 32.5 ± 1.1 | 0.47 |
Data reported as means ± SE. Comparisons were performed by independent 2-tailed t test.
At 17 wk of age, mice were euthanized and tissues were collected. Interscapular brown adipose tissue (BAT) was similarly sized between groups, with or without normalization to body mass (Table 2). Inguinal adipose mass was significantly reduced in the Na-depleted group, but this effect was abrogated by normalization for body mass. Similarly, kidneys were smaller in the Na-depleted group; however, this size was proportional to their reduced body mass. Adrenal glands were increased relative to body mass in the Na-depleted group. Serum osmolality and Na concentration were indistinguishable between groups.
Table 2.
Tissue masses and serum chemistries of Continuous Na-depleted male mice at 17 wk of age
| End Point | 0.04% Na (n = 8) | 0.30% Na (n = 7) | P Value |
|---|---|---|---|
| Body mass, g | 22.77 ± 0.51 | 25.18 ± 0.56 | <0.01 |
| Interscapular brown adipose, mg | 89.4 ± 8.8 | 92.9 ± 8.2 | 0.78 |
| Interscapular brown adipose, mg/g body mass | 3.9 ± 0.3 | 3.7 ± 0.3 | 0.66 |
| Inguinal adipose, mg | 194.5 ± 13.0 | 258.8 ± 19.9 | 0.02 |
| Inguinal adipose, mg/g body mass | 8.6 ± 0.6 | 10.2 ± 0.7 | 0.07 |
| Kidneys, mg | 292.9 ± 18.8 | 352.0 ± 19.6 | <0.05 |
| Kidneys, mg/g body mass | 12.8 ± 0.7 | 14.0 ± 0.8 | 0.28 |
| Adrenal glands, mg | 4.6 ± 0.50 | 3.1 ± 0.7 | 0.08 |
| Adrenal glands, mg/g body mass | 0.20 ± 0.02 | 0.12 ± 0.03 | 0.04 |
| Serum osmolality, mosmol/kgH2O | 342 ± 5 | 345 ± 3 | 0.68 |
| Serum Na, mmol/L | 147.9 ± 2.3 | 153.7 ± 2.6 | 0.12 |
Data reported as means ± SE. Comparisons were performed by independent 2-tailed t test.
Collectively, these results support the conclusion that chronic Na depletion reduced growth trajectory through a robust increase in basal metabolism. Next, we sought to determine whether this effect was mediated through the sustained reduction in Na supply or if early-life Na depletion was sufficient to program persistent changes in energy homeostasis.
Body Composition Normalizes Rapidly after Na Supply Is Restored
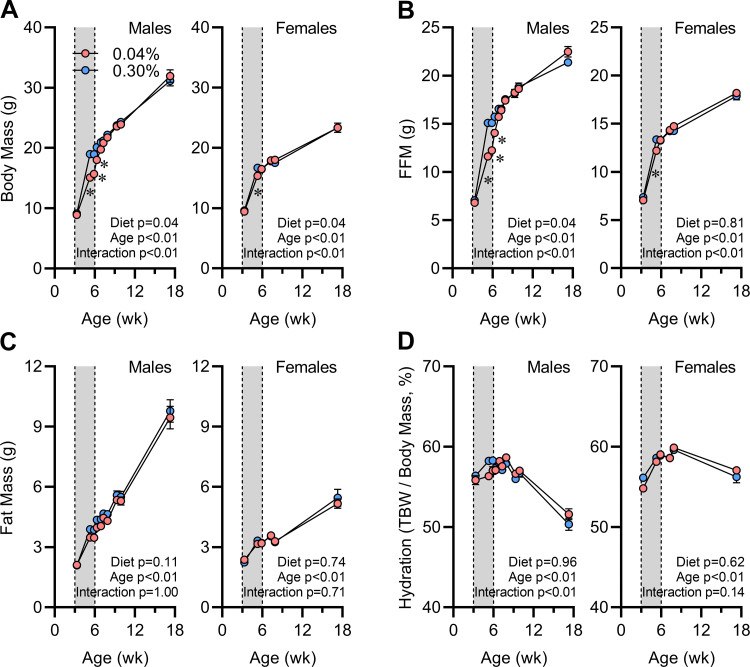
To explore the effects of early-life Na depletion to program long-term changes in energy homeostasis, we next used the Postwean dietary intervention model, in which mice were supplied low (0.04%)- or elevated (0.30%)-Na custom versions of the soy-free 2920x diet from weaning (at 3 wk of age) to 6 wk of age and then returned to standard (0.15% Na) 2920x diet for the remainder of the study (Fig. 1B). At weaning, mice were randomized between groups, and the body size and composition of each group were indistinguishable within each sex (Fig. 3). During the 3 wk of dietary intervention, mice supplied the low-Na diet exhibited reduced FFM and TBW gains but minimal suppression of fat gain or modulation of total body hydration. Similar phenotypes were observed in both sexes, though the magnitude of effects was much greater in males than in females.
Figure 3.
Body composition effects of early-life Na depletion are not sustained. A: body mass vs. age. B: fat-free mass (FFM) vs. age. C: fat mass vs. age. D: total body hydration calculated as total body water (TBW) per body mass vs. age. For all panels, 0.04% n = 6 male + 8 female vs. 0.30% n = 8 male + 8 female, Data analyzed by 2-way repeated-measures ANOVA, and data presented as means ± SE.
At 6 wk of age (when mice were switched to the standard diet formulation), body composition, tissue masses, and blood chemistries were evaluated in a cohort of mice that were subjected to the low- or elevated-Na diets. Briefly, mice on the low (0.04%)-Na diet exhibited lower total body mass and FFM and reduced total body water regardless of sex (Table 3). Total fat mass was reduced in males but not females. Masses of individual organs including heart, kidney, and liver were reduced in the low-Na group; however, these reductions were proportional to the total body mass reduction, as the effect of diet was not maintained after normalization of these tissue masses to body mass. Perigonadal fat mass was significantly reduced in the low-Na mice, even after correction for total body mass. Plasma Na, ionized calcium (iCa), and creatinine concentrations were not modified by diet. Plasma K and blood urea nitrogen (BUN) concentrations were increased in mice fed the low-Na diet, and plasma chloride (Cl) was reduced in females fed the high-Na diet. Finally, a minor effect of the low-Na diet to reduce hematocrit was noted in females.
Table 3.
Tissue masses and blood chemistries of ad libitum-fed mice at 6 wk of age (P42) supplied either low (0.04%)- or high (0.30%)-Na versions of 2920x soy-free chow since weaning (P21)
| End Point | Male 0.04% Na (n = 5) | Male 0.30% Na (n = 5) | Female 0.04% Na (n = 5) | Female 0.30% Na (n = 5) | Sex P Value | Diet P Value | Sex × Diet Interaction P Value |
|---|---|---|---|---|---|---|---|
| Total body mass, g | 16.9 ± 0.5* | 21.9 ± 0.4 | 14.7 ± 0.4* | 17.3 ± 0.4 | <0.01 | <0.01 | 0.01 |
| FFM, g | 13.1 ± 0.3* | 17.4 ± 0.4 | 10.9 ± 0.3* | 13.6 ± 0.3 | <0.01 | <0.01 | 0.02 |
| Fat mass, g | 3.9 ± 0.2* | 4.5 ± 0.1 | 3.8 ± 0.2 | 3.7 ± 0.1 | <0.01 | 0.06 | 0.02 |
| Fat mass, % total | 22.8 ± 0.5 | 20.6 ± 0.6 | 25.6 ± 1.1* | 21.4 ± 0.4 | 0.01 | <0.01 | 0.16 |
| Total body water, g | 9.6 ± 0.2* | 12.8 ± 0.3 | 8.0 ± 0.2* | 10.0 ± 0.2 | <0.01 | <0.01 | 0.02 |
| Hydration, % | 56.5 ± 0.4 | 58.1 ± 0.4 | 54.5 ± 0.8* | 57.5 ± 0.3 | 0.01 | <0.01 | 0.16 |
| Heart | |||||||
| mg | 80 ± 3* | 101 ± 2 | 68 ± 1 | 83 ± 7 | <0.01 | <0.01 | 0.49 |
| mg/g | 4.7 ± 0.1 | 4.6 ± 0.1 | 4.6 ± 0.1 | 4.8 ± 0.4 | 0.82 | 0.86 | 0.46 |
| Average kidney | |||||||
| mg | 99 ± 3* | 132 ± 2 | 83 ± 3* | 101 ± 2 | <0.01 | <0.01 | <0.01 |
| mg/g | 5.9 ± 0.0 | 6.0 ± 0.1 | 5.7 ± 0.1 | 5.8 ± 0.1 | 0.09 | 0.10 | 0.93 |
| Adrenal glands | |||||||
| mg | 4.0 ± 0.5 | 4.0 ± 0.5 | 4.6 ± 0.4 | 6.4 ± 0.7 | 0.01 | 0.13 | 0.13 |
| mg/g | 0.23 ± 0.03 | 0.18 ± 0.02 | 0.31 ± 0.03 | 0.37 ± 0.04 | <0.01 | 0.97 | 0.09 |
| Liver | |||||||
| g | 0.83 ± 0.01* | 1.06 ± 0.02 | 0.70 ± 0.02* | 0.88 ± 0.03 | <0.01 | <0.01 | 0.39 |
| mg/kg | 49.3 ± 0.8 | 48.4 ± 0.5 | 47.5 ± 1.4 | 51.3 ± 2.6 | 0.71 | 0.35 | 0.14 |
| Interscapular fat | |||||||
| mg | 48.3 ± 5.7 | 52.7 ± 6.9 | 56.8 ± 7.0 | 55.1 ± 8.2 | 0.45 | 0.85 | 0.67 |
| mg/g | 2.89 ± 0.41 | 2.41 ± 0.32 | 3.89 ± 0.52 | 3.22 ± 0.52 | 0.06 | 0.22 | 0.84 |
| Inguinal fat | |||||||
| mg | 106.4 ± 21.8 | 139.7 ± 28.1 | 130.9 ± 49.6 | 140.2 ± 35.1 | 0.73 | 0.55 | 0.74 |
| mg/g | 6.35 ± 1.32 | 6.38 ± 1.28 | 8.90 ± 3.35 | 8.22 ± 2.17 | 0.33 | 0.88 | 0.87 |
| Perigonadal fat | |||||||
| mg | 172.0 ± 10.5* | 305.5 ± 15.2 | 107.2 ± 21.4 | 140.4 ± 3.4 | <0.01 | <0.01 | <0.01 |
| mg/g | 10.2 ± 0.6* | 14.0 ± 0.8 | 7.2 ± 1.3 | 8.1 ± 0.3 | <0.01 | 0.01 | 0.10 |
| Plasma Na, mmol/L | 144.8 ± 1.3 | 144.0 ± 1.6 | 142.3 ± 1.2 | 144.0 ± 1.4 | 0.42 | 0.77 | 0.42 |
| Plasma K, mmol/L | 6.4 ± 0.2 | 5.9 ± 0.1 | 6.3 ± 0.02 | 5.8 ± 0.1 | 0.51 | <0.01 | 0.79 |
| Plasma Cl, mmol/L | 107.8 ± 1.1 | 108.4 ± 1.0 | 108.0 ± 1.0 | 104.0 ± 0.9 | 0.07 | 0.13 | <0.05 |
| Plasma ionized Ca, mmol/L | 1.15 ± 0.13 | 1.01 ± 0.08 | 0.86 ± 0.17 | 1.16 ± 0.09 | 0.56 | 0.50 | 0.08 |
| Blood urea nitrogen, mg/dL | 29.0 ± 2.0 | 24.2 ± 0.8 | 37.3 ± 3.0* | 26.4 ± 0.5 | <0.01 | <0.01 | 0.07 |
| Creatinine, mg/dL | 0.84 ± 0.05 | 0.98 ± 0.07 | 0.97 ± 0.18 | 0.96 ± 0.05 | 0.51 | 0.42 | 0.37 |
| Hematocrit, % | 42.8 ± 0.5 | 43.0 ± 0.4 | 42.7 ± 1.3* | 45.4 ± 0.7 | 0.14 | 0.06 | 0.10 |
Data presented as means ± SE and analyzed by 2-way ANOVA. FFM, fat-free mass. *P < 0.05 vs. 0.30% Na group within sex, by Šídák's multiple comparisons test.
Upon return to standard (0.15% Na) 2920x diet at 6 wk of age, Na-depleted mice of both sexes exhibited rapid catch-up growth, and body composition was indistinguishable between diet groups by 8 wk of age (Fig. 3). Body composition remained similar between groups for the remainder of the study.
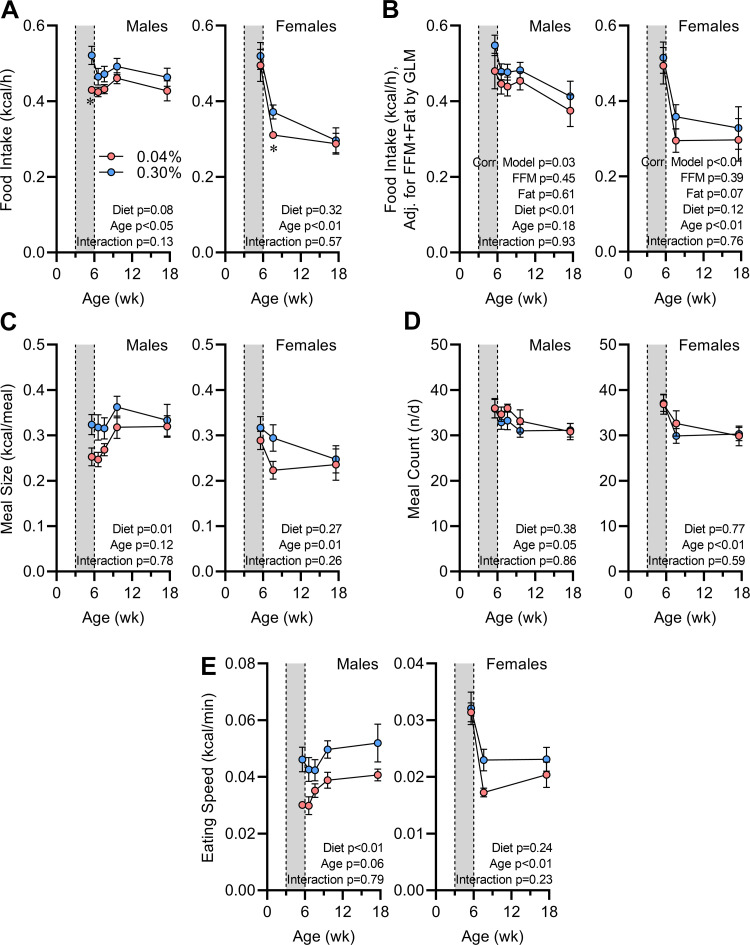
Early-Life Na Depletion Programs Reduced Food Intake due to Smaller Meal Sizes
Mice with early-life Na depletion exhibited reduction in food intake that was dependent on age and sex, with much more robust differences observed in males (Fig. 4A). Correction of food intake rates for FFM and fat mass with generalized linear modeling (GLM) did not diminish the difference between groups, and thus we conclude that the reduced intake in males was not due to changes in body composition (Fig. 4B). Meal patterning analyses indicate that the reduced food intake in males was associated with reduced individual meal sizes (Fig. 4C), without any change in the number of meals consumed per day (Fig. 4D). Interestingly, the speed with which males consumed food during a meal was lower in mice with history of Na depletion (Fig. 4E), prompting concerns that the animals may alter food intake because of differences in the hardness of the food sources, changes in muscle function, or changes in perception or palatability.
Figure 4.
Food intake behaviors are programmed by early-life Na depletion. A: 24-h average rate of food intake vs. age. B: food intake corrected for fat-free mass (FFM; males 17.67 g, females 15.03 g) and fat mass (males 5.27 g, females 3.79 g) by general linear modeling (GLM). C: average meal size vs. age. D: average number of meals per day (n/d) vs. age. E: average rate of food consumption within a meal vs. age. For all panels, 0.04% n = 6 male + 8 female vs. 0.30% n = 8 male + 8 female, and data presented as means ± SE. A and C–E analyzed by 2-way repeated-measures ANOVA followed by Šídák's multiple comparisons test (*P < 0.05). B analyzed by GLM.
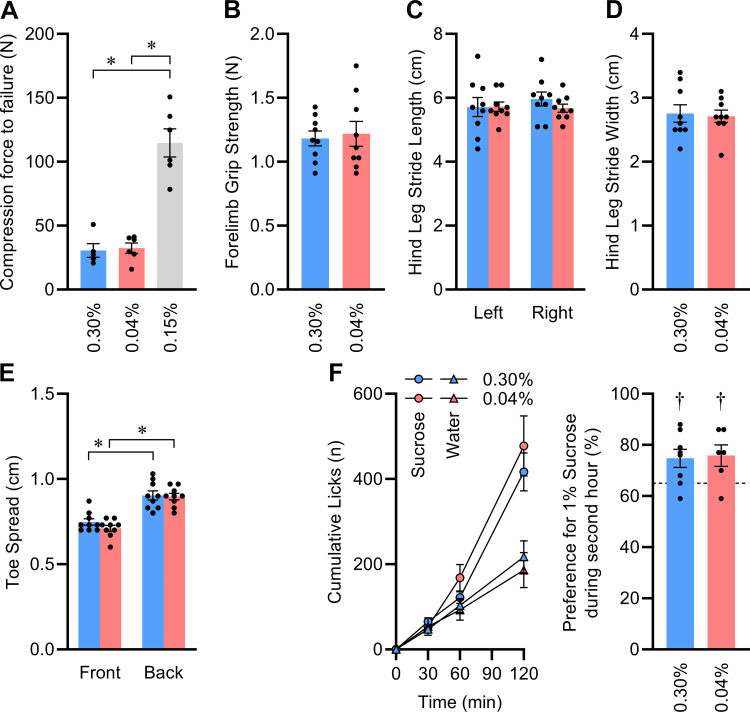
To explore these concepts, we first examined the hardness of the food sources. The compression force to failure of the customized 0.04% and 0.30% Na versions of the 2920x diet were indistinguishable, and both were substantially softer than the standard 0.15% Na version of the diet (Fig. 5A). Second, we examined muscle function, using a battery of tests on only male mice at 12 wk of age, owing to their more robust food intake phenotypes. No differences in forelimb grip strength (Fig. 5B), hindlimb stride length (Fig. 5C), hindlimb stride width (Fig. 5D), or toe spread (Fig. 5E) were noted between diet groups. Next, we used a simple two-bottle choice lickometer assay to examine the behaviors of the mice toward a caloric tastant solution. During a 2-h access period at 12 wk of age, male mice exhibited a significant preference for 1% sucrose solution versus deionized water, and this preference did not differ between dietary groups (Fig. 5F). Combined with NMR data indicating that the groups had indistinguishable total FFM (Fig. 3B), these findings lead us to conclude that the difference in food intake was unlikely to be explained by an inability of the formerly depleted group to physically consume the food or any gross alteration in the ability of the animals to detect and choose to consume a caloric substance.
Figure 5.
No evidence of muscle deficits or taste changes to explain reduced meal size and rate after early-life Na depletion. A: compression force to failure of representative pellets of each food type; n = 5 or 6 per diet, analyzed by 1-way ANOVA followed by Šídák's multiple comparisons test (*P < 0.05). B: forelimb grip strength. C: hind leg stride length. D: hind leg stride width. E: toe spread. F: 2-h, 2-bottle intake preference test by lickometry, assessing relative intake of 1% sucrose solution vs. deionized water, with 0.04% n = 6 vs. 0.30% n = 8, at 16 wk of age. For B–E, n = 9 per group at 12 wk of age. B, D, and F analyzed by independent 2-tailed t test. C and E analyzed by 2-way ANOVA followed by Šídák's multiple comparisons test (*P < 0.05). In F, †P < 0.05 determined by 1-sample t test vs. 65% (illustrated by dashed line), as a threshold to define an intake preference. For all panels, data presented as means ± SE.
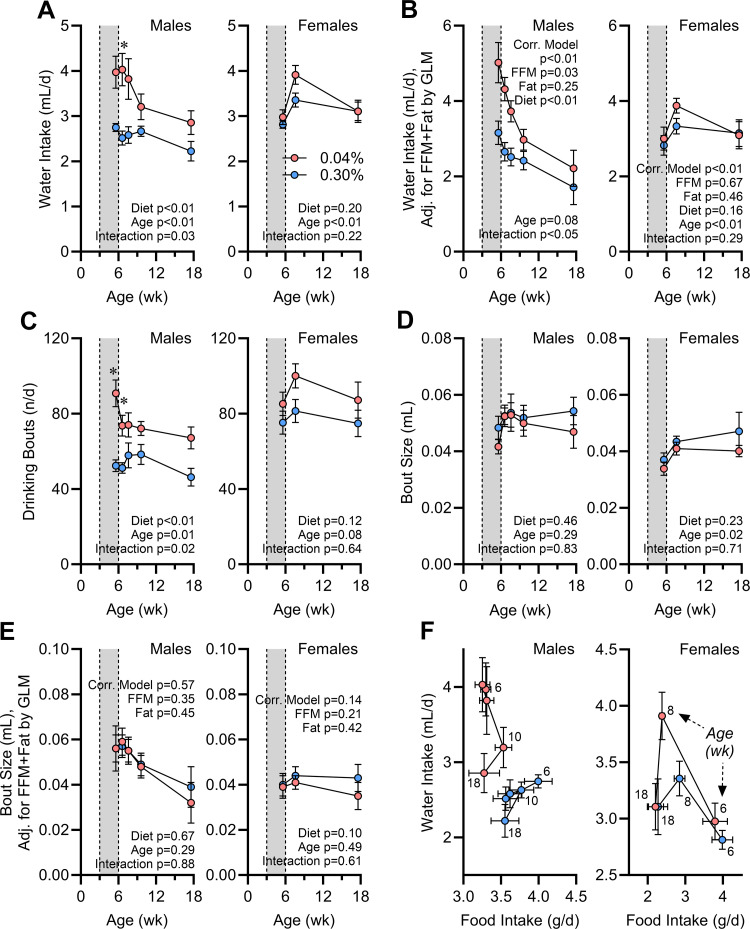
Early-Life Na Depletion Programs Increased Water Intake via Increased Drink Bout Initiations
Water intake was increased in Na-depleted males both during the intervention and after return to normal diet (Fig. 6A). Once again, these programming effects of early-life Na depletion were not formally detected in females. Correction for FFM and fat mass by GLM clarified that the differences in water intake in males were not driven simply by body composition (Fig. 6B). In contrast to food intake behaviors, this change in water intake behavior was associated with changes in bout initiation (Fig. 6C) rather than changes in bout size (Fig. 6D). Bout size was not modified even after correction for FFM and fat mass (Fig. 6E). Interestingly, although food and water intake are typically correlated in rodents (34), this association was altered in an age-dependent manner in males with a history of Na depletion (Fig. 6F). We conclude that food versus fluid consummatory behaviors are distinctly controlled and that the programming effects of early-life Na depletion on these two end points are dissociable. Furthermore, the effects of early-life Na depletion on feeding behaviors is dependent on sex and much more robust in male mice.
Figure 6.
Water intake behaviors are programmed by early-life Na depletion. A: total daily water intake vs. age. d, Day. B: water intake corrected for fat-free mass (FFM; males 17.67 g, females 14.79 g) and fat mass (males 5.27 g, females 3.71 g) by general linear modeling (GLM). C: total daily drinking bouts vs. age. D: average volume of individual drinking bouts vs. age. E: average drinking bout volume corrected for FFM (males 17.67 g, females 15.03 g) and fat mass (males 5.27 g, females 3.79 g) by GLM. F: correlation of water intake vs. food intake per day at each age. For all panels, 0.04% n = 6 males + 8 females vs. 0.30% n = 8 males + 8 females, and data presented as mean or estimated marginal means ± SE. For A, C, and D, data analyzed by 2-way repeated-measures ANOVA, followed by Šídák's multiple comparisons test (*P < 0.05). For B and E, data analyzed by GLM.
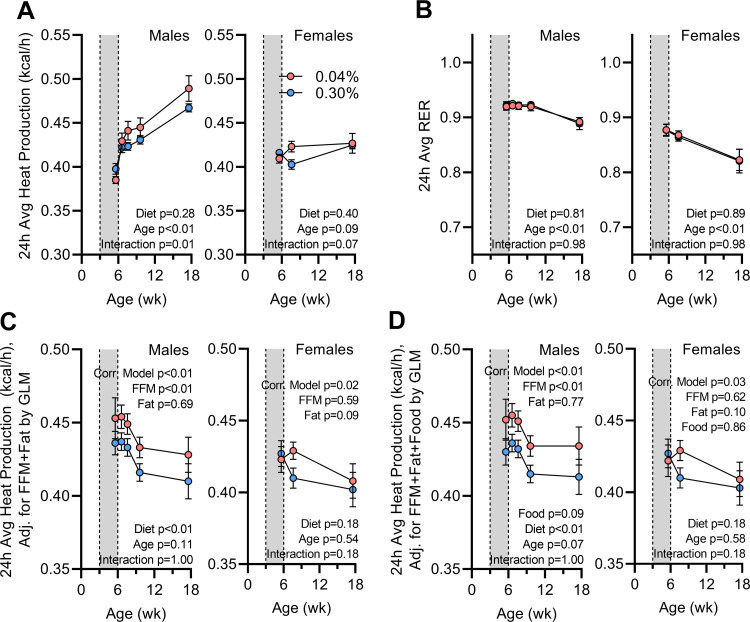
Early-Life Na Depletion Programs Increases in Total Energy Expenditure via Increased Resting Metabolic Rate
Total 24-h heat production was similar or reduced in the Na-depleted mice during the diet intervention stage but rapidly increased and surpassed 24-h heat production after restoration of the standard 2920x diet (Fig. 7A). Once again, this phenotype was more robust and sustained over time in males. The average daily respiratory exchange ratio (RER), however, was indistinguishable between diet groups at any age (Fig. 7B). To understand the influence of body composition changes on energy expenditure, heat production values were corrected for FFM and fat mass by GLM. Correction for body composition clarified that early-life Na depletion causes a disproportionate increase in total daily energy expenditure in males (Fig. 7C). In addition, acknowledging that food intake (through modulation of caloric supply but also the thermic effect of food) impacts total energy expenditure, we also corrected total daily energy expenditure for total daily food intake, and this correction further clarified the dominant effect of early-life Na depletion to stimulate energy expenditure in males (Fig. 7D).
Figure 7.
Energy expenditure is programmed by early-life Na depletion. A: 24-h average heat production vs. age. B: 24-h average respiratory exchange ratio (RER) vs. age. C: 24-h average heat production corrected for fat-free mass (FFM; males 17.67 g, females 15.03 g) and fat mass (males 5.27 g, females 3.79 g) by general linear modeling (GLM). D: 24-h average heat production corrected for FFM (males 17.67 g, females 15.03 g), fat mass (males 5.27 g, females 3.79 g), and 24-h average food intake (males 0.462 kcal/h, females 0.386 kcal/h) by GLM. For all panels, 0.04% n = 6 males + 8 females vs. 0.30% n = 8 males + 8 females. For A and B, data analyzed by 2-way repeated-measures ANOVA, followed by Šídák's multiple comparisons test. For C and D, data analyzed by GLM. For all panels, data presented as mean or estimated marginal means ± SE.
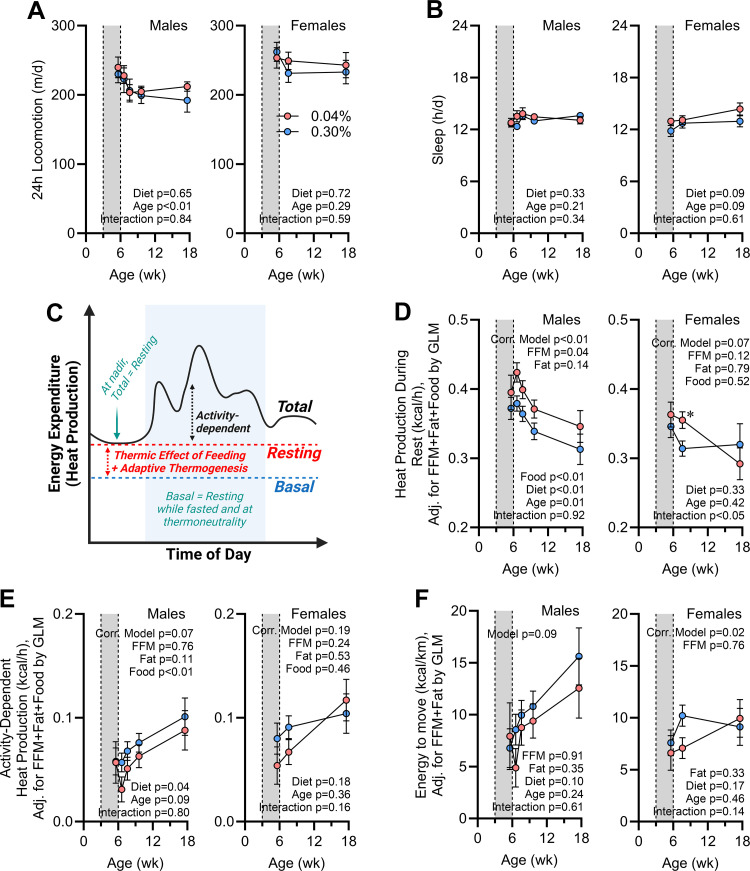
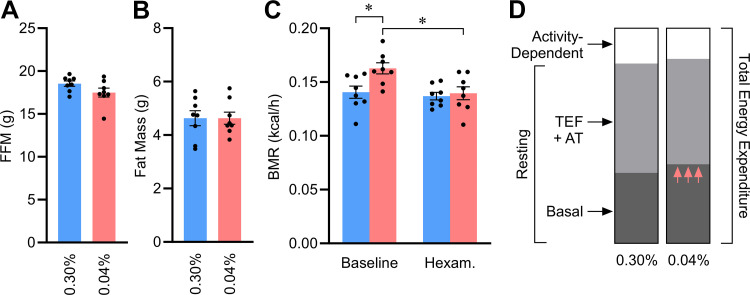
Total daily energy expenditure is dictated by multiple factors, including both activity-dependent and -independent forms of energy output. To delineate these contributors, we first examined patterns of physical activity. Total daily locomotion (Fig. 8A) and time spent completely immobile (as an index of hours spent sleeping; Fig. 8B) were both indistinguishable between diet groups. Together, these data support the conclusion that early-life Na depletion has no effect on total locomotion and imply that changes in total expenditure are likely related to changes in resting metabolism.
Figure 8.
Increases in energy expenditure due to early-life Na depletion are primarily the result of increased expenditure during rest. A: total daily locomotion vs. age. d, Day. B: total hours sleeping per day vs. age. C: cartoon illustrating distinct components contributing to total energy expenditure (Total), including both activity-dependent energy expenditure and resting metabolic rate (Resting). Resting metabolic rate comprises basal metabolic rate (Basal), the thermic effect of feeding, and adaptive thermogenesis. D: energy expenditure during rest corrected for fat-free mass (FFM; males 17.67 g, females 15.03 g), fat mass (males 5.27 g, females 3.79 g), and 24-h food intake (males 0.462 kcal/h, females 0.386 kcal/h) by general linear modeling (GLM). E: activity-dependent energy expenditure, calculated as the difference between total daily expenditure and resting expenditure and corrected for FFM, fat, and food intake as in D. F: efficiency of activity-dependent energy expenditure, reflecting the energy invested per unit distance moved, corrected for FFM and fat as in D. For all panels, 0.04% n = 6 males + 8 females vs. 0.30% n = 8 males + 8 females. For A and B, data analyzed by 2-way repeated-measures ANOVA, followed by Šídák's multiple comparisons test. For C and D, data analyzed by GLM. For all panels, data presented as mean or estimated marginal means ± SE. C created with BioRender.
To clarify the effects of early-life Na depletion on RMR, we next compared the metabolic rates of mice while resting (evaluated by the Sable Systems International Promethion system as “QR_EE_30”). Conceptually, RMR reflects the total metabolic rate when the animal is at rest, which represents the sum of basal metabolism (BMR), the thermic effect of feeding (TEF), and adaptive thermogenesis (AT) energy expenditure mechanisms (Fig. 8C). Thus, because animals were not fasted or housed at thermoneutral temperatures during these analyses and as mice are known to prefer a warmer ambient temperature (32), TEF and AT are expected to contribute a significant proportion of RMR in these testing conditions. RMR values were significantly greater in male mice with history of Na depletion, and this effect was not due to differences in body composition or food intake (Fig. 8D). A significant interaction was observed in females between RMR and age, and multiple comparison testing shows that at 8 wk of age a difference in RMR between diet groups was observed. Energy expenditure due to activity, representing the difference between total energy expenditure and RMR, was significantly suppressed by early-life Na depletion in males but essentially unchanged in females (Fig. 8E). Finally, the efficiency of locomotion was estimated by dividing activity-dependent energy expenditure per day by distance traveled per day. Early-life Na depletion had no significant modulatory effect on the efficiency of locomotion (Fig. 8F). Thus, the programming effects of early-life Na depletion to increase total energy expenditure are mediated entirely through an increase in resting metabolism. This effect is consistently sustained in males as they age to 18 wk; however, this effect is only sustained for a few weeks in females.
At 18 wk of age, male mice were euthanized. At this age, no differences in tissue masses were noted between treatment groups (Table 4), confirming results determined by NMR (Fig. 3) and paralleling results obtained with the Continuous model (Table 2). We conclude that despite normalization of body mass and composition, early-life Na restriction is sufficient to program long-term changes in ingestive behaviors and energy expenditure even after normalization of body size and composition and that these effects are much more robust in male versus female C57BL/6J mice.
Table 4.
Tissue masses of Postwean Na-depleted male mice at 18 wk of age
| End Point | 0.04% Na (n = 8) | 0.30% Na (n = 8) | P Value |
|---|---|---|---|
| Body mass, g | 29.64 ± 1.44 | 30.59 ± 0.89 | 0.58 |
| Interscapular brown adipose, mg | 128 ± 18 | 112 ± 12 | 0.47 |
| Interscapular brown adipose, mg/g body mass | 4.2 ± 0.5 | 3.7 ± 0.4 | 0.44 |
| Inguinal adipose, mg | 268 ± 47 | 292 ± 60 | 0.75 |
| Inguinal adipose, mg/g body mass | 8.8 ± 1.5 | 9.6 ± 1.9 | 0.75 |
| Kidneys, mg | 173 ± 8 | 179 ± 3 | 0.54 |
| Kidneys, mg/g body mass | 5.9 ± 0.2 | 5.9 ± 0.2 | 0.93 |
| Adrenal glands, mg | 6.2 ± 0.5 | 6.2 ± 0.9 | 0.98 |
| Adrenal glands, mg/g body mass | 0.21 ± 0.02 | 0.20 ± 0.03 | 0.79 |
Data reported as means ± SE. Comparisons were performed by independent 2-tailed t test.
Early-Life Na Depletion Programs Increased Basal Metabolic Rate via Increased Autonomic Activity
To further explore the mechanism of programmed increases in RMR, a separate cohort of male mice were subjected to the Postwean model of early-life Na depletion (Fig. 1B). These mice were returned to standard diet at 6 wk of age, and then BMR was examined at 8 wk of age. At this age, body mass and composition had normalized between groups (Fig. 9, A and B). BMR was determined in fasted animals at rest in thermoneutral temperatures, as previously in the Continuous model (Fig. 2). Under these conditions, mice with early-life Na depletion exhibited a very large (16%) increase in BMR relative to control littermates (Fig. 9C). After baseline differences were established, mice were briefly removed from the chamber and injected with the ganglionic blocker hexamethonium. Mice were then immediately returned to the chamber to again measure BMR. After hexamethonium injection, control littermates exhibited no change in BMR, which is consistent with the concept that BMR normally reflects metabolic rate in the absence of autonomically driven increases in metabolism. In contrast, hexamethonium caused previously Na-depleted mice to reduce metabolic rate to levels indistinguishable from control littermates. Finally, the relative contributions of activity-dependent, resting, and basal energy expenditures were compared within male mice at 8–9 wk of age, and a modest redistribution was noted, with a greater proportional contribution of BMR to total expenditure noted in the previously Na-restricted group (Fig. 9D). Together, these results indicate that early-life Na depletion causes programmed increases in BMR and thereby total energy expenditure, which is mediated through programmed elevations in autonomic activity.
Figure 9.
Early-life Na depletion programs increased basal metabolic rate via increased autonomic activity. A and B: fat-free mass (FFM; A) and fat mass (B) of mice at 8 wk of age. C: basal metabolic rate (BMR) before and after acute injection of hexamethonium (30 mg/kg ip). For A–C, n = 8 for each group and data presented as mean ± SE. A and B analyzed by 2-tailed independent t test, and C analyzed by 2-way ANOVA, followed by Šídák's multiple comparisons test (*P < 0.05). D: comparison of relative contributions of activity-dependent energy expenditure, the thermic effect of food (TEF) plus adaptive thermogenesis (AT), and basal metabolic rate to total energy expenditure. Fractional contributions estimated with week 9 energy expenditure data from male mice studied in Promethion [with ad libitum food access at room temperature (22°C)] as shown in Figs. 7 and 8, plus basal metabolic rate from fasted 8-wk-old males studied at thermoneutrality (30°C) as shown in C.
DISCUSSION
This study aimed to evaluate the effects of both chronic and early-life decreased dietary Na intake on growth, body composition, and metabolism. Our data indicate that prolonged Na depletion leads to growth failure, altered body composition, and increased heat production. Of clinical relevance, Na depletion for a limited period of time early in life, followed by resumption of a standard dietary Na intake, resulted in a period of decreased somatic growth followed by catch-up growth and programmed (long term) changes in ingestive behaviors and aerobic energy expenditure. The programmed increase in heat production through 18 wk of age in animals exposed to early-life Na depletion appeared to be mediated by the autonomic nervous system, as administration of hexamethonium abolished the increased BMR phenotype. These findings highlight the importance of early-life Na homeostasis and support the concept that inadequate Na supply in early life contributes to reduced somatic growth and may impart long-lasting effects on energy expenditure and metabolism.
We and others have previously demonstrated the importance of dietary Na on somatic growth in humans and various animal models (8, 17, 35–37). That work has shown that Na deficiency results in decreased weight and length gain, food energy intake utilization, nitrogen retention, and muscle protein synthesis. Additionally, NaCl supplementation to Na-depleted animals restores normal rates of weight and length growth and protein synthesis. Haycock (6) suggested that growth failure resulting from Na deficiency results from inhibition of membrane Na+/H+ antiporter activity, with acidification of the intracellular space and resultant decreased cellular responsiveness to growth factors. However, to our knowledge, no studies have utilized in vivo approaches to extensively explore potential physiological mechanisms linking Na depletion with impaired energy balance and growth. Our work thus extends this body of literature, suggesting that a Na-deficient diet, even for a limited period of time early in life, results in sustained changes in autonomic function and energy homeostasis. We hypothesize that such changes may underlie the increased risk of cardiometabolic diseases in prematurely born humans as they reach adulthood (7, 38, 39).
Although not explored in the present study, we speculate that the renin-angiotensin system (RAS) may be intimately involved in mediating the metabolic changes observed in response to low Na supply. Na homeostasis is a key regulator of RAS activity, which in turn is a vital modulator of body fluid homeostasis, neuroendocrine, and cardiometabolic functions across the life span. Components of the RAS are expressed in multiple brain regions early in development and are thus susceptible to disruption by early postnatal factors, including nutrition and Na homeostasis (40). There is growing evidence for the role of the brain RAS in energy homeostasis, and in particular control of basal and resting metabolic rates (reviewed in Refs. 41–43). Both pharmacological and genetic alterations of the brain RAS impact body metabolism. Our team and others have demonstrated that intracerebroventricular (icv) infusion of angiotensin (ANG) II increases energy expenditure in rats (44) and mice (45), and this effect is dependent on a losartan-sensitive [i.e., angiotensin II type 1 receptor (AT1R)] mechanism (46). Similarly, we have shown that activation of the endogenous brain RAS via chronic deoxycorticosterone acetate (DOCA)-salt treatment stimulates RMR through a mechanism dependent on AT1R in the brain (46, 47). We have also shown that transgenic activation of the brain RAS in the double-transgenic “sRA” mouse model causes growth failure and lifelong increases in RMR starting within the first week after birth (48, 49). Importantly, we have demonstrated that the brain RAS stimulates RMR through activation of sympathetic nerve activity (SNA) to thermogenic tissues, which causes β-adrenergic-mediated stimulation of heat production (48). Thus, it follows that the programming effects of early-life Na restriction may be mediated through stimulation of the brain RAS. Because RAS inhibitors cannot be applied during pregnancy (because of teratogenic effects on the kidney) and surgical manipulations of the brain in the neonatal period are unrealistic, future studies utilizing the Continuous and Postwean interventions described here (Fig. 1) in combination with mice with genetic targeting of the RAS (e.g., AT1R and associated second-messenger cascades) within selected neuronal or glial populations are required to explore these working hypotheses.
The observation that animals with early-life Na restriction display rapid catch-up growth after being placed back onto a standard-Na diet while also exhibiting a sustained increase in thermogenesis and aerobic energy expenditure appears somewhat contradictory. Increased food energy utilization and/or efficiency are unlikely to contribute to this finding, as we previously found these to be similar in both groups of animals (17). Rather, we speculate that early-life Na restriction may additionally modulate anaerobic forms of energy expenditure. Previously we and others have demonstrated that anaerobic energy expenditure contributes between 0 and 40% of total energy expenditure in adult mice, humans, quail, doves, and kangaroo rats, depending on body composition, sex, diet, bariatric surgeries, genetics, antibiotics, and anesthesia (26, 29, 50–56). With the use of the total aerobic expenditure, energy intake, and body composition change data presented here and an algebraic method we recently reported (26, 29) to estimate anaerobic caloric flux, it appears that anaerobic expenditure is lower in mice that experienced early-life Na restriction. For example, at 7–10 wk of age, this method estimates that anaerobic metabolism is ≈0.06 kcal/h lower in previously Na-restricted male mice. Because anaerobic metabolism in mice is a function primarily of the gut microbiota (50), it is interesting to speculate that differences in early-life Na intake may impact and program the energy utilization of the gut microbiota. Preclinical and clinical studies have shown that Na intake can regulate the microbiome diversity and composition (57). Given the known link between microbiota, energy expenditure, and risk for obesity, examination of this hypothesis is also warranted. Programmed changes in the gut microbiota would similarly be hypothesized to contribute to a wide array of other functional changes and increased risks for various diseases in children born prematurely, including but not limited to gut and immune system development and infections such as necrotizing enterocolitis (58).
Mice with early-life Na restriction displayed changes in food and water intake behaviors until completion of the study at 18 wk of age. Reductions in food intake were specifically related to differences in the size of meals and speed of consumption during a meal (metrics of satiation) rather than frequency of meal initiation (a metric of satiety). This distinction is important, as different neural and hormonal signals regulate these distinct aspects of feeding (59). Thus, the present dataset prompts the hypothesis that early-life Na restriction may modify mechanisms that specifically mediate satiation, including gastric emptying kinetics, release of various incretin hormones, or the sensitivity of relevant hypothalamic structures to those signals.
The immediate and sustained increase in water intake in Na-restricted animals could appear counterintuitive, as reduced salt intake might be expected to decrease urine output and therefore reduce the total fluid needs of the animal. However, multiple factors impact the relationship between Na and fluid intake. Na depletion may be associated with an acute decrease in extracellular fluid volume, and thus thirst may increase to replace the volume of fluid lost from the extracellular space. We previously reported that early-life Na restriction was associated with a decrease in TBW over the period of Na restriction (P21–P42) that quickly returned to normal on return to a normal-Na diet (17). This decrease in total body water resulted primarily from a decrease in extracellular fluid. Although the decrease in extracellular fluid would be a potent stimulus for the release of humoral factors that act at the brain to increase thirst and water intake (e.g., volemic thirst), the finding that extracellular and total body fluid volumes returned to normal values soon after return to the standard-Na diet might argue against this mechanism. Interestingly, in human spaceflight simulation studies, a low salt intake (6 g/day) was associated with increased daily water intake (60, 61). The authors speculated that a higher-salt diet resulted in glucocorticoid-mediated endogenous water production with resultant decrease in thirst. It is notable that the increase in fluid intake observed in the present study was primarily the result of an increased number of drinking bout initiations rather than larger bout volumes. First, this is opposite to the pattern of intake that drove changes in food intake. Second, this pattern of increased initiations parallels what is observed in Na-replete adult animals with intracerebroventricular infusion of angiotensin II (45). Thus, this observation provides another line of evidence supporting the concept that early-life Na restriction may program increased activity of (or sensitivity to) the brain RAS.
A number of previous studies have identified that the various physiological challenges during sensitive periods of development modify fluid intake patterns in adulthood (reviewed in Ref. 62). With regard to perinatal Na exposure, the majority of these studies have focused on the impact of high Na intake, with fewer studies exploring long-term effects of low Na intake. Specifically, Mouw et al. (63) identified in rats that offspring of mothers on a Na-free diet during pregnancy and early postpartum period displayed increased water intake at 3 mo of age without a change in preference for NaCl. Adult offspring of dams fed 0.1% NaCl throughout gestation and lactation have increased water intake compared with adults exposed to maternal 1% but not 3% NaCl diet. Using a different paradigm of prenatal maternal Na depletion, Galaverna et al. (64) identified that offspring of Na-depleted mothers had increased Na but not water intake. This effect was abolished by maternal administration of captopril, again supporting an important role for activation of the RAS in programing long-term changes in fluid intake behavior. The present study complements and extends those studies by exploring the role of Na supply during the immediate postweaning period to program long-term changes in behaviors and metabolism.
The timing of Postwean dietary intervention in the present study (weeks 3–6 of life) was specifically selected to target critical periods of development of relevant brain structures of the mouse, while taking advantage of the fact that the timing of these dietary Na manipulations will not adversely affect renal development in the mouse, which would confound interpretation of resulting data. The ontogeneses of individual tissues (e.g., kidney vs. brain) differ. Ontogenesis of individual brain structures (anatomical projections and functional synaptic connections) also differs between species (e.g., human vs. mouse). Nephrogenesis completes within the first week after birth, and the maturation of the mouse kidney is essentially complete by weaning at P21 (18). In contrast, in mice the functional maturation of forebrain, midbrain, and hindbrain structures extends far after birth, beyond P21. Surprisingly little is known about the developmental timeline of major thirst-mediating centers such as the subfornical organ (SFO) and lamina terminalis regardless of species, though details have been established for other circumventricular organs (reviewed in Ref. 65). Some insights from rats indicate that major structural development occurs by P5, though nuanced aspects continue to P30 (66). Various nutritional and hemodynamic antenatal (in utero) and perinatal (<P21) insults program adult changes in SFO neuron activity and AT1R binding that correlate with and contribute to programmed cardiovascular dysfunctions (67–69). Thus, the rat SFO may be structurally formed and sensitive to ante-/perinatal programming, but the ontogenesis and plasticity of this region to postweaning insults in mice remain unexplored. In contrast to the SFO, ontogenesis of major energy balance centers such as the arcuate nucleus of the hypothalamus (ARC) in mice has been documented in some detail (recently reviewed in Ref. 20). For example, anatomical projections of melanocortin system neurons involved in the control of energy balance (including proopiomelanocortin and Agouti-related peptide neurons) are largely completed by P21, yet the functionality of these connections (e.g., establishment of functional synapses and establishment of melanocortin receptor-mediated effects in postsynaptic cells) remains ongoing up to 6 wk of age (20–22, 70–74). Many additional studies of the impact of Na supply on the development and function of different brain structures at distinct ages are warranted, to comprehensively understand these processes and to maximize the translational significance of findings from studies using nonhuman species.
It is notable in the present study that continuous Na restriction had a different effect on drinking behavior from only early-life Na restriction. Fluid intake by male mice in the Continuous model was not statistically different between diet groups at 12 wk of age (8% greater in the low-Na group, P = 0.51), but intake of low-Na males in the Postwean model displayed increased intake at 10 (20%) and 18 (29%) wk of age compared with age-matched high-Na males. This discrepancy may reflect differences in the physiological functions induced by the two models, along with differences in methodologies employed to study intake in the two models. With regard to the physiology, it is possible that continuous Na restriction may result in a distinct pattern or intensity of activation of hormonal and renal mechanisms to retain Na and water, or desensitization of mechanisms that drive fluid intake in response to such signals, resulting in reduced fluid-seeking behavior compared with mice that experienced Na depletion only in early life. Alternatively or in addition, the use of metabolic caging to evaluate fluid and energy flux in the Continuous model may result in altered behaviors. For example, individual housing in metabolic cages at room temperature prevents normal thermoregulatory behaviors (nesting, burrowing, huddling) and therefore may stimulate adaptive thermogenic mechanisms to maintain core temperature (25). Activation of such mechanisms may alter ingestive behaviors (food and fluid) and thereby confound interpretation of the effects of Na restriction. Additional study of ingestive behaviors of mice exposed to the Continuous model of Na restriction would be required to clarify this issue.
There are inherent limitations in our study. First, our Postwean model of Na deprivation limited Na intake between P21 and P42. This represents a relatively late period for organ development in mice, and restriction earlier in life through maternal Na restriction during gestation and/or lactation may result in different physiological and behavioral effects. This time frame was specifically chosen for the present study, however, both because we previously noted behavioral and metabolic effects that were programmed by this treatment (17) and because this time frame follows the completion of nephrogenesis in the mouse, therefore minimizing confounding effects of altered renal development. Second, we studied energy balance phenotypes at room temperature (22–24°C), which is below the preferred thermoneutral temperature for mice (typically near 30°C). Studying the effects of Na depletion in mice maintained at higher ambient temperatures may additionally alter findings related to growth, food and water intake, and energy expenditure. Third, the present studies were performed with customized versions of the soy-free 2920x diet. This diet was chosen to minimize dietary phytoestrogens, but other nonsodium components of the diet may contribute to phenotype development in this model (75). Fourth, for the present study we examined phenotype development up to 18 wk of age, which represents a full-grown adult but is not reflective of advanced age. It may be informative to extend the study period beyond 40–50 wk of age, to determine how early-life Na manipulations impact various end points in later life. Fifth, although both sexes were examined in this study, females were only studied at a subset of the ages studied in males. Therefore the exact time course of phenotype development and amelioration in females deserves additional study, and direct statistical comparisons between males and females may be confounded by the difference in sampling frequency. Finally, the present study focused on the effects of early-life Na manipulations on cardiometabolic phenotype development and programming in mice that were otherwise unperturbed, yet the impact of early-life Na manipulation to modify sensitivity to additional “second-hit” stimuli, such as obesogenic diets, remains untested. Future studies to examine the cardiometabolic responses of these animals to dietary, surgical, and pharmacological manipulations are warranted.
Perspectives and Significance
In summary, data presented here demonstrate prolonged, programmed effects of early-life Na restriction on cardiometabolic functions in C57BL/6J mice including reductions in food intake secondary to slower eating speeds and smaller individual meals, increases in fluid intake secondary to increased frequency of drinking bouts, and increases in basal metabolism that were mediated through increased autonomic stimulation of energy expenditure. Where differences were observed between sexes, these effects were more robust in male mice. These findings are translationally significant, as prematurely born human infants exhibit Na depletion due to renal insufficiency until they reach the equivalent of 37 wk of gestation. The present data therefore support the concept that resulting Na depletion mechanistically contributes to the programmed increases in risk for cardiometabolic dysfunction in these children as they reach adulthood. Understanding Na needs and optimizing dietary Na supply to prematurely born humans thus promises to improve growth trajectories and improve outcomes of these vulnerable patients.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by the National Institutes of Health (DK133121 to J.L.G. and J.L.S., HL134850 to J.L.G., HL084207 to J.L.G., HL007852 to A.A.Z., HL153101 to P.N., HL072483 to S.B.R.L) and the American Heart Association (18EIA33890055 to J.LG.), the Medical College of Wisconsin Clinical and Translational Science Institute “Obesity” Ensemble (UL1TR001436 to J.L.G. and J.L.S.), the Children’s Research Institute (CRI22700 to J.L.S.), and the Advancing a Healthier Wisconsin Endowment to Medical College of Wisconsin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.Z., J.L.G., and J.L.S. conceived and designed research; A.A.Z., S.B.R.L., C.C.G., J.J.R., B.P.F., C.M.L.B., J.L.G., and J.L.S. performed experiments; A.A.Z., S.B.R.L., C.C.G., J.J.R., B.P.F., C.M.L.B., J.L.G., and J.L.S. analyzed data; A.A.Z., C.C.G., J.J.R., P.N., J.L.G., and J.L.S. interpreted results of experiments; A.A.Z. and J.L.G. prepared figures; A.A.Z. and J.L.S. drafted manuscript; A.A.Z., C.C.G., J.J.R., B.P.F., C.M.L.B., P.N., J.L.G., and J.L.S. edited and revised manuscript; A.A.Z., S.B.R.L., C.C.G., J.J.R., B.P.F., C.M.L.B., P.N., J.L.G., and J.L.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge assistance provided by the Medical College of Wisconsin Comprehensive Rodent Metabolic Phenotyping Core, Neuroscience Research Center Behavior Core, Engineering Core, Biomedical Resource Center, and Genotyping Service Center.
Schematics in Fig. 1 were made with BioRender.com.
REFERENCES
- 1.World Health Organization. https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
- 2. Fenton TR, Cormack B, Goldberg D, Nasser R, Alshaikh B, Eliasziw M, Hay WW, Hoyos A, Anderson D, Bloomfield F, Griffin I, Embleton N, Rochow N, Taylor S, Senterre T, Schanler RJ, Elmrayed S, Groh-Wargo S, Adamkin D, Shah PS. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J Perinatol 40: 704–714, 2020. doi: 10.1038/s41372-020-0658-5. [DOI] [PubMed] [Google Scholar]
- 3. Gultom E, Doyle LW, Davis P, Dharmalingam A, Bowman E. Changes over time in attitudes to treatment and survival rates for extremely preterm infants (23-27 weeks’ gestational age). Aust N Z J Obstet Gynaecol 37: 56–58, 1997. doi: 10.1111/j.1479-828X.1997.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 4. Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol 27: 302–310, 2003. doi: 10.1016/S0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 5. Hay WW Jr. Aggressive nutrition of the preterm infant. Curr Pediatr Rep 1: 229–239: 2013. doi: 10.1007/s40124-013-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haycock GB. The influence of sodium on growth in infancy. Pediatr Nephrol 7: 871–875, 1993. doi: 10.1007/BF01213376. [DOI] [PubMed] [Google Scholar]
- 7. Sipola-Leppanen M, Vaarasmaki M, Tikanmaki M, Matinolli HM, Miettola S, Hovi P, Wehkalampi K, Ruokonen A, Sundvall J, Pouta A, Eriksson JG, Jarvelin MR, Kajantie E. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol 181: 861–873, 2015. doi: 10.1093/aje/kwu443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell HH, Carman GG. Does the addition of sodium chloride increase the value of a corn ration for growing animals? J Biol Chem 68: 165–181, 1926. doi: 10.1016/S0021-9258(18)84683-X. [DOI] [Google Scholar]
- 9. Vanpée M, Herin P, Broberger U, Aperia A. Sodium supplementation optimizes weight gain in preterm infants. Acta Paediatr 84: 1312–1314, 1995. doi: 10.1111/j.1651-2227.1995.tb13556.x. [DOI] [PubMed] [Google Scholar]
- 10. Kenny LC, Lavender T, McNamee R, O’Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One 8: e56583, 2013. doi: 10.1371/journal.pone.0056583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A, Moore L, Singh G, Hoy WE, Black MJ. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol 307: F149–F158, 2014. doi: 10.1152/ajprenal.00439.2013. [DOI] [PubMed] [Google Scholar]
- 12. Segar JL, Grobe CC, Grobe JL. Fetal storage of osmotically inactive sodium. Am J Physiol Regul Integr Comp Physiol 318: R512–R514, 2020. doi: 10.1152/ajpregu.00336.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perrin MT, Belfort MB, Hagadorn JI, McGrath JM, Taylor SN, Tosi LM, Brownell EA. The nutritional composition and energy content of donor human milk: a systematic review. Adv Nutr 11: 960–970, 2020. doi: 10.1093/advances/nmaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gates A, Marin T, De Leo G, Waller JL, Stansfield BK. Nutrient composition of preterm mother’s milk and factors that influence nutrient content. Am J Clin Nutr 114: 1719–1728, 2021. doi: 10.1093/ajcn/nqab226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Segar DE, Segar EK, Harshman LA, Dagle JM, Carlson SJ, Segar JL. Physiological approach to sodium supplementation in preterm infants. Am J Perinatol 35: 994–1000, 2018. doi: 10.1055/s-0038-1632366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schafflhuber M, Volpi N, Dahlmann A, Hilgers KF, Maccari F, Dietsch P, Wagner H, Luft FC, Eckardt KU, Titze J. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol 292: F1490–F1500, 2007. doi: 10.1152/ajprenal.00300.2006. [DOI] [PubMed] [Google Scholar]
- 17. Segar JL, Grobe CC, Balapattabi K, Ritter ML, Reho JJ, Grobe JL. Dissociable effects of dietary sodium in early life upon somatic growth, fluid homeostasis, and spatial memory in mice of both sexes. Am J Physiol Regul Integr Comp Physiol 320: R438–R451, 2021. doi: 10.1152/ajpregu.00281.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMahon AP. Development of the mammalian kidney. Curr Top Dev Biol 117: 31–64, 2016. doi: 10.1016/bs.ctdb.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, Bertram JF, McMahon AP, Little MH, Moore L, Black MJ. Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27: 275–283, 2018. doi: 10.1016/j.ebiom.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bouret SG. Developmental programming of hypothalamic melanocortin circuits. Exp Mol Med 54: 403–413, 2022. doi: 10.1038/s12276-021-00625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouret SG. Development of hypothalamic circuits that control food intake and energy balance. In: Appetite and Food Intake: Central Control, edited by Harris RB. Boca Raton, FL: CRC Press/Taylor & Francis, 2017, p. 135–154. [PubMed] [Google Scholar]
- 22. Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24: 2797–2805, 2004. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swaab DF. Development of the human hypothalamus. Neurochem Res 20: 509–519, 1995. doi: 10.1007/BF01694533. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 25. Ziegler AA, Grobe CC, Reho JJ, Jensen ES, Thulin JD, Segar JL, Grobe JL. Short-term housing in metabolic caging on measures of energy and fluid balance in male C57BL/6J mice (Mus musculus). J Am Assoc Lab Anim Sci 61: 132–139, 2022. doi: 10.30802/AALAS-JAALAS-21-000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reho JJ, Nakagawa P, Mouradian GC Jr, Grobe CC, Saravia FL, Burnett CML, Kwitek AE, Kirby JR, Segar JL, Hodges MR, Sigmund CD, Grobe JL. Methods for the comprehensive in vivo analysis of energy flux, fluid homeostasis, blood pressure, and ventilatory function in rodents. Front Physiol 13: 855054, 2022. doi: 10.3389/fphys.2022.855054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Segar JL, Balapattabi K, Reho JJ, Grobe CC, Burnett CM, Grobe JL. Quantification of body fluid compartmentalization by combined time-domain nuclear magnetic resonance and bioimpedance spectroscopy. Am J Physiol Regul Integr Comp Physiol 320: R44–R54, 2021. doi: 10.1152/ajpregu.00227.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soto JE, Burnett CM, Ten Eyck P, Abel ED, Grobe JL. Comparison of the effects of high-fat diet on energy flux in mice using two multiplexed metabolic phenotyping systems. Obesity (Silver Spring) 27: 793–802, 2019. doi: 10.1002/oby.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neely CL, Pedemonte KA, Boggs KN, Flinn JM. Nest building behavior as an early indicator of behavioral deficits in mice. J Vis Exp 152: e60139, 2019. doi: 10.3791/60139. [DOI] [PubMed] [Google Scholar]
- 31. Gordon CJ. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiol Behav 179: 55–66, 2017. doi: 10.1016/j.physbeh.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gordon CJ. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol 37: 654–685, 2012. doi: 10.1016/j.jtherbio.2012.08.004. [DOI] [Google Scholar]
- 33. Gordon CJ. Temperature Regulation in Laboratory Rodents. Cambridge: Cambridge University Press, 2009. [Google Scholar]
- 34. Smith JC. Microstructure of the rat’s intake of food, sucrose and saccharin in 24-hour tests. Neurosci Biobehav Rev 24: 199–212, 2000. doi: 10.1016/S0149-7634(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 35. Wassner SJ. The effect of sodium repletion on growth and protein turnover in sodium-depleted rats. Pediatr Nephrol 5: 501–504, 1991. doi: 10.1007/BF01453690. [DOI] [PubMed] [Google Scholar]
- 36. Wassner SJ. Altered growth and protein turnover in rats fed sodium-deficient diets. Pediatr Res 26: 608–613, 1989. doi: 10.1203/00006450-198912000-00019. [DOI] [PubMed] [Google Scholar]
- 37. Fine BP, Ty A, Lestrange N, Levine OR. Sodium deprivation growth failure in the rat: alterations in tissue composition and fluid spaces. J Nutr 117: 1623–1628, 1987. doi: 10.1093/jn/117.9.1623. [DOI] [PubMed] [Google Scholar]
- 38. Sumanasena SP, Vipulaguna DV, Mendis MM, Gunawardena NS. Beyond survival: 5-year neurodevelopmental follow-up of a cohort of preterm infants in Colombo, Sri Lanka. Paediatr Int Child Health 38: 128–136, 2018. doi: 10.1080/20469047.2017.1380944. [DOI] [PubMed] [Google Scholar]
- 39. Hovi P, Vohr B, Ment LR, Doyle LW, McGarvey L, Morrison KM, Evensen KA, van der Pal S, Grunau RE; Adults Born Preterm International Collaboration, Brubakk AM, Andersson S, Saigal S, Kajantie E. Blood pressure in young adults born at very low birth weight: Adults Born Preterm International Collaboration. Hypertension 68: 880–887, 2016. doi: 10.1161/HYPERTENSIONAHA.116.08167. [DOI] [PubMed] [Google Scholar]
- 40. Alexander BT, South AM, August P, Bertagnolli M, Ferranti EP, Grobe JL, Jones EJ, Loria AS, Safdar B, Sequeira-Lopez ML. Appraising the preclinical evidence of the role of the renin-angiotensin-aldosterone system in antenatal programming of maternal and offspring cardiovascular health across the life course: moving the field forward: a Scientific Statement From the American Heart Association. Hypertension 80: e75–e89, 2023. doi: 10.1161/HYP.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Claflin KE, Grobe JL. Control of energy balance by the brain renin-angiotensin system. Curr Hypertens Rep 17: 38, 2015. doi: 10.1007/s11906-015-0549-x. [DOI] [PubMed] [Google Scholar]
- 42. Littlejohn NK, Grobe JL. Opposing tissue-specific roles of angiotensin in the pathogenesis of obesity, and implications for obesity-related hypertension. Am J Physiol Regul Integr Comp Physiol 309: R1463–R1473, 2015. doi: 10.1152/ajpregu.00224.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oliveira V, Kwitek AE, Sigmund CD, Morselli LL, Grobe JL. Recent advances in hypertension: intersection of metabolic and blood pressure regulatory circuits in the central nervous system. Hypertension 77: 1061–1068, 2021. doi: 10.1161/HYPERTENSIONAHA.120.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, Seeley RJ, Woods SC. Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab 301: E1081–E1091, 2011. doi: 10.1152/ajpendo.00307.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oliveira V, Reho JJ, Balapattabi K, Ritter ML, Mathieu NM, Opichka MA, Lu KT, Grobe CC, Silva SD Jr, Wackman KK, Nakagawa P, Segar JL, Sigmund CD, Grobe JL. Chronic intracerebroventricular infusion of angiotensin II causes dose- and sex-dependent effects on intake behaviors and energy homeostasis in C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol 323: R410–R421, 2022. doi: 10.1152/ajpregu.00091.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, Pearson NA, Morgan DA, Gibson-Corley KN, Rahmouni K, Grobe JL. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest 127: 1414–1424, 2017. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Littlejohn NK, Keen HL, Weidemann BJ, Claflin KE, Tobin KV, Markan KR, Park S, Naber MC, Gourronc FA, Pearson NA, Liu X, Morgan DA, Klingelhutz AJ, Potthoff MJ, Rahmouni K, Sigmund CD, Grobe JL. Suppression of resting metabolism by the angiotensin AT2 receptor. Cell Rep 16: 1548–1560, 2016. doi: 10.1016/j.celrep.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Riedl RA, Burnett CM, Pearson NA, Reho JJ, Mokadem M, Edwards RA, Kindel TL, Kirby JR, Grobe JL. Gut microbiota represent a major thermogenic biomass. Function (Oxf) 2: zqab019, 2021. doi: 10.1093/function/zqab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burnett CM, Grobe JL. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Mol Metab 3: 460–464, 2014. doi: 10.1016/j.molmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burnett CM, Grobe JL. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. Am J Physiol Endocrinol Metab 305: E916–E924, 2013. doi: 10.1152/ajpendo.00387.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walsberg GE, Hoffman TC. Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. J Exp Biol 208: 1035–1043, 2005. doi: 10.1242/jeb.01477. [DOI] [PubMed] [Google Scholar]
- 54. Pittet P, Chappuis P, Acheson K, De Techtermann F, Jequier E. Thermic effect of glucose in obese subjects studied by direct and indirect calorimetry. Br J Nutr 35: 281–292, 1976. doi: 10.1079/BJN19760033. [DOI] [PubMed] [Google Scholar]
- 55. Pittet P, Gygax PH, Jequier E. Thermic effect of glucose and amino acids in man studied by direct and indirect calorimetry. Br J Nutr 31: 343–349, 1974. doi: 10.1079/BJN19740042. [DOI] [PubMed] [Google Scholar]
- 56. Ye Y, Abu El Haija M, Morgan DA, Guo D, Song Y, Frank A, Tian L, Riedl RA, Burnett CML, Gao Z, Zhu Z, Shahi SK, Zarei K, Couvelard A, Poté N, Ribeiro-Parenti L, Bado A, Noureddine L, Bellizzi A, Kievit P, Mangalam AK, Zingman LV, Le Gall M, Grobe JL, Kaplan LM, Clegg D, Rahmouni K, Mokadem M. Endocannabinoid receptor-1 and sympathetic nervous system mediate the beneficial metabolic effects of gastric bypass. Cell Rep 33: 108270, 2020. doi: 10.1016/j.celrep.2020.108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trautmann T, Bang C, Franke A, Vincent D, Reinshagen K, Boettcher M. The impact of oral sodium chloride supplementation on thrive and the intestinal microbiome in neonates with small bowel ostomies: a prospective cohort study. Front Immunol 11: 1421, 2020. doi: 10.3389/fimmu.2020.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thänert R, Keen EC, Dantas G, Warner BB, Tarr PI. Necrotizing enterocolitis and the microbiome: current status and future directions. J Infect Dis 223: S257–S263, 2021. doi: 10.1093/infdis/jiaa604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology 148: 1219–1233, 2015. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rakova N, Kitada K, Lerchl K, Dahlmann A, Birukov A, Daub S, Kopp C, Pedchenko T, Zhang Y, Beck L, Johannes B, Marton A, Müller DN, Rauh M, Luft FC, Titze J. Increased salt consumption induces body water conservation and decreases fluid intake. J Clin Invest 127: 1932–1943, 2017. doi: 10.1172/JCI88530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kitada K, Daub S, Zhang Y, Klein JD, Nakano D, Pedchenko T, Lantier L, LaRocque LM, Marton A, Neubert P, Schröder A, Rakova N, Jantsch J, Dikalova AE, Dikalov SI, Harrison DG, Muller DN, Nishiyama A, Rauh M, Harris RC, Luft FC, Wassermann DH, Sands JM, Titze J. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest 127: 1944–1959, 2017. doi: 10.1172/JCI88532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mecawi AS, Macchione AF, Nuñez P, Perillan C, Reis LC, Vivas L, Arguelles J. Developmental programing of thirst and sodium appetite. Neurosci Biobehav Rev 51: 1–14, 2015. doi: 10.1016/j.neubiorev.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 63. Mouw DR, Vander AJ, Wagner J. Effects of prenatal and early postnatal sodium deprivation on subsequent adult thirst and salt preference in rats. Am J Physiol Renal Physiol 234: F59–F63, 1978. doi: 10.1152/ajprenal.1978.234.1.F59. [DOI] [PubMed] [Google Scholar]
- 64. Galaverna O, Nicolaidis S, Yao SZ, Sakai RR, Epstein AN. Endocrine consequences of prenatal sodium depletion prepare rats for high need-free NaCl intake in adulthood. Am J Physiol Regul Integr Comp Physiol 269: R578–R583, 1995. doi: 10.1152/ajpregu.1995.269.3.R578. [DOI] [PubMed] [Google Scholar]
- 65. Kiecker C. The origins of the circumventricular organs. J Anat 232: 540–553, 2018. doi: 10.1111/joa.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dellmann HD, Stahl SJ. Fine structural cytology of the rat subfornical organ during ontogenesis. Brain Res Bull 13: 135–145, 1984. doi: 10.1016/0361-9230(84)90015-7. [DOI] [PubMed] [Google Scholar]
- 67. Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res 55: 1042–1049, 2004. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- 68. Dadam FM, Amigone JL, Vivas L, Macchione AF. Comparison of dipsogenic responses of adult rat offspring as a function of different perinatal programming models. Brain Res Bull 188: 77–91, 2022. doi: 10.1016/j.brainresbull.2022.07.015. [DOI] [PubMed] [Google Scholar]
- 69. Macchione AF, Beas C, Dadam FM, Caeiro XE, Godino A, Ponce LF, Amigone JL, Vivas L. Early free access to hypertonic NaCl solution induces a long-term effect on drinking, brain cell activity and gene expression of adult rat offspring. Neuroscience 298: 120–136, 2015. doi: 10.1016/j.neuroscience.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 70. Melnick I, Pronchuk N, Cowley MA, Grove KL, Colmers WF. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron 56: 1103–1115, 2007. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 71. Nilsson I, Johansen JE, Schalling M, Hökfelt T, Fetissov SO. Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Brain Res Dev Brain Res 155: 147–154, 2005. doi: 10.1016/j.devbrainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 72. Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J Neurosci 28: 9218–9226, 2008. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci USA 105: 2687–2692, 2008. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310: 683–685, 2005. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 75. Patil CN, Ritter ML, Wackman KK, Oliveira V, Balapattabi K, Grobe CC, Brozoski DT, Reho JJ, Nakagawa P, Mouradian GC Jr, Kriegel AJ, Kwitek AE, Hodges MR, Segar JL, Sigmund CD, Grobe JL. Cardiometabolic effects of DOCA-salt in male C57BL/6J mice are variably dependent on sodium and nonsodium components of diet. Am J Physiol Regul Integr Comp Physiol 322: R467–R485, 2022. doi: 10.1152/ajpregu.00017.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.