Abstract
Summary
The RICO study indicated that most patients would like to receive information regarding their fracture risk but that only a small majority have actually received it. Patients globally preferred a visual presentation of fracture risk and were interested in an online tool showing the risk.
Purpose
The aim of the Risk Communication in Osteoporosis (RICO) study was to assess patients’ preferences regarding fracture risk communication.
Methods
To assess patients’ preferences for fracture risk communication, structured interviews with women with osteoporosis or who were at risk for fracture were conducted in 11 sites around the world, namely in Argentina, Belgium, Canada at Hamilton and with participants from the Osteoporosis Canada Canadian Osteoporosis Patient Network (COPN), Japan, Mexico, Spain, the Netherlands, the UK, and the USA in California and Washington state. The interviews used to collect data were designed on the basis of a systematic review and a qualitative pilot study involving 26 participants at risk of fracture.
Results
A total of 332 women (mean age 67.5 ± 8.0 years, 48% with a history of fracture) were included in the study. Although the participants considered it important to receive information about their fracture risk (mean importance of 6.2 ± 1.4 on a 7-point Likert scale), only 56% (i.e. 185/332) had already received such information. Globally, participants preferred a visual presentation with a traffic-light type of coloured graph of their FRAX® fracture risk probability, compared to a verbal or written presentation. Almost all participants considered it important to discuss their fracture risk and the consequences of fractures with their healthcare professionals in addition to receiving information in a printed format or access to an online website showing their fracture risk.
Conclusions
There is a significant communication gap between healthcare professionals and patients when discussing osteoporosis fracture risk. The RICO study provides insight into preferred approaches to rectify this communication gap.
Supplementary information
The online version contains supplementary material available at 10.1007/s00198-023-06955-9.
Keywords: Fracture, FRAX®, Osteoporosis, Patient-healthcare professional communication, Risk communication, Shared decision-making, Visual aids
Introduction
Osteoporotic fractures represent a global public health burden [1–3]. During the last two decades, several tools have been developed to determine the individual fracture risk of patients [4]. For example, the fracture risk assessment tool (FRAX)® [5] was developed in 2008 and shows patients’ 10-year probability of hip fracture and major osteoporotic fractures based on their bone mineral density (BMD) and clinical risk factors. Nowadays, such tools are increasingly used in clinical decision-making and osteoporosis treatment reimbursement policies. Demonstration of the output fracture risk can also be used by healthcare professionals as a tool when discussing a therapeutic plan with the patient.
Effective communication of fracture risk between healthcare professionals and patients with osteoporosis is an essential aspect of patient-centred care and shared decision-making [6]. Indeed, poor communication between patients and healthcare professionals is one of the several determinants of treatment non-initiation and non-adherence to osteoporosis medications [7, 8]. Available data revealed that osteoporosis patients are frequently dissatisfied with the information they receive from health professionals [9]. Studies also reported that patients often have a poor understanding of osteoporosis, the risk and consequences of fractures, and strategies for managing their disease in everyday life [7, 10].
A recent scoping review provided a summary of the existing evidence for effective communication regarding fracture risk [4]. The quality of fracture risk communication between patient and healthcare professional involves different factors, such as the way in which the information is presented by healthcare professionals or the capacity of the healthcare professionals to modify language to meet the patient’s needs. Equally important is the patient perspective, which includes the patient’s relationship with their healthcare professional, patient health literacy (that is, how the patient understands the information and applies it in the perspective of their own health), numeracy, and their emotions and experiences. Communication about the risks of osteoporosis medication and its side effects is significant as well.
The scoping review found that tools such as visual aids can improve the quality of communication between healthcare professionals and patients. Unfortunately, despite the availability of individualized risk assessment tools, performance measures, and educational tools to better understand and identify fracture risk, the benefits of these advances are not being effectively realised. Little is known about how patients value the communication of fracture risk, how much of the information they understand, and about their preferences and wishes for fracture risk communication. Therefore, the Risk Communication in Osteoporosis (RICO) project, endorsed by the International Osteoporosis Foundation (IOF) Epidemiology/Quality of Life (EpiQol) working group, was set up to improve fracture risk communication, with the ultimate goal of improving osteoporosis treatment and management, reducing fractures, and improving quality of life. In order to facilitate greater patient involvement in clinical decision-making, the RICO study aims to assess patients’ preferences regarding fracture risk communication.
Methods
Study design and objectives
The RICO study is a survey of women with osteoporosis or at risk of fracture. Structured interviews were used to assess (1) patient preferences in presentations of fracture risk in osteoporosis, (2) the impact of presentations on patient understanding and willingness to take medication, and (3) whether patient risk presentation preferences and risk perceptions differ by country.
A protocol describing the RICO study in detail was developed and approved by all sites involved in the study. The protocol has not been published but is available on request from the corresponding author. The present study follows good practice in the conduct and reporting of survey research [11].
Study sites, participants, and enrolment procedure
The study was conducted at eleven sites from around the world, namely Argentina, Belgium, Canada at Hamilton and the Osteoporosis Canada Canadian Osteoporosis Patient Network (COPN), Japan, Mexico, Spain, the Netherlands, the UK, and the states of California and Washington in the USA. The participants recruited for this study were women 60 + years old diagnosed with osteoporosis or postmenopausal women at risk of fractures. They were drawn from a number of sources, including the local community, the site investigator’s clinical practice (often an osteoporosis specialty clinic), and a national osteoporosis patient network such as the COPN or the Royal Osteoporosis Society (ROS) in the UK. As there were no specific hypotheses, a sample size calculation was not applicable. However, analogous to qualitative research that tends to include up to 30 participants, often corresponding to data saturation, a pragmatic sample target of 30 participants per site (total of 330) was targeted. Each site was encouraged to include at least 10 women with a previous fracture, 10 without a previous fracture, at least 10 women with college and/or graduate degree, 10 without college and/or graduate degree, and at least 10 women taking medication for osteoporosis and 10 not on osteoporosis medication. Once the target of 30 participants was reached by each site, these recruitment objectives were checked and, if not achieved, the sample size was increased.
Development of the interview guide
First, a structured workbook was developed, including the findings of a scoping literature review and experts’ insight. Details on the methodology and findings of the scoping review have been previously published [4]. A pilot study was subsequently organized with 26 women at risk of fractures, enrolled in five recruitment sites located in four different countries (i.e. Liège, Belgium; Hiroshima, Japan; Maastricht, the Netherlands; and the states of California and Washington in the USA). The pilot study aimed at checking the acceptability of the structured interview format and the content of the survey. Throughout the interviews, participants were offered an opportunity to provide suggestions to improve the presentation. The characteristics of the participants included in the pilot, the results, and quotes from the qualitative analysis are reported in Appendix 1 (Table A1, Table A2).
The final survey (available in Appendix 2) was developed on the basis of the above-mentioned scoping review, experts’ insights, and the results of the pilot study. The survey was first developed in English and then translated into French, Japanese, European Spanish, Latin American Spanish, and Mexican Spanish by one or two local translator(s), depending on the site, and reviewed by a second local translator. Cultural adaptations were also made, taking care to respect the content of the survey, and each translation was piloted before being approved by the co-ordination centre and used for data collection.
The survey was organised into four parts: (1) introduction, (2) participants’ knowledge/interpretation about fracture risk, (3) participants’ preferences for fracture risk presentation, and (4) additional information when communicating fracture risk. The survey introduction included an explanation of the objective of the study and role of participants, the collection of socio-demographic data (e.g. age, education level, history of fracture, osteoporosis), and five questions designed to estimate each participant’s numeracy. The numeracy section of the validated questionnaire was limited to just five questions to reduce the burden of survey administration. The five numeracy questions included two that evaluated how comfortable participants were with numbers and three that allowed for categorisation of participant numeracy levels as low, moderate, or high. Chosen on the basis of guidance by experts in the literature [12, 13], the three questions assessing levels of numeric literacy worked by observing the number of correct answers given. Participants who correctly answered all three questions were considered to be highly numerate. Those who could only answer one or two questions correctly were deemed to have moderate numeric literacy, while those who were unable to respond to any of the three questions correctly were characterised as having a low level of numeracy. The remaining two questions of the five determined whether participants were comfortable with the use of numbers to inform their understanding of a situation. The two questions were “How good are you at calculating a 15% tip?” and “When people tell you the chances of something happening, do you prefer that they use words or numbers?” Participants were scored on a scale of 1 to 7, where a score of 7 was given to those who were most able to calculate the tip correctly and who expressed a preference for evaluating risk in terms of ratios or percentages. A score of 1 was given to participants who were unable to calculate the tip or who were uncomfortable with numerical calculation and preferred words. Participants were considered to be comfortable with numbers if they scored a 6 or 7 on the scale.
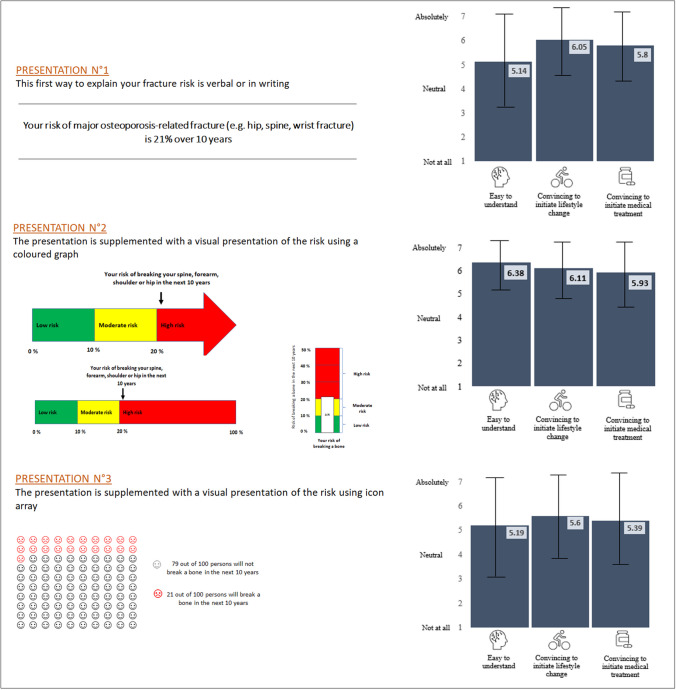
Part 2 of the survey explored how much participants knew about fracture risk and how they interpreted the applicability of that risk to themselves, for example, by examining the history of how that risk was communicated to them and how significant they considered that risk to be. Part 3 examined participants’ preferences for fracture risk presentation (Fig. 1). Using a hypothetical fracture risk of 21% in the next 10 years, three presentations were used to guide these interviews: presentation 1 consisted of a written/verbal presentation of fracture risk without any visual support; presentation 2 consisted of a visual presentation of the risk using a traffic-light system coloured graph; presentation 3 consisted of a visual presentation of the risk using an icon array. For each presentation, participants were asked to rate, using 7-point Likert scales, its clarity, the importance of confronting the risk of fracture without/with a medical treatment (using a hypothetical relative fracture risk reduction of about 30%, which is in line with the osteoporosis treatment effect highlighted by the recent network meta-analyses published on this topic [14, 15]), and the motivation to initiate treatment if the fracture risk was presented using each presentation. At the end, participants were invited to rank the three presentation formats in order of preference, firstly from the easiest to understand to the most difficult, and then from the most effective at convincing them to initiate medical treatment to the least convincing. Part 4 of the survey included additional information when communicating fracture risk, such as the importance of specifying the consequences of fractures in patient-clinician communications, the necessity of verbal explanation by a health professional, and the relevance of a web-based tool to measure fracture risk.
Fig. 1.
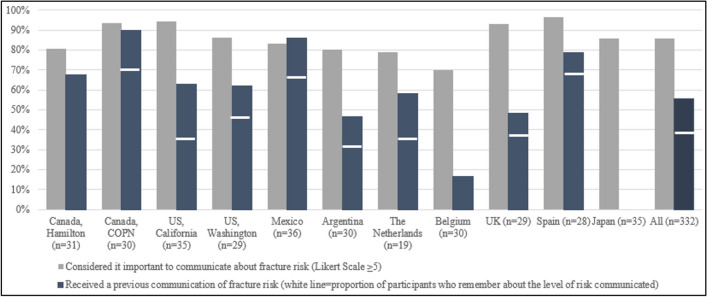
Communication of fracture risk; expectation versus reality. NB. The white lines indicates, among the participants who reported having received a previous communication of their fracture risk, the proportion who remembered the level of fracture that was communicated (e.g. for the entire population, n = 156, 47%)
All interviews were supported by a slide presentation developed to ensure a global structure to the interviews. To further ensure standardisation, the principal investigator gave a training webinar to all interviewers at each site. Interviews were conducted in person or online, depending on the participant’s preference, and the audio was recorded.
Ethical considerations.
Ethics Committee approval was obtained from Advarra, a central IRB in the USA. It was also obtained for all sites that required additional local approval.
Data entry and statistical analyses
Data from the multi-centre survey were entered by each investigator using the platform “Online Clinical Trials” (www.essaionline.com). Each site was allocated unique and protected access to the platform for data entry. Quality control procedures were developed to ensure there was no missing data or errors in data entry. Moreover, the system allowed co-ordinators to upload survey data as well as an electronic copy of the case report form, so that the core team could monitor the accuracy of data entry. The data was then processed using the SPSS Statistics 24 software package (IBM Corporation, Armonk, NY, USA). The normality of the variables was checked by examining the histogram, the quantile–quantile plot, the Shapiro–Wilk test, and the difference between the mean and the median values [16]. Continuous variables were expressed as mean and standard deviation or median and interquartile range, depending on their distributions. Binary and categorical variables were described by absolute (n) and relative (%) frequencies. A multi-step approach was developed for statistical analysis. First, the socio-demographic characteristics of the population were analysed for the full sample of participants and by site. Then, the preferences for the risk presentations were analysed for all participants and by site. Differences in characteristics and preferences between sites were tested using a Chi2 or Fisher exact test for categorical/binary variables and using a one-way ANOVA for quantitative variables. Finally, five subgroup analyses were performed according to participant characteristics (i.e. history of fracture yes/no, college degree yes/no, osteoporosis medication yes/no, osteoporosis status yes/no, numeric literacy low/medium/high). The differences between groups were tested using a Chi2 or Fisher exact test for categorical/binary variables and using a Student T test for quantitative variables. A Bonferroni-adjusted alpha of 0.01 (for five comparisons) was used to assess differences between subgroups.
Results
Socio-demographic characteristics
A total of 332 participants completed the survey (52% online interviews and 48% face-to-face interviews). The population characteristics are shown in Table 1. The mean age of participants (± SD) was 67.5 ± 8.0 years, with 83% reporting having been diagnosed with osteoporosis. The prevalence of osteoporosis across sites ranged from 30% in Belgium to 100% in the Netherlands, Canada, and Spain, (p < 0.001). About 48% of all participants had a prevalent fracture, ranging from 30% in Argentina to 95% in the Netherlands (p = 0.001); and 50% were undergoing pharmacological osteoporosis treatment, ranging from 23% in California in the USA to 95% in the Netherlands (p < 0.001). About a third of the total population (35%) was classified as having a low numeric literacy level, with differences between sites ranging from 13% in Canada (COPN) to 71% in Spain. Fifty-nine per cent of the population was reported to be comfortable with numbers, ranging from 37% in Japan to 79% in Washington state in the USA (p = 0.004).
Table 1.
Socio-demographic characteristics of the included population
| All (n = 332) | Canada, Hamilton (n = 31) | Canada, COPN (n = 30) | USA, California (n = 35) | USA, Washington (n = 29) | Mexico (n = 36) | Argentina (n = 30) | The Netherlands (n = 19) | Belgium (n = 30) | UK (n = 29) | Spain (n = 28) | Japan (n = 35) | p-value1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Format of interviews | < 0.001 | ||||||||||||
| Online | 173 (52.1) | 6 (19.4) | 30 (100.0) | 35 (100.0) | 29 (100.0) | 26 (72.2) | 0 (0.00) | 19 (100.0) | 14 (46.7) | 29 (100.0) | 1 (3.6) | 3 (8.6) | |
| Face-to-face | 159 (47.9) | 25 (80.6) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 10 (27.8) | 30 (100.0) | 0 (0.00) | 16 (53.3) | 0 (0.00) | 27 (96.4) | 32 (91.4) | |
| Age (years) | 67.5 ± 8.02 | 68.9 ± 8.18 | 67.9 ± 5.33 | 63.9 ± 7.1 | 65.4 ± 7.79 | 66.5 ± 7.36 | 68.2 ± 8.55 | 67.0 ± 10.5 | 67.1 ± 7.62 | 65.8 ± 8.28 | 69.4 ± 8.69 | 71.8 ± 7.47 | 0.007 |
| Diploma | |||||||||||||
| Primary school | 17 (5.1) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 4 (11.1) | 2 (6.7) | 1 (5.3) | 0 (0.0) | 0 (0.00) | 10 (35.7) | 0 (0.00) | < 0.001 |
| Lower secondary | 24 (7.2) | 2 (6.5) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 6 (16.7) | 2 (6.7) | 1 (5.3) | 6 (20.0) | 2 (6.9) | 3 (10.7) | 2 (5.7) | |
| Upper secondary | 77 (23.2) | 11 (35.5) | 5 (16.7) | 2 (5.7) | 7 (24.1) | 4 (11.1) | 9 (30.0) | 6 (31.6) | 12 (40.0) | 5 (17.2) | 3 (10.7) | 13 (37.1) | |
| Post-secondary (non U) | 60 (18.1) | 8 (25.8) | 3 (10.0) | 6 (17.1) | 3 (10.3) | 2 (5.6) | 8 (26.7) | 8 (42.1) | 11 (36.7) | 1 (3.4) | 0 (0.00) | 10 (28.6) | |
| Post-secondary (U) | 154 (46.4) | 10 (32.3) | 22 (73.3) | 27 (77.1) | 19 (65.5) | 20 (55.6) | 9 (30.0) | 3 (15.8) | 1 (3.3) | 21 (72.4) | 12 (42.9) | 10 (28.6) | |
| Diagnosis of OP | |||||||||||||
| Yes | 284 (85.5) | 31 (100.0) | 30 (100.0) | 30 (85.7) | 26 (89.7) | 27 (75.0) | 22 (73.3) | 19 (100.0) | 9 (30.0) | 28 (96.6) | 28 (100.0) | 34 (97.1) | < 0.001 |
| Currently on osteoporosis treatment | |||||||||||||
| Yes | 167 (50.3) | 13 (41.9) | 14 (46.7) | 8 (22.9) | 14 (48.3) | 14 (38.9) | 15 (50.0) | 18 (94.7) | 8 (26.7) | 21 (72.4) | 21 (75.0) | 21 (60.0) | < 0.001 |
| History of fracture | |||||||||||||
| Yes | 160 (48.2) | 15 (48.4) | 15 (50.0) | 14 (40.0) | 11 (37.9) | 15 (41.7) | 9 (30.0) | 18 (94.7) | 12 (40.0) | 21 (72.4) | 14 (50.0) | 17 (48.6) | 0.001 |
| Site of previous fractures | |||||||||||||
| Spine | 48 (14.5) | 2 (6.5) | 2 (6.7) | 1 (2.9) | 10 (28.6) | 0 (0.00) | 1 (3.3) | 8 (42.1) | 0 (0.0) | 13 (44.8) | 7 (25.0) | 7 (25.0) | < 0.001 |
| Hip | 17 (5.1) | 0 (0.0) | 0 (0.00) | 0 (0.0) | 2 (6.9) | 15 (41.7) | 3 (10.0) | 4 (21.1) | 1 (3.3) | 2 (6.9) | 2 (7.1) | 2 (5.7) | 0.18 |
| Wrist | 43 (13.0) | 5 (16.1) | 4 (13.3) | 0 (0.0) | 2 (6.9) | 8 (22.2) | 1 (3.3) | 5 (26.3) | 2 (6.7) | 10 (34.5) | 4 (14.3) | 2 (5.7) | 0.03 |
| Humerus | 18 (5.4) | 2 (6.5) | 3 (10.0) | 0 (0.0) | 0 (0.0) | 4 (11.1) | 3 (10.0) | 2 (10.5) | 1 (3.3) | 1 (3.4) | 2 (7.1) | 0 (0.0) | 0.10 |
| Other | 92 (27.7) | 10 (32.3) | 7 (23.3) | 13 37.1) | 9 (31.0) | 6 (16.7) | 2 (6.7) | 9 (47.4) | 11 (36.7) | 11 (37.9) | 5 (17.9) | 9 (25.7) | 0.005 |
| Numeric literacy | |||||||||||||
| Low (0, 1 point) | 116 (34.9) | 9 (29.0) | 4 (13.3) | 9 (25.7) | 12 (41.4) | 13 (36.1) | 18 (60.0) | 7 (36.8) | 10 (33.3) | 5 (17.2) | 20 (71.4) | 9 (25.7) | 0.001 |
| Medium (2 points) | 132 (39.8) | 18 (58.1) | 13 (43.3) | 17 (48.6) | 10 (34.5) | 13 (36.1) | 8 (26.7) | 5 (26.3) | 14 (46.7) | 14 (48.3) | 6 (21.4) | 14 (40.0) | |
| High (3 points) | 83 (25.0) | 3 (9.7) | 13 (43.3) | 9 (25.7) | 7 (24.1) | 10 (27.8) | 4 (13.3) | 7 (36.8) | 6 (20.0) | 10 (34.5) | 2 (7.1) | 12 (34.3) | |
| Comfortable with numbers | |||||||||||||
| Yes | 197 (59.3) | 18 (58.1) | 20 (66.7) | 25 (71.4) | 23 (79.3) | 24 (66.7) | 14(46.7) | 12 (63.2) | 13 (43.3) | 22 (75.9) | 13 (46.4) | 13 (37.1) | 0.004 |
NB. Quantitative variables are expressed in mean ± SD; binary and categorical variables are expressed in absolute and relative frequencies, n (%)
FR fracture risk, U University, OP Osteoporosis
1p-values assessing statistical differences between countries were obtained using a Chi2 test (or exact Fisher test) for categorical/binary variables and using a one-way ANOVA for quantitative variables
Risk communication information
The participants considered it important to communicate about fracture risk (mean of 6.22 ± 1.40 on a Likert scale of 1–7). Of the total sample, 86% gave a score of importance ≥ 5 (Fig. 1), ranging from 70% in Belgium to 96% in Spain. However, just over half of participants (n = 185, 56%) reported having been informed about their fracture risk in the past. A significant difference between sites was observed since none of the participants from Japan reported having been informed about their fracture risk, whereas in Canada (COPN) 90% of the participants had previously been given information on their 10-year fracture risk in the past. Moreover, among those who received this communication (n = 185), 156 (84%) remembered the level of fracture risk (i.e. low, moderate, or high) that was communicated by their healthcare professional (Table 2).
Table 2.
Profile of participants regarding their wishes, experience, and self-assessment of fracture risk
| All (n = 332) | Canada, Hamilton (n = 31) | Canada, COPN (n = 30) | USA, California (n = 35) | USA, Washington (n = 29) | Mexico (n = 36) | Argentina (n = 30) | The Netherlands (n = 19) | Belgium (n = 30) | UK (n = 29) | Spain (n = 28) | Japan (n = 35) | p-value2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Importance to know FR (1–7)1 | 6.22 ± 1.40 | 6.39 ± 1.28 | 6.60 ± 1.08 | 6.6 ± 1.06 | 6.25 ± 1.27 | 6.19 ± 1.89 | 6.23 ± 1.30 | 5.79 ± 1.69 | 4.97 ± 1.6 | 6.34 ± 1.11 | 6.79 ± 0.63 | 6.11 ± 1.39 | < 0.001 |
| Importance to know FR > 5 | 285 (85.8) | 25 (88.6) | 28 (93.3) | 33 (94.3) | 25 (86.2) | 30 (83.3) | 24 (80.0) | 15 (78.9) | 21 (70.0) | 27 (93.1) | 27 (96.4) | 30 (85.7) | 0.08 |
| Previous communication of FR | |||||||||||||
| Yes | 185 (55.7) | 21 (67.7) | 27 (90.0) | 22 (62.9) | 18 (62.1) | 31 (86.1) | 14 (46.7) | 11 (57.9) | 5 (16.7) | 14 (48.3) | 22 (78.6) | 0 (0.0) | < 0.001 |
| Remembered level of risk communicated | 156 (47.0) | 21 (67.7) | 22 (73.3) | 15 (42.8) | 15 (51.7) | 25 (80.6) | 12 (40.0) | 8 (42.1) | 5 (16.7) | 13 (44.8) | 20 (71.4) | 0 (0.0) | |
| No | 124 (37.3) | 7 (22.6) | 3 (10.0) | 11 (31.4) | 11 (37.9) | 3 (8.3) | 11 (36.7) | 4 (21.1) | 19 (63.3) | 15 (51.7) | 5 (17.9) | 35 (100.0) | |
| Unsure | 23 (6.9) | 3 (9.7) | 0 (0.00) | 2 (5.7) | 0 (0.00) | 2 (5.6) | 5 (16.7) | 4 (21.1) | 6 (20.0) | 0 (0.00) | 1 (3.6) | 0 (0.0) | |
| Self-assessment of FR | |||||||||||||
| Low | 62 (18.7) | 14 (45.2) | 5 (16.7) | 10 (28.6) | 5 (17.2) | 2 (5.6) | 7 (23.3) | 1 (5.3) | 10 (33.3) | 1 (3.4) | 2 (7.1) | 5 (14.3) | < 0.001 |
| Moderate | 100 (30.1) | 10 (32.3) | 11 (36.7) | 11 (31.4) | 9 (31.0) | 4 (11.1) | 12 (40.0) | 4 (21.1) | 9 (30.0) | 7 (24.1) | 10 (35.7) | 13 (37.1) | |
| High | 110 (33.1) | 6 (19.4) | 12 (40.0) | 8 (22.9) | 9 (31.0) | 7 (19.4) | 6 (20.0) | 11 (57.9) | 4 (13.3) | 18 (62.1) | 14 (50.0) | 15 (42.9) | |
| Do not know | 59 (17.8) | 1 (3.2) | 2 (6.7) | 6 (17.1) | 5 (17.2) | 23 (63.9) | 5 (16.7) | 3 (15.8) | 7 (23.3) | 3 (10.3) | 2 (7.1) | 2 (5.7) | |
NB. Quantitative variables are expressed in mean ± SD; binary and categorical variables are expressed in absolute and relative frequencies, n (%)
FR fracture risk
1Scale for importance to know fracture risk: not important l (1), very important (7)
2p-values assessing statistical differences between countries were obtained using a Chi2 test (or exact Fisher test) for categorical/binary variables and using a one-way ANOVA for quantitative variables
FR fracture risk
Preferences for presentation of fracture risk
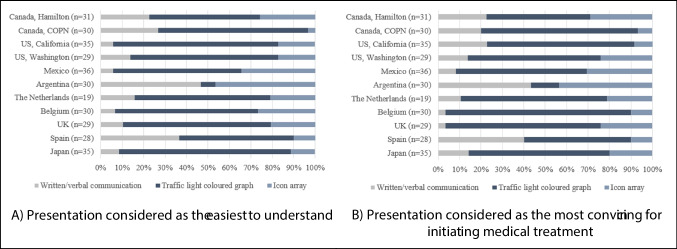
Considerations for the three presentations of fracture risk varied between the study sites (Table 3). About 61% of the participants agreed that, overall, the coloured traffic-light graph (presentation 2) was not only the most understandable format of communication but also the most convincing for initiating treatment with osteoporosis medication. Only participants from Argentina considered coloured traffic-light graph as the least understandable format. Of the two other formats—the non-visual textual format and the icon array—the icon array was consistently found to be the least acceptable. The non-visual textual presentation, on the other hand, reflected some interesting distinctions. In terms of comprehension of fracture risk, the participants in all study sites, except Spain, found the non-visual presentation to be least understandable format. Spanish participants, in contrast, preferred that method of communication over icon arrays (p < 0.001). When it came to encouraging participants to initiate medical treatment, non-visual presentation was again considered the least convincing in most study sites, with three noteworthy exceptions. Participants in the USA (California), Spain, and Canada (COPN) considered non-visual communication to be more convincing than icon arrays (p < 0.001) (Fig. 2 and Fig. 3). For all presentations, participants found it very helpful to compare the fracture risk without medical treatment to the fracture risk treated medically (5.8 ± 1.6 point on a Likert scale, 1–7 for written/verbal communication; 5.93 ± 1.53 for coloured traffic-light graph; and 5.39 ± 1.84 for icon arrays).
Table 3.
Preferences for fracture risk communication by study site
| All (n = 332) | Canada, Hamilton (n = 31) | Canada, COPN (n = 30) | USA, California (n = 35) | USA, Washington (n = 29) | Mexico (n = 36) | Argentina (n = 30) | The Netherlands (n = 19) | Belgium (n = 30) | UK (n = 29) | Spain (n = 28) | Japan (n = 35) | p-value2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Verbal/written communication1 | |||||||||||||
| Easy to understand (1–7) | 5.14 ± 1.92 | 5.68 ± 1.49 | 5.03 ± 1.96 | 4.97 ± 2.22 | 5.28 ± 1.91 | 5.56 ± 1.95 | 4.7 ± 2.10 | 6.05 ± 1.31 | 4.83 ± 1.82 | 4.52 ± 1.86 | 5.71 ± 1.92 | 4.54 ± 1.85 | 0.026 |
| Feel concerned (1–7) | 3.36 ± 1.77 | 3.00 ± 1.71 | 2.90 ± 1.69 | 3.06 ± 1.68 | 3.59 ± 1.57 | 3.44 ± 2.13 | 2.93 ± 1.68 | 3.89 ± 1.66 | 4.67 ± 1.35 | 3.76 ± 1.59 | 2.46 ± 1.53 | 3.46 ± 1.84 | < 0.001 |
| Add FR with treatment (1–7) | 6.10 ± 1.41 | 6.39 ± 1.09 | 6.03 ± 1.30 | 6.20 ± 1.21 | 6.14 ± 1.43 | 6.42 ± 1.46 | 6.0 ± 1.62 | 5.89 ± 1.85 | 5.60 ± 1.48 | 6.07 ± 1.33 | 6.64 ± 0.87 | 5.63 ± 1.63 | 0.10 |
| Lifestyle change (1–7) | 6.05 ± 1.45 | 6.26 ± 1.39 | 6.90 ± 0.40 | 6.2 ± 1.57 | 6.07 ± 1.46 | 6.50 ± 1.13 | 6.0 ± 1.51 | 5.84 ± 1.21 | 5.30 ± 1.37 | 5.79 ± 1.89 | 6.11 ± 1.37 | 5.49 ± 1.58 | 0.001 |
| Medical treatment (1–7) | 5.8 ± 1.60 | 5.52 ± 1.50 | 5.73 ± 1.95 | 4.86 ± 2.05 | 5.52 ± 1.55 | 6.64 ± 0.89 | 6.23 ± 1.30 | 6.37 ± 1.01 | 4.97 ± 1.63 | 6.03 ± 1.55 | 6.5 ± 1.00 | 5.69 ± 1.60 | < 0.001 |
| Coloured traffic-light graph1 | |||||||||||||
| Easy to understand (1–7) | 6.38 ± 1.23 | 6.19 ± 1.49 | 6.53 ± 1.25 | 6.63 ± 1.00 | 6.59 ± 0.91 | 6.64 ± 1.15 | 5.97 ± 1.67 | 6.32 ± 1.25 | 5.93 ± 1.05 | 6.72 ± 0.53 | 6.89 ± 0.31 | 5.77 ± 1.61 | 0.001 |
| Feel concerned (1–7) | 2.74 ± 1.87 | 2.74 ± 1.82 | 1.97 ± 1.52 | 2.03 ± 1.25 | 2.21 ± 1.42 | 3.86 ± 2.29 | 2.27 ± 1.76 | 2.95 ± 1.81 | 4.56 ± 1.59 | 2.48 ± 1.59 | 1.89 ± 1.13 | 3.06 ± 2.14 | < 0.001 |
| Add FR with treatment (1–7) | 6.12 ± 1.53 | 5.94 ± 1.63 | 6.43 ± 1.28 | 6.34 ± 1.06 | 6.59 ± 0.95 | 6.64 ± 1.39 | 5.70 ± 1.93 | 5.58 ± 2.09 | 5.53 ± 1.28 | 6.17 ± 1.34 | 5.61 ± 2.18 | 6.37 ± 1.16 | 0.01 |
| Lifestyle change (1–7) | 6.11 ± 1.42 | 6.10 ± 1.25 | 6.87 ± 0.57 | 6.54 ± 0.89 | 6.45 ± 0.91 | 6.39 ± 1.40 | 6.17 ± 1.46 | 5.58 ± 1.54 | 5.07 ± 1.34 | 6.14 ± 1.50 | 5.71 ± 1.76 | 5.86 ± 1.80 | < 0.001 |
| Medical treatment (1–7) | 5.93 ± 1.53 | 5.52 ± 1.84 | 5.80 ± 1.69 | 5.54 ± 1.61 | 6.07 ± 1.19 | 6.67 ± 0.76 | 5.73 ± 1.98 | 5.89 ± 1.33 | 5.5 ± 1.31 | 6.45 ± 0.98 | 5.71 ± 1.92 | 6.20 ± 1.49 | 0.02 |
| Usefulness of classification1 (1–7) | 6.11 ± 1.33 | 5.84 ± 1.39 | 6.17 ± 1.31 | 6.31 ± 1.08 | 6.31 ± 1.39 | 6.44 ± 1.42 | 6.0 ± 1.58 | 5.84 ± 1.61 | 5.30 ± 1.53 | 6.28 ± 0.99 | 6.61 ± 0.78 | 6.03 ± 1.07 | 0.01 |
| Usefulness of colours (1–7) | 6.48 ± 1.06 | 6.32 ± 1.35 | 6.43 ± 1.16 | 6.71 ± 0.71 | 6.79 ± 0.67 | 6.72 ± 1.11 | 6.23 ± 1.48 | 6.37 ± 1.42 | 5.63 ± 0.97 | 6.59 ± 0.63 | 6.82 ± 0.48 | 6.57 ± 0.92 | < 0.001 |
| Being in the red Sect. (1–7) | 2.0 ± 1.32 | 1.87 ± 1.02 | 1.57 ± 1.04 | 1.83 ± 1.01 | 1.83 ± 1.07 | 2.11 ± 1.85 | 1.70 ± 1.12 | 2.79 ± 1.47 | 2.80 ± 1.32 | 1.90 ± 1.20 | 1.89 ± 1.07 | 2.00 ± 1.59 | 0.005 |
| Preference for three graphs | |||||||||||||
| Horizontal with arrow | 142 (42.8) | 9 (29.0) | 9 (30.0) | 15 (42.9) | 11 (37.9) | 14 (38.9) | 10 (33.3) | 5 (26.3) | 17 (56.7) | 14 (48.3) | 22 (78.6) | 16 (45.7) | < 0.001 |
| Horizontal scaled on 100 | 106 (31.9) | 12 (38.7) | 12 (40.0) | 13 (37.1) | 13 (44.8) | 1 (2.8) | 8 (26.7) | 10 (52.6) | 9 (30.0) | 9 (31.0) | 4 (14.3) | 15 (42.9) | |
| Vertical | 84 (25.3) | 10 (32.3) | 9 (30.0) | 7 (20.0) | 5 (17.2) | 21 (58.3) | 12 (33.3) | 4 (21.1) | 4 (13.3) | 6 (20.7) | 2 (7.1) | 4 (11.4) | |
| Icon array1 | |||||||||||||
| Easy to understand (1–7) | 5.19 ± 2.09 | 5.58 ± 2.05 | 4.80 ± 2.31 | 5.14 ± 2.17 | 5.66 ± 1.95 | 4.42 ± 2.50 | 5.53 ± 2.14 | 5.95 ± 1.68 | 5.1 ± 1.75 | 5.41 ± 1.90 | 5.71 ± 1.76 | 4.40 ± 2.05 | 0.04 |
| Feel concerned (1–7) | 3.71 ± 1.91 | 4.16 ± 1.75 | 3.47 ± 1.83 | 3.86 ± 1.54 | 4.14 ± 1.57 | 3.17 ± 2.29 | 3.07 ± 2.26 | 3.79 ± 1.90 | 4.17 ± 1.64 | 3.97 ± 1.64 | 3.00 ± 2.05 | 4.06 ± 2.04 | 0.06 |
| Add FR with treatment (1–7) | 5.63 ± 1.84 | 5.77 ± 1.80 | 5.83 ± 2.05 | 5.23 ± 1.89 | 5.52 ± 1.82 | 4.89 ± 2.62 | 6.37 ± 1.24 | 5.63 ± 1.61 | 5.2 ± 1.45 | 5.76 ± 1.57 | 6.86 ± 0.45 | 5.20 ± 1.88 | 0.001 |
| Lifestyle change (1–7) | 5.60 ± 1.74 | 5.81 ± 1.70 | 5.93 ± 1.76 | 5.66 ± 1.66 | 5.76 ± 1.55 | 5.08 ± 2.43 | 5.97 ± 1.49 | 5.74 ± 1.56 | 5.0 ± 1.23 | 5.24 ± 2.06 | 6.39 ± 1.03 | 5.23 ± 1.75 | 0.04 |
| Medical treatment (1–7) | 5.39 ± 1.84 | 5.32 ± 2.04 | 5.17 ± 1.95 | 4.57 ± 1.88 | 5.21 ± 1.54 | 5.42 ± 2.27 | 6.0 ± 1.64 | 5.84 ± 1.64 | 4.83 ± 1.77 | 5.38 ± 1.78 | 6.54 ± 0.99 | 5.4 ± 1.73 | 0.003 |
| Preference for arrays | |||||||||||||
| Add a scale | 155 (46.7) | 13 (41.9) | 19 (63.3) | 18 (51.4) | 17 (58.6) | 8 (22.2) | 20 (66.7) | 4 (21.1) | 7 (23.3) | 12 (41.4) | 19 (67.9) | 18 (51.4) | < 0.001 |
| Invert the scale | 64 (19.3) | 4 (12.9) | 3 (10.0) | 9 (25.7) | 7 (24.1) | 3 (8.3) | 13 (43.3) | 3 (15.8) | 6 (20.0) | 5 (17.2) | 5 (17.9) | 6 (17.1) | 0.002 |
| Use a lower scale | 125 (37.7) | 13 (41.9) | 13 (43.3) | 8 (22.9) | 13 (44.8) | 7 (19.4) | 21 (70.0) | 6 (31.6) | 11 (36.7) | 8 (27.6) | 16 (57.1) | 9 (25.7) | < 0.001 |
| Use colours | 267 (80.4) | 23 (74.2) | 20 (66.7) | 29 (82.9) | 24 (82.8) | 24 (66.7) | 22 (73.3) | 17 (89.5) | 27 (90.0) | 23 (79.3) | 26 (92.9) | 32 (91.4) | 0.01 |
| Use other icon arrays | |||||||||||||
| Preference for faces | 162 (48.8) | 17 (54.8) | 8 (26.7) | 18 (51.4) | 12 (41.4) | 9 (25.0) | 11 (36.7) | 11 (57.9) | 21 (70.0) | 17 (58.6) | 15 (53.6) | 23 (65.7) | < 0.001 |
| Preference for bones | 25 (7.5) | 2 (6.5) | 1 (3.3) | 0 (0.0) | 1 (3.4) | 10 (27.8) | 4 (13.3) | 2 (10.5) | 1 (3.3) | 0 (0. 0) | 1 (3.6) | 3 (8.6) | |
| Preference for persons | 83 (25.0) | 4 (12.9) | 16 (53.3) | 11 (31.4) | 11 (37.9) | 4 (11.1) | 8 (26.7) | 3 (15.8) | 2 (6.7) | 7 (24.1) | 8 (28.6) | 9 (25.7) | |
| General preference (easier to understand) | |||||||||||||
| Simple verbal/written presentation of % | 59 (17.8) | 7 (22.6) | 8 (26.7) | 2 (5.7) | 4 (13.8) | 2 (5.6) | 14 (46.7) | 3 (15.8) | 2 (6.7) | 3 (10.3) | 11 (39.3) | 3 (8.6) | < 0.001 |
| Coloured traffic-light graphs | 203 (61.1) | 16 (51.6) | 21 (70.0) | 27 (77.1) | 20 (69.0) | 21 (58.3) | 2 (6.7) | 12 (63.2) | 20 (66.7) | 20 (69.0) | 16 (57.1) | 28 (80.0) | < 0.001 |
| Icon arrays | 71 (21.4) | 8 (25.8) | 1 (3.3) | 6 (17.1) | 5 (17.2) | 12 (33.3) | 14 (46.7) | 4 (21.1) | 8 (26.7) | 6 (20.7) | 3 (10.7) | 4 (11.1) | 0.004 |
| General preference (for treatment initiation) | |||||||||||||
| Simple verbal/written presentation of % | 62 (18.7) | 7 (22.6) | 6 (20.0) | 8 (22.9) | 4 (13.8) | 3 (8.3) | 13 (43.3) | 2 (10.5) | 1 (3.3) | 1 (3.4) | 12 (42.9) | 5 (14.3) | < 0.001 |
| Coloured traffic-light graphs | 203 (61.1) | 15 (48.4) | 22 (73.3) | 24 (68.6) | 18 (62.1) | 22(61.1) | 4 (13.3) | 13 (68.4) | 26 (86.7) | 21 (72.4) | 15 (53.6) | 23 (65.7) | < 0.001 |
| Icon arrays | 69 (20.8) | 9 (29.0) | 2 (6.7) | 3 (8.6) | 7 (24.1) | 11 (30.6) | 13 (43.3) | 4 (21.1) | 3 (10.0) | 7 (24.1) | 3 (10.7) | 7 (20.0) | 0.008 |
NB. Quantitative variables are expressed in mean ± SD; binary and categorical variables are expressed in absolute and relative frequencies, n (%)
1Scale used (1–7): from negative (very difficult, very concerned, not useful, not motivated for change) (1), to positive (very easy, not concerned at all, very useful, very motivated) (7)
2p-values assessing statistical differences between countries were obtained using a Chi2 test (or exact Fisher test) for categorical/binary variables and using a one-way ANOVA for quantitative variables
Fig. 2.
Three presentations of fracture risk. Presentation 1, written/verbal presentation of fracture risk without any visual support; presentation 2, visual presentation of the risk using a coloured traffic-light graph (three versions, horizontal with arrow, horizontal scale 0–100, and vertical); presentation 3, visual presentation of the risk using icon array. The blue bars in the graph represent the participants' ratings for each presentation (mean on a 7-point Likert scale). The black lines represent the SD around the mean. For each presentation, participants were asked to indicate their level of agreement on a Likert scale ranging from 1 (not at all) to 7 (absolutely) regarding their perception of the presentation's ease of understanding, persuasiveness for initiating lifestyle changes, and persuasiveness for initiating medical treatment
Fig. 3.
Preference for presentations of risk
Additional considerations
Participants considered it important to integrate the consequences of fractures into the fracture risk communication, preferably using pictures. However, large differences between study sites were observed (Table 4). When informed about the increased risk of dying following a fracture, a little more than half of participants (55%) considered it a significant concern, although this percentage ranged from 17% of participants in Argentina to 83% of participants in Canada (COPN). More than 70% of participants thought it was also important to receive information about the other consequences of osteoporotic fractures (i.e. inability to walk, loss of independence, reduced quality of life, and the risk of kyphosis), but once again with large variability across study sites. In terms of ranking, all of the other consequences, except kyphosis, were considered equally important to present during a discussion about fracture risk. Kyphosis seemed to be less important to participants except in Argentina, where 33% considered kyphosis to be an essential consequence to discuss. In contrast, kyphosis was only selected by a maximum of three participants (p < 0.001) in the other sites as the most important consequence.
Table 4.
Additional considerations regarding the fracture risk communication by study site
| All (n = 332) | Canada, Hamilton (n = 31) | Canada, COPN (n = 30) | USA, California (n = 35) | USA, Washington (n = 29) | Mexico (n = 36) | Argentina (n = 30) | The NL (n = 19) | Belgium (n = 30) | UK (n = 29) | Spain (n = 28) | Japan (n = 35) | p-value2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Add consequences of fractures | |||||||||||||
| Risk of dying | |||||||||||||
| Yes | 183 (55.1) | 17 (54.8) | 25 (83.3) | 25 (71.4) | 18 (62.1) | 20 (55.6) | 5 (16.7) | 6 (31.6) | 16 (53.3) | 14 (48.3) | 27 (96.4) | 10 (28.6) | < 0.001 |
| Unable to walk | |||||||||||||
| Yes | 260 (78.3) | 24 (77.4) | 27 (90.0) | 27 (77.1) | 23 (79.3) | 33 (91.7) | 8 (26.7) | 11 (57.9) | 29 (96.7) | 19 (65.5) | 28 (100.0) | 31 (88.6) | < 0.001 |
| Losing independence | |||||||||||||
| Yes | 262 (78.9) | 25 (80.6) | 27 (90.0) | 27 (77.1) | 23 (79.3) | 31 (86.1) | 12 (40.0) | 11 (57.9) | 29 (96.7) | 21 (72.4) | 28 (100.0) | 28 (80.0) | < 0.001 |
| Losing QoL | |||||||||||||
| Yes | 253 (76.2) | 27 (87.1) | 27 (90.0) | 27 (77.1) | 25 (86.2) | 32 (88.9) | 5 (16.7) | 16 (84.2) | 28 (93.3) | 22 (75.9) | 28 (100.0) | 16 (45.7) | < 0.001 |
| Being kyphotic | |||||||||||||
| Yes | 238 (71.7) | 23 (74.2) | 27 (90.0) | 26 (74.3) | 21 (72.4) | 31 (86.1) | 3 (10.0) | 12(63.2) | 28 (93.3) | 19 (65.5) | 27 (96.4) | 21 (60.0) | < 0.001 |
| Ranking of consequences | |||||||||||||
| Ranked as the most important | |||||||||||||
| Risk of dying | 72 (21.7) | 6 (19.4) | 10 (33.3) | 16 (45.7) | 7 (24.1) | 7 (19.4) | 6 (20.0) | 1 (5.3) | 5 (16.7) | 4 (13.8) | 7 (25.0) | 4 (11.4) | 0.003 |
| Unable to walk | 71 (21.4) | 6 (19.4) | 2 (6.7) | 3 (8.6) | 4 (13.8) | 9 (25.0) | 5 (16.7) | 6 (31.6) | 9 (30.0) | 5 (17.2) | 1 (3.6) | 21 (60.0) | < 0.001 |
| Losing independence | 75 (22.6) | 7 (22.6) | 11 (36.7) | 2 (5.7) | 6 (20.7) | 6 (16.7) | 5 (16.7) | 2 (10.5) | 12 (40.0) | 4 (13.8) | 13 (46.4) | 7 (20.0) | 0.005 |
| Losing QoL | 80 (24.1) | 9 (29.0) | 7 (23.3) | 9 (25.7) | 8 (27.6) | 13 (36.1) | 4 (13.3) | 9 (47.4) | 3 (10.0) | 12 (41.4) | 5 (17.9) | 1 (2.9) | 0.001 |
| Being kyphotic | 26 (7.8) | 3 (9.7) | 2 (6.7) | 1 (2.9) | 2 (6.9) | 1 (2.8) | 10 (33.3) | 2 (10.5) | 1 (3.3) | 1 (3.4) | 2 (7.1) | 3 (8.6) | < 0.001 |
| Relevance of pictures | |||||||||||||
| Yes | 230 (69.3) | 21 (67.7) | 20 (66.7) | 17 (48.6) | 21 (72.4) | 31 (86.1) | 25 (83.3) | 12 (63.2) | 18 (60.0) | 12 (41.4) | 21 (75.0) | 32 (91.4) | < 0.001 |
| No | 70 (21.1) | 8 (25.8) | 7 (23.3) | 16 (45.7) | 6 (20.7) | 4 (11.1) | 1 (3.3) | 1 (5.3) | 6 (20.0) | 16 (55.2) | 3 (10.7) | 2 (5.7) | |
| Do not have opinion | 32 (9.6) | 2 (6.5) | 3 (10.0) | 2 (5.7) | 2 (6.9) | 1 (2.8) | 4 (13.3) | 6 (31.6) | 6 (20.0) | 1 (3.4) | 4 (14.3) | 1 (2.9) | |
| Verbal explanation from caregivers (1–7)1 | 6.55 ± 1.13 | 5.97 ± 1.64 | 6.97 ± 0.18 | 6.77 ± 0.55 | 6.48 ± 1.15 | 5.92 ± 2.25 | 6.80 ± 0.61 | 6.63 ± 0.76 | 6.43 ± 0.73 | 6.86 ± 0.44 | 7.00 ± 0.00 | 6.43 ± 0.95 | < 0.001 |
| Information printed (1–7)1 | 6.27 ± 1.41 | 6.39 ± 0.99 | 6.97 ± 0.18 | 6.31 ± 1.37 | 6.48 ± 0.95 | 5.92 ± 2.26 | 6.40 ± 1.13 | 6.21 ± 1.51 | 5.67 ± 1.71 | 6.66 ± 0.55 | 6.11 ± 1.73 | 5.91 ± 1.44 | 0.02 |
| Development of an online website (1–7)1 | 5.55 ± 1.82 | 5.42 ± 1.94 | 6.27 ± 1.62 | 6.37 ± 1.65 | 6.14 ± 1.41 | 5.81 ± 1.95 | 5.33 ± 1.83 | 5.00 ± 2.21 | 3.83 ± 1.68 | 6.28 ± 1.13 | 5.39 ± 1.55 | 4.97 ± 1.54 | < 0.001 |
NB. Quantitative variables are expressed in mean ± SD; binary and categorical variables are expressed in absolute and relative frequencies, n (%)
QoL quality of life
1Scale for (1–7): Not relevant (1), very relevant (7)
2p-values assessing statistical differences between countries were obtained using a Chi2 test (or exact Fisher test) for categorical/binary variables and using a one-way ANOVA for quantitative variables
Participants considered it necessary to receive a verbal explanation of their fracture risk from their healthcare professional (6.55 ± 1.13 on a Likert scale of 1–7 points) as well as printed information (6.27 ± 1.41 on a Likert scale of 1–7 points). Most participants were also willing to use website to obtain this information (5.55 ± 1.82 on a Likert scale of 1–7 points), although participants in Belgium considered online information less relevant than participants from Canada (COPN), the USA (California), and the UK (difference between groups < 0.001).
Subgroup analyses
Considerations and preferences for fracture risk communication according to the history of fractures, educational level, osteoporotic treatment, osteoporotic status, and numeric literacy level are presented in Appendix 3 (Tables A3-A7). Even if presented solely with a non-visual communication of the risk, participants with a history of fractures (n = 160) were more inclined to choose a medical treatment to reduce their fracture risk than participants without history of fractures (n = 172) (p = 0.001). Not surprisingly, we also observed that participants who were currently taking osteoporotic medication (n = 167) were also more inclined to initiate medical treatment to reduce their fracture risk, regardless of the type of communication used (visual vs. nonvisual, all p < 0.001). No other differences between groups were observed. Regarding educational level, merging all sites together, it appears that when using a non-visual form alone to communicate the fracture risk, participants with a higher education level (university degree, n = 214) were more likely to initiate lifestyle changes compared to participants with a lower level of education (n = 118) (p = 0.01). Participants with a higher education level also viewed the idea of developing an online website for the assessment of fracture risk more favourably (p = 0.002). No other significant differences were observed between groups. Subgroup analysis of participant numeracy revealed that the level of numeric literacy did not influence risk presentations preferences (all p > 0.01).
Participants with osteoporosis (n = 284), and therefore a higher fracture risk, differed from participants without osteoporosis (n = 48) in several aspects of risk presentations preferences: they were more concerned about the fracture risk, regardless of whether the information presented was non-visual (p = 0.01) or visual (i.e. coloured traffic-light graphs p < 0.001). If a visual communication, such as the coloured graph, was the only format used to present information, osteoporotic individuals expressed a preference for presentations that showed the risk of fractures both with and without medical treatment (non-visual presentation p = 0.03; coloured traffic-light graph p = 0.001; icon array p = 0.01). Moreover, whatever the mode of communication used, osteoporotic individuals were more likely to initiate lifestyle changes or initiate medical treatment to reduce their fracture risk (all p < 0.01).
Discussion
The Risk Communication in Osteoporosis, or RICO, study aimed to understand patient preferences regarding the communication of fracture risk. Consideration of patient preferences in communications by healthcare professionals is crucial to increasing and facilitating patient involvement in clinical decision-making [17]. In the RICO study, a sample of 332 postmenopausal women with osteoporosis or at risk of fracture were recruited from 11 different sites around the world. Despite a great willingness to be informed about their fracture risk, only 56% of the women remembered having received such information from healthcare professionals. Although these results should be interpreted with caution, as some participants may not have remembered receiving information, this nevertheless highlights a significant communication gap between healthcare professionals and patients when discussing osteoporosis fracture risk. Despite the tools already available to estimate an individual’s fracture risk (i.e. FRAX® tool, Garvan Fracture Risk Calculator, and QFracture) [4] and the increasing importance of such tools in healthcare decision-making, the retention of information by patients and the quality of fracture risk communications remain suboptimal. Previous studies have already highlighted a lack of quality in fracture risk communication. In a qualitative study conducted in 2014, Sale et al. [7, 18] reported that patients often experienced a lack of interest by fracture clinics, receiving minimal communications. What messages patients did receive were not understood properly (e.g. patients could remember that they were at high risk, but did not believe it or did not know what they were at high risk for).
In 2018, Jakobsen et al. [19] also highlighted the importance of a patient’s medical history in understanding fracture risk. The authors reported, for example, that people who had never experienced a fracture could have substantial difficulty in appreciating the risk involved. This aspect was confirmed in our study, since—regardless of the method of communication—participants with osteoporosis or with a history of fracture were more concerned about their fracture risk as compared to those without such experiences. Granted that this result might be obvious, but it is still important for studies to be developed that test and confirm assumptions. A previous scoping review on fracture risk communication [4] provided some recommendations to healthcare professionals for improving communication to achieve an optimal patient-centred approach. It was suggested, among other things, that long-term knowledge of patients might be improved if healthcare professionals were to adapt their language to suit the patient’s history, needs, and health literacy. This suggestion is consistent with the qualitative findings reported by Jakobsen et al. [19].
The close and strong relationship between the quality of fracture risk communication and the initiation of osteoporosis treatment and adherence [7, 8, 20] compels the need for efforts to improve this communication. Decision aids are tools that support the implementation of shared decision-making in practice [17]. A Cochrane systematic review published by Stacey et al. [21] showed that people exposed to decision aids, using icon arrays, felt more knowledgeable, better informed, clearer about their values, and more active in their decision-making role. In the field of osteoporosis, Paskins et al. [22] used a systematic review and environmental scan to identify eleven different decision aids for fracture risk communication and treatment decision-making. These decision aids were found to be helpful in increasing the accuracy of risk perception, but only limited evidence was offered for improving treatment adherence in osteoporosis using these decision aids. In addition, the authors found that the available decision aids did not comprehensively meet international quality standards and patient needs. Later, Nogues et al. [23] reported that only five decision aids in the field of osteoporosis could be deemed to be completely comprehensive, as they effectively deliver pertinent information about the disease and present viable treatment options to patients. Therefore, the development of new tools and new decision aids is crucial for the field of osteoporosis and fracture prevention. Another noteworthy aspect to consider is the computer literacy of individuals with osteoporosis, who may struggle to use online tools. However, as the use of e-health technology and the publication of online information by healthcare professionals gradually becomes more common, patients are likely to find themselves engaging more frequently with such e-tools.
The results of our study revealed a strong preference for visual presentation of fracture risk versus a verbal/written communication without visual support. Sixty-one percent of the participants perceived the coloured traffic-light style graphs as the most understandable presentation of fracture risk. The coloured traffic-light graph was also the presentation most associated with motivating the initiation of medical treatment. Participants expressed an interest in the use of colours to inform them about the level of fracture risk (i.e. low, moderate, or high). Another study also found that this visual presentation of risk, using a stoplight colour system, was regarded by patients as the most clear and easy to read. Face arrays and pictograms, on the other hand, were considered more difficult to understand, as these formats make it harder for people to quickly ascertain their individual risk category [24]. Our study highlighted patient preferences for receiving information about the potential for reduction of their fracture risk by initiating treatment with osteoporosis medication. Therefore, to better reflect the benefits of treatment and encourage treatment initiation and adherence, it is advisable to contrast the outcome of fracture risk with treatment and without in patient communications. Last but not least, participants also expressed their desire to be informed about the more devastating consequences of fractures. They seemed particularly focused on the risks of losing the ability to walk, their independence, and, especially, their quality of life.
An awareness of all these patient preferences in fracture risk communications could help in the progress of new patient-decision tools. Participants were interested in the development of an online tool that could visually represent their individual fracture risk, as measured with fracture risk calculators such as the FRAX® tool [5, 10]. Because participants also expressed a willingness to talk directly with their healthcare professionals, the use of such an online tool should take place in an environment where patients and healthcare professionals work together to ensure a thorough understanding of the patient’s fracture risk and develop a treatment plan appropriate to the individual patient's situation, needs, and preferences.
An important aspect highlighted by the RICO study is the variation of patient preferences observed between countries. Such heterogeneity could be explained by differences from centre to centre, which could signal a need to adapt communication tools to local preferences. In our survey, we used US-specific cutoffs to define low, intermediate, and high fracture risk. However, the thresholds used for classification may be country- or age-specific, as suggested for the FRAX® tool, and it could be helpful to identify the thresholds at which a risk might be considered as low, moderate, or high. Furthermore, online tools have the advantage of having automated systems that can be adapted to accommodate patient or country characteristics. Given the results of our RICO study, for example, the use of colours is strongly recommended (apart from cases of colour blindness) to define risks as low, medium, or high. However, the interpretation of colours may be country-specific. For example, while populations in Western countries tend to view the colour red negatively as a warning, the colour red is viewed positively as an indicator of something good in countries like Japan.
Heterogeneity in preferences based on certain clinical characteristics was also observed between individuals. For instance, we observed that participants with osteoporosis and those with a history of fracture were more receptive to measures to reduce their fracture risk (i.e. lifestyle changes, medical treatment), regardless of the mode of communication used, whether visual or not. The use of visual aids may therefore be more useful for participants without osteoporosis or a history of fractures, as those patients may be more unaware of their fracture risk and less responsive communications concerning the consequences and benefits of treatment with osteoporosis medication.
Strengths and limitations
Our study has several strengths. To the best of our knowledge, this is one of the first studies to investigate patient preferences for fracture risk communication, and the large number of participants from eleven sites around the world increases the external validity of the results. Moreover, we included patients with different educational backgrounds, and examined the role of numeric literacy on the results. In addition, the methodology used—that is, structured interviews with participants—ensures standardisation of data collection and reduces potential systematic bias.
Nevertheless, some limitations should be noted. First, the RICO study only included women, although osteoporosis also affects men, with approximately one in five men over 50 years of age suffering from this disease worldwide [25]. Men’s preferences for fracture risk communications should therefore also be considered. Second, although we used purposive sampling approach, the final sample may not be representative of the target population. Individuals who voluntarily participate in this type of research often have a different profile compared to the global population, as they may be more interested in the topic, more concerned about the risk, and have higher health literacy. Additionally, it is essential to acknowledge the variation in enrolment across sites. Although having a high-risk fracture was the primary inclusion criterion, women with low bone density who were interested and or concerned about their fracture risk were also included at some sites. Consequently, disparities have been observed between sites in the prevalence of osteoporosis, fractures, and medication usage. The discrepancies in preferences can therefore be partially attributed to these differences in enrolment procedures. Furthermore, significant variations have also been observed between sites in demographic characteristics (e.g. age, educational level, numeric literacy level). Therefore, some caution must be exercised when interpreting preference results, especially regarding differences between sites.
Third, the sample size within each study site is limited. This considerably diminishes the generalizability of the results for each country. It also prevents us from conducting subgroup analyses to better highlight participant characteristics that may influence preferences. Fourth, data analysed in the study were only based on patient-reported information and could thus be subject to bias at that level. For example, 83% of the population reported a diagnosis of osteoporosis but we were not able to verify this prevalence with accurate medical data. Fifth, the format of interviews may have impacted data collection, as some study sites performed exclusively online interviews whereas others performed exclusively face-to-face interviews, and still others carried out both. The choice of online or face to-face interviews was left to the local investigator and participants. It also depended on the computer literacy of the participants. Online interviews were tested during the pilot study and were shown to be feasible, although the real impact on results is difficult to ascertain.
Sixth, in this survey, we did not test participants’ actual understanding of presentations, but rather their perception of how easy they found it to understand. Therefore, we still do not know if the visual images/graphs were accurately interpreted [26]. Seventh, the guide was translated into different languages by one or two translators and checked by another researcher. However, no back translation was performed. While we do not expect this aspect to have influenced the results in any way, this limitation should be acknowledged for the sake of transparency. Finally, the differences observed between countries could not be fully explained by the methodology used in our study. For example, the fact that all participants, with the exception of those from Argentina, preferred the coloured traffic light system of graphs to other communication formats remains unexplained. Participants from Argentina do not present any particular characteristics that might allow us to develop hypotheses to explain this difference in fracture risk communication preference.
Conclusion
The Risk Communication in Osteoporosis (RICO) study highlights the significant communication gap that remains between healthcare professionals and patients when discussing osteoporosis fracture risk, despite the availability of tools such as FRAX®, which incorporates both bone mineral density and fracture risk factors to provide a 10-year fracture risk estimate. The willingness of patients to receive communications about their fracture risk needs to be encouraged, and we believe this will occur through improvements in the quality of communication which will in turn lead to better management of osteoporosis. RICO demonstrated that visual presentations using graphs with a coloured traffic-light system is the preferred way to communicate fracture risk, as well as the most persuasive way to convince patients at risk of fracture to initiate treatment. RICO also revealed that an online visual decision aid that takes into account patients’ preferences for fracture risk communication would be a significant added benefit. Because heterogeneity in preferences for fracture risk presentation was observed between countries, country-specific visual aids will be even more relevant. Improved communication between at-risk patients and their healthcare professionals will undoubtedly heighten awareness of osteoporosis, the consequences of fractures, and the significance of fracture prevention, ultimately leading to an improved quality of life.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Osteoporosis Canada and its Canadian Osteoporosis Patient Network and the UK Royal Osteoporosis Society for helping to recruit participants. We also thank all of the participants included in this study for their time dedicated to this research. As compensation for their time, some participants were paid an honorarium. The decision to compensate participants was at the discretion of each study site investigator.
Funding
The RICO (Risk Communication in Osteoporosis) project received funding from Amgen US/UCB. Amgen had no role in design, data collection, analysis, or interpretation. ZP is funded by the National Institute for Health Research (NIHR) (Clinician Scientist Award (CS-2018–18-ST2-010)/NIHR Academy). The views represent those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care.
Data availability
Upon request.
Code availability
NA.
Declarations
Ethics approval and consent to participate
This entire study was subject to an initial central IRB (US: Advarra for California and New Mexico) and then local IRB (country and institution specific) review and approval (if and when required by applicable law). Informed consent was obtained for all phases of the project. All subjects received either a patient information sheet or informed consent, depending on the local IRB of the site. Subjects were informed of their ability to withdraw at any time by the co-ordinator and by the patient information sheet or informed consent.
Conflicts of interest
John A. Kanis is the founder of FRAX®; Zoé Paskins has sat on an advisory board for UCB Pharma about risk communication; E. Michael Lewiecki is an Amgen investigator, consultant, and speaker as well as a Radius investigator and consultant; Lynn A. Kohlmeier is an Amgen speaker as well as a Radius consultant and speaker. Charlotte Beaudart, Mitali Sharma, Patricia Clark, Saeko Fujiwara, Jonathan D. Adachi, Osvaldo D. Messina, Suzanne N. Morin, Caroline B. Sangan, Xavier Nogues, Griselda Adriana Cruz Priego, Andrea Cavallo, Fiona Cooper, Jamie Grier, Carolyn Leckie, Diana Montiel-Ojeda, Alexandra Papaioannou, Nele Raskin, Leonardo Yurquina, Michelle Wall, Olivier Bruyère, Annelies Boonen, Elaine Dennison, Nicholas C. Harvey, Jean-François Kaux, Oscar Lopez-Borbon, Jean-Yves Reginster, Stuart Silverman, and Mickaël Hiligsmann all declare that they have no conflict of interest with regard to the content of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stuart Silverman and Mickaël Hiligsmann are senior co-authors.
References
- 1.Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporos Int 16 Suppl 2:S3–7. 10.1007/S00198-004-1702-6 [DOI] [PubMed]
- 2.Kanis JA, Cooper C, Rizzoli R, Reginster JY. Executive summary of European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Aging Clin Exp Res. 2019;31:15–17. doi: 10.1007/S40520-018-1109-4. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, McCloskey EV, Harvey NC, et al. Intervention thresholds and diagnostic thresholds in the management of osteoporosis. Aging Clin Exp Res. 2022;34:3155. doi: 10.1007/S40520-022-02216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudart C, Hiligsmann M, Li N, et al. Effective communication regarding risk of fracture for individuals at risk of fragility fracture: a scoping review. Osteoporos Int. 2022;33:13–26. doi: 10.1007/S00198-021-06151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanis JA, McCloskey EV, Johansson H, et al. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010;21(Suppl 2):S407–S413. doi: 10.1007/s00198-010-1253-y. [DOI] [PubMed] [Google Scholar]
- 6.Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20:600–607. doi: 10.1111/j.1745-7599.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- 7.Sale JEM, Hawker G, Cameron C, et al. Perceived messages about bone health after a fracture are not consistent across healthcare providers. Rheumatol Int. 2015;35:97–103. doi: 10.1007/s00296-014-3079-y. [DOI] [PubMed] [Google Scholar]
- 8.Dewan N, MacDermid JC, MacIntyre NJ, Grewal R. Therapist’s practice patterns for subsequent fall/osteoporotic fracture prevention for patients with a distal radius fracture. J Hand Ther. 2019;32:497–506. doi: 10.1016/j.jht.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Lewiecki EM. The role of risk communication in the care of osteoporosis. Curr Osteoporos Rep. 2011;9:141–148. doi: 10.1007/s11914-011-0056-1. [DOI] [PubMed] [Google Scholar]
- 10.Zoccarato F, Ceolin C, Trevisan C, et al. Comparison between real-world practice and application of the FRAX algorithm in the treatment of osteoporosis. Aging Clin Exp Res. 2022;34:2807. doi: 10.1007/S40520-022-02212-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003;15:261–266. doi: 10.1093/INTQHC/MZG031. [DOI] [PubMed] [Google Scholar]
- 12.Galesic M, Garcia-Retamero R. Graph literacy: a cross-cultural comparison. Med Decis Making. 2011;31:444–457. doi: 10.1177/0272989X10373805. [DOI] [PubMed] [Google Scholar]
- 13.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, et al. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27:672–680. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 14.Ayers C, Kansagara D, Lazur B, et al. Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: a living systematic review and network meta-analysis for the American college of physicians. Ann Intern Med. 2023;176:182–195. doi: 10.7326/M22-0684/SUPPL_FILE/M22-0684_SUPPLEMENT.PDF. [DOI] [PubMed] [Google Scholar]
- 15.Simpson EL, Martyn-St James M, Hamilton J et al (2020) Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: a systematic review and network meta-analysis. Bone 130:115081. 10.1016/J.BONE.2019.115081 [DOI] [PubMed]
- 16.Mishra P, Pandey CM, Singh U, et al. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. 2019;22:67–72. doi: 10.4103/aca.ACA_157_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Wit M, Cooper C, Tugwell P, et al. Practical guidance for engaging patients in health research, treatment guidelines and regulatory processes: results of an expert group meeting organized by the World Health Organization (WHO) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) Aging Clin Exp Res. 2019;31:905–915. doi: 10.1007/S40520-019-01193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sale JEM, Gignac MA, Hawker G, et al. Patients do not have a consistent understanding of high risk for future fracture: a qualitative study of patients from a post-fracture secondary prevention program. Osteoporos Int. 2016;27:65–73. doi: 10.1007/s00198-015-3214-y. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen PR, Hermann AP, Søndergaard J, et al. Left in limbo – experiences and needs among postmenopausal women newly diagnosed with osteoporosis without preceding osteoporotic fractures: A qualitative study. Post Reprod Health. 2018;24:26–33. doi: 10.1177/2053369118755189. [DOI] [PubMed] [Google Scholar]
- 20.Gonnelli S, Caffarelli C, Rossi S, et al. How the knowledge of fracture risk might influence adherence to oral therapy of osteoporosis in Italy: the ADEOST study. Aging Clin Exp Res. 2016;28:459–468. doi: 10.1007/S40520-016-0538-1. [DOI] [PubMed] [Google Scholar]
- 21.Stacey D, Légaré F, Lewis K, et al (2017) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 4(4):CD001431 [DOI] [PMC free article] [PubMed]
- 22.Paskins Z, Torres Roldan VD, Hawarden AW, et al. Quality and effectiveness of osteoporosis treatment decision aids: a systematic review and environmental scan. Osteoporos Int. 2020;31:1837–1851. doi: 10.1007/s00198-020-05479-w. [DOI] [PubMed] [Google Scholar]
- 23.Nogués X, Carbonell MC, Canals L, et al. Current situation of shared decision making in osteoporosis: a comprehensive literature review of patient decision aids and decision drivers. Health Sci Rep. 2022;5:e849. doi: 10.1002/HSR2.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edmonds SW, Cram P, Lu X, et al. Improving bone mineral density reporting to patients with an illustration of personal fracture risk. BMC Med Inform Decis Mak. 2014;14:1–12. doi: 10.1186/s12911-014-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adler RA (2014) Osteoporosis in men: A review. Bone Res 2:14001 [DOI] [PMC free article] [PubMed]
- 26.Trevena LJ, Zikmund-Fisher BJ, Edwards A, et al. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13:1–15. doi: 10.1186/1472-6947-13-S2-S7/FIGURES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request.
NA.