Abstract
Objective
To examine if perioperative blood transfusion affects overall survival (OS) and recurrence‐free survival (RFS) in head and neck cancer patients who undergo free tissue reconstruction.
Design
Retrospective cohort study.
Methods
The medical records of free tissue flaps between 2007 and 2010 were reviewed. Differences in demographics and clinical factors based on the level of transfused packed red blood cells (PRBC) were examined using chi‐squared tests, Kruskal–Wallis tests, and/or ANOVA tests. Survival time was compared using a Cox proportional hazard model.
Results
Data were available for 183 patients. Patients who had PRBC transfusion significantly differed from the non‐transfused group by flap type, flap with bone, Charlson Comorbidity Index (CCI), and hemoglobin and hematocrit. When stratified into three groups based on units of PRBC; flap type, flap with bone, CCI, preoperative hemoglobin, and hematocrit were found to differ significantly. The 2‐year Kaplan–Meier plot demonstrated improved OS for those who did not receive any PRBC transfusion. The use of more than 3 units of blood decreased 2‐year OS significantly when compared to the non‐transfused group. Finally, after adjusting for CCI using a Cox proportional hazard model, survival was significantly affected by CCI.
Conclusion
After controlling for patient age, oncologic stage, cancer subsite, histology, type of free flap, vascularized bone‐containing flap, recurrence type, CCI, and preoperative hemoglobin and hematocrit, patients who received 3 or more units of PRBC in the perioperative period had significantly decreased OS. RFS did not differ between the transfused versus non‐transfused groups.
Level of Evidence
Level 4.
Keywords: blood transfusion, free flap, free tissue transfer, head and neck cancer
This study examines if perioperative blood transfusion (PBT) affects overall survival (OS) and recurrence‐free survival (RFS) in HN cancer patients who undergo free tissue reconstruction. One hundred eighty‐three patients were examined, and those who had packed red blood cell (PRBC) transfusion significantly differed from the non‐transfused group. After controlling important variables, patients who received 3 or more units of PRBC in the perioperative period had significantly decreased OS.
1. INTRODUCTION
Free tissue transfer has fundamentally changed the oncologic and reconstructive range of head and neck surgeons. Numerous studies have confirmed that reconstruction of critical head and neck defects with vascularized tissue optimizes patient function postoperatively. 1 , 2 , 3
Despite these advantages, the decision to pursue free tissue transfer can complicate simple ablative surgery, and an increased reliance on blood transfusion after free tissue transfer may also adversely affect outcomes in head and neck cancer patients. 4
Blood transfusion has changed the course of medicine and remains a life‐saving intervention for many patients. Blood transfusion commonly occurs during the treatment of cancer, with oncology services utilizing approximately 15% of transfused blood products in the United States annually. 5 , 6 , 7
Regarding head and neck cancer, multiple studies have shown that 50%–75% of oral, oropharyngeal, and laryngeal cancers require perioperative blood transfusion (PBT) during surgical ablation. 8 , 9 , 10 , 11 , 12 On average, patients who require extirpation of a head and neck cancer with free tissue reconstruction are treated with 2 units of blood within a 2‐ to 3‐day perioperative period. 13
While often beneficial to many patients with head and neck cancer, host immunosuppression and inflammation after a blood transfusion may affect overall survival (OS), recurrence, and recurrence‐free survival (RFS). Despite the use of leucocyte‐reduced blood products, transfused products still contain cytokines, growth factors, and inflammatory mediators that affect cancer outcomes, wound healing, and infection after surgery. 11 , 12 , 14 , 15 , 16
Few studies have specifically examined blood transfusion in the head and neck free flap population, 15 , 17 , 18 and results from these studies were conflicting. From the published literature to date, only two studies demonstrated adverse oncologic outcomes independently related to blood transfusion. 15 , 18 The purpose of this study is to evaluate the effect of PBT after head and neck cancer ablation and free tissue reconstruction and how this affects OS and RFS.
2. METHODS
All patients in this study received leuko‐depleted blood and were treated at a single institution by a single reconstructive surgeon. These were consecutive head and neck cancer patients whose postoperative care was managed using a published clinical care pathway agreed upon by a multidisciplinary team. 19 Transfusion protocols and records were standardized and available for all patients. The transfusion criteria for free flaps surgery at the time of collected data was a hemoglobin level lower than 10 g/dL. Finally, prior studies on this specific population only captured and/or defined PBT as occurring intraoperatively or during hospital admission. 10 , 15 , 17 This approach potentially excluded patients who developed anemia beyond the index admission for surgery. In this study, we captured all blood transfusion products that occurred after initial surgical ablation and for a 90‐day postoperative period.
After academic institutional review board approval, a retrospective chart review was performed on consecutive patients who underwent flap reconstruction with the senior author. Microvascular free tissue transfers performed by the senior author between March 2007 and March 2010 were queried. A total of 303 free tissue transfers were identified. Any free tissue transfers that were performed as part of an ablative surgery for malignancy of the head and neck were included in the cohort. One hundred eighty‐three patients were identified and their medical records were examined for age, race, gender, comorbidities using the age‐adjusted Charlson Comorbidity Index (CCI), preoperative hemoglobin and hematocrit (which were not available for all patients), cancer site and stage, type of malignancy, tissue (mucosal), type of free flap, vascularized bone‐containing flap, presence of infection or fistula, and administration of blood transfusion including packed red blood cell (PRBC), platelets, fresh‐frozen plasma, and cryoprecipitate up to 90 days postoperatively. In addition, OS and RFS at 2 years, recurrence type (local or regional/distant metastatic), and diagnosis date were determined. Cervical wound infection was defined as wound purulence and salivary leakage.
Descriptive statistics were calculated and reported using means with standard deviation or medians with interquartile range for continuous variables as well as frequency and percentage for categorical variables. Differences in patient characteristics across levels of blood transfusion were examined, initially as any transfusion versus none, and further categorized into levels 0, 1–2, and ≥3 units of blood. Chi‐squared tests, Kruskal–Wallis tests, and ANOVA were used to test differences among patient characteristics across levels of transfusion. Kaplan–Meier curves were used to examine OS, as well as RFS at 2 years. The log rank test was used to test for differences among groups. p Values were adjusted for multiple comparisons using the Tukey–Kramer method. Finally, a Cox proportional hazard model was used to compare survival times among groups while simultaneously adjusting for relevant covariates. Adjusted hazard ratios (HR) with 95% confidence intervals (CIs) were reported. p < .05 were considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC) was used to conduct all analyses.
3. RESULTS
One hundred eighty‐four consecutive microvascular free tissue transfers for 183 patients were performed. Patient and tumor characteristics, free flap type, survival and recurrence details, CCI, transfusion details, and preoperative hemoglobin and hematocrit are summarized in Table 1.
TABLE 1.
Patient characteristics and their association with 90‐day postoperative transfusion.
| PRBCs 90 days | ||||
|---|---|---|---|---|
| None | Any | p Value* | ||
| N = 60 | N = 123 | |||
| Covariate | n (%) | n (%) | ||
| Age | Mean (SD) | 57.53 (SD 13.09) | 60.54 (SD 11.78) | .120 |
| TNM | 3–4 | 45 (75) | 103 (83.74) | .158 |
| 1–2 | 15 (25) | 20 (16.26) | ||
| SCC | Yes | 56 (93.33) | 117 (95.12) | .731** |
| No | 4 (6.67) | 6 (4.88) | ||
| Cancer subsite | Oral cavity | 36 (60) | 61 (49.59) | .197 |
| Oropharynx | 3 (5) | 19 (15.45) | ||
| Larynx/hypopharynx | 10 (16.67) | 18 (14.63) | ||
| Skin/other | 11 (18.33) | 25 (20.33) | ||
| Tissue | Mucosa | 51 (85) | 98 (79.67) | .385 |
| Non‐mucosa | 9 (15) | 25 (20.33) | ||
| Free flap | Radial | 40 (66.67) | 51 (41.46) | .012** |
| Fibula | 8 (13.33) | 26 (21.14) | ||
| Scapular | 2 (3.33) | 8 (6.5) | ||
| Latissimus | 3 (5) | 7 (5.69) | ||
| ALT | 3 (5) | 1 (0.81) | ||
| OCR | 1 (1.67) | 6 (4.88) | ||
| Rad/fibula | 0 (0) | 2 (1.63) | ||
| Rectus | 3 (5) | 22 (17.89) | ||
| Free flap with bone | Yes | 11 (18.33) | 42 (34.15) | .027 |
| No | 49 (81.67) | 81 (65.85) | ||
| Death at 2 years | Yes | 20 (33.33) | 57 (46.34) | .094 |
| No | 40 (66.67) | 66 (53.66) | ||
| Wound/fistula | Yes | 15 (25) | 26 (21.14) | .556 |
| No | 45 (75) | 97 (78.86) | ||
| Smoking | Yes | 49 (81.67) | 100 (81.3) | .952 |
| No | 11 (18.33) | 23 (18.7) | ||
| Local recurrence | Yes | 14 (23.33) | 34 (27.64) | .534 |
| No | 46 (76.67) | 89 (72.36) | ||
| Regional/distant recurrence | Yes | 3 (5) | 13 (10.57) | .211 |
| No | 57 (95) | 110 (89.43) | ||
| Age adj CCI | Median (IQR) | 6 (4–7) | 7 (4–8) | .032** |
| Hemoglobin, g/dL a | N | 48 | 116 | <.001 |
| Mean (SD) | 13.58 (1.64) | 12.48 (1.99) | ||
| Hematocrit, % a | N | 48 | 116 | .003 |
| Mean | 39.71 (4.77) | 36.94 (5.61) | ||
Abbreviations: age adj CCI, age‐adjusted Charlson Comorbidity Index; ALT, anterolateral thigh; IQR, interquartile range; OCR, osteocutaneous radial forearm; PRBCs 90, packed red blood cell in 90 days; SD, standard deviation; SSC, squamous cell carcinoma.
Hemoglobin and hematocrit are preoperative values only for 164 patients.
The p value is calculated by ANOVA for numerical covariates and chi‐square test for categorical covariates.
The p value is calculated by the Kruskal–Wallis test.
Overall, 83% of the transfused group and 75% of the non‐transfused group had advanced Stage 3–4 cancers. The oral cavity was the most common head and neck subsite in the transfused and non‐transfused groups. Radial forearm free flap represented 49.7% of the free tissue transfers in this cohort. Fifty‐three (28.9%) of 183 patients required vascularized bone‐containing free flaps. Twenty‐two percent of patients developed fistula and/or wound infection. Twenty‐six percent (n = 49) of patients required reoperation, and no patients experienced flap loss. One patient required two free flaps for reconstruction, and this was performed during the initial cancer ablation. The median CCI for the non‐transfused versus transfused cohorts was 6 (4–7) and 7 (4–8), respectively. One hundred twenty‐three (67.2%) patients received PRBC within the 90‐day postoperative period, 42 patients received 1–2 units of blood, and 81 received ≥3 transfused units. 33.4% of patients received no blood products in a 90‐day perioperative period. Only 3 (1.6%) received platelets, 10 (5.4%) received fresh‐frozen plasma, and 1 (0.54%) patient received cryoprecipitate. Preoperative hemoglobin and hematocrit values were available for 164 patients and were statistically different and higher in the non‐transfused group (Table 1).
Patients with any PRBC transfusion significantly differed when compared to non‐transfused patients based on the type of free tissue transfer, the inclusion of vascularized bone, CCI, and preoperative hemoglobin and hematocrit levels, while distributions of age, TNM stage, cancer subsite, histology, and recurrence type were similar (Table 1). When patients were stratified based on units of PRBC transfused, flap type, flap with bone, CCI, hemoglobin, and hematocrit were significantly different (Table 2). The 2‐year Kaplan–Meier curve indicates significantly better OS for those who did not receive any PRBC transfusion than those with PRBC transfusion (p = .032) (Figure 1). When looking at levels of 0, 1–2, and ≥3 units of PRBCs transfused, OS differed among groups (log rank test p = .0046) (Figure 2). OS at 2 years for patients who received ≥3 units of PRBC was lower than for patients with no transfusion (adjusted p = .003) but did not significantly differ when compared to patients who received 1–2 units of PRBC (adjusted p = .114). Patients transfused with 1–2 units PRBCs did not differ in OS at 2 years compared to patients without transfusion (adjusted p = .184) (Figure 2). Local RFS did not differ among patients with transfusion versus non‐transfused PRBCs (p = .442). Regional and distant metastatic survival did not differ between the transfused versus non‐transfused groups (p = .125). Local RFS (p = .348) and regional and distant metastatic RFS (p = .309) did not differ among patients who received 0, 1–2, or ≥3 units of PRBCs. After adjusting for worsening CCI scores using a Cox proportional hazard model, the HR (95% CI) for patients who received ≥3 units compared to 0 units PRBCs was 2.34 (95% CI: 1.21–4.52). Patients who received 1–2 PRBC units compared to 0 units did not significantly differ (HR 1.73 [95% CI: 0.81–3.71]) (Figure 3).
TABLE 2.
Patient characteristics and their association with number of PRBC units transfused.
| PRBCs 90 days | |||||
|---|---|---|---|---|---|
| Characteristic | 0 | 1–2 | ≥3 | p Value* | |
| N = 60 | N = 42 | N = 81 | |||
| n (%) | n (%) | n (%) | |||
| Free flap | Radial | 40 (66.67) | 24 (57.14) | 27 (33.33) | .021 |
| Fibula | 8 (13.33) | 5 (11.9) | 21 (25.93) | ||
| Scapular | 2 (3.33) | 3 (7.14) | 5 (6.17) | ||
| Latissimus | 3 (5) | 1 (2.38) | 6 (7.41) | ||
| ALT | 3 (5) | 0 (0) | 1 (1.23) | ||
| OCR | 1 (1.67) | 2 (4.76) | 4 (4.94) | ||
| Rad/fibula | 0 (0) | 0 (0) | 2 (2.47) | ||
| Rectus | 3 (5) | 7 (16.67) | 15 (18.52) | ||
| Free flap with bone | Yes | 11 (18.33) | 10 (23.81) | 32 (39.51) | .016 |
| No | 49 (81.67) | 32 (76.19) | 49 (60.49) | ||
| Death at 2 years | Yes | 20 (33.33) | 18 (42.86) | 39 (48.15) | .210 |
| No | 40 (66.67) | 24 (57.14) | 42 (51.85) | ||
| Local recurrence | Yes | 14 (23.33) | 17 (40.48) | 17 (20.99) | .055 |
| No | 46 (76.67) | 25 (59.52) | 64 (79.01) | ||
| Regional/distant recurrence | Yes | 3 (5) | 5 (11.9) | 8 (9.88) | .425 |
| No | 57 (95) | 37 (88.1) | 73 (90.12) | ||
| Age adj CCI | Median (IQR) | 6 (4–7) | 6 (4–8) | 7 (4–9) | .030** |
| Hemoglobin, g/dL a | N | 48 | 40 | 76 | .004 |
| Mean (SD) | 13.58 (1.64) | 12.62 (1.96) | 12.41 (2.02) | ||
| Hematocrit, % a | N | 48 | 40 | 76 | .011 |
| Mean | 39.71 (4.77) | 37.35 (5.39) | 36.73 (5.75) | ||
Abbreviations: age adj CCI, age‐adjusted Charlson Comorbidity Index; ALT, anterolateral thigh; IQR, interquartile range; OCR, osteocutaneous radial forearm; PRBCs 90, packed red blood cell in 90 days; SD, standard deviation.
Hemoglobin and hematocrit are preoperative values only for 164 patients.
The p value is calculated by ANOVA for numerical covariates and chi‐square test for categorical covariates.
The p value is calculated by the Kruskal–Wallis test.
FIGURE 1.
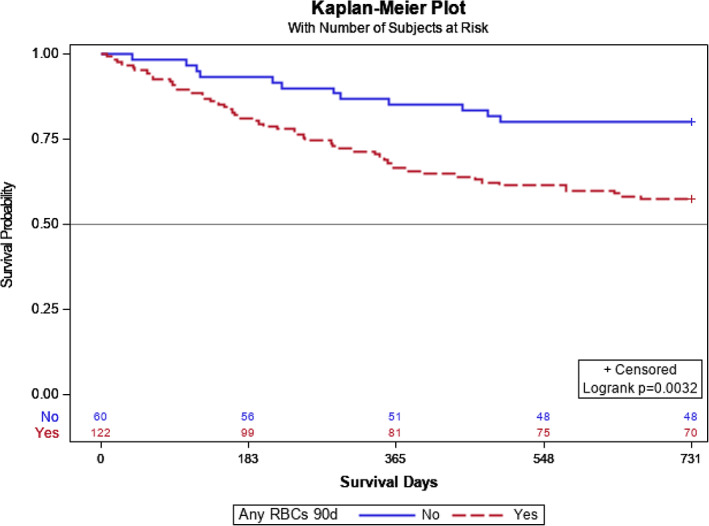
Overall survival with transfusion versus no transfusion. RBC's 90, any packed red blood cell in 90 days.
FIGURE 2.
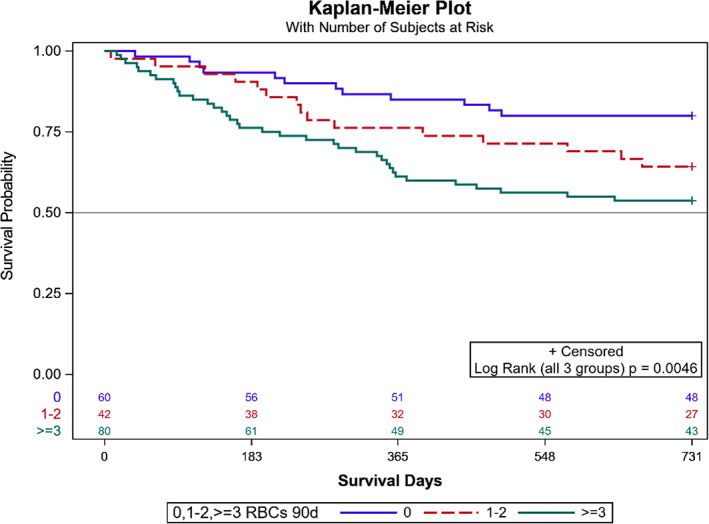
Overall survival by transfusion group. RBC's 90, any packed red blood cell in 90 days.
FIGURE 3.
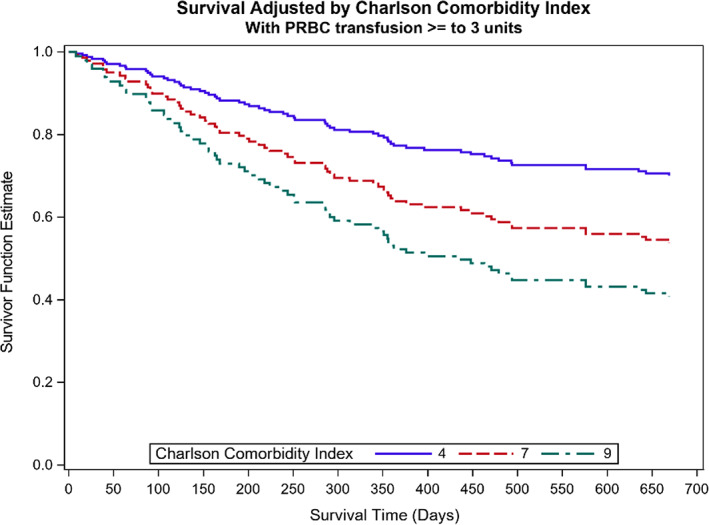
Plot showing how CCI affects the survival probability. PRBC, packed red blood cell in 90 days.
4. DISCUSSION
In this study, we found that the transfusion of ≥3 units of PRBCs following surgical resection of head and neck cancer and free tissue reconstruction was associated with decreased OS. Patients who received transfusion did not differ significantly from the non‐transfused cohort when age, stage, cancer subsite, or mucosal involvement were examined. On closer examination of patient subgroups based on the number of perioperative transfusions, we found that OS was significantly decreased in patients who received 3 or more units of PRBC when compared to those who required no transfusions. Moreover, no survival difference was noted in patients who received less than 3 units of PRBC when compared to the non‐transfused group. Finally, although worsening CCI scores significantly affected survival, PBT of 3 or more units of PRBC was independently associated with decreased OS.
On review of the head and neck surgery literature, several studies have previously examined how PBT affected OS in non‐free flap patients. These studies vary based on head and neck subsites and malignancies that are included. Taniguchi et al. studied how PBT affects outcomes in oral cancer. They found that patients who received 3 or more units of blood had an OS of 58.3% compared with 83.5% in the non‐transfused group. 12 Baumeister et al. studied 354 patients with squamous cell carcinoma of the upper aerodigestive tract who were treated with open surgical resection. They noted that PBT significantly decreased OS but did not alter RFS. 20 Finally, Leon et al. studied 269 patients with cancer of the larynx or hypopharynx treated surgically. Their study examined how perioperative allogenic blood given 1 week before surgery or up to 2 weeks after surgery affected survival. At 5 years, there was no significant difference between the transfused and non‐transfused groups. PBT did not affect recurrence in this study either. 21
Several recent studies examine transfusion and cancer outcomes within the head and neck. These studies do not specifically focus on free flap patients and show conflicting results. Schuller et al. reported on 217 patients surgically treated for SCC of the upper aerodigestive tract. No association between transfused blood up to 72 h after surgery and survival was noted. Furthermore, transfusion did not appear to affect locoregional control. 22 On the other hand, Chau et al. examined the effect of leuko‐depleted PBT in a cohort of 520 patients with malignancies of head and neck who underwent cancer ablation with and without free tissue transfer. They specifically studied how PBT within 21 days of surgery impacted OS and recurrence and found a significant difference in survival of 64% in the transfused group and 42% in the non‐transfused group. 23
In our study population, local recurrence and local RFS were not different among transfused and non‐transfused patients. Regional and distant metastases and regional and distant metastatic RFS were not different in the transfused versus non‐transfused groups. No difference in RFS based on the level of transfused PRBC was observed in this cohort. Numerous studies have examined the association between transfusion and recurrence of malignancy and found conflicting results. Alun‐Jones et al. studied PBT in a cohort of 69 larynx cancer patients treated surgically. 11 They concluded that per‐operative blood transfusion was associated with decreased survival after laryngectomy. Woolley et al. studied their experience with 143 patients with supraglottic or hypopharyngeal squamous cell carcinoma. They examined how PBT within 2 weeks of the surgery affected outcomes. In this study, the overall odds ratio for recurrence was 2.65 for patients receiving more than 2 units of blood. They recommended a policy of blood conservation to enhance cancer control. 24 Moir et al. examined 165 patients who were treated surgically for upper aerodigestive tract SCC. Patients receiving both autologous and allogenic blood were analyzed separately. Patients with previously treated cancer, nodal disease, and allogenic blood transfusion were found to have significantly higher recurrence rates. The number of units of transfused blood was not significantly associated with the risk for recurrence. Although this study only included 18 patients who received autologous blood, the authors concluded that self‐donation should be considered when possible. 25 Finally, von Doersten et al. examined 104 mucosal and paranasal malignancies treated with primary surgical resection. They concluded that PBT did not affect recurrence. 26
A brief overview of the current head and neck cancer literature that specifically examines cancer outcomes after transfusion in the microvascular free tissue transfer population shows conflicting results as well. Danan et al. performed a retrospective review of all patients who underwent free tissue transfer for head and neck cancer at their institution in a 6‐year period. They examined OS, disease‐free survival, and infections. They included 167 patients and concluded that patients who receive more than 3 units of PRBC during extirpative surgery and flap reconstruction have significantly lower OS, relapse‐free survival, and higher wound infection rates when compared to patients who receive less than 3 units of PRBC. In this study, they included multiple upper aerodigestive tract subsites as well as skin cancer and other non‐mucosal sites. 15 Fenner et al. studied how PBT affects outcomes in previously untreated oral cancer patients who underwent FTT as part of their surgical care. All patients had biopsy proven SCC. Only 13% of patients did not require intraoperative blood transfusion and only six patients did not receive any transfusion during the entire hospital stay. In this cohort of 223 patients, PBT was not associated with poorer OS or RFS. 17 Szakmany et al. in a retrospective cohort study of 559 patients undergoing free flap primary surgery for untreated oral and oropharyngeal head and neck SCC between 1992 and 2002 at their institution. Leuko‐depleted blood was introduced during the midpoint of this study period. Seventy‐seven percent were transfused with blood, and 68% of those received 3 or more units of blood. PBT was found to negatively impact OS, disease‐free survival, and disease‐specific survival. 10
In our study, average preoperative hemoglobin and hematocrit were significantly higher in the non‐transfused versus transfused group, 13.58 g/dL and 39.7% versus 12.48 g/dL and 36.9%, respectively. Each unit of PRBC is expected to raise hemoglobin by 1 g/dL and increase hematocrit by 3%. Seventy‐seven percent of the transfused group and 48% of the non‐transfused group were reconstructed with flaps harvested under tourniquet control. Flaps raised under tourniquet control comprised the majority of both groups, suggesting that donor‐site bleeding is not a significant contributor to overall blood loss. Finally, more bone‐containing flaps were harvested in the transfused group, and this reached statistical significance. This suggests that cancers requiring bony reconstruction needed blood transfusion and this is related to surgical ablation rather than flap harvest. This finding was previously noted by Puram et al. 4 Based on our results, even small differences in preoperative hemoglobin and subsequent blood transfusion based on symptomatic anemia or transfusion protocols may affect survival.
Despite good evidence that asymptomatic anemia does not adversely affect free tissue transfer and that receipt of blood transfusion may adversely affect cancer survival, many flap surgeons hold on to the belief that transfusion for a hematocrit below 30% is vital to maintain tissue oxygenation and healing. This idea dates back to older studies that examined tissue perfusion in animal models. 27 , 28 This belief that liberal transfusion may help flap survival was not refuted by a 1988 NIH consensus statement on the subject. 29 Puram et al. estimated that nearly 50% of free flap patients receive a blood transfusion. On average, they found that 2.5 units of PRBC are required to maintain hemoglobin above 7 g/dL. 4 The effect of transfusion on free flap survival has been studied by Kim et al. They concluded that the lowest hemoglobin and age were correlated with flap loss. Transfusion was not associated with flap loss. 30 Rossemiller et al. studied how a restrictive transfusion policy affected head and neck free flap patients in the immediate perioperative period. They noted no increase in flap death and fewer transfusions, fewer fistula and respiratory failure, and fewer deaths and wound infections in patients who were treated with a restrictive transfusion policy. Despite good evidence that a restrictive blood transfusion policy has potential benefits in the immediate perioperative period in flap patients, very few studies have evaluated the effect of blood transfusion on cancer outcomes in this population. 31
The detrimental effect of PBT seems to be most pronounced and well‐studied in the gastrointestinal literature. 5 Studies with large cohort sizes, as well as meta‐analyses, are still lacking in the head and neck literature. PBT during the treatment of colorectal cancer (CRC) has been studied extensively. Amato et al. concluded that PBT clearly had a detrimental effect on CRC recurrence. 32 Liu et al. studied the effect of allogenic PBT on patients with hepatocellular carcinoma. OS and disease‐free survival were significantly decreased in patients who had an allogenic blood transfusions. 33 Similar conclusions are also seen in gastric cancer patients who undergo blood transfusion after oncologic resection. In a meta‐analysis of published studies, a dose dependent effect of PBT on all‐cause survival was noted as well as an increased recurrence in transfused patients was observed. 34
5. LIMITATIONS
Despite its retrospective nature, our study discusses the potential deleterious effects of transfusion on OS in cancer patients. All patients in this study were managed by a multidisciplinary team using a codified clinical care pathway, and this may have limited the use of PBT. The author used portions of this cohort and database from 2007 to 2010 in a previously published article, 18 and this current study uses an updated database. Future studies with a larger sample size should examine if transfusion influences recurrence in our population and if there is something that we can modify in these patients to improve their outcomes.
6. CONCLUSION
Patients who received 3 units or more of PRBC during head and neck cancer ablation and free tissue reconstruction have decreased OS at 2 years after controlling for comorbidity and tumor characteristics. Transfusion at any level did not appear to adversely affect locoregional control, distant metastases, or wound infection and fistula. In this study, there is no effect on disease related survival and recurrence. Patients who needed blood transfusion were noted to have lower preoperative hemoglobin. Future studies should examine how restrictive transfusion protocols affect cancer outcomes in head and neck cancer patients.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank Helen Hancock, MT and Laura DeMartino MT from Blood Bank & Transfusion Medicine, for helping us to collect data for this project.
Patil YJ, Yakoub M, Moreno KF, et al. The effect of transfusion on survival in head and neck cancer after free tissue reconstruction. Laryngoscope Investigative Otolaryngology. 2024;9(1):e1215. doi: 10.1002/lio2.1215
This manuscript has been submitted and approved as a Triological Society Thesis with the number 2021‐24.
REFERENCES
- 1. Chepeha DB, Annich G, Pynnonen MA, et al. Pectoralis major myocutaneous flap vs revascularized free tissue transfer: complications, gastrostomy tube dependence, and hospitalization. Arch Otolaryngol Head Neck Surg. 2004;130:181‐186. [DOI] [PubMed] [Google Scholar]
- 2. Dziegielewski PT, Ho ML, Rieger J, et al. Total glossectomy with laryngeal preservation and free flap reconstruction: objective functional outcomes and systematic review of the literature. Laryngoscope. 2013;123:140‐145. [DOI] [PubMed] [Google Scholar]
- 3. Genden EM, Okay D, Stepp MT, et al. Comparison of functional and quality‐of‐life outcomes in patients with and without palatomaxillary reconstruction: a preliminary report. Arch Otolaryngol Head Neck Surg. 2003;129:775‐780. [DOI] [PubMed] [Google Scholar]
- 4. Puram SV, Yarlagadda BB, Sethi R, et al. Transfusion in head and neck free flap patients: practice patterns and a comparative analysis by flap type. Otolaryngol Head Neck Surg. 2015;152:449‐457. [DOI] [PubMed] [Google Scholar]
- 5. Iqbal N, Haider K, Sundaram V, et al. Red blood cell transfusion and outcome in cancer. Transfus Apher Sci. 2017;56:287‐290. [DOI] [PubMed] [Google Scholar]
- 6. Jones JM, Sapiano MRP, Savinkina AA, et al. Slowing decline in blood collection and transfusion in the United States—2017. Transfusion. 2020;60(suppl 2):s1‐s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benson K. Transfusion medicine issues pertaining to patients with cancer. Cancer Control. 2015;22:4‐5. [DOI] [PubMed] [Google Scholar]
- 8. Schrijvers D. Management of anemia in cancer patients: transfusions. Oncologist. 2011;16(Suppl 3):12‐18. [DOI] [PubMed] [Google Scholar]
- 9. de Almeida JP, Vincent JL, Galas FR, et al. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology. 2015;122:29‐38. [DOI] [PubMed] [Google Scholar]
- 10. Szakmany T, Dodd M, Dempsey GA, et al. The influence of allogenic blood transfusion in patients having free‐flap primary surgery for oral and oropharyngeal squamous cell carcinoma. Br J Cancer. 2006;94:647‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alun‐Jones T, Clarke PJ, Morrissey S, Hill J. Blood transfusion and laryngeal cancer. Clin Otolaryngol Allied Sci. 1991;16:240‐244. [DOI] [PubMed] [Google Scholar]
- 12. Taniguchi Y, Okura M. Prognostic significance of perioperative blood transfusion in oral cavity squamous cell carcinoma. Head Neck. 2003;25:931‐936. [DOI] [PubMed] [Google Scholar]
- 13. Kolbenschlag J, Schneider J, Harati K, et al. Predictors of intraoperative blood transfusion in free tissue transfer. J Reconstr Microsurg. 2016;32:706‐711. [DOI] [PubMed] [Google Scholar]
- 14. Goubran H, Seghatchian J, Prokopchuk‐Gauk O, et al. Reflections on multiple strategies to reduce transfusion in cancer patients: a joint narrative. Transfus Apher Sci. 2017;56:322‐329. [DOI] [PubMed] [Google Scholar]
- 15. Danan D, Smolkin ME, Varhegyi NE, Bakos SR, Jameson MJ, Shonka DC Jr. Impact of blood transfusions on patients with head and neck cancer undergoing free tissue transfer. Laryngoscope. 2015;125:86‐91. [DOI] [PubMed] [Google Scholar]
- 16. Vamvakas EC, Carven JH, Hibberd PL. Blood transfusion and infection after colorectal cancer surgery. Transfusion. 1996;36:1000‐1008. [DOI] [PubMed] [Google Scholar]
- 17. Fenner M, Vairaktaris E, Nkenke E, Weisbach V, Neukam FW, Radespiel‐Troger M. Prognostic impact of blood transfusion in patients undergoing primary surgery and free‐flap reconstruction for oral squamous cell carcinoma. Cancer. 2009;115:1481‐1488. [DOI] [PubMed] [Google Scholar]
- 18. Feng A, Zhang J, Lu X, Fang Q. Effect of blood transfusion on short‐ and long‐term outcomes in Oral squamous cell carcinoma patients undergoing free flap reconstruction. Front Surg. 2021;8:666768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mhawej R, Harmych BM, Houlton JJ, Tabangin ME, Meinzen‐Derr J, Patil YJ. The impact of a post‐operative clinical care pathway on head and neck microvascular free tissue transfer outcomes. J Laryngol Otol. 2020;134:150‐158. [DOI] [PubMed] [Google Scholar]
- 20. Baumeister P, Canis M, Reiter M. Preoperative anemia and perioperative blood transfusion in head and neck squamous cell carcinoma. PloS One. 2018;13:e0205712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leon X, Quer M, Luz Maestre M, Burgues J, Muniz E, Madoz P. Blood transfusions in laryngeal cancer: effect on prognosis. Head Neck. 1996;18:218‐224. [DOI] [PubMed] [Google Scholar]
- 22. Schuller DE, Scott C, Wilson KM, et al. The effect of perioperative blood transfusion on survival in head and neck cancer. Arch Otolaryngol Head Neck Surg. 1994;120:711‐716. [DOI] [PubMed] [Google Scholar]
- 23. Chau JK, Harris JR, Seikaly HR. Transfusion as a predictor of recurrence and survival in head and neck cancer surgery patients. J Otolaryngol Head Neck Surg. 2010;39:516‐522. [PubMed] [Google Scholar]
- 24. Woolley AL, Hogikyan ND, Gates GA, Haughey BH, Schechtman KB, Goldenberg JL. Effect of blood transfusion on recurrence of head and neck carcinoma. Retrospective review and meta‐analysis. Ann Otol Rhinol Laryngol. 1992;101:724‐730. [DOI] [PubMed] [Google Scholar]
- 25. Moir MS, Samy RN, Hanasono MM, Terris DJ. Autologous and heterologous blood transfusion in head and neck cancer surgery. Arch Otolaryngol Head Neck Surg. 1999;125:864‐868. [DOI] [PubMed] [Google Scholar]
- 26. von Doersten P, Cruz RM, Selby JV, Hilsinger RL Jr. Transfusion, recurrence, and infection in head and neck cancer surgery. Otolaryngol Head Neck Surg. 1992;106:60‐67. [DOI] [PubMed] [Google Scholar]
- 27. Messmer K, Lewis DH, Sunder‐Plassmann L, Klövekorn WP, Mendler N, Holper K. Acute normovolemic hemodilution. Changes of central hemodynamics and microcirculatory flow in skeletal muscle. Eur Surg Res. 1972;4:55‐70. [DOI] [PubMed] [Google Scholar]
- 28. Earle AS, Fratianne RB, Nunez FD. The relationship of hematocrit levels to skin flap survival in the dog. Plast Reconstr Surg. 1974;54:341‐344. [DOI] [PubMed] [Google Scholar]
- 29. Greenwalt TJ, Buckwalter JA, Desforges J, et al. Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700‐2703. [PubMed] [Google Scholar]
- 30. Kim MJ, Woo KJ, Park BY, Kang SR. Effects of transfusion on free flap survival: searching for an optimal hemoglobin threshold for transfusion. J Reconstr Microsurg. 2018;34:610‐615. [DOI] [PubMed] [Google Scholar]
- 31. Rossmiller SR, Cannady SB, Ghanem TA, Wax MK. Transfusion criteria in free flap surgery. Otolaryngol Head Neck Surg. 2010;142:359‐364. [DOI] [PubMed] [Google Scholar]
- 32. Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;2006(1):CD005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu L, Wang Z, Jiang S, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta‐analysis. PloS One. 2013;8:e64261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta‐analysis. Int J Surg. 2015;13:102‐110. [DOI] [PubMed] [Google Scholar]


