Abstract
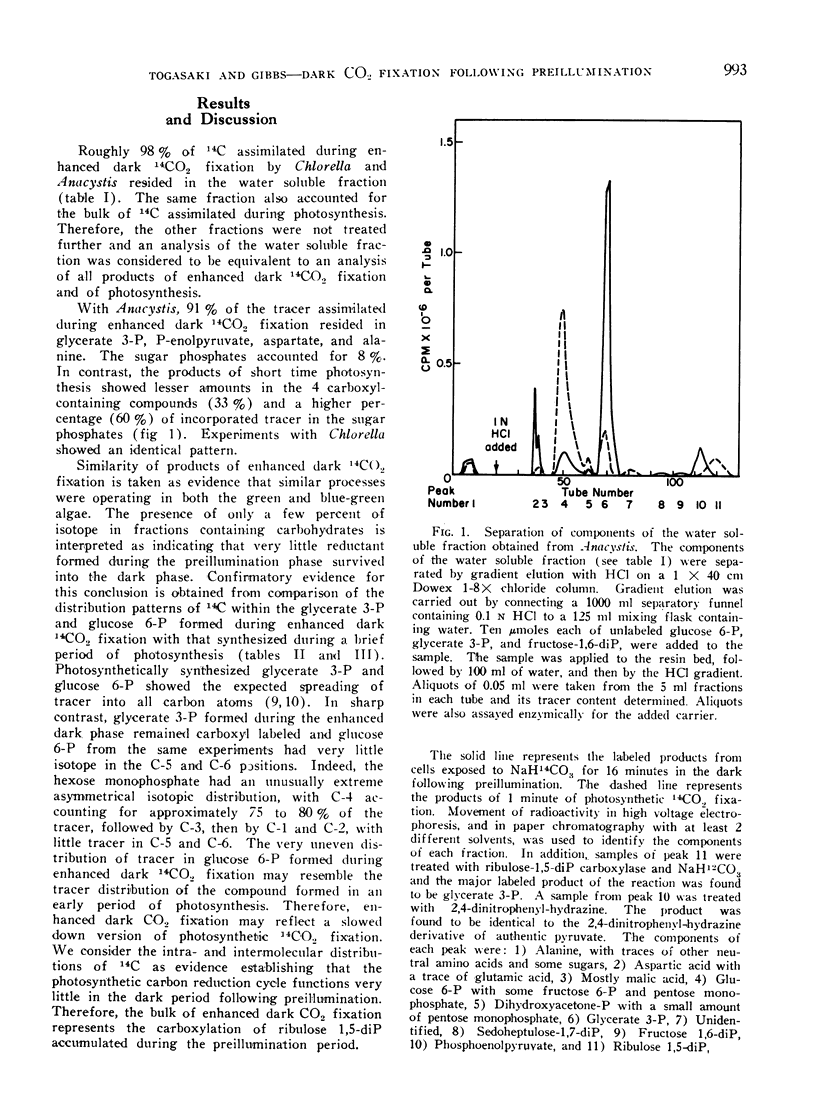
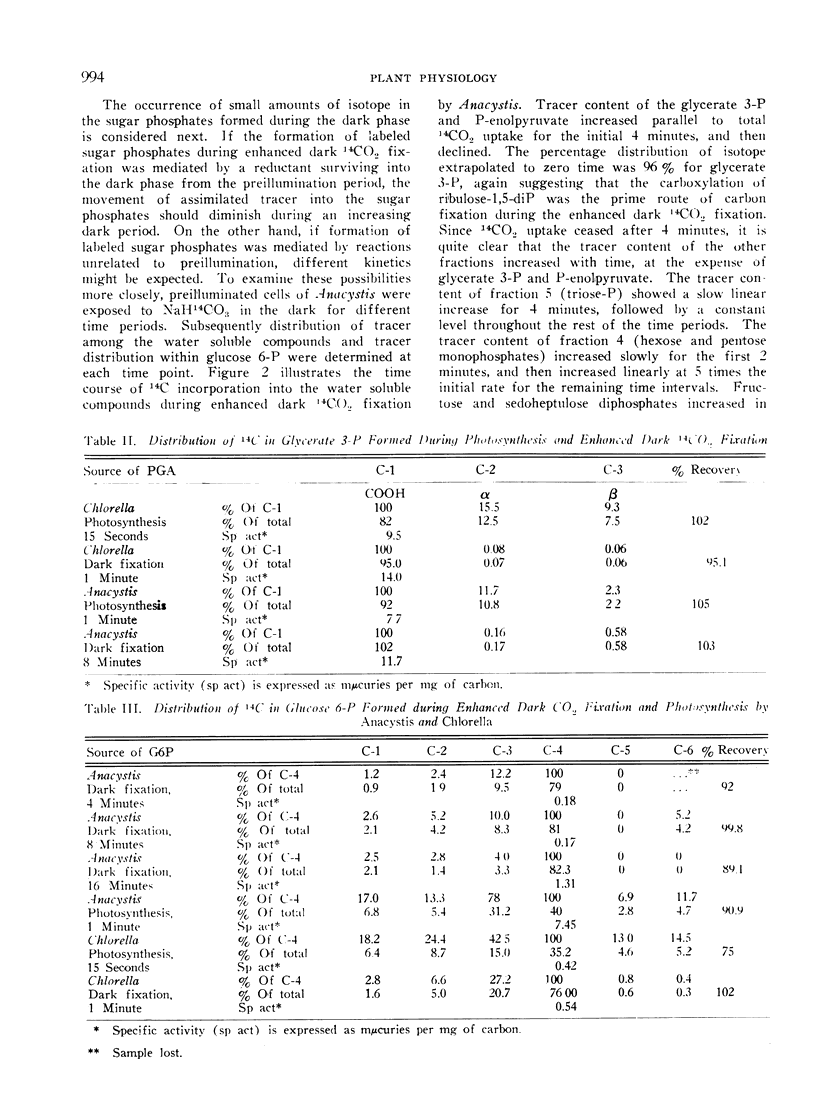
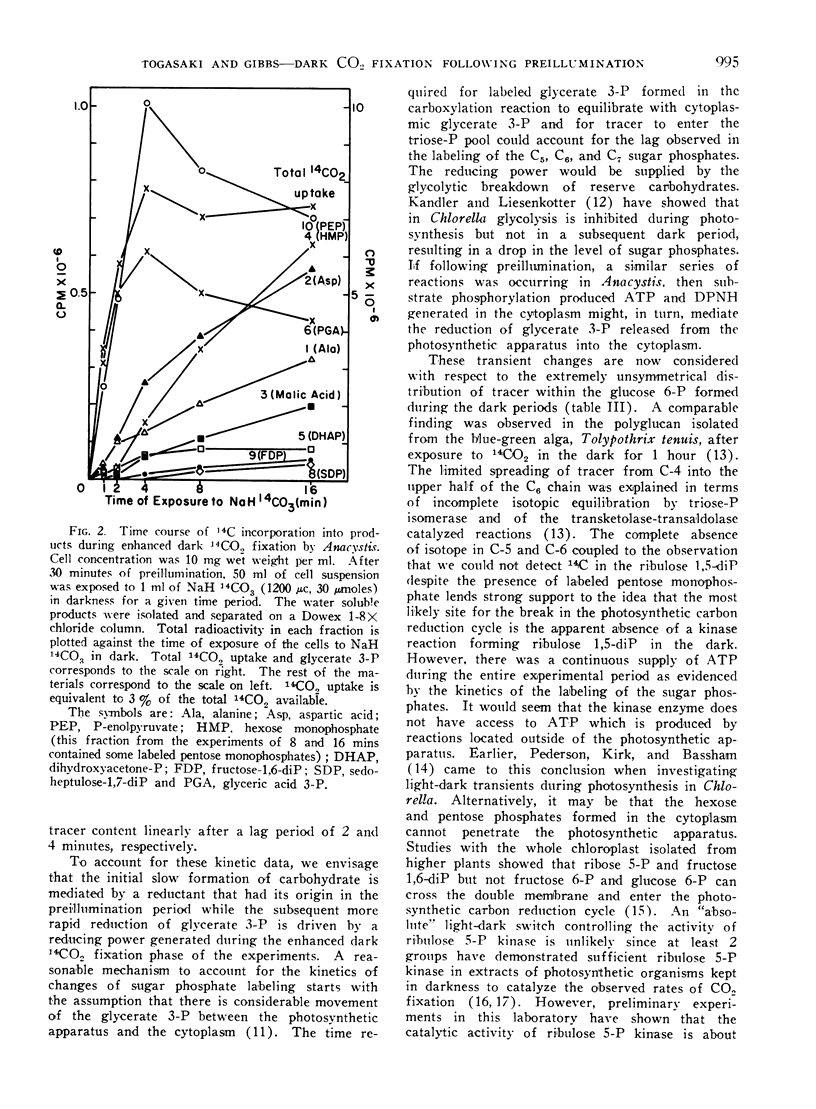
The products of short time photosynthesis and of enhanced dark 14CO2 fixation (illumination in helium prior to addition of 14CO2 in dark) by Chlorella pyrenoidosa and Anacystis nidulans were compared. Glycerate 3-phosphate, phosphoenolpyruvate, alanine, and aspartate accounted for the bulk of the 14C assimilated during enhanced dark fixation while hexose and pentose phosphates accounted for the largest fraction of isotope assimilated during photosynthesis. During the enhanced dark fixation period, glycerate 3-phosphate is carboxyl labeled and glucose 6-phosphate is predominantly labeled in carbon atom 4 with lesser amounts in the upper half of the C6 chain and traces in carbon atoms 5 and 6. Tracer spread throughout all the carbon atoms of photosynthetically synthesized glycerate 3-phosphate and glucose 6-phosphate. During the enhanced dark fixation period, there was a slow formation of sugar phosphates which subsequently continued at 5 times the initial rate long after the cessation of 14CO2 uptake. To explain the kinetics of changes in the labelling patterns and in the limited formation of the sugar phosphates during enhanced dark CO2 fixation, the suggestion is made that most of the reductant mediating these effects did not have its origin in the preillumination phase.
It is concluded that a complete photosynthetic carbon reduction cycle operates to a limited extent, if at all, in the dark period subsequent to preillumination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvin M., Benson A. A. The Path of Carbon in Photosynthesis. Science. 1948 May 7;107(2784):476–480. doi: 10.1126/science.107.2784.476. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Gibbs M. Dark and photometabolism of sugars by a blue green alga: Tolypothrix tenuis. Plant Physiol. 1966 Apr;41(4):731–737. doi: 10.1104/pp.41.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAGER E. W., ROSENBERG J. L., GAFFRON H. Intermediates in photosynthesis. Fed Proc. 1950 Jun;9(2):535–542. [PubMed] [Google Scholar]
- Gibbs M., Kandler O. ASYMMETRIC DISTRIBUTION OF C IN SUGARS FORMED DURING PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1957 Jun 15;43(6):446–451. doi: 10.1073/pnas.43.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Racker E. The reductive pentose phosphate cycle. III. Enzyme activities in cell-free extracts of photosynthetic organisms. Plant Physiol. 1961 Jul;36(4):409–414. doi: 10.1104/pp.36.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Urbach W., Hudson M. A., Ullrich W., Santarius K. A., Heber U. Verteilung und Wanderung von Phosphoglycerat zwischen den Chloroplasten und dem Cytoplasma während der Photosynthese. Z Naturforsch B. 1965 Sep;20(9):890–898. [PubMed] [Google Scholar]


