Abstract
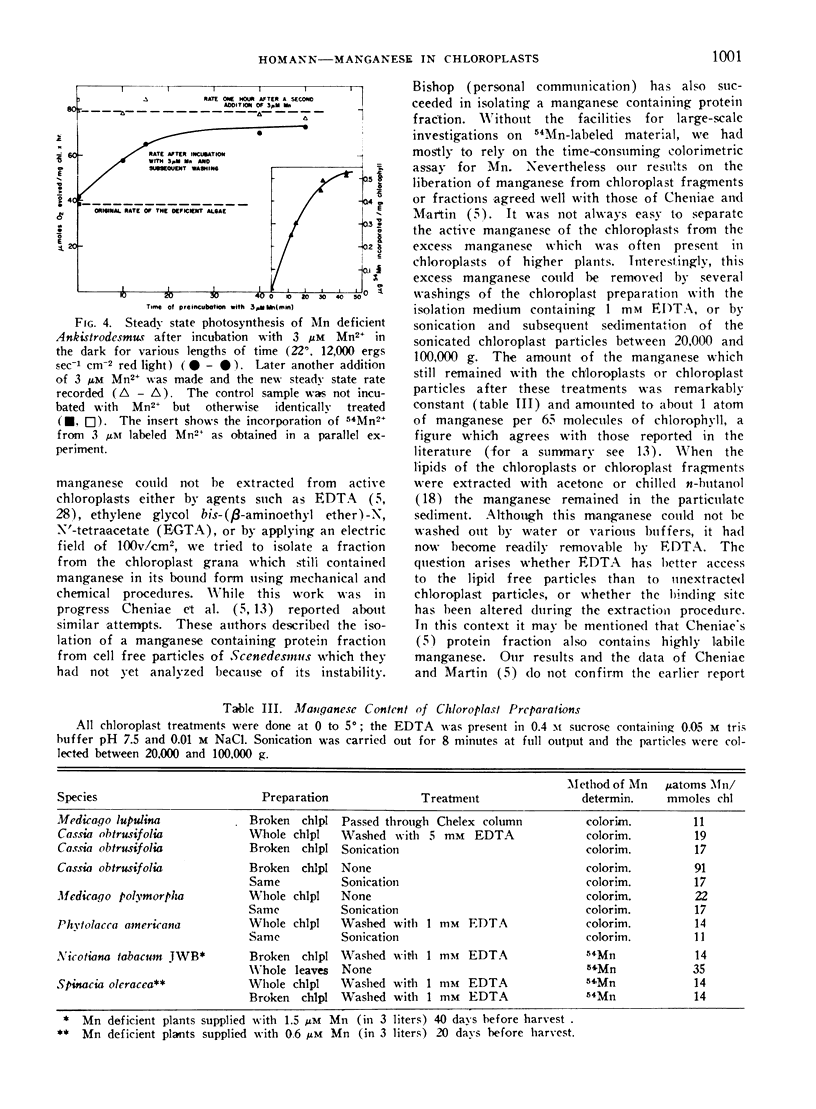
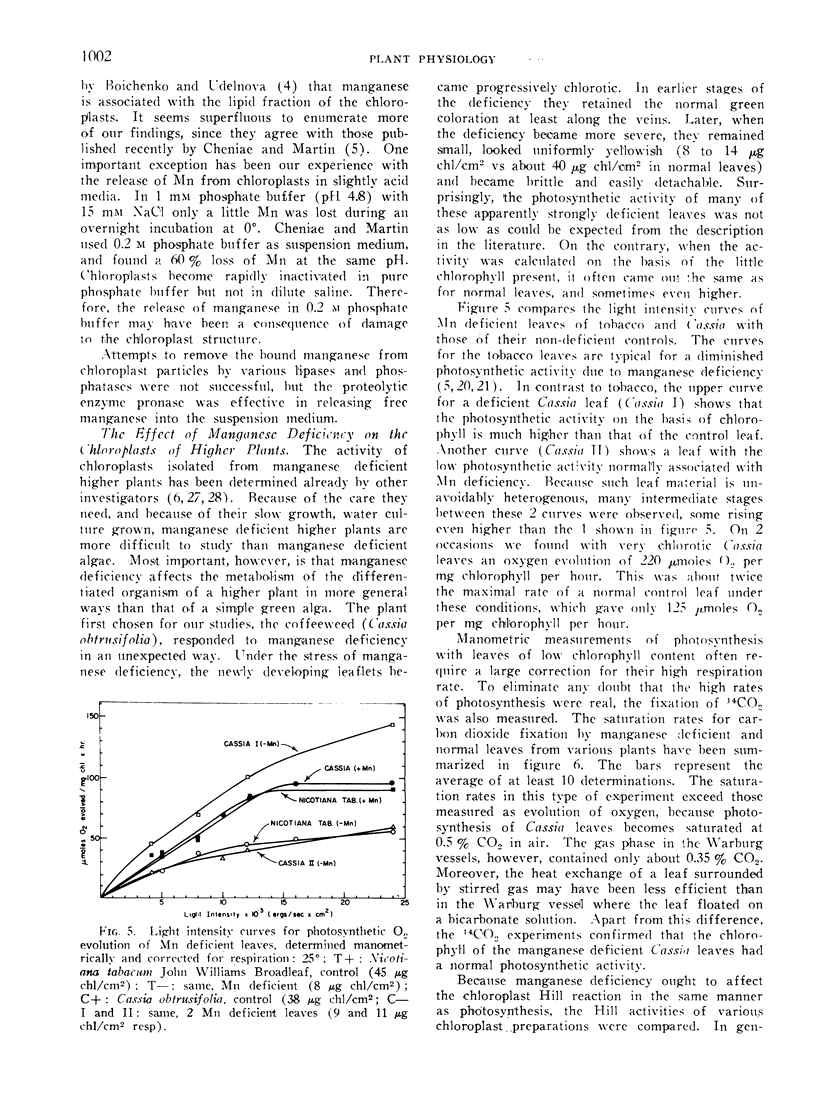
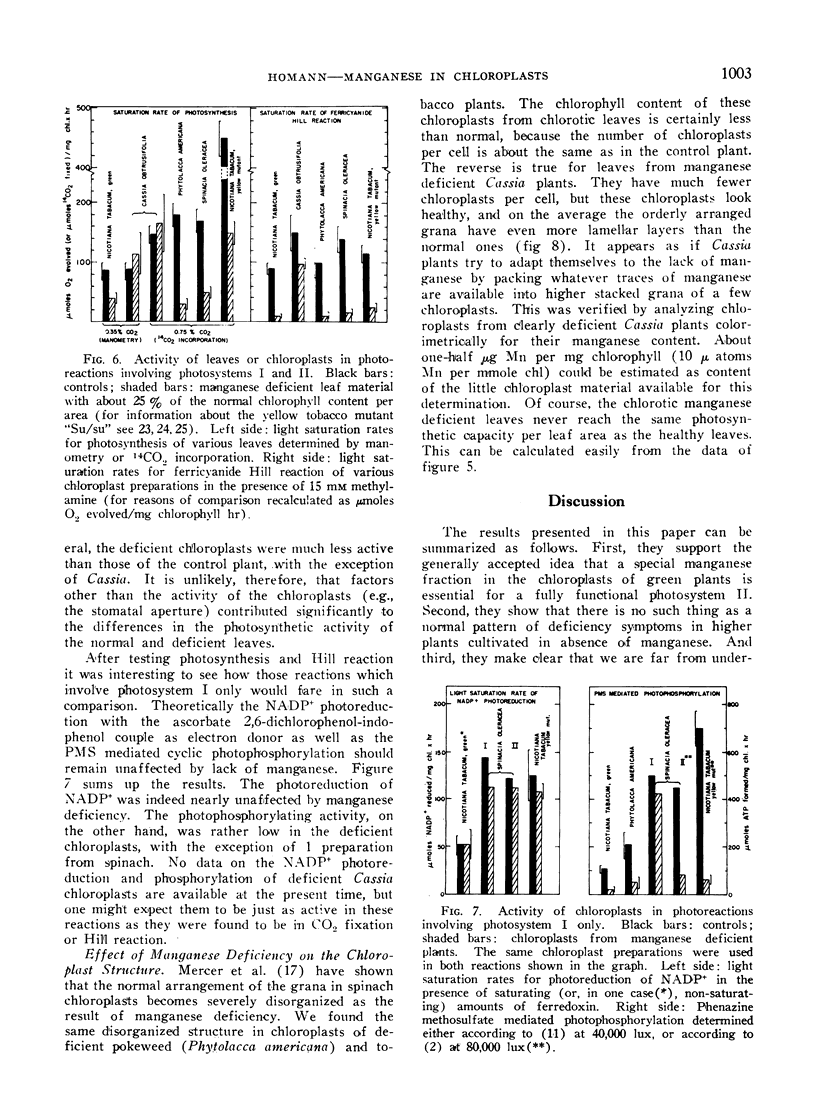
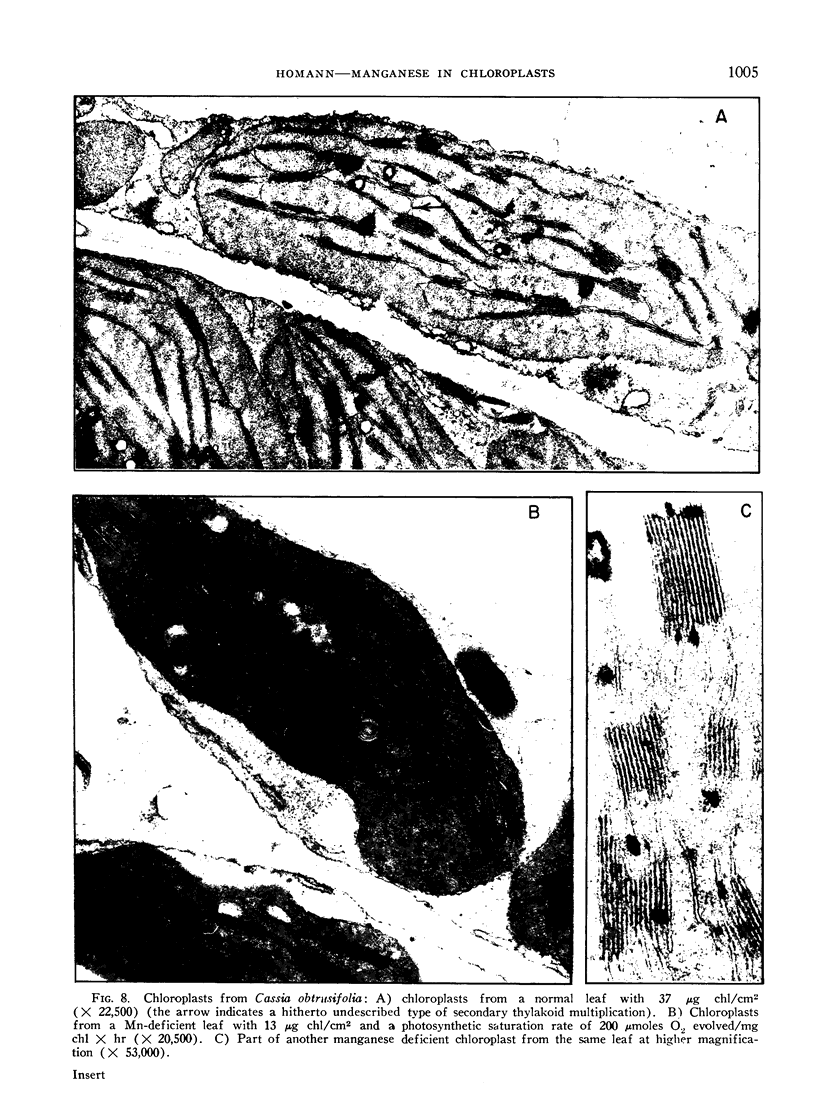
Manganese deficiency of green plants is known to affect preferentially the activity of the oxygen evolving system in the photosynthetic apparatus. Our studies showed that the time needed to reactivate photosynthesis in Mn-deficient algae varies with each culture, and is often very short when Mn is added not before illumination but during the light period. The recent finding by Cheniae and Martin that the reactivation requires light, is confirmed. The plain incorporation of 54Mn into deficient algae as distinguished from reactivation was barely affected by light, yet was inhibited by uncouplers of phosphorylation. Higher plants responded to manganese deficiency either by adjusting the number of chloroplasts per cell to the limited Mn supply, or by forming disorganized chloroplasts with low chlorophyll content. These 2 types of responses produced chlorotic plants which had either a few photosynthetically active or many disabled chloroplasts. Photosystem I mediated photophosphorylation turned out to be much more sensitive to manganese deficiency than the system I dependent photoreduction of NADP+.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Izawa S., Good N. E. The number of sites sensitive to 3-(3,4-dichlorophenyl)-1,1-dimethylurea,3-(4-chlorophenyl)-1,1-dimethylurea and 2-chloro-4-(2-propylamino)-6-ethylamino-s-triazine in isolated chloroplasts. Biochim Biophys Acta. 1965 May 25;102(1):20–38. doi: 10.1016/0926-6585(65)90200-1. [DOI] [PubMed] [Google Scholar]
- JAGENDORF A. T., AVRON M. Cofactors and rates of photosynthetic phosphorylation by spinach chloroplasts. J Biol Chem. 1958 Mar;231(1):277–290. [PubMed] [Google Scholar]
- Jacobson L. MAINTENANCE OF IRON SUPPLY IN NUTRIENT SOLUTIONS BY A SINGLE ADDITION OF FERRIC POTASSIUM ETHYLENEDIAMINE TETRA-ACETATE. Plant Physiol. 1951 Apr;26(2):411–413. doi: 10.1104/pp.26.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER E. On the role of manganese in the oxygen-evolving system of photosynthesis. Arch Biochem Biophys. 1955 Dec;59(2):527–529. doi: 10.1016/0003-9861(55)90519-1. [DOI] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T. A Spectrophotometric Assay of the Hill Reaction with Ferricyanide. Plant Physiol. 1957 Jul;32(4):373–374. doi: 10.1104/pp.32.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERCER F. V., NITTIM M., POSSINGHAM J. V. The effect of manganese deficiency on the structure of spinach chloroplasts. J Cell Biol. 1962 Nov;15:379–381. doi: 10.1083/jcb.15.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L., Barnard A. C., Deamer D. W. Ultrastructural and Photometric Evidence for Light-Induced Changes in Chloroplast Structure in vivo. Plant Physiol. 1967 Feb;42(2):283–293. doi: 10.1104/pp.42.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- Walker D. A. Correlation between Photosynthetic Activity and Membrane Integrity in Isolated Pea Chloroplasts. Plant Physiol. 1965 Nov;40(6):1157–1161. doi: 10.1104/pp.40.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier T. E., Stocking C. R., Shumway L. K. The photosynthetic apparatus in chloroplasts of higher plants. Brookhaven Symp Biol. 1966;19:353–374. [PubMed] [Google Scholar]
- Wiessner W. Relative quantum yields for anaerobic photoassimilation of glucose. Nature. 1966 Oct 22;212(5060):403–404. doi: 10.1038/212403a0. [DOI] [PubMed] [Google Scholar]