Abstract
Invasion of host cells is believed to be an important strategy utilized by a number of pathogens, which affords them protection from the host immune system. The connective tissues of the periodontium are extremely well vascularized, which allows invading microorganisms, such as the periodontal pathogen Porphyromonas gingivalis, to readily enter the bloodstream. However, the ability of P. gingivalis to actively invade endothelial cells has not been previously examined. In this study, we demonstrate that P. gingivalis can invade bovine and human endothelial cells as assessed by an antibiotic protection assay and by transmission and scanning electron microscopy. P. gingivalis A7436 was demonstrated to adhere to and to invade fetal bovine heart endothelial cells (FBHEC), bovine aortic endothelial cells (BAEC), and human umbilical vein endothelial cells (HUVEC). Invasion efficiencies of 0.1, 0.2, and 0.3% were obtained with BAEC, HUVEC, and FBHEC, respectively. Invasion of FBHEC and BAEC by P. gingivalis A7436 assessed by electron microscopy revealed the formation of microvillus-like extensions around adherent bacteria followed by the engulfment of the pathogen within vacuoles. Invasion of BAEC by P. gingivalis A7436 was inhibited by cytochalasin D, nocodazole, staurosporine, protease inhibitors, and sodium azide, indicating that cytoskeletal rearrangements, protein phosphorylation, energy metabolism, and P. gingivalis proteases are essential for invasion. In contrast, addition of rifampin, nalidixic acid, and chloramphenicol had little effect on invasion, indicating that bacterial RNA, DNA, and de novo protein synthesis are not required for P. gingivalis invasion of endothelial cells. Likewise de novo protein synthesis by endothelial cells was not required for invasion by P. gingivalis. P. gingivalis 381 was demonstrated to adhere to and to invade BAEC (0.11 and 0.1% efficiency, respectively). However, adherence and invasion of the corresponding fimA mutant DPG3, which lacks the major fimbriae, was not detected. These results indicate that P. gingivalis can actively invade endothelial cells and that fimbriae are required for this process. P. gingivalis invasion of endothelial cells may represent another strategy utilized by this pathogen to thwart the host immune response.
Adult periodontitis is a bacterially induced chronic inflammatory disease that is the major cause of tooth loss in the adult population (28). The disease has recently been associated with cardiovascular disease and preterm delivery of low birth weight infants (2, 10, 23). Interactions of the periodontal pathogen Porphyromonas gingivalis with the host immune system are believed to be the basis for the destructive inflammatory response which is characteristic of this disease, and the intimate interaction of P. gingivalis with the host has become the subject of intense investigation. The innate host defense system has been postulated to function in limiting the spread of P. gingivalis within the periodontal pocket by maintaining an intact epithelial cell barrier. However, several studies have documented that P. gingivalis can be found within gingival tissues in vivo, suggesting that it may pass through the epithelial barrier (24, 26). Invasion of oral epithelial cells by P. gingivalis in vitro has also been demonstrated by a number of investigators (8, 14, 16, 22). Reports by Madianos et al. (16) and by Lamont et al. (14) have confirmed the ability of P. gingivalis to replicate within KB cells and within primary gingival epithelial cells. P. gingivalis has also been shown to advance into deeper epithelial layers (24, 26), a process that could play a role in the systemic spread of the organism.
Ulcerations and disruptions of the basement membrane commonly occur in periodontitis (20) and may result from the production of potent P. gingivalis proteases that can degrade type IV collagen present in the basement membrane (33). The connective tissues of the periodontium are extremely well vascularized (9), allowing the invading microorganisms to readily enter the bloodstream. Thus, in addition to invasion of epithelial cells, the ability of P. gingivalis to actively invade endothelial cells could represent an additional mechanism evolved by this pathogen to evade the host response. The interactions between P. gingivalis outer membrane components and endothelial cells (EC) have been investigated by Darveau and colleagues, who examined the ability of P. gingivalis lipopolysaccharide (LPS) to stimulate an inflammatory response (5). These investigators found that unlike Escherichia coli LPS, P. gingivalis LPS did not stimulate the expression of E-selectin in human umbilical cord EC nor did it stimulate neutrophil adhesion to these cells (5). These observations are consistent with a role of P. gingivalis in suppressing the innate host inflammatory response to bacteria. Although these studies demonstrate that P. gingivalis components can interact with EC and cause a direct effect on the expression of adhesion molecules, the ability of viable P. gingivalis whole cells to interact with EC (active invasion process) has not been previously examined. The goal of this study was to determine whether P. gingivalis invades vascular EC in vitro. Our results indicate that P. gingivalis can actively invade EC and can replicate intracellularly. Expression of the major fimbriae is required for both adherence to and invasion of EC. We have also characterized the metabolic requirements for invasion of EC by P. gingivalis.
MATERIALS AND METHODS
Bacterial strains.
P. gingivalis A7436 was originally isolated from a patient with refractory periodontitis and was characterized in our laboratory; strain 381 and its fimA mutant DPG3 have been described previously (17). P. gingivalis fimA mutant DPG3 was constructed following allelic exchange of P. gingivalis 381 with the insertionally inactivated fimA gene (17). As seen previously, P. gingivalis 381 cultures were electron dense and produced long slender fimbriae (ca. 15 nm in diameter) that were easily seen in negatively stained preparations (22). By contrast, we did not detect these long fimbriae on the surface of the corresponding P. gingivalis fimA mutant DPG3 (22), indicating that the insertion mutation in DPG3 blocked fimbria production. P. gingivalis A7436 and 381 were grown on anaerobic blood agar (ABA) plates (BBL media; Becton Dickinson Co., Cockeysville, Md.), and DPG3 was grown on ABA containing erythromycin (10 μg/ml) under anaerobic conditions in an anaerobic chamber (with 85% N2, 10% CO2, and 5% H2) for 3 to 5 days, inoculated into fresh Schaedler broth (Difco) (containing erythromycin for DPG3), and grown for 24 h until the optical density reached 1.0.
Interaction of P. gingivalis with EC.
Adherence and invasion of P. gingivalis was assessed in bovine aortic endothelial cells (BAEC), fetal bovine heart endothelial cells (FBHEC), and human umbilical vein endothelial cells (HUVEC) as described previously with EC (22). Invasion of KB oral EC (GIBCO-BRL Life Technologies, Grand Island, N.Y.) was as previously described (22). BAEC (Repository no. AG08592, N.I.A. Aging Cell Culture Repository, Coriell Institute for Medical Research, Camden, N.J.) and FBHEC (ATCC CRL 1395, American Type Culture Collection [ATCC], Rockville, Md.) were maintained in Dulbecco’s modified Eagle medium (DMEM) (GIBCO-BRL Life Technologies) supplemented with 10% heat-inactivated newborn calf serum (NCS [GIBCO-BRL]) and penicillin-streptomycin-glutamine (GIBCO-BRL). FBHEC were supplemented with 100 ng of fibroblast growth factor (GIBCO-BRL)/ml. HUVEC (ATCC CRL 1730; ATCC) were maintained in F-12K medium (ATCC) supplemented with heparin (100 μg/ml; Sigma Chemical Co., St. Louis, Mo.) and EC growth supplement (50 μg/ml; Sigma) in addition to normal human serum and antibiotic solution as described for BAEC. Subcultivation was performed on confluent cultures that had been plated 24 h earlier. All EC were plated at a concentration of 106 cells per ml, as determined by cell counting on a Coulter Counter (8). For all invasion assays, we used Falcon six-well flat-bottom plates with a volume of 15.5 ml/well and a surface area of 9.6 cm2/well. The multiplicity of infection (MOI) was calculated based on the number of cells per well at confluence. P. gingivalis strains grown to an optical density of 1.0 were centrifuged, washed with phosphate-buffered saline (PBS), and resuspended in DMEM containing 1 mM MgCl2 and 0.2 mM CaCl2 at a final concentration of 108 cells per ml in the absence of serum. Bacterial suspensions (1.0 ml) were added to confluent EC monolayers (MOI = 100) and incubated at 37°C in 5% CO2 for 2 h. After incubation, unattached bacteria were removed following washing of the monolayers twice with PBS. EC were lysed in 1 ml of sterile distilled water (dH2O) per well and incubated for 30 min, during which they were disrupted by repeated pipetting. Control experiments demonstrated that exposure to water for 30 min did not affect bacterial viability. Lysates were serially diluted, plated on ABA plates, and incubated anaerobically at 37°C for 7 days to determine the number of organisms that adhered to EC. All assays were performed in triplicate.
Invasion of EC monolayers by P. gingivalis strains was quantified by determining the number of CFU recovered following antibiotic treatment. Confluent EC monolayers were infected with 1.0 ml of a bacterial suspension (final concentration, 108 cells), and tissue culture plates were centrifuged at 2,000 × g for 10 min at 4°C to enhance contact between EC and bacteria. Infected monolayers were incubated at 37°C for 1 to 4 h in a 5% CO2 incubator. After every hour, unattached bacteria were removed following washing of the monolayers twice with PBS. External adherent cells were killed by incubating the infected monolayers with DMEM containing 100 μg of metronidazole/ml. This concentration of antibiotic was sufficient to completely kill 108 bacteria per ml in 1 h (data not shown). The antibiotic did not affect the morphology or viability of the EC as detected by their ability to exclude trypan blue. Controls for antibiotic killing of P. gingivalis were included in all experiments. After exposure to antibiotic, monolayers were washed twice with PBS, and EC were lysed in 1 ml of sterile dH2O per well and incubated for 30 min. Lysates were diluted and plated on ABA plates and incubated anaerobically at 37°C for 7 days. CFU of invasive organisms were then enumerated. Invasion was expressed as the percentage of the initial inoculum recovered after antibiotic treatment and EC lysis. All assays were performed in triplicate. E. coli JM109 and HB101 were used as appropriate negative controls for invasion assays with BAEC.
Since our results indicated that cytochalasin D treatment inhibited P. gingivalis invasion of EC (see below), we also examined the adherence of P. gingivalis to EC in cytochalasin D-treated cultures. When adherence was measured without cytochalasin D treatment, we obtained an adherence efficiency of 0.16%. When adherence was measured in cytochalasin D-treated cultures, we observed an adherence efficiency of 0.14%. Thus, the adherence assay described here is a good readout of the number of bacteria adhering.
To address the possibility of intracellular replication of P. gingivalis within the EC, P. gingivalis A7436 was incubated with BAEC for 2 h after which the extracellular bacteria were killed by antibiotic treatment. After washing of the wells to remove the antibiotic, the EC were maintained in culture medium for an additional 6 h to allow replication of intracellular bacteria. Lysate was prepared as described above, diluted appropriately, and plated on ABA plates, and CFU were enumerated after incubation under anaerobic conditions as described above.
Inhibitors of bacterial and EC functions.
The effect of a variety of inhibitors of procaryotic and eucaryotic cell functions on P. gingivalis invasion of BAEC was investigated. All chemicals were obtained from Sigma. The following inhibitors, in the solvent and at the final concentration indicated, were used: cytochalasin D, 1 μg/ml in dimethyl sulfoxide (DMSO); nocodazole, 10 μg/ml in DMSO; staurosporine, 1 μM in DMSO; sodium azide, 50 mM in PBS; cycloheximide, 100 μg/ml in ethanol; chloramphenicol, 5 μg/ml in DMEM; rifampin, 0.25 μg/ml in acetone; nalidixic acid, 5 μg/ml in 1 N NaOH; and a cocktail of protease inhibitors containing aprotonin, 2 μg/ml in dH2O; phenylmethylsulfonyl fluoride, 0.1 mM in methanol; pepstatin, 0.7 μg/ml in methanol; and benzamidine, 1 mM in methanol. Cytochalasin D and staurosporine were preincubated with the EC for 30 min prior to the addition of the bacteria and remained present throughout the invasion assay. Nocodazole was preincubated with the EC for 1 h on ice and then at 37°C for 30 min prior to reacting with the bacteria. The drug was present during the invasion assay. Cycloheximide was preincubated with the EC for 4 h before bacterial addition and was present during the assay. Sodium azide was preincubated with the EC for 4 h and then removed by washing three times in DMEM prior to bacterial addition. Sodium azide was also preincubated with P. gingivalis for 4 h and then removed by washing prior to the assay. Chloramphenicol, rifampin, and nalidixic acid were preincubated with the bacteria for 4 h before reaction with the EC and were present during the assay. Protease inhibitors were preincubated with the bacteria for 30 min prior to the assay. All potential inhibitors were tested at the concentrations used for possible adverse effects on the EC (compared with cells without the inhibitor) by examining the morphology of the cells and the confluency of the monolayer and by trypan blue exclusion. Cytochalasin D, nocodazole, staurosporine, cycloheximide, and the protease inhibitors were examined for any toxic effects on P. gingivalis as determined by viable cell counting and were found to have no adverse effect on the viability at the concentrations used. Ethanol, acetone, DMSO, methanol, and NaOH, which were used as solvents, were tested at the appropriate concentrations and found to produce no reduction in P. gingivalis numbers.
Electron microscopy.
Transmission and scanning electron microscopies (TEM and SEM) were performed on designated samples to confirm adherence to EC and internalization within the cells. For TEM, EC were cultured in six-well plates and treated as described for the invasion experiments, with the following modifications. Following a 2-h incubation with bacteria, monolayers were washed four times in PBS and detached from the plastic surface with trypsin. The cell slurry was then immediately centrifuged for 4 min at 15,000 × g. The cell pellet was washed twice with PBS and fixed with 2.5% gluteraldehyde in 0.1 M sodium cacodylate. Cells were pelleted and postfixed in 1% OsO4 in 0.1 M sodium cacodylate for 1 h, and the ultrathin sections were contrasted with lead citrate and uranyl acetate before examination by TEM with a 1200EX electron microscope (JEOL, Tokyo, Japan). For SEM, EC were incubated with P. gingivalis for 1 h and washed with PBS, and bacteria attached to EC were observed with a scanning electron microscope (JSM-820; JEOL).
RESULTS
Invasion of EC by P. gingivalis.
P. gingivalis A7436 was found to invade BAEC, FBHEC, and HUVEC at invasion efficiencies of 0.1, 0.3, and 0.2%, respectively (Table 1). Adherence of P. gingivalis A7436 to KB epithelial cells was approximately one log higher than that observed with EC. However, the invasion efficiencies observed for P. gingivalis A7436 with EC (0.1 to 0.3%) were approximately one log higher than that observed for P. gingivalis invasion of epithelial cells (0.01%) (Table 1). As expected, we did not observe invasion of BAEC with E. coli JM109 or HB101, which were used as negative controls. The invasion efficiencies observed for E. coli JM109 and HB101 were 0.0007 and 0.005%, respectively (data not shown).
TABLE 1.
Invasion of epithelial and endothelial cells by P. gingivalis A7436
| Cell type | Bacteria adhering and invading (%)a | Invasion efficiency (%)b |
|---|---|---|
| KB epithelial | 1.1 ± 0.3 | 0.01 ± 0.003 |
| Bovine aortic endothelial | 0.12 ± 0.02 | 0.1 ± 0.03 |
| Bovine heart endothelial | 0.35 ± 0.04 | 0.3 ± 0.04 |
| Human umbilical vein endothelial | 0.5 ± 0.06 | 0.2 ± 0.05 |
Defined as the percentage of CFU input that bound to epithelial or endothelial cells; represents both bacteria adhering and invading. Values are the means of triplicate independent determinations from a typical experiment ± standard deviations.
Percentage of the inoculum (P. gingivalis) protected from metronidazole killing after the infection period relative to the number of bacteria adhering. Values are the means of triplicate independent determinations from a typical experiment ± standard deviations.
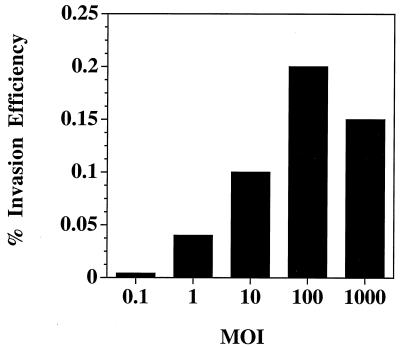
The number of input P. gingivalis cells was also observed to influence the invasion efficiency of P. gingivalis for BAEC. The invasion efficiency of P. gingivalis for BAEC at an MOI of 1:1 was 0.04% and increased to 0.1% at an MOI of 1:10 (Fig. 1). The maximum invasion efficiency of 0.2% was observed at an MOI of 1:100. We obtained a slightly lower level of invasion of BAEC by P. gingivalis A7436 at an MOI of 1:1,000, and thus for the remainder of the studies described below we utilized an MOI of 1:100. At an MOI of 1:100, optimal invasion of BAEC by P. gingivalis A7436 was observed at 2 h with no further increase in invasion with an extended incubation period up to 4 h (data not shown). We also found that a majority of the P. gingivalis organisms that had adhered to the EC were found to invade as assessed by an antibiotic protection assay (Table 1). Centrifugation of P. gingivalis cultures on the EC monolayers was observed to slightly enhance the invasion efficiency but was not required for active invasion (data not shown).
FIG. 1.
MOI of P. gingivalis for BAEC. P. gingivalis A7436 was grown as described in Materials and Methods, and 105 to 109 CFUs were added to 106 BAEC and incubated as described for the invasion assays. The invasion efficiency is defined as the percentage of the inoculum (P. gingivalis) protected from metronidazole killing after the infection period. For MOI of 0.1, 1.0, 10, 100, and 1,000, 105, 106, 107, 108, and 109 P. gingivalis CFU, respectively, were used.
Adherence and invasion efficiencies of the P. gingivalis wild-type strains 381 and A7436 to BAEC were similar (Table 2). In contrast to the results obtained with the P. gingivalis wild-type strain 381, we did not detect adherence of the P. gingivalis fimA mutant DPG3 to BAEC. In agreement with the results obtained in the adherence assay, we did not detect invasion of BAEC by P. gingivalis DPG3. These results are in agreement with our previous observations in a KB oral epithelial cell line (22) and indicate that the major fimbriae are required for invasion of EC.
TABLE 2.
Invasion of BAEC by P. gingivalis
| Strain | Bacteria adhering and invading (%)a | Invasion efficiency (%)b |
|---|---|---|
| A7436 | 0.12 ± 0.02 | 0.10 ± 0.03 |
| 381 | 0.11 ± 0.01 | 0.10 ± 0.01 |
| DPG3 | 0.001 ± 0.0003 | 0.001 ± 0.0004 |
Defined as the percentage of CFU input that bound to EC; represents both bacteria adhering and invading. Values are the means of triplicate independent determinations from a typical experiment ± standard deviations.
Percentage of the inoculum (P. gingivalis) protected from metronidazole killing after the infection period. Values are the means of triplicate independent determinations from a typical experiment ± standard deviations.
We also found that P. gingivalis was capable of replicating within BAEC, increasing from a mean of 4.2 × 106 CFU detected following a 2-h incubation to a mean of 6.2 × 107 CFU detected following a 6-h incubation period (data not shown). The BAEC were viable following the 6-h incubation period with P. gingivalis as determined by trypan blue dye exclusion (data not shown). We were unable to accurately assess the growth of P. gingivalis in EC for longer periods of time due to alterations in the morphology of the BAEC monolayer following long-term incubation with P. gingivalis A7436.
Electron microscopy.
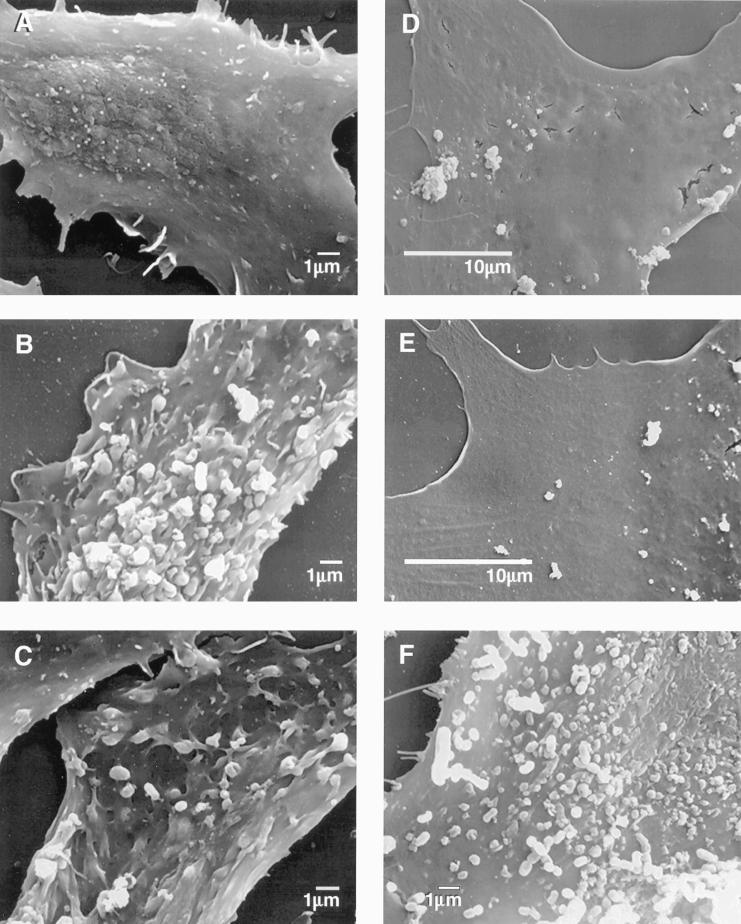
We next examined the nature of the interaction of P. gingivalis with EC by electron microscopy. P. gingivalis wild-type strain A7436 was seen adhering to BAEC and could be seen entering an individual cell (Fig. 2A and B). P. gingivalis A7436 could be seen intimately attached to both BAEC and to FBHEC (Fig. 2A, B, E, and F). This intimate attachment included the presence of microvilli protruding from the EC and surrounding the attached bacteria; this process may represent the initial formation of a cytoplasmic vacuole. P. gingivalis A7436 was also visible within the cytoplasm of BAEC and FBHEC and was detected within what appeared to be vacuoles (Fig. 2B and F). Similar results were observed in HUVEC infected with P. gingivalis A7436 (data not shown). We also observed the presence of microvilli protruding from the cell surface in BAEC infected with P. gingivalis wild-type strain 381 (Fig. 2C). In contrast, we did not observe microvilli on the surface of BAEC infected with the fimA mutant strain, nor did we detect alterations in the BAEC surface (Fig. 2D). Likewise, P. gingivalis DPG3 was not detected within EC.
FIG. 2.
Transmission electron micrographs demonstrating P. gingivalis invasion of EC. (A and B) BAEC with P. gingivalis A7436. (A) At the cell surface, bacteria appear to induce EC structural rearrangements consistent with an endocytic mechanism. (B) Internalized bacteria are found within vacuole. (C) BAEC with P. gingivalis 381. Note the apparent contact between microfilamentous cellular components and surface-adhering P. gingivalis. (D) BAEC with P. gingivalis fimA mutant DPG3. Absence of intimate interaction between EC surface and bacteria. (E and F) FBHEC incubated with P. gingivalis A7436. Surface adherence (E) and engulfment in vacuole (F). Arrows in all panels point to P. gingivalis. Bars on each image are 0.5 μm unless otherwise specified. Composite image was constructed with Adobe Photoshop 3.0.
We also examined the interactions of P. gingivalis A7436, 381, and DPG3 with BAEC and FBHEC by SEM. Following the addition of P. gingivalis wild-type strain A7436 or 381, profound changes in the normal architecture of the EC surface occurred with the appearance of long microvilli surrounding large bacterial clumps (Fig. 3B, C, and F). However, no such change was observed with the fimA mutant DPG3 (Fig. 3D). Taken together, these results suggest that the major fimbriae may trigger a sequence of events that can lead to the reorganization of cytoskeletal components, giving rise to long microvilli which facilitate fimbrial contact with the EC. In the absence of fimbria expression, P. gingivalis does not appear to come into contact with the EC nor does it trigger host signaling events.
FIG. 3.
Adherence of P. gingivalis to EC examined by SEM. (A) Uninfected BAEC. (B) BAEC infected with P. gingivalis A7436. (C) BAEC infected with P. gingivalis 381. (D) BAEC infected with P. gingivalis fimA mutant DPG3. (E) Uninfected FBHEC. (F) FBHEC infected with P. gingivalis A7436.
Metabolic requirements for invasion.
Inhibition of invasion of BAEC by P. gingivalis with compounds that impede various metabolic functions of eucaryotic and procaryotic cells was examined as shown in Table 3. Bacterial DNA and RNA synthesis does not appear to be required for invasion, as nalidixic acid and rifampin did not inhibit P. gingivalis invasion of BAEC. Similarly, bacterial and BAEC de novo protein synthesis was not required for invasion since both chloramphenicol and cycloheximide did not reduce the invasion of BAEC by P. gingivalis. All these compounds were also tested at higher concentrations (five times the concentration indicated in the Materials and Methods section) and were found to have no effect on the invasion of BAEC by P. gingivalis (data not shown). Cytochalasin D, an inhibitor of actin polymerization, and nocodazole, an inhibitor of microtubule formation, substantially reduced invasion, indicating that both microfilaments and microtubule activity are required for invasion. Staurosporine, a broad-spectrum inhibitor of protein kinases, inhibited invasion, suggesting that protein phosphorylation is involved in the EC signaling pathway that results in bacterial entry into the cell. EC as well as P. gingivalis energy metabolism is also required for active invasion of P. gingivalis, as sodium azide (which reduced the proton motive force and inhibits cytochrome oxidase) inhibited invasion when bacteria or BAEC were preincubated with this compound. The cocktail of protease inhibitors, aprotonin, phenylmethylsulfonyl fluoride, pepstatin, and benzamidine, at concentrations predetermined not to be lethal to the BAEC monolayers or to P. gingivalis, inhibited P. gingivalis invasion of BAEC by 99% (Table 3).
TABLE 3.
Effects of metabolic inhibitors on P. gingivalis A7436 invasion of BAEC
| Inhibitor | Targeta | % Inhibitionb |
|---|---|---|
| Cytochalasin D | EC (actin) | 97.5 ± 1.2 |
| Nocodazole | EC (microtubule) | 87.4 ± 1.3 |
| Staurosporine | EC (protein kinase) | 86.6 ± 4.2 |
| Cycloheximide | EC (protein synthesis) | 2.5 ± 0.8 |
| Sodium azide | EC (energy metabolism) | 80.5 ± 2.1 |
| Sodium azide | P. gingivalis (energy metabolism) | 99.1 ± 0.1 |
| Chloramphenicol | P. gingivalis (protein synthesis) | 3.2 ± 0.4 |
| Rifampin | P. gingivalis (RNA synthesis) | 4.1 ± 1.9 |
| Nalidixic acid | P. gingivalis (DNA synthesis) | 3.3 ± 1.1 |
| Protease inhibitors | P. gingivalis (proteases) | 99.1 ± 0.2 |
Inhibitors were preincubated with either the endothelial cells or P. gingivalis as described in the text.
Reduction in invasion in the presence of inhibitors as compared with that of a control with no inhibitor present. Values are means ± standard deviations (n = 3).
DISCUSSION
Invasion of epithelial cells by P. gingivalis has been reported by a number of investigators (8, 14, 16, 22). However, this is the first report on the invasion of EC by P. gingivalis. We have demonstrated that P. gingivalis can invade FBHEC and BAEC as well as HUVEC. We found that P. gingivalis was capable of replicating within EC, suggesting that it has the capacity to persist within this host cell and possibly to alter the integrity of the EC. We also observed that a majority of the P. gingivalis organisms that had adhered to the EC were able to actively invade, suggesting a highly efficient endocytic uptake of surface-adherent organisms. Similar results have been observed during invasion of brain microvascular EC by group B streptococci (21). Whether P. gingivalis uses an endocytic pathway for uptake into EC has not yet been established; however, analysis of the invasion of EC by P. gingivalis by electron microscopy supports the endocytic pathway of engulfment. In addition, studies of P. gingivalis invasion of epithelial cells have shown that treatment of epithelial cells with monodansylcadaverine and ouabain, substances that inhibit the formation of coated pits, results in reduction in the number of invading P. gingivalis (27). Thus it is possible that P. gingivalis utilizes a similar pathway for the invasion of EC.
The major fimbria of P. gingivalis appears to play a major role in adherence and invasion of BAEC, since we did not detect invasion by the fimA mutant DPG3. Previous studies have established that P. gingivalis DPG3 does not produce fimbriae detectable by electron microscopy or Western blot analysis (17, 22). The involvement of P. gingivalis major fimbriae in invasion of epithelial cells has been reported previously (23, 32). We cannot rule out the possibility that molecules other than fimbriae may also be required for the adherence of P. gingivalis to EC. Our recent results indicate that digestion of P. gingivalis A7436 with amyloglucosidase, which partially dissolves the polysaccharide capsule, increases threefold the invasion efficiency of P. gingivalis for BAEC (6). It is well documented that in other bacteria the production of a polysaccharide capsule may interfere with attachment of bacteria to epithelial and endothelial cells (30, 31). It has been demonstrated that polysaccharide capsule-deficient mutants of Haemophilus influenzae type B exhibit enhanced adherence to and invasion of human cells (30). Whether the partial digestion of P. gingivalis capsule helped to unmask or expose adhesive ligands and fimbriae present on P. gingivalis cell surface remains to be determined.
It appears that invasion of EC by P. gingivalis shares some similar characteristics with the invasion process for epithelial cells, while other characteristics are unique to the EC. Electron microscopic analysis confirmed the intimate interaction of P. gingivalis A7436 and 381 with BAEC. The interaction of P. gingivalis with BAEC appears to involve attachment to BAEC and subsequent engulfment of the bacteria and is similar to what has been previously observed during invasion of epithelial cells by P. gingivalis (22). Invasion of EC by P. gingivalis does not require bacterial DNA, RNA, or de novo protein synthesis, while these bacterial functions are reported to be essential for epithelial cell invasion by P. gingivalis (14). Endothelial and epithelial cell invasion, however, seems to require the energy metabolism of both BAEC and bacteria, as well as the expression of the P. gingivalis proteases (14). In agreement with observations of invasion of epithelial cells by P. gingivalis (27), our results indicate that cytoskeletal rearrangements and protein phosphorylation are required for invasion of BAEC by P. gingivalis. It appears that the receptors required for adherence of P. gingivalis may be present on the EC surface since de novo protein synthesis of EC is not required for invasion.
The interactions between P. gingivalis outer membrane components and EC have been studied extensively by Darveau and colleagues (5). These investigators have shown that LPS of P. gingivalis does not stimulate E-selectin expression and that it can block E-selectin expression by LPS from other gram-negative bacteria (5). However, it remains to be determined how the active invasion of EC by viable P. gingivalis cells affects the regulation of specific adhesion molecules as well as the cytokine response of the EC. A number of microorganisms have been observed to actively invade EC by a process that stimulates adhesion molecule expression and cytokine production (7, 11–13, 15). Chlamydia pneumoniae, which has recently been linked to coronary heart disease, has been demonstrated to cause an upregulation of E-selectin, intercellular adhesion molecule (ICAM-1), and vascular cell adhesion molecule (VCAM-1) (13). Rickettsia rickettsii infection of EC stimulates these cells to produce interleukin-1α (IL-1α) (29). Rickettsia conorii infection of EC has also been demonstrated to enhance the expression of the adhesive molecules E-selectin, ICAM-1, and VCAM-1 (7). Active invasion of R. conorii was also required for production of IL-6 and IL-8 from cultured human EC (12). Likewise, active invasion of epithelial cells by several microorganisms has been demonstrated to modulate endogenous cytokine production (1, 4). Aihara et al. (1) found that only viable Helicobacter pylori in direct contact with gastric epithelial cells induces IL-8 formation. Killed bacteria, culture supernatants, and juxtaposition of a cell-impermeable barrier between the epithelial cells and bacteria abrogated the effect. Recent studies indicate that invasion of epithelial cells by P. gingivalis results in the inhibition of IL-8 secretion (4). Thus, growing evidence suggests an important role for direct bacteria cell interactions for the modulation of expression of cell adhesion molecules and cytokines.
Several reports have described an association between periodontal disease and coronary artery disease. These include case control studies which demonstrated significant associations after correction for cholesterol, smoking, hypertension, social class, and body mass index (2, 10, 18, 19). Infection with P. gingivalis and the biological consequences for increased risk for cardiovascular disease have recently received considerable attention (10). P. gingivalis infection can cause local inflammation which leads to ulceration of the gingivae and local vascular changes which increase the incidence and the severity of transient bacteremias when the gingivae are traumatized. Procedures such as dental extraction, periodontal surgery, tooth scaling, and even tooth brushing can lead to the presence of oral bacteria in circulating blood (3). The gingival sulcus is believed to be the most likely area of entry of oral bacteria into the bloodstream, and the integrity of the basement membrane of the oral mucosa, and particularly the gingival sulcus, is paramount in the protection of patients at risk for cardiovascular disease (3). The results presented here indicate that P. gingivalis can actively invade and multiply within EC, indicating that P. gingivalis has evolved mechanisms for survival and replication within vascular EC. Injured or activated EC (during infection) may show various artherogenic properties, including increased procoagulant activity, secretion of vasoactive and inflammatory mediators, and expression of adhesion molecules. E-selectin, ICAM-1, and VCAM-1 have been detected in atherosclerotic areas of human arteries (25), and inflammatory cells are abundant at these sites. Studies are currently under way in our laboratory to examine the regulation of adhesion molecule expression as well as the cytokine production in response to P. gingivalis invasion of EC. Invasion of vascular and heart EC by P. gingivalis after entry into the bloodstream may contribute to the pathology of cardiovascular disease.
ACKNOWLEDGMENTS
This work was supported by Research Centers for Minority Institutions (RCMI) grant RR/AI03034 and by DE9161 from the National Institutes of Health.
The authors would like to thank Lawrence Brako for the electron microscopy, Aurellia James for the illustrations, and Yvonne Powers for typing the manuscript. We also acknowledge Hakim Sojar and Robert J. Genco for stimulating discussions regarding this work.
REFERENCES
- 1.Aihara M, Tsuchimot D, Takizawa H, Azuma A, Wakebe H, Ohmoto Y, Imagawa K, Kikuchi M, Mukaida N, Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line. Infect Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck J, Garcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 3.Daly C, Mitchell D, Grossberg D, Highfield J, Stewart D. Bacteremia caused by periodontal probing. Aust Dent J. 1997;42:77–80. doi: 10.1111/j.1834-7819.1997.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 4.Darveau R P, Belton C M, Reife R A, Lamont R J. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun. 1998;66:1660–1665. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darveau R P, Cunningham M D, Bailey T, Seachord C, Ratcliffe K, Bainbridge B, Dietsch M, Page R C, Aruffo A. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect Immun. 1995;63:1311–1317. doi: 10.1128/iai.63.4.1311-1317.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande, R. G. Unpublished data.
- 7.Dignat-George F, Teysseire N, Mutin M, Bardin N, Lesaule G, Raoult D, Sampol J. Rickettsia conorii infection enhances vascular cell adhesion molecule-1- and intercellular adhesion molecule-1-dependent mononuclear cell adherence to endothelial cells. J Infect Dis. 1997;175:1142–1152. doi: 10.1086/520353. [DOI] [PubMed] [Google Scholar]
- 8.Duncan M J, Nakao S, Skobe Z, Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egelberg J. The blood vessels of the dento-gingival junction. J Periodont Res. 1966;1:163–179. doi: 10.1111/j.1600-0765.1966.tb01857.x. [DOI] [PubMed] [Google Scholar]
- 10.Genco R J. Periodontal disease and risk for myocardial infarction and cardiovascular disease. Cardiovasc Rev Rep. 1998;19:34–40. [Google Scholar]
- 11.Huang S N, Wass C A, Fu Q, Prasadarao N V, Stins M F, Kim K S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplanski G, Teysseire N, Farnarier C, Kaplanski S, Lissitzky J-C, Durand J-M, Soubeyrand J, Dinnarello C A, Bongrand P. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1 alpha dependent pathway. J Clin Investig. 1995;96:2839–2844. doi: 10.1172/JCI118354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaukaranta-Tolvanen S-S E, Ronni T, Leinonen M, Saikku P, Laitinen K. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog. 1996;21:407–411. doi: 10.1006/mpat.1996.0071. [DOI] [PubMed] [Google Scholar]
- 14.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Sturrock A, Weis J J. Intracellular localization of Borrelia burgdorferi with human endothelial cells. Infect Immun. 1991;59:671–678. doi: 10.1128/iai.59.2.671-678.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madianos P N, Papapanou P N, Nannmark U, Dahlen G, Sandros J. Porphyromonas gingivalis FDC381 multiplies and persists within human oral epithelial cells in vitro. Infect Immun. 1996;64:660–664. doi: 10.1128/iai.64.2.660-664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malek R, Fisher G, Caleca A, Stinson M, Van Oss C J, Lee J-Y, Cho M-I, Genco R J, Evans R T, Dyer D W. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J Bacteriol. 1994;176:1052–1059. doi: 10.1128/jb.176.4.1052-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matilla K J, Valle M S, Nieminen M S, Valtonen V V, Hietaniemi K L. Dental infections and coronary atherosclerosis. Atherosclerosis. 1993;103:205–211. doi: 10.1016/0021-9150(93)90263-t. [DOI] [PubMed] [Google Scholar]
- 19.Nery E B, Meister F, Ellinger R F, Eslami A, McNamara T J. Prevalence of medical problems in periodontal patients obtained from three different populations. J Periodontol. 1987;58:564–568. doi: 10.1902/jop.1987.58.8.564. [DOI] [PubMed] [Google Scholar]
- 20.Nisengard R J, Peng T K. Basement membrane alterations in periodontitis. J Periodontol. 1987;58:331–332. [Google Scholar]
- 21.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Njoroge T, Genco R J, Sojar H T, Hamada N, Genco C A. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect Immun. 1997;65:1980–1984. doi: 10.1128/iai.65.5.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 24.Papapanou P N, Sandros J, Lindberg K, Duncan M J, Niederman R, Nannmark U. Porphyromonas gingivalis may multiply and advance within stratified human junctional epithelium in vitro. J Periodont Res. 1994;29:374–375. doi: 10.1111/j.1600-0765.1994.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 26.Saglie F R, Marfany A, Camargo P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J Periodontol. 1988;59:259–265. doi: 10.1902/jop.1988.59.4.259. [DOI] [PubMed] [Google Scholar]
- 27.Sandros J, Madianos P N, Papapanou P N. Cellular events concurrent with Porphyromonas gingivalis invasion of oral epithelium in vitro. Eur J Oral Sci. 1996;104:363–371. doi: 10.1111/j.1600-0722.1996.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 28.Socransky, S. S., and A. D. Haffajee. 1992. The bacterial etiology of destructive periodontal disease: current concepts. J. Periodontol. 63(Suppl.):322–331. [DOI] [PubMed]
- 29.Sporn L A, Marder V J. Interleukin-1α production during Rickettsia rickettsii infection of cultured endothelial cells: potential role in autocrine cell stimulation. Infect Immun. 1996;64:1609–1613. doi: 10.1128/iai.64.5.1609-1613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St. Geme J W, III, Falkow S. Loss of capsule expression by Haemophilus influenzae type B results in enhanced adherence to and invasion of human cells. Infect Immun. 1991;59:1325–1333. doi: 10.1128/iai.59.4.1325-1333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virji M, Makepeace K, Peak I R A, Ferguson D P J, Jennings M P, Moxon E R. Opc- and pilus-dependent interactions of meningococci with human epithelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol Microbiol. 1995;18:741–754. doi: 10.1111/j.1365-2958.1995.mmi_18040741.x. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg A, Belton C M, Park Y, Lamont R J. Role of Porphyromonas gingivalis fimbriae in invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkler J R, Matarese V, Hoover C I, Kramer R H, Murray P A. An in vitro model to study bacterial invasion of periodontal tissues. J Periodontol. 1988;59:40–45. doi: 10.1902/jop.1988.59.1.40. [DOI] [PubMed] [Google Scholar]