Abstract
Objectives
Laryngeal cartilage defects are a major problem that greatly impacts structural integrity and function. Cartilage repair is also a challenging issue. This study evaluated the efficacy of a collagen scaffold enveloped by amniotic membrane (AM/C) on laryngeal cartilage repair.
Study Design
Experimental animal study.
Methods
Fourteen Dutch rabbits were enrolled in the study. A 5 mm cartilage defect was created in the right and left thyroid lamina. The animals were divided into two groups randomly. Group 1 collagen scaffolds and group 2 AM/C were applied to the right side defects. Left side defects were not repaired, serving as control. Histologic evaluation was done 45 and 90 days following collagen and AM/C application with criteria of tissue and cell morphology, lacuna formation, vascularization, and inflammation.
Results
Significant improvement in cartilage repair was observed in the AM/C side compared to the control side in all histologic criteria after 45 days (p<.05). After 90 days, cartilage repair improved in cell morphology, lacuna formation, and inflammation significantly (p<.05).
Conclusion
The combination of amniotic membrane and collagen scaffolds provides a promising treatment modality for improving the repair of laryngeal cartilage defects.
Level of Evidence
NA.
Keywords: amniotic membrane, animal model, cartilage regeneration, collagen, laryngeal repair, scaffold
Our article evaluated the efficacy of collagen scaffolds enveloped in amniotic membrane (AM/C) on cartilage repair in an animal model. Our findings showed that AM/C promotes cartilage healing and can be a promising treatment modality in future.
1. INTRODUCTION
Cartilaginous defects of the larynx, which are mainly caused by trauma and procedures to remove tumors, have a great impact on the framework integrity and subsequent laryngeal function. Due to the avascular nature of cartilage, self‐repair is poor. Aberrant repair can also lead to scar formation and stenosis in the larynx. 1
Various treatment modalities, materials, and drugs have been introduced to facilitate laryngeal cartilaginous healing and prevent complications. Several clinical and experimental studies have shown different degrees of success and limitations of these treatment modalities, such as the use of amniotic membrane (AM), 2 adipose‐derived stem cells (ASCs), 3 seeded scaffolds, 4 and platelet‐rich plasma (PRP) 5 in laryngeal defects repair.
Scaffolds are tissue‐engineered materials that have a special place in regenerative medicine investigations. They are made of natural, synthetic, or a combination of both (hybrid) materials. 6 Scaffolds provide structural support for cell adhesion, migration, and growth in tissue regeneration. Scaffold materials should be biocompatible with minimal immunogenic and inflammatory adverse effects. 7
Collagen is one of the materials used in making natural scaffolds. Due to its similarity with the extracellular cartilage matrix, collagen scaffolds alone or with natural and synthetic materials are widely used in cartilage regeneration. 7 , 8 They provide a low immunogenic, highly biocompatible, and biodegradable material for cellular proliferation, migration, and differentiation. 9 Studies have demonstrated that collagen scaffolds promote mesenchymal stromal cells and connective tissues regenerative properties with restoration of function. 10 , 11
The amniotic membrane (AM) is the innermost part of the placenta. It comprises an inner single epithelial layer, a thick basement membrane, and an avascular stroma. 12 The amniotic membrane acts as a source of stem cells. Amniotic epithelial cells (AECs) and amniotic mesenchymal stromal cells (AMSCs) are the two types of stem cells detected in AM. The latter can differentiate into chondrocytes, osteocytes, myocytes, and adipocytes. 13 , 14
Investigations have shown that cells release some growth factors 15 , 16 and cytokines, 17 , 18 such as epithelial growth factor, fibroblast growth factor, transforming growth factor, keratinocyte growth factor, IL‐6, and IL‐10. These mediators promote cellular proliferation, migration, and differentiation and reduce tissue inflammation and infection. 19 , 20
In this study, we investigated the efficacy of collagen‐based scaffolds enveloped by amniotic membrane (AM/C) on regeneration of cartilaginous laryngeal defects in an experimental model in rabbits. We used decellularized hyaline cartilage as a scaffold, mainly consisting of collagen type II and glycosaminoglycan. The contents of these biomaterials are very similar to the cartilaginous extracellular matrix. In contrast to similar studies, we did not use any types of stem cells. Instead, we applied AM for its potential ability to transform into stem cells and have growth factors that enhance tissue regeneration.
2. MATERIALS AND METHODS
2.1. Animal preparation
Fourteen male Dutch rabbits, aged 5–6 months, were enrolled in this study. The rabbits were housed individually in a controlled environment regarding temperature, humidity, and full access to food and water. The study protocols and procedures were approved by the local Ethics Committee of SUMS (IR.SUMS.MED.REC.1401.013) and according to Shiraz University's regulations on animal care.
The rabbits were divided into two equal groups. The first group (group 1) was considered cartilage repair with collagen scaffolds alone, and the second group (group 2)was repaired with AM and collagen scaffolds.
In the operating room, animals were anesthetized using intramuscular ketamine (44 mg/kg) and xylazine (10 mg/kg). After preparing the neck area, a short incision was made over the thyroid cartilage, and the strap muscles were separated in the midline. After exposure to thyroid cartilage, two symmetric cartilage defects (5 mm in diameter) were created on both sides of the thyroid lamina. On the right side, the defect was covered with collagen scaffolds in group 1 and scaffolds enveloped by the amniotic membrane in group 2. On the left side, no intervention was done in either group, and it was considered as a control. The inner perichondrium of thyroid cartilage remained intact in two groups. Finally, the strap muscles were approximated, and the skin incision was sutured with nylon 5‐0. All rabbits received streptomycin (3 × 106 units/day) for three days to prevent infection following intervention.
At the predetermined times of 45 and 90 days following the intervention, seven rabbits were sacrificed each time, and laryngeal specimens were harvested. Laryngeal specimens were preserved in 10% formalin and sent for histopathologic examination.
2.2. Histological criteria
Histologic features were studied in paraffin‐embedded sections using hematoxylin and eosin and Masson's trichrome stains at a magnification of ×40 to ×400.
Many scores have been used for the histopathological assessment of cartilage repair. Most recently, Mainil‐Varlet et al. validated a new histology scoring system for the assessment of the quality of cartilage repair named ICRS II (International Cartilage Research Society), which is an attempt to approve many of the defects of prior scores. 21 Therefore, we used a modified scoring system (Table 1) for histologic evaluation based on this scoring system. The main histologic findings included tissue morphology, cell morphology, lacuna formation, new vascularization, and inflammation in new cartilage. The definition of each histologic index is listed below, and criteria gradings are shown in Table 1.
TABLE 1.
Grading of histologic indexes for cartilage regeneration.
| Histologic index | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Tissue morphology |
Fibrous tissue (Poor quality repair tissue, characterizedby collagen fibers throughout the tissue and the presence of blood vessels) |
Fibrocartilage (Collagen fibers can be seen throughout the immature cartilage tissue) |
Mixed hyaline and fibrocartilage |
Hyaline cartilage (Good‐qualityrepair tissue, represented by homogeneous (glass‐like) tissue with no obvious collagen fibers) |
| Cell Morphology | Mostly spindle‐shaped cells | Both spindle‐shaped (dominant) and round/oval cells | Both spindle‐shaped and round/oval cells (dominant) | Mostly round/oval cells |
| Lacuna formation (%) | <25 | 25–50 | 51–75 | >75 |
| New vascularization (%) | <25 | 25–50 | 51–75 | >75 |
| Inflammation | None | Scant | Moderate | Abundant |
Tissue morphology: The distribution of collagen fibers.
Cell Morphology: Cells associated with hyaline cartilage are oval. Cells associated with fibrocartilage are more elongated and spindle‐shaped.
Lacuna formation: Estimated by the percentage of chondrocytes in the new cartilage that contains lacuna formation.
Percentage of new vascularization: Estimated by the percentage of new cartilage tissue containing blood vessels.
Inflammation: Defined as the presence of inflammatory cells (mainly chronic cell types such as lymphocytes and plasma cells) in the new cartilage tissue.
We also used Masson's trichrome stains to increase the accuracy of detection of collagen bundles and so better fibrosis scoring. Histologic examination was done by an expert pathologist masked as to the treatment side and type of intervention.
2.3. Scaffold preparation
Following the previously published procedure, hyaline cartilage was collected from the calf nose and decellularized. 22 In brief, minced cartilage was incubated in 10 mMTris–HCl, pH 8.0, then frozen at −80°C and thawed at 37°C for six cycles. Trypsin 0.25% was applied for 24 h at 37°C. After trypsinization of cartilage, it was rinsed with a hypertonic buffer solution containing 150 mMNaCl in 50 mMTris‐HCl, pH 7.6, and subsequently treated with 50 U/mL DNAse solution, 10 mMTris‐HCl, pH 7.5, for 4 h at 37°C. To vacate enzyme residues, the slurry was treated with hypotonic Tris–HCl for 20 hours, followed by incubation with 1% Triton X‐100 for 48 h, and the solution was refreshed every 24 h. PBS was rinsed several times after each step to remove cell remnants and chemicals. The decellularized cartilage was rinsed for 3 days in distilled water to wash away all detergent. In the next step, decellularized extracellular matrix (dECM) was lyophilized and, in liquid nitrogen, crushed. To digest dECM powder, 50 mg/mL was weighed and digested in 0.5 M acetic acid with pepsin (P7125 by Sigma‐Aldrich) for 48 h. Each 100 mg dECM was treated with 10 mg pepsin. The sponge‐shaped scaffolds were formed by lyophilizing dECM pre‐gel (Christ, Alpha 1–2 LD, Germany). In subsequent steps, scaffolds were chemically cross‐linked with 50 mM sterile 2‐N‐morphlino ethanesulfonic (MES, Sigma, USA) in 70% ethanol (pH 5.4) containing 0.059 mol/L of 1‐ethyl‐3‐[3‐dimethylaminopropyl] carbodiimide (EDC, Sigma, USA) and 0.04 mol/L N‐hydroxysulfosuccinimide (NHS, Sigma, USA) for 12 h at 37°C. The scaffolds were rinsed with Na2HPO4 (0.1 M) to inhibit cross‐linking for 2 h. In preparation for transplantation, sponges were washed several times with sterile deionized water, sterilized using 70% ethanol, and rinsed thoroughly with distilled water.
2.4. AM preparation
Due to the lack of immunogenicity, we used human AM in rabbits as a xenograft. AMs were taken from women who did not have a history of pregnancy problems such as premature rupture of membrane and endometritis and had undergone elective cesarean section. In a sterile condition, blunt dissection separates the amnion from the chorion. The amnion was washed three times with phosphate buffer saline (PBS) containing penicillin, streptomycin, and amphotericin B. The amnion was spread on a nitrocellulose membrane with the epithelial surface up and cut into 5 × 5 pieces. The amnion pieces were immersed in 4%, 8%, and 12% dimethyl sulphoxide (DMSO) PBS for 5 min. Finally, the membranes were placed in vials containing 12% DMSO and stored at −80°C before application.
2.5. Statistical analysis
Data presented as mean± SD and one‐way analysis of variance was used to compare groups followed by Tukey post hoc test. All the statistical analyses were performed in SPSS version 23.0, and p < .05 was considered statistically significant.
3. RESULTS
Effects of AM/C and collagen scaffolds on cartilage repair in 45 and 90 days following application are summarized in Tables 2 and 3.
TABLE 2.
Comparison of histopathological variables among three understood groups after 45 days.
| Variable | Control | Scaffold | AM/C | LSD TEST | ||
|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | mean ± SD | Scaffold versus control | Am/C versus control | AM/C versus scaffold | |
| Tissue morphology | 0.86 ± 0.38 | 1.33 ± 0.58 | 2.25 ± 0.50 | 0.16 | <0.001 | 0.02 |
| Cell morphology | 1.14 ± 0.38 | 2.00 ± 0.00 | 1.75 ± 0.50 | 0.01 | 0.03 | 0.41 |
| lacuna formation | 0.14 ± 0.38 | 1.00 ± 0.00 | 1.00 ± 0.82 | 0.03 | 0.02 | 0.99 |
| New vascularization | 3.14 ± 0.69 | 2.00 ± 0.00 | 1.25 ± 0.50 | 0.01 | <0.001 | 0.11 |
| Inflammation | 2.86 ± 0.69 | 2.33 ± 0.58 | 1.50 ± 0.58 | 0.26 | 0.01 | 0.12 |
Values less than <0.05 are in red.
TABLE 3.
Comparison of histopathological variables among three understood groups after 90 days.
| Variable | Control | Scaffold | AM/C | LSD TEST | ||
|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | mean ± SD | Scaffold versus control | AM/C versus control | AM/C versus scaffold | |
| Tissue morphology | 1.67 ± 0.52 | 2.25 ± 0.50 | 2.50 ± 0.71 | 0.13 | 0.09 | 0.60 |
| Cell morphology | 1.33 ± 0.52 | 2.50 ± 0.58 | 2.50 ± 0.71 | 0.01 | 0.03 | 0.99 |
| lacuna formation | 0.33 ± 0.52 | 2.25 ± 0.50 | 2.00 ± 0.00 | <0.001 | 0.002 | 0.56 |
| New vascularization | 1.83 ± 0.75 | 1.00 ± 0.82 | 0.50 ± 0.71 | 0.13 | 0.06 | 0.47 |
| Inflammation | 2.17 ± 0.75 | 1.00 ± 0.82 | 0.50 ± 0.71 | 0.04 | 0.03 | 0.47 |
Values less than <0.05 are in red.
After 45 days, tissue morphology was significantly higher in AM/C compared to the control (p < .001) and scaffolds (p = .02) groups. The cell was significantly lower in the control group compared to the scaffolds (p = .01) and AM/C (p = 0.03) groups. Also, lacuna formation was significantly lower in the control group compared to the scaffolds (p = .001 and p = .01) and AM/C (p < .001 and p < .001) groups, respectively. Moreover, the observed inflammation was significantly lower in the AM/C group compared to the control group (p = .01, Table 2) (Figure 1).
FIGURE 1.
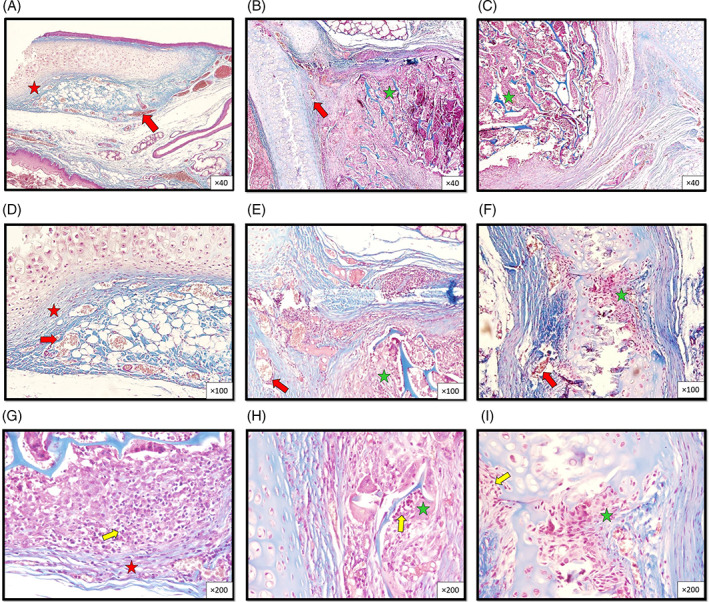
Histopathologic micrographs from sections of the thyroid cartilage, 45 days after wounding in rabbit groups at different magnifications show the presence of moderate vascularization (red arrow) and moderate inflammatory cellar infiltration in the control group (yellow arrow) compared to the other two groups (scaffold, and scaffold + AMT). New cartilage formation in the grafted scaffold shows more fibrous tissue in the control group (red asterisk) compared to more fibrohyaline cartilage in the other two groups (scaffold and AM/C) (green asterisk). (Masson's trichrome. Control group: A, D, G, scaffold group: B, E, H, and scaffold + AMT group: C, F, I. Magnification ×40 (A, B, C), ×100 (D, E, F), and ×200 (G, H, I).)
After 90 days, cell morphology was significantly lower in the control group compared to the scaffolds (p = .01) and AM/C (p = 0.03) groups. Also, lacuna formation was significantly lower in the control group compared to the scaffolds (p < .001) and AM/C (p = .002) groups, respectively. Moreover, the observed inflammation was significantly higher in the control group compared to the scaffolds (p = .04) and AM/C groups (p = .03, Table 3) (Figure 2).
FIGURE 2.
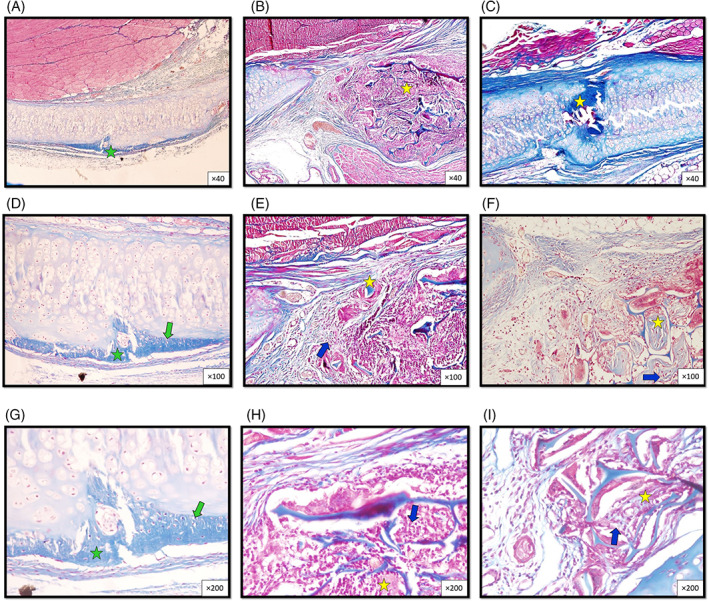
Histopathologic micrographs from sections of the thyroid cartilage, 90 days after wounding in rabbit groups at different magnifications show more fibrohyaline cartilage formation in the control group(green asterisk) compared to more hyaline cartilage in grafted scaffold between scaffold trabeculae in other two groups (scaffold, and AM/C) (yellow asterisk). The control group shows a single chondrocyte within a single lacuna (green arrows) compared to the other two groups, which show more lacuna formation and more chondrocyte maturation (blue arrow). (Masson's trichrome. Control group: A, D, G, scaffold group: B, E, H, and scaffold + AMT group: C, F, I. Magnification ×40 (A, B, C), ×100 (D, E, F), and ×200 (G, H, I).)
In 45 days, except for the tissue morphology criteria, there is no significant difference in the effect of AM/C and collagen scaffolds on cartilage repair. After 90 days, there is no significant difference between AM/C and collagen scaffolds in all histological parameters of cartilage repair.
4. DISCUSSION
Cartilaginous defects of the larynx and its repair are challenging issues that greatly impact the quality of life. This study revealed that AM/C can significantly improve cartilaginous laryngeal defect repair. This study also showed that the collagen scaffolds alone, although to a lesser extent, can have similar effects on cartilage repair compared to the AM/C.
In recent years, collagen scaffolds have had various clinical and research applications. Recent investigations have shown the effect of collagen on the repair of connective tissue defects, including tendons and ligaments. 23 , 24 Investigations also showed that in addition to improving the damaged tendon healing, its function returns to normal. 25 , 26 Evidence has shown that collagen scaffolds affect the differentiation of chondrocytes by increasing the expression of cartilage‐specific genes of aggrecan and the sex‐determining region Y‐box (SOX90). 27 , 28
Type II collagen is one factor that plays a role in the differentiation and maturation of chondrocytes. Type II collagen also enhances cartilage regeneration through the immune system and activation of anti‐inflammatory macrophages. The effect on the immune system also causes the production of pro‐chondrogenic cytokines, such as IL‐10, IL‐1RA, and transforming growth factor beta (TGF‐b), which play a role in wound healing. 29 , 30
Despite the natural type of the collagen scaffolds, which makes it highly biocompatible, it has limitations. As mentioned in studies, the limitations of using collagen scaffolds include weak mechanical properties and low chondrogenic capacity. 31 , 32 Due to these shortcomings in using collagen scaffolds alone, many researchers use collagen together with mesenchymal stem cells in cartilage repair. Due to the similarity of the collagen scaffolds with the extracellular matrix of cartilage, the chondrogenic capacity of seeded mesenchymal stem cells is enhanced. This similarity in composition of the extracellular matrix and collagen scaffolds causes more cellular attachment and better stem cell proliferation and differentiation. It is considered that collagen scaffolds induce chondrogenic induction through β1 integrin‐mediated Rho A/Rock signaling on mesenchymal stem cells. 33 , 34 , 35
The physical properties of collagen in terms of its stiffness, fibrillar, and porous structure also provide suitable conditions for cellular seeding, adhesion, growth, secretion, and differentiation. The three‐dimensional properties of collagen scaffolds create a microenvironment that promotes cell attachment and chondrogenic differentiation. The effect of this microenvironment on cell morphology and differentiation causes secretion of aggrecan and collagen type II, which in turn provides an environment similar to cartilage extracellular matrix. 36 , 37
Because of having mesenchymal stem cells, the AM can be used as a source of stem cells in regenerative medicine. In the study by Hortensius et al., the AM was used with collagen scaffolds to repair damaged tendons. 38 Cao et al. used AM/collagen scaffolds with ASCs to repair laryngeal cartilage defects in rabbits. 39 In another study, collagen scaffolds fabricated with AM matrix were used to enhance the differentiation of ASCs to craniofacial bones under inflammatory conditions. 40 The AM is a source of mesenchymal stem cells and also a natural scaffold in tissue regeneration. The two anti‐inflammatory and anti‐fibrosis properties of the AM have highlighted its role in tissue regeneration. AM downregulated the expression of pro‐inflammatory cytokines in healing tissues. 17 , 41 , 42
Human AM matrix is one of the sources of mesenchymal stem cells. The human AM matrix detects some growth factors, such as fibroblast growth factor, epidermal growth factor, and transforming growth factor beta (TGF‐b). Among these, the role of TGF‐b is more obvious than others in chondrogenesis. TGF‐b, through activation of intracellular kinase A and C and mitogen‐activated protein kinase, up‐regulates the chondrogenic transcriptional factors of SOX9 and COL2A1. This transcriptional activation enhances the synthesis of collagen type 2 and aggrecan, promoting chondrogenic proliferation and differentiation. 43 , 44
Studies have shown the superiority of amniotic MSCs over other types of MSCs, such as those derived from the bone marrow and adipose tissue. This superiority is in the higher proliferation capacity, lower immunogenicity, and even lower mutation incorporation. Amniotic MSCs have lower HLA class 1 expression and can secrete cytokines that modulate the immune response. 45 , 46 These characteristics of the AM have created a promising future for use in regenerative medicine. For this reason, in this study, we used AM with collagen scaffolds and without MSCs.
One of the interesting findings of this study was that although the collagen scaffolds have a lower effect on cartilage regeneration according to histological criteria than the AM/C, this difference is not statistically significant (except for tissue morphology at 45 days). Among the reasons for this effect, we can mention the similarity of the collagen scaffolds to the extracellular matrix of cartilage and the presence of collagen type II and pro‐chondrogenic mediators such as IL‐10, IL‐1RA, and TGF‐b. The US Food and Drug Administration also approved the clinical use of decellularized matrix scaffolds, which can play an important role in regenerative medicine in the future. 47
The strength of this study is the use of specific histopathological criteria for cartilage repair in an animal model. Detailed assessment of histopathological changes in laryngeal cartilage repair can be the basis of clinical trials in the future. One limitation of this study is using human AM in an animal study. However, considering the AM's non‐immunogenicity, we think this cannot be a major concern for this study.
5. CONCLUSION
Cartilaginous defects of the larynx are one of the problems that greatly impact the functioning of the larynx and, as a result, the quality of life. Our experimental study showed that based on histological criteria, collagen scaffolds enveloped by amniotic membrane (AM/C) have a significant effect on promoting the repair of laryngeal cartilage defects. The findings of this pilot preclinical study can be used in future clinical trials.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The present article was financially supported by Shiraz University of Medical Sciences (grant No. 24762). The authors thank Shiraz University of Medical Sciences, Shiraz, Iran.
Iravani K, Mousavi S, Owji SM, Sani M, Owji SH. Effect of amniotic membrane/collagen scaffolds on laryngeal cartilage repair. Laryngoscope Investigative Otolaryngology. 2024;9(1):e1222. doi: 10.1002/lio2.1222
This work was done in the Department of Otolaryngology with the collaboration of the animal laboratory, Shiraz University of Medical Sciences, Shiraz, Iran.
REFERENCES
- 1. Lorenz RR. Adult laryngotracheal stenosis: etiology and surgical management. Curr Opin Otolaryngol Head Neck Surg. 2003;11(6):467‐472. [DOI] [PubMed] [Google Scholar]
- 2. Iravani K, Mehravar S, Bahador M, Azarpira N. The healing effect of amniotic membrane in laryngeal defects in rabbit model. Laryngoscope. 2021;131(2):E527‐e533. [DOI] [PubMed] [Google Scholar]
- 3. Brookes S, Zhang L, Puls TJ, Kincaid J, Voytik‐Harbin S, Halum S. Laryngeal reconstruction using tissue‐engineered implants in pigs: A pilot study. Laryngoscope. 2021;131(10):2277‐2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herrmann P, Ansari T, Southgate A, et al. In vivo implantation of a tissue engineered stem cell seeded hemi‐laryngeal replacement maintains airway, phonation, and swallowing in pigs. J Tissue Eng Regen Med. 2019;13(11):1943‐1954. [DOI] [PubMed] [Google Scholar]
- 5. Reksodiputro MH, Hutauruk SM, Widodo DW, Fardizza F, Mutia D. Platelet‐rich fibrin enhances surgical wound healing in total laryngectomy. Facial Plast Surg. 2021. Jun;37(3):325‐332. [DOI] [PubMed] [Google Scholar]
- 6. Kalkan R, Nwekwo CW, Adali T. The use of Sca_olds in cartilage regeneration. Eukaryot Gene Expr. 2018;28:343‐348. [DOI] [PubMed] [Google Scholar]
- 7. Wasyłeczko M, Sikorska W, Chwojnowski A. Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes (Basel). 2020;10(11):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irawan V, Sung TC, Higuchi A, Ikoma T. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng Regen Med. 2018;15(6):673‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tonndorf R, Aibibu D, Cherif C. Isotropic and anisotropic scaffolds for tissue engineering: collagen, conventional, and textile fabrication technologies and properties. Int J Mol Sci. 2021;22(17):9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rashedi I, Talele N, Wang XH, Hinz B, Radisic M, Keating A. Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS One. 2017;12(10):e0187348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie X, Xu J, Lin J, et al. A regeneration process‐matching scaffold with appropriate dynamic mechanical properties and spatial adaptability for ligament reconstruction. Bioact Mater. 2021;12(13):82‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salah RA, Mohamed IK, El‐Badri N. Development of decellularizedamniotic membrane as a bioscafold for bone marrow‐derived mesenchymal stem cells: ultrastructural study. J Mol Histol. 2018;49:289‐301. [DOI] [PubMed] [Google Scholar]
- 13. Cai J, Li W, Su H, et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J Biol Chem. 2010;285(15):11227‐11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu J, Cai Z, Zhou Z. Progress in studies on the characteristics of human amnion mesenchymal cells. Prog Nat Sci. 2009;19(9):1047‐1052. [Google Scholar]
- 15. Yoon BS, Moon J‐H, Jun EK, et al. Secretory profiles and wound healing effects of human amniotic fluid‐derived mesenchymal stem cells. Stem Cells Dev. 2009;19(6):887‐902. [DOI] [PubMed] [Google Scholar]
- 16. Grzywocz Z, Pius‐Sadowska E, Klos P, et al. Growth factors and their receptors derived from human amniotic cells in vitro. Folia Histochem Cytobiol. 2014;52(3):163‐170. [DOI] [PubMed] [Google Scholar]
- 17. Elkhenany H, El‐Derby A, AbdElkodous M, Salah RA, Lotfy A, El‐Badri N. Applications of the amniotic membrane in tissue engineering and regeneration: the hundred‐year challenge. Stem Cell Res Ther. 2022;13(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu B, Gao F, Lin J, Lu L, Xu H, Xu GT. Conditioned medium of human amniotic epithelial cells alleviates experimental allergic conjunctivitis mainly by IL‐1ra and IL‐10. Front Immunol. 2021;22(12):774601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han SH, Chae DS, Kim SW. Dual CXCR4/IL‐10 gene‐edited human amniotic mesenchymal stem cells Exhibit robust therapeutic properties in chronic wound healing. Int J Mol Sci. 2022;23(23):15338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ragni E, Papait A, PeruccaOrfei C, et al. Amniotic membrane‐mesenchymal stromal cells secreted factors and extracellular vesicle‐miRNAs: Anti‐inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl Med. 2021;10(7):1044‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mainil‐Varlet P, van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38(5):880‐890. [DOI] [PubMed] [Google Scholar]
- 22. Sani M, Hosseinie R, Latifi M, et al. Engineered artificial articular cartilage made of decellularized extracellular matrix by mechanical and IGF‐1 stimulation. Biomater Adv. 2022;1(139):213019. [DOI] [PubMed] [Google Scholar]
- 23. Gabler C, Saß J‐O, Gierschner S, Lindner T, Bader R, Tischer T. In vivo evaluation of different collagen scaffolds in an achilles tendon defect model. BioMed Res Int. 2018;2018:6432742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray MM, Kalish LA, Fleming BC, et al. Bridge‐enhanced anterior cruciate ligament repair: two‐year results of a first‐in‐human study. Orthop J Sports Med. 2019;7:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng Z, Ran J, Chen W, et al. Alignment of collagen fiber in knitted silk scaffold for functional massive rotator cuff repair. Acta Biomater. 2017;15(51):317‐329. [DOI] [PubMed] [Google Scholar]
- 26. Kiapour AM, Ecklund K, Murray MM, et al. Changes in cross‐sectional area and signal intensity of healing anterior cruciate ligaments and grafts in the first 2 years after surgery. Am J Sports Med. 2019;47(8):1831‐1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang RF, Zhou XZ, Niu L, Qi YY. Type I collagen scaffold with WNT5A plasmid for in situ cartilage tissue engineering. Biomed Mater Eng. 2022;33(1):65‐76. [DOI] [PubMed] [Google Scholar]
- 28. Cao R, Zhan A, Ci Z, et al. A biomimetic biphasic scaffold consisting of decellularized cartilage and decalcified bone matrixes for osteochondral defect repair. Front Cell Dev Biol. 2021;19(9):639006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fernandes TL, Gomoll AH, Lattermann C, Hernandez AJ, Bueno DF, Amano MT. Macrophage: a potential target on cartilage regeneration. Front Immunol. 2020;11(11):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tiruvannamalai Annamalai R, Mertz DR, Daley EL, Stegemann JP. Collagen type II enhances chondrogenic differentiation in agarose‐based modular microtissues. Cytotherapy. 2016;18(2):263‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu S, Zhou C, Mou S, et al. Biocompatible graphene oxide‐collagen composite aerogel for enhanced stiffness and in situ bone regeneration. Mater Sci Eng C Mater Biol Appl. 2019;105:110137. [DOI] [PubMed] [Google Scholar]
- 32. Jiang YH, Lou YY, Li TH, et al. Cross‐linking methods of type I collagen‐based scaffolds for cartilage tissue engineering. Am J Transl Res. 2022;14(2):1146‐1159. [PMC free article] [PubMed] [Google Scholar]
- 33. Jung H, McClellan P, Welter JF, Akkus O. Chondrogenesis of mesenchymal stem cells through local release of TGF‐β3 from heparinized collagen biofabric. Tissue Eng Part A. 2021;27(21‐22):1434‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang W, Zheng Y, Chen J, et al. Preparation and characterization of the collagen/cellulose nanocrystals/USPIO scaffolds loaded kartogenin for cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 2019;99:1362‐1373. [DOI] [PubMed] [Google Scholar]
- 35. Lu Z, Doulabi BZ, Huang C, Bank RA , Helder MN. Collagen type II enhances chondrogenesis in adipose tissue‐derived stem cells by affecting cell shape. Tissue Eng Part A. 2010;16(1):81‐90. [DOI] [PubMed] [Google Scholar]
- 36. Baek J, Lee KI, Ra HJ, Lotz MK, D'Lima DD. Collagen fibrous scaffolds for sustained delivery of growth factors for meniscal tissue engineering. Nanomedicine (Lond). 2022;17(2):77‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meyer M. Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online. 2019;18(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hortensius RA, Ebens JH, Dewey MJ, Harley BAC. Incorporation of the amniotic membrane as an immunomodulatory design element in collagen scaffolds for tendon repair. ACS Biomater Sci Eng. 2018;4(12):4367‐4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cao L, Tong Y, Wang X, et al. Effect of amniotic membrane/collagen‐based scaffolds on the chondrogenic differentiation of adipose‐derived stem cells and cartilage repair. Front Cell Dev Biol. 2021;25(9):647166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dewey MJ, Johnson EM, Slater ST, Milner DJ, Wheeler MB, Harley BAC. Mineralized collagen scaffolds fabricated with amniotic membrane matrix increase osteogenesis under inflammatory conditions. Regen Biomater. 2020. Jun;7(3):247‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Umezawa A, Hasegawa A, Inoue M, et al. Amnion‐derived cells as a reliable resource for next‐generation regenerative medicine. Placenta. 2019;1(84):50‐56. [DOI] [PubMed] [Google Scholar]
- 42. Fénelon M, Catros S, Meyer C, et al. Applications of human amniotic membrane for tissue engineering. Membranes (Basel). 2021;11(6):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo Y, Wang AT, Zhang QF, Liu RM, Xiao JH. RASL11B gene enhances hyaluronic acid‐mediated chondrogenic differentiation in human amniotic mesenchymal stem cells via the activation of Sox9/ERK/smad signals. Exp Biol Med (Maywood). 2020;245(18):1708‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lefebvre V, Angelozzi M, Haseeb A. SOX9 in cartilage development and disease. Curr Opin Cell Biol. 2019;61:39‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deuse T, Stubbendorff M, Tang‐Quan K, et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20(5):655‐667. [DOI] [PubMed] [Google Scholar]
- 46. Ma J, Wu J, Han L, et al. Comparative analysis of mesenchymal stem cells derived from amniotic membrane, umbilical cord, and chorionic plate under serum‐free condition. Stem Cell Res Ther. 2019;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17(8):424‐432. [DOI] [PubMed] [Google Scholar]


