Abstract
Vaccine adjuvants exert critical and unique influences on the quality of immune responses induced during active immunizations. We investigated the mechanisms of action of immunological adjuvants in terms of their requirements for cytokine-mediated pathways for adjuvanticity. Antibody responses potentiated by several adjuvants to a Plasmodium falciparum MSP1-19 (C-terminal 19-kDa processing fragment of MSP1) vaccine were studied in gamma interferon (IFN-γ) or interleukin (IL-4) knockout mice. The levels of anti-MSP1-19 antibodies and the induction of Th1- and Th2-type antibodies were analyzed. Results revealed a spectrum of requirements for cytokine-mediated pathways in the potentiation of immunogenicity, and such requirements were influenced by interactions among individual components of the adjuvant formulations. One adjuvant strictly depended on IFN-γ to induce appreciable levels of anti-MSP1-19 antibodies, while some formulations required IFN-γ only for the induction of Th1-type antibodies. Other formulations induced exclusively Th2-type antibodies and were not affected by IFN-γ knockout. There were three patterns of requirements for IL-4 by various adjuvants in the induction of Th2-type anti-MSP1-19 antibodies. Moreover, the induction of Th1-type anti-MSP1-19 antibodies by adjuvants showed two distinct patterns of regulation by IL-4. The utilization of an IL-4 regulated pathway(s) for the induction of Th2-type antibodies by the same adjuvant differed between mouse strains, suggesting that animal species variability in responses to vaccine adjuvants may be due, at least in part, to differences in the utilization of immune system pathways by an adjuvant among animal hosts.
Effective induction of immune responses to whole, subunit, or synthetic peptide vaccines often requires coadministration of adjuvants or immunomodulators. Recent research has led to the identification of a number of clinically acceptable adjuvants which are more potent and efficacious than the alum-type adjuvants (16, 17). Many of these compounds are comparable to the “gold standard,” i.e., complete Freund’s adjuvant (CFA), in inducing strong and/or protective immunities against many infectious diseases in animal models (16, 17).
While the discoveries of potentially effective adjuvants for human vaccines are encouraging, little is known regarding the mechanisms of action of vaccine adjuvants in inducing a particular immune response during in vivo, active immunizations. This is of particular concern since vaccine adjuvants can have profound effects on the qualities of immune responses induced. As examples, studies on animals immunized with malaria vaccine antigens (i.e., sporozoite, merozoite, and gametocyte antigens) show that adjuvants influence the specificities of immune responses induced, the major histocompatibility complex-regulated responsiveness to epitopes, and the induction of protective immunity (4, 11, 13, 17, 18, 20, 28, 35, 41). Furthermore, the responses to adjuvants often vary with animal species or subspecies (17). Such differential influences on immune responses occur despite the pleomorphic effects of adjuvants on a variety of immune cells, and such effects often overlap among different classes of adjuvants (16, 17, 22, 38, 39).
We hypothesize that during active immunizations, vaccine adjuvants selectively or preferentially utilize different immune pathways for the potentiation of an immune response. These may be in the form of cytokine-potentiated pathways, selective costimulatory interactions, and/or preferential activation of subpopulations of immune cells. To begin to address this issue, we investigated the requirement of gamma interferon (IFN-γ)- and interleukin-4 (IL-4)-mediated immune pathways for the potentiation of immunogenicity to a well-known Plasmodium falciparum blood-stage malaria vaccine antigen, the major merozoite surface protein 1 (MSP1) (8). Mice with homozygotic disruption of the IFN-γ or IL-4 gene were immunized with a recombinant MSP1 antigen (24) in several previously described (18–20) adjuvant formulations. Past studies have shown that protective immunity against MSP1 is primarily antibody mediated (5, 8, 10), and thus we examined the induction of anti-MSP1 antibodies by adjuvants. Our results revealed a spectrum of requirements for cytokine-mediated pathways for immunopotentiation, and such requirements were subjected to dynamic influences among components of the adjuvant formulations. Furthermore, utilization of immune pathways by an adjuvant differed among mouse strains and subspecies.
MATERIALS AND METHODS
Mouse strains.
BALB/c mice with homozygotic disruption of the IFN-γ gene (IFN-γ−/− mice; described in reference 9) were bred from heterozygotic breeding pairs obtained from Genentech Inc. Genotyping for the wild-type and disrupted IFN-γ genes was performed by PCR analyses of genomic DNA from tail biopsies. Eight- to ten-week-old female IFN-γ−/− mice and their sex- and age-matched heterozygous littermates (IFN-γ+/−) were used.
BALB/c and C57BL/6 mice with homozygotic disruption of the IL-4 gene, described elsewhere (23), were BALB/c-II4tm1Nnt and C57BL/6-II4tmlCgn mice from The Jackson Laboratory (Bar Harbor, Maine) and are hereafter referred to as BALB/c IL-4−/− and C57BL/6 IL-4−/− mice, respectively. Eight- to ten-week-old females were used. Controls were sex- and age-matched BALB/c or C57BL/6 mice (IL-4+/+).
Immunogen.
The yeast-expressed, recombinant MSP1 protein corresponding to the C-terminal 19-kDa processing fragment of MSP1, P2P30-MSP1-19, has been previously described and shown to induce protective immunity in Aotus monkeys (24). The immunogen was a kind gift from David Kaslow (National Institute of Allergy and Infectious Diseases, National Institutes of Health). This clinical-grade antigen was produced and purified under GMP conditions.
Adjuvant formulations.
The five adjuvant formulations were CFA or incomplete Freund’s adjuvant (IFA), alum (Alhydrogel; Accurate Chemical Science Corp., New York, N.Y.), multilamellar liposomes, monophosphoryl lipid A (LA-15-PH) in liposomes (LA-15-PH/liposomes) and muramyl dipeptide (B30-MDP) in liposomes (B30-MDP/liposomes). The characteristics of these adjuvant formulations in inducing antibody responses to MSP1 were described previously (18–20). Multilamellar liposomes were prepared from l-α-phosphatidylcholine dimyristoyl–cholesterol–dicetyl phosphate at a 1.0:0.75:0.11 ratio (19). These components were dissolved in chloroform-methanol (4:1) and shell evaporated under vacuum on the wall of a Wheaton 50-ml rotoflask at 40 to 45°C. Liposomes were formed, after the addition of antigen, by adding 10 glass beads and shaking on ice for 30 min. To prepare LA-15-PH/liposomes and B30-MDP/liposomes, LA-15-PH and B30-MDP were first dissolved with the lipid components, and the liposomes were formed as described above. Antigens were incorporated into the adjuvant formulations as follows: for the CFA (or IFA) formulation, equal volumes of antigen and CFA (or IFA) were emulsified; for the alum formulation, alum was mixed with an equal volume of antigen and let stand at 25°C for 30 min before use. For formulations containing liposomes, antigen solutions were added to the rotoflask coated with lipid components and were then entrapped in multilamellar liposomes as the latter were formed.
Immunization.
BALB/c IFN-γ−/− and IFN-γ+/− mice (five per group) were immunized intraperitoneally with P2P30-MSP1-19 (10 μg/mouse/injection) formulated with the five adjuvant formulations described above. Three injections were given at 4-week intervals. BALB/c IL-4−/− and IL-4+/+ mice were similarly immunized. In addition, C57BL/6 IL-4−/− and IL-4+/+ mice were immunized with P2P30-MSP1-19 in LA-15-PH/liposomes or with CFA or IFA. Mice were bled 3 weeks after the tertiary immunization.
ELISAs.
Mouse sera were assayed for anti-MSP1-19 antibodies by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (18). Briefly, ELISA plates were coated with the recombinant MSP1 antigen at 1.0 μg/ml and blocked with 1% bovine serum albumin (BSA). Sera were diluted 1/250 in 1% BSA–0.5% yeast extract. Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) plus IgM (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) served as the secondary antibody. ELISA optical densities (ODs) were measured at 410 nm, after the addition of the peroxidase substrates H2O2 and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Kirkegaard & Perry Laboratories). For immunoglobulin isotype analyses, peroxidase-conjugated rabbit anti-mouse IgG1, IgG2a, IgG2b, IgG3, and IgM (Zymed Laboratories Inc., South San Francisco, Calif.) were used as secondary antibodies. The Student t test was used to determine significant differences in OD levels; results are presented as average ± standard deviation (SD).
RESULTS
Immunogenicity of P2P30-MSP1-19 in IFN-γ−/− and IFN-γ+/− mice.
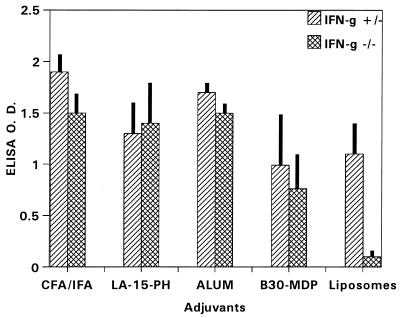
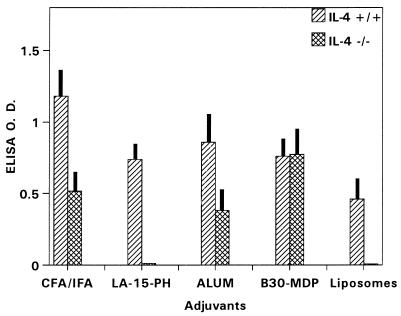
As shown in Fig. 1, P2P30-MSP1-19 in liposomes showed a strong dependency for IFN-γ to induce anti-MSP1-19 antibodies (IFN-γ−/− versus IFN-γ+/−, P < 0.001). Addition of LA-15-PH or B30-MDP to the liposome formulation abrogated the dependency of IFN-γ for immunopotentiation, with no significant changes in antibody levels. The absence of IFN-γ has little effect on the levels of anti-MSP1-19 antibodies induced by CFA and alum.
FIG. 1.
ELISA antibody levels to MSP1-19 in BALB/c IFN-γ−/− and IFN-γ+/− mice immunized with P2P30-MSP1-19 in liposomes, LA-15-PH/liposomes, B30-MDP/liposomes, CFA, and alum. Tertiary 21-day sera at a 1/250 dilution were used; data represent average OD ± SD from five mice per group.
Isotypes of anti-MSP1-19 antibodies in IFN-γ−/− and IFN-γ+/− mice.
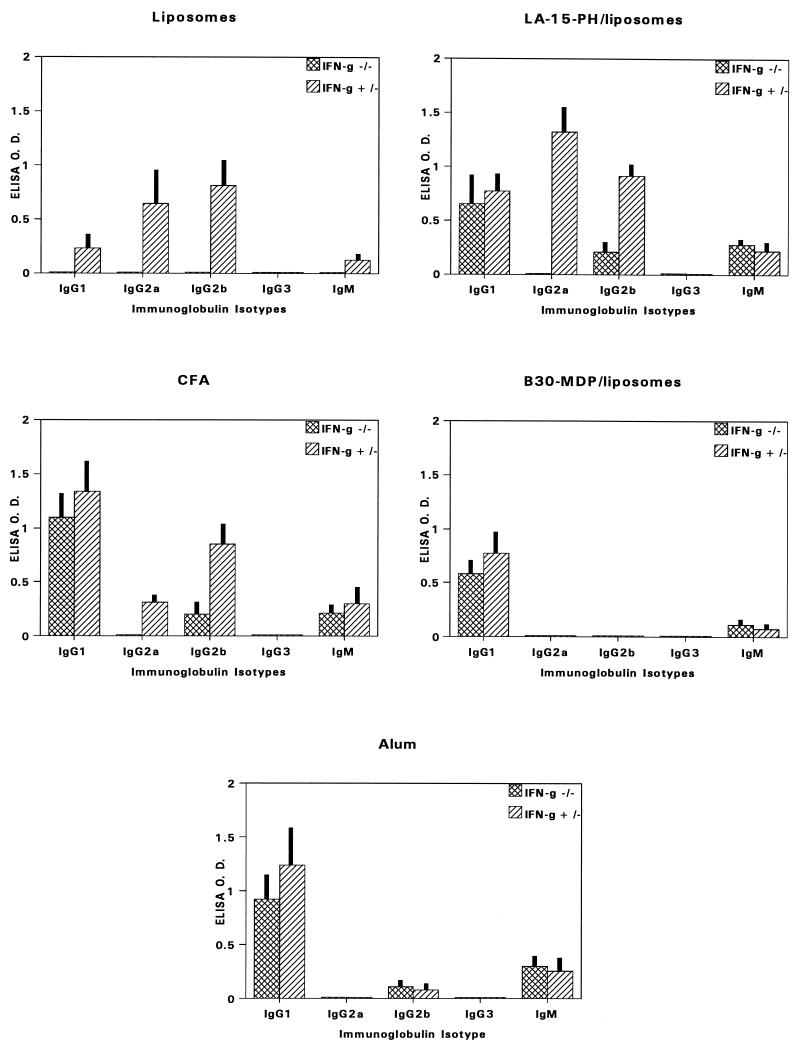
In IFN-γ+/− mice, both liposomes and LA-15-PH/liposomes potentiated a predominance of Th1-type (IgG2a) antibodies (Fig. 2). However, only LA-15-PH/liposomes, not liposomes, induced Th2-type (IgG1) anti-MSP1-19 antibodies in IFN-γ−/− mice. Freund’s adjuvants produced isotypes similar to those produced by LA-15-PH/liposomes in IFN-γ−/− and IFN-γ+/− mice, with a predominance of IgG1. Anti-MSP1-19 antibodies induced by B30-MDP/liposomes were exclusively IgG1, irrespective of the presence or absence of IFN-γ. Thus, the ability of the liposomal components in this formulation to induce IgG2a antibodies in IFN-γ+/− mice was abrogated. Alum induced an isotype profile very similar to that induced by B30-MDP/liposomes.
FIG. 2.
Immunoglobulin isotype-specific antibody levels to MSP1-19 in BALB/c IFN-γ−/− and IFN-γ+/− mice immunized with P2P30-MSP1-19 in various adjuvants. Tertiary 21-day bleeds from five mice per group were used; data represent average OD ± SD.
Immunogenicity of P2P30-MSP1-19 in BALB/c IL-4−/− and BALB/c IL-4+/+ mice.
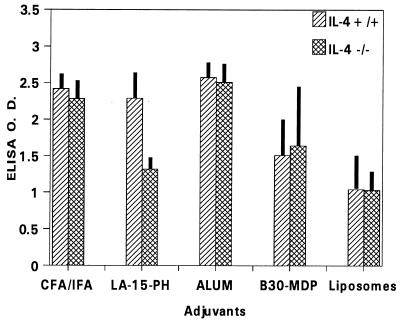
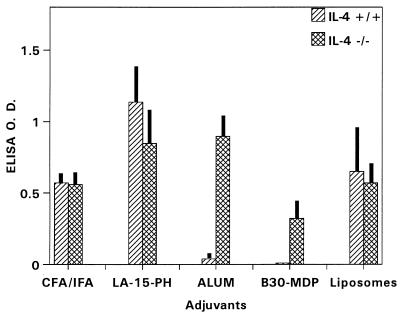
For CFA, alum, B30-MDP/liposomes, and liposomes, deficiency in IL-4 had little or no effect on the immunogenicity of P2P30-MSP1-19 (Fig. 3). However, IL-4−/− mice receiving LA-15-PH/liposomes had significantly lower anti-MSP1-19 antibody levels than controls (P < 0.01), suggesting that this adjuvant requires IL-4 for full immunopotentiating activity.
FIG. 3.
ELISA antibody levels to MSP1-19 in BALB/c IL-4−/− and IL-4+/+ mice immunized with P2P30-MSP1-19 in liposomes, LA-15-PH/liposomes, B30-MDP/liposomes, CFA, and alum. Tertiary 21-day sera at a 1/250 dilution were used; data represent average OD ± SD from five mice per group.
Isotypes of anti-MSP1-19 antibodies in BALB/c IL-4−/− and BALB/c IL-4+/+ mice.
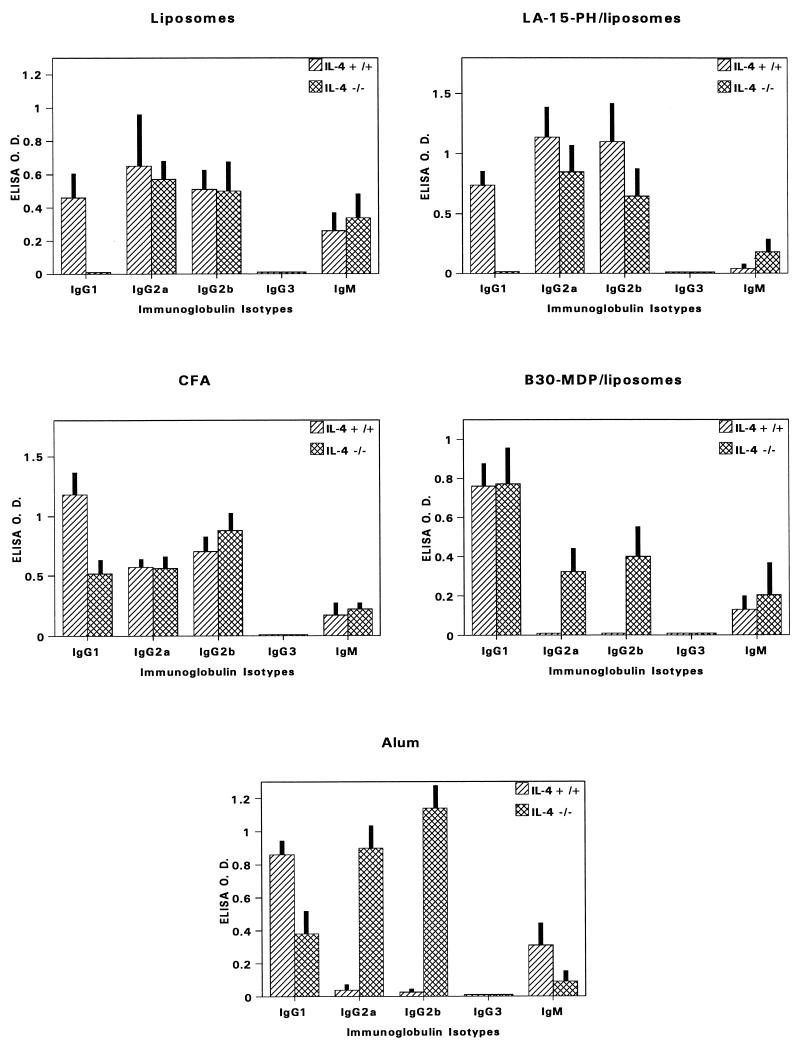
Figure 4 details the isotype distribution of anti-MSP1-19 antibodies induced by different adjuvants. With respect to IgG1 (Th2-type) responses, results revealed three different effects of IL-4 deficiency that were adjuvant dependent (Fig. 5). For LA-15-PH/liposomes and liposomes, IL-4 knockout caused almost complete abrogation of IgG1 responses (P < 0.001). By contrast, the potentiation of IgG1 by B30-MDP/liposomes was clearly IL-4 independent. In CFA and alum groups, IL-4 knockout caused partial but significant reductions in IgG1 (P < 0.01 for CFA or alum). The effects of IL-4 deficiency on the Th1-type (IgG2a) antibody response fell into two categories (Fig. 6). For CFA, LA-15-PH/liposomes, and liposomes, the production of Th1-type antibodies was relatively independent of IL-4. By contrast, IL-4 knockout caused significant IgG2a or IgG2b production in the mice receiving B30-MDP/liposomes and alum, while these isotypes were undetectable or minimally produced in IL-4+/+ mice (P < 0.001) (Fig. 4 and 6).
FIG. 4.
Immunoglobulin isotype-specific antibody levels to MSP1-19 in BALB/c IL-4−/− and IL-4+/+ mice immunized with P2P30-MSP1-19 in various adjuvants. Tertiary 21-day bleeds from five mice per group were used; data represent average OD ± SD.
FIG. 5.
ELISA MSP1-19-specific IgG1 antibody levels in BALB/c IL-4−/− and IL-4+/+ mice immunized with P2P30-MSP1-19 in different adjuvant formulations. Tertiary 21-day bleeds from five mice per group were used; data represent average OD ± SD.
FIG. 6.
ELISA MSP1-19-specific IgG2a antibody levels in BALB/c IL-4−/− and IL-4+/+ mice immunized with P2P30-MSP1-19 in different adjuvant formulations. Tertiary 21-day bleeds from five mice per group were used; data represent average OD ± SD.
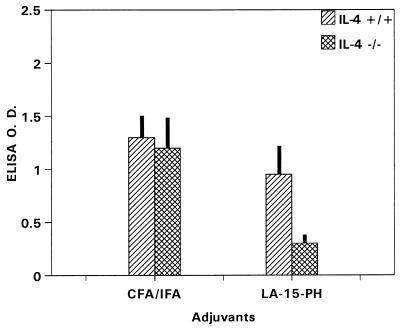
Immunogenicity of P2P30-MSP1-19 in C57BL/6 IL-4−/− and C57BL/6 IL-4+/+ mice.
Anti-MSP1-19 antibody responses were unaffected by IL-4 knockout in CFA immunizations (Fig. 7). However, the antibody levels were significantly lower in IL-4−/− mice receiving LA-15-PH/liposomes than in controls (P < 0.05) (Fig. 7).
FIG. 7.
ELISA antibody levels to MSP1-19 in C57BL/6 IL-4−/− and IL-4+/+ mice immunized with P2P30-MSP1-19 in CFA and in LA-15-PH/liposomes. Tertiary 21-day sera at a 1/250 dilution were used; data represent average OD ± SD from five mice per group.
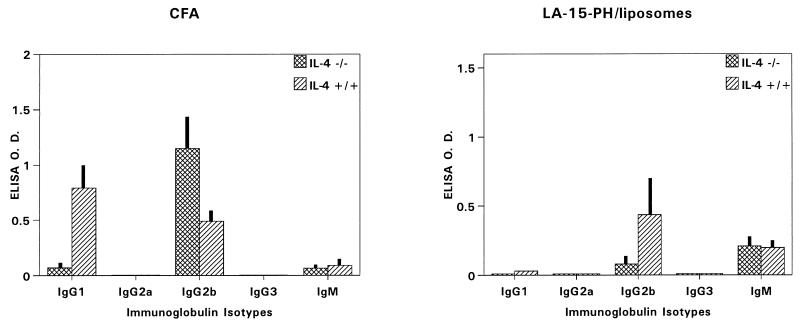
Isotypes of anti-MSP1-19 antibodies in C57BL/6 IL-4−/− and C57BL/6 IL-4+/+ mice.
A prominent effect of IL-4 knockout was the near-complete abrogation of IgG1 antibodies after CFA immunization (Fig. 8). For LA-15-PH/liposomes, IgG1 was only minimally detected in IL-4+/+ mice and undetectable in IL-4−/− mice (Fig. 8). There were also increases in IgG2b production in IL-4−/− mice for both adjuvants. Thus, the IL-4-dependent pathway of anti-MSP1-19 IgG1 production was utilized in C57BL/6 mice immunized with CFA but not LA-15-PH/liposomes. On the other hand, the absence of IgG2a antibodies suggests that both adjuvants did not utilize Th1-mediated pathways for the production of anti-MSP1-19 antibodies.
FIG. 8.
Immunoglobulin isotype-specific antibody levels to MSP1-19 in C57BL/6 IL-4−/− and IL-4+/+ mice immunized with P2P30-MSP1-19 in CFA and in LA-15-PH/liposomes. Tertiary 21-day bleeds from five mice per group were used; data represent average OD ± SD.
Basis for animal species differences in responses to adjuvants.
Animal species differences in responses to an adjuvant may result from disparate usages of cytokine-mediated immune pathways by an adjuvant. This is seen in the IL-4-regulated IgG1 responses induced by CFA and LA-15-PH/liposomes in BALB/c and C57BL/6 mice (Table 1). In the BALB/c background, CFA and LA-15-PH/liposomes induced IgG1 responses, and these antibodies were dominant in IFN-γ−/− mice. The IgG1 responses induced by both adjuvants were regulated by IL-4, as seen in BALB/c IL-4−/− mice. In the C57BL/6 background, only CFA, not LA-15-PH/liposomes, was able to induce IL-4-dependent IgG1 responses. The inability of C57BL/6 mice to produce appreciable levels of anti-MSP1-19 IgG1 in response to LA-15-PH/liposomes was not due to an inherent defect of this mouse strain since CFA readily induced these antibodies in C57BL/6 mice.
TABLE 1.
Mouse strain dependency of production of Th2-type (IgG1) antibodies to MSP1-19 by CFA and by LA-15-PH/liposomes
| Adjuvant | Mouse strain background | Cytokine genotype | Th2-type IgG1 levela |
|---|---|---|---|
| LA-15-PH/liposomes | BALB/c | IFN-γ+/− | + |
| BALB/c | IFN-γ−/− | ++ | |
| BALB/c | IL-4+/+ | + | |
| BALB/c | IL-4−/− | − | |
| C57BL/6 | IL-4+/+ | − | |
| C57BL/6 | IL-4−/− | − | |
| CFA | BALB/c | IFN-γ+/− | ++ |
| BALB/c | IFN-γ−/− | ++ | |
| BALB/c | IL-4+/+ | ++ | |
| BALB/c | IL-4−/− | + | |
| C57BL/6 | IL-4+/+ | ++ | |
| C57BL/6 | IL-4−/− | − |
++, highest of all isotypes; +, significant; −, very low or insignificant.
DISCUSSION
The results of this study suggest that adjuvants utilize different cytokine-mediated immune pathways to potentiate the immunogenicity of an antigen and/or to uniquely affect the quality or specificity of immune responses. Our results further suggest that the in vitro or in vivo immunoactivities of adjuvants do not always predict or correspond to the adjuvants’ requirements of immune mediators for immunopotentiation. For example, both LA-15-PH and B30-MDP induce IFN-γ, -α, and -β (22, 38, 39), but they do not depend on IFN-γ for the immunopotentiation of MSP1-19.
Comparisons of the Th1- and Th2-type antibodies offered clues to the mode of action of these adjuvants. The induction of Th2-type (IgG1) antibodies exclusively by B30-MDP/liposomes or predominantly by alum would explain why a deficiency in IFN-γ (Th1 cytokine) may not interfere with the immunopotentiation of these adjuvants via Th2 pathways. The enhancement of Th1-type (IgG2a) anti-MSP1-19 antibodies by liposomes or LA-15-PH/liposomes is consistent with previous studies which show preferential stimulation of this isotype during immunizations with BSA in liposomes (32). Indeed, it has been shown that ovalbumin (OVA) encapsulated liposomes preferentially stimulate production of IFN-γ from splenocytes (1). Our results for liposome-mediated immunizations extend the role of IFN-γ as an obligatory immune mediator for immunopotentiation. One such role may be the activation of macrophages as antigen-presenting cells (12). Studies have shown a role of macrophages in the uptake of liposome-trapped antigens (42), and this may require activation by IFN-γ (12). Since LA-15-PH/liposomes did not depend on IFN-γ to potentiate anti-MSP1-19 antibodies, the adjuvant may activate macrophages independent of IFN-γ. In this regard, lipid A derivatives have been shown to activate macrophages (39) and enhance the antigen-processing capabilities of macrophages (43).
It is possible that compensatory immune mechanisms were utilized by some adjuvants in IFN-γ−/− mice for antibody production. Recent studies suggest that IL-12 plays a major role in the development of antibody responses (27). In these studies, IL-12 exerts its influence via up-regulation of IFN-γ production, resulting in enhanced IgG2a production. Most importantly, in the absence of IFN-γ, IL-12 also potentiates antibody responses by enhancing IgG1 and IgG2b production, perhaps via direct or indirect effects on B cells. It is possible that LA-15-PH/liposomes or other adjuvants in our study utilize IL-12 for antibody production in IFN-γ−/− mice. Since little or no anti-MSP1-19 antibodies were produced in IFN-γ−/− mice immunized with P2P30-MSP1-19/liposomes, this adjuvant formulation would not have utilized such IL-12-mediated pathways.
In our IL-4 studies, only LA-15-PH/liposomes required IL-4 to potentiate full immunogenicity to P2P30-MSP1-19. Thus, this adjuvant may depend on IL-4 for activities additional to IgG1 responses. These may be through IL-4’s effects on B-cell growth and activation, thymocyte maturation, and/or selective activation of monocytes (2). There were two extremes in terms of dependency for IL-4 to induce IgG1 (Th2) to MSP1-19. The LA-15-PH/liposomes characterized an absolute requirement for IL-4, while potentiation by B30-MDP/liposomes was IL-4 independent. Several studies on the immune responses to schistosomal, mycobacterial, and filarial proteins suggest that IL-13, IL-5, MCP-3, and/or B7-2 costimulation can substitute for IL-4 in the generation of Th2 responses (6, 21, 26, 29, 37), and IL-13 has been shown to promote antibody isotype switching (34). Whether B30-MDP/liposomes utilizes these immune mediators for IgG1 production remains to be determined. Equally important is to determine if the IL-4-independent pathway(s) is utilized by this adjuvant formulation principally or as a compensatory mechanism in the absence of IL-4. Immunization studies of IL-4+/+ mice with genetic disruption of such pathways may clarify these issues. Previous studies of IL-4−/− mice immunized with OVA in alum or CFA show moderate decreases in IgG1 responses compared to IL-4+/+ mice (3). These results are similar to our findings with P2P30-MSP1-19 in alum or CFA. This partial requirement of IL-4 for IgG1 response may reflect the abilities of alum and CFA to drive both IL-4-dependent and IL-4-independent pathways, as represented by LA-15-PH/liposomes and B30-MDP/liposomes, respectively. However, disruption of IL-4-dependent pathways in IL-4−/− mice was not fully compensated for by IL-4-independent mechanisms induced by CFA or alum.
IL-4 may affect an adjuvant’s ability to induce Th1-like, IgG2a responses. In IL-4−/− mice immunized with OVA in alum, a vigorous IgG2a response was produced, whereas no detectable IgG2a was observed in IL-4+/+ mice (3). Our results for IL-4−/− mice immunized with P2P30-MSP1-19 in alum were in agreement with these studies. Moreover, our findings that immunization with B30-MDP/liposomes was also able to induce IgG2a in IL-4−/− but not IL-4+/+ mice clearly demonstrate that this is not a phenomenon peculiar to alum. Thus, the immunopotentiating activities of adjuvants such as alum and B30-MDP/liposomes may be regulated by the cytokine (e.g., IL-4) milieu of the host.
Our findings regarding the IL-4-regulated IgG1 responses induced by CFA and LA-15-PH/liposomes in BALB/c and C57BL/6 mice suggest that animal species differences in response to an adjuvant may result from disparate usages of cytokine-mediated immune pathways by the adjuvant. We hypothesize that specific immune cells in one mouse strain which mediate a particular pathway may be more sensitive or permissive to stimulation by an adjuvant formulation than the analogous cell populations from another strain. On the other hand, the types of immune cells stimulated by an adjuvant may be different among mouse strains. Alternatively, nonimmune factors unique to the genetic background of one mouse strain may influence the activities of adjuvants.
This investigation focused on the antigen P2P30-MSP1-19. A logical question is whether the immune pathways utilized by an adjuvant vary with the particular immunogen. Studies have shown that for antigens that are weak or neutral in their abilities to polarize immune responses (e.g., Th1 versus Th2), adjuvants play a critical role in determining the profiles of immune responses (7). In these scenarios, the immune pathways utilized by an adjuvant may be identical or similar irrespective of the antigen used. On the other hand, some antigens possess intrinsic adjuvanticities, and administration of these antigens alone can polarize the profile of immune responses. The Leishmania protein LelF and the Schistosoma mansoni soluble egg antigens are examples which polarize for Th1 and Th2 responses, respectively (25, 31, 33, 36). Immunization with these antigens may enhance or limit the effectiveness of adjuvants, depending on whether the profiles of immune responses associated with the immunogen and with the adjuvant are opposing or complementary. Polarization of immune responses by an antigen may limit the availability of an immune pathway that is principally utilized by an adjuvant, which may then default to other pathways for immunopotentiation. These hypotheses are readily testable in appropriate gene knockout models. Finally, it is known that the affinity of an antigen for the T-cell receptor, the strength of T-cell receptor signalling, and the density of major histocompatibility complex class II ligands may influence immune response profiles (15, 40). Whether these factors affect the immune mediator usage by adjuvants remains to be determined.
ACKNOWLEDGMENTS
We thank Shozo Kotani, Shoichi Kusumoto, and Daiichi Pharmaceutical, Tokyo, Japan, for providing the adjuvants LA-15-PH and B30-MDP. We are also grateful to David Kaslow for providing the P2P30-MSP1-19 and MSP1-19 recombinant antigens and to Genentech Inc. for providing the IFN-γ+/− mice.
This work was supported by Public Health Service grant AI31058.
REFERENCES
- 1.Aramaki Y, Suda H, Tsuchuya S. Interferon-gamma inductive effect of liposomes as an immunoadjuvant. Vaccine. 1995;13:1809–1814. doi: 10.1016/0264-410x(95)00117-j. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Greene W C. Interleukin-4. In: Thomson A W, editor. The cytokine handbook. New York, N.Y: Academic Press; 1994. pp. 99–126. [Google Scholar]
- 3.Brewer J M, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to Freund’s complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–2066. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 4.Burghaus P A, Wellde B T, Hall T, Richards R L, Egan A F, Riley E M, Ballou W R, Holder A A. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum adjuvant does not induce protection against a challenge infection. Infect Immun. 1996;64:3614–3619. doi: 10.1128/iai.64.9.3614-3619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns J M, Jr, Daly T M, Vaidya A B, Long C A. The 3′ portion of the gene for a Plasmodium yoelii merozoite surface antigen encodes the epitope recognized by a protective monoclonal antibody. Proc Natl Acad Sci USA. 1988;85:602–606. doi: 10.1073/pnas.85.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chensue S W, Warmington K, Ruth J H, Lukacs N, Kunkel S L. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-γ and IL-4 knockout mice. J Immunol. 1997;159:3565–3573. [PubMed] [Google Scholar]
- 7.Comoy E E, Capron A, Thyphronitis G. In vivo induction of type 1 and 2 immune responses against protein antigens. Int Immunol. 1997;9:523–531. doi: 10.1093/intimm/9.4.523. [DOI] [PubMed] [Google Scholar]
- 8.Cooper J A. Merozoite surface antigen-1 of Plasmodium. Parasitol Today. 1993;9:50–54. doi: 10.1016/0169-4758(93)90031-a. [DOI] [PubMed] [Google Scholar]
- 9.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted IFN-gamma genes. Science. 1993;259:1739–1745. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 10.Daly T M, Long C A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J Immunol. 1995;155:236–243. [PubMed] [Google Scholar]
- 11.Daly T M, Long C A. Influence of adjuvants on protection induced by a recombinant fusion protein against malarial infection. Infect Immun. 1996;64:2602–2608. doi: 10.1128/iai.64.7.2602-2608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Maeyer E, De Maeyer-Guignard J. Interferons. In: Thomson A W, editor. The cytokine handbook. New York, N.Y: Academic Press; 1994. pp. 265–288. [Google Scholar]
- 13.de Souza J B, Ling I R, Ogun S A, Holder A A, Playfair J H L. Cytokines and antibody subclass associated with protective immunity against blood-stage malaria in mice vaccinated with the C terminus of merozoite surface protein 1 plus a novel adjuvant. Infect Immun. 1996;64:3532–3536. doi: 10.1128/iai.64.9.3532-3536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMolfetto L, Neal H A, Wu A, Reilly C, Lo D. The density of the class II MHC T cell receptor ligand influences IFN-gamma/IL-4 ratios in immune responses in vivo. Cell Immunol. 1998;183:70–79. doi: 10.1006/cimm.1997.1231. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R K, Relyveld E H, Lindblad E B, Bizzini B, Ben-Efraim S, Gupta C K. Adjuvants—a balance between toxicity and adjuvanticity. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 17.Hui, G. S. N. 1994. Liposomes, muramyl dipeptide derivatives, and nontoxic lipid A derivatives as adjuvants for human malaria vaccines. Am. J. Trop. Med. Hyg. 50(Suppl.):41–51. [DOI] [PubMed]
- 18.Hui G S N, Chang S P, Gibson H, Hashimoto A, Hashiro C, Barr P J, Kotani S. Influence of adjuvants on the antibody specificity to the Plasmodium falciparum major merozoite surface protein, gp195. J Immunol. 1991;147:3935–3941. [PubMed] [Google Scholar]
- 19.Hui G S N, Tam L Q, Chang S P, Case S E, Hashiro C, Siddiqui W A, Shiba T, Kusumoto S, Kotani S. Synthetic low-toxicity muramyl dipeptide and monophosphoryl lipid A replace Freund’s complete adjuvant in inducing growth inhibitory antibodies to the Plasmodium falciparum major merozoite surface protein, gp195. Infect Immun. 1991;59:1585–1591. doi: 10.1128/iai.59.5.1585-1591.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui G S N, Chang S P. Plasmodium falciparum: induction of biologically active antibodies to gp195 is dependent on the choice of adjuvants. Exp Parasitol. 1992;75:155–157. doi: 10.1016/0014-4894(92)90131-s. [DOI] [PubMed] [Google Scholar]
- 21.King C L, Malhotra I, Jia X. Schistosoma mansoni: protective immunity in IL-4-deficient mice. Exp Parasitol. 1996;84:245–252. doi: 10.1006/expr.1996.0110. [DOI] [PubMed] [Google Scholar]
- 22.Kotani S. Bacterial cell surface biological response modifiers and their synthetic counterparts. Adv Exp Med Biol. 1992;319:145–164. doi: 10.1007/978-1-4615-3434-1_16. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Yadava A, Keister D B, Tian J H, Ohl M, Perdue-Greenfield K A, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:1–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Maron R, Palanivel V, Weiner H L, Harn D A. Oral administration of schistosome egg antigens and insulin B-chain generates and enhances Th2-type responses in NOD mice. Clin Immunol Immunopathol. 1998;87:85–92. doi: 10.1006/clin.1997.4506. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie A N, Culpepper J A, de Waal Malefyt R, Briere F, Punnonen J, Aversa G, Sato A, Dang W, Cocks B G, Menon S, de Vries J E, Banchereau J, Zurawski G. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci USA. 1993;90:3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzger D W, McNutt R M, Collins J T, Buchanan J M, Van Cleave V H, Dunnick W A. Interleukin-12 acts as an adjuvant for humoral immunity through interferon-gamma-dependent and -independent mechanisms. Eur J Immunol. 1997;27:1958–1965. doi: 10.1002/eji.1830270820. [DOI] [PubMed] [Google Scholar]
- 28.Millet P, Kalish M L, Collins W E, Hunter R L. Effect of adjuvant formulations on the selection of B-cell epitopes expression by a malaria peptide vaccine. Vaccine. 1992;10:547–550. doi: 10.1016/0264-410x(92)90355-n. [DOI] [PubMed] [Google Scholar]
- 29.Minty A, Chalon P, Derocq J M, Dumont X, Guillemot J C, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B, Minty C, Caselas P, Loison G, Lupker J, Shire D, Ferrara P, Caput D. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann T R. Interleukin-10. In: Thomson A W, editor. The cytokine handbook. New York, N.Y: Academic Press; 1994. pp. 223–238. [Google Scholar]
- 31.Palanivel V, Posey C, Horauf A M, Solbach W, Piessens W F, Harn D A. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp Parasitol. 1996;84:168–177. doi: 10.1006/expr.1996.0102. [DOI] [PubMed] [Google Scholar]
- 32.Philips N C, Emili A. Enhanced antibody response to liposome-associated protein antigens: preferential stimulation of IgG2a/b production. Vaccine. 1992;10:151–158. doi: 10.1016/0264-410x(92)90004-4. [DOI] [PubMed] [Google Scholar]
- 33.Probst P, Sheiky Y A, Steeves M, Gervassi A, Grabstein K H, Reed S G. A Leishmania protein that modulates interleukin (IL)-12, IL-10, and tumor necrosis factor-alpha production and expression of B7-1 in human monocyte-derived antigen-presenting cells. Eur J Immunol. 1997;27:2634–2642. doi: 10.1002/eji.1830271024. [DOI] [PubMed] [Google Scholar]
- 34.Punnonen J, Aversa G, Cocks B G, McKenzie A N, Menon S, Zurawski G, Malefyt R D W, De Vries J E. Interleukin 13 induces interleukin-4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawlings D J, Kaslow D C. Adjuvant-dependent immune response to malarial transmission-blocking vaccine candidate antigens. J Exp Med. 1992;176:1483–1487. doi: 10.1084/jem.176.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skeiky Y A, Guderian J A, Benson D R, Bacelar O, Carvalho E M, Kubin M, Badaro R, Trinchieri G, Reed S G. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian G, Kazura J W, Pearlman E, Jia X, Malhotra I, King C L. B7-2 requirement for helminth-induced granuloma formation and CD4 type 2 T helper cell cytokine expression. J Immunol. 1997;158:5914–5920. [PubMed] [Google Scholar]
- 38.Takada H, Kotani S. Muramyl dipeptide and derivatives. In: Stewart-Tull D E S, editor. The theory and practical application of adjuvants. New York, N.Y: John Wiley & Sons; 1995. pp. 171–201. [Google Scholar]
- 39.Takada H, Kotani S. Structure-function relationships of lipid A. In: Morrison D C, Ryan J L, editors. Molecular biochemistry and cellular biology. 1. Bacterial endotoxic lipopolysaccharides. Ann Arbor, Mich: CRC Press; 1992. pp. 108–134. [Google Scholar]
- 40.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956–5963. [PubMed] [Google Scholar]
- 41.ten Hagen T M L, Sulzer A J, Kidd M R, Lal A A, Hunter R L. Role of adjuvants in the modulation of antibody isotype, specificity, and induction of protection by whole blood-stage Plasmodium yoelii vaccines. J Immunol. 1993;151:7077–7085. [PubMed] [Google Scholar]
- 42.Verma J N, Wassef N M, Wirtz R A, Atkinson C T, Aikawa M, Loomis L D, Alving C R. Phagocytosis of liposomes by macrophages: intracellular fate of liposomal malaria antigen. Biochim Biophys Acta. 1991;1066:229–238. doi: 10.1016/0005-2736(91)90191-a. [DOI] [PubMed] [Google Scholar]
- 43.Verma J N, Rao M, Amselem S, Krzych U, Alving C R, Green S J, Wassef N M. Adjuvant effects of liposomes containing lipid A: enhancement of liposomal antigen presentation and recruitment of macrophages. Infect Immun. 1992;60:2438–2444. doi: 10.1128/iai.60.6.2438-2444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]