Abastract
Bullous pemphigoid (BP) is a common autoimmune bullous disease affecting mainly the elderly, with rising incidence due to increased life expectancy. This disease is characterized by tense bullous lesions on normal or erythematous skin, accompanied by pruritus. BP pathogenesis involves autoantibodies against hemidesmosomal proteins BP180 and BP230, leading to detachment at the dermo-epidermal junction as well as blister formation. BP is associated with coexisting comorbidities and drug exposure, and its management often requires high doses or chronic use of systemic glucocorticoids, posing risks of adverse effects. This review focuses on novel treatment options for BP, exploring therapies targeting different immune pathways. Rituximab, a CD20 monoclonal antibody, depletes B-lymphocytes and has shown efficacy in severe cases. Dupilumab, targeting interleukin (IL)-4 receptor α and thus blocking IL-4 and IL-13, downregulates type 2 helper (Th2) responses and has demonstrated promising results. Targeting eosinophil-related molecules using bertilimumab and AKST4290 has yielded positive results in clinical trials. Omalizumab, an immunoglobulin (Ig) E antibody, can reduce disease severity and allows corticosteroid tapering in a number of cases. Complement inhibitors such as nomacopan and avdoralimab are being investigated. IL-17 and IL-23 inhibitors such as secukinumab and tildrakizumab have shown potential in a limited number of case reports. Neonatal Fc receptor antagonists such as efgartigimod are under investigation. Additionally, topical therapies and Janus kinase inhibitors are being explored as potential treatments for BP. These novel therapies offer promising alternatives for managing BP, with potential to improve outcomes and reduce high cumulative doses of systemic corticosteroids and related toxicities. Further research, including controlled clinical trials, is needed to establish their efficacy, safety, and optimal dosing regimens for BP management.
Key Points
| Evolving therapies This review explores novel treatments for bullous pemphigoid (BP), focusing on innovative approaches targeting various immune pathways. |
| Promising options Rituximab, dupilumab, eosinophil-targeted molecules, omalizumab, complement inhibitors, interleukin (IL)-17 and IL-23 inhibitors, neonatal Fc receptor antagonists, topical therapies, and Janus kinase inhibitors are among the promising therapies being investigated for the management of BP. |
| Need for further research Despite these potential treatments, controlled clinical trials are crucial to establish the efficacy, safety, and optimal dosing regimens for managing BP, aiming to improve outcomes while minimizing systemic steroid use and associated toxicities. |
Introduction
Prevalence
Bullous pemphigoid (BP) is one of the most frequent autoimmune bullous diseases that affects mainly the elderly (eighth decade of life) without gender predilection [1]. As life expectancy raises in recent years, so does the BP incidence in Europe, ranging from 2.5 to 42.8 cases per million per year [2]. Although the incidence is low, the risk of developing BP increases with age, and people over 90 years of age have a 300-fold increased risk compared with people under 60 years of age [3]. A possible cause may be that in older populations, coexisting comorbidities as well as multiple drug exposure can trigger the disease.
Prognosis
In a meta-analysis by Kridin et al., the pooled estimate of 1-year mortality rate was 23.5% (95% confidence interval 20.2–26.8, I2 = 81%, p < 0.001) worldwide [4]. Compared with individuals in the general population of the same age, patients with BP face a 3.6-fold higher risk of death. This elevated risk can be attributed to the disease burden, adverse effects of pharmacological treatments, and the presence of severe multimorbidities [5]. Moreover, BP is a disease that severely affects patients’ quality of life.
Clinical Manifestations and Comorbid Conditions
BP is clinically manifested with tense bullous lesions on normal or erythematous/edematous skin, mainly located on the groin, axillary folds, thighs and the lower abdomen, as well as intense itching [6]. BP patients may also present with a non-bullous prodromic phase with eczematous, excoriated, urticaria-like, or nodular lesions with varying duration [2]. Oral, esophageal or genital mucosal lesions may present in 10–20% of cases [7]. Comorbid conditions, such as neurological disorders (e.g. multiple sclerosis, dementia, Parkinson’s disease, epilepsy, stroke), as well as cardiovascular diseases (e.g. diabetes, hypertension, pneumonia, pulmonary embolism), have an increased prevalence among patients with BP [2, 8–10]. In a retrospective cohort study involving 335 BP patients there was no significant difference in malignancy rates between BP patients and the general population [11]. Consequently, a comprehensive malignancy work-up might not be necessary for BP patients However, the study revealed that BP patients faced a higher risk of melanoma (10.7% vs. 4.3%, p < 0.001), indicating the potential benefit of routine skin screenings [11].
Drug-Induced BP
Drug-induced/associated BP has been rising in the last years. A variety of systemic agents may cause BP, such as diuretics (furosemide, bumetanide, spironolactone), analgesics, D-penicillamine, antibiotics (amoxicillin, ciprofloxacin), psycholeptics (phenothiazines), potassium iodide, captopril, antidiabetic agents (metformin and dipeptidyl peptidase IV inhibitors such as vildagliptin, sildagliptin, saxagliptin, linagliptin, alogliptin) and programmed death-1 (PD-1) inhibitors [12–14]. BP may also occur after either severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or related vaccines [15, 16].
Pathogenesis
The development of BP is rooted in the production of autoantibodies targeting hemidesmosomal proteins BP180 (collagen XVII) and BP230, both situated at the basement membrane zone and crucial for the dermo-epidermal junction (DEJ) [17]. When immunotolerance is lost, detachment of the DEJ occurs, leading to the formation of subepidermal blisters, usually characterized by a negative Nikolsky sign [18]. Histopathologically, the condition is marked by a separation between the dermis and epidermis, accompanied by infiltration of inflammatory cells, primarily lymphocytes and eosinophils [19].
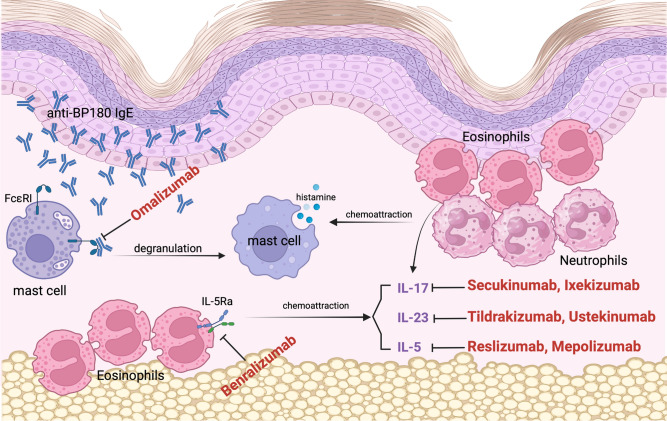
Disease activity correlates with the titers of circulating BP180 NC16A immunoglobulin (Ig) G antibodies measured by enzyme-linked immunosorbent assay [20]. Anti-BP180 IgG can be found in 95% of patients with BP, while anti-BP230 is detected in 70% of patients with BP [21]. The incidence of anti-BP180 IgE varies widely among patients [22]. Anti-BP180 IgE can bind to FcεRI receptors in mast cells, leading to a crosslink with hemidesmosomal BP180 in the DEJ, degranulation of mast cells, and histamine release, amplifying the chemotaxis of eosinophils and neutrophils (Fig. 1) [23]. Moreover, IgE may directly bind to the soluble 120-kd BP180 ectodomain on keratinocytes, get internalized and stimulate the release of interleukin (IL)-6 and IL-8, which both have a chemotactic effect. These two mechanisms may lead to bullae formation [23].
Fig. 1.
BP patients demonstrated elevated serum levels of IgE and eosinophilia. Anti-BP180 IgE can bind to the FcεRI receptors in mast cells, leading to a crosslink with hemidesmosome, degranulation of mast cells and histamine release, amplifying the chemotaxis of eosinophils and neutrophils. Omalizumab is a monoclonal antibody against the Cε3 domain of IgE, blocking its interaction with the specific FcεRI receptor, leading to a reduction of total IgE levels and eosinophilia. IL-5 is a Th2 cell-induced cytokine that contributes to eosinophilic maturation, activation and chemotactic activity. Reslizumab and mepolizumab are both monoclonal antibodies that target IL-5. Benralizumab, a monoclonal antibody against IL-5RA, can cause direct apoptosis of eosinophils and basophils. IL-17 also enhances eosinophilia. Secukinumab and ixekizumab both target IL-17A. IL-23 is an important cytokine in IgE-mediated responses, which promotes and maintains IL-17 activity and therefore eosinophilia. Tildrakizumab targets IL-23. Ustekinumab is an IL-12 and IL-23 inhibitor (targets their common p40 subunit). BP bullous pemphigoid, IL interleukin, IL-5RA alpha subunit of the IL-5 receptor, IgE immunoglobulin E autoantibodies targeting hemidesmosomal protein BP180 (collagen XVII), Th2 type 2 helper
Therapy
BP treatment can be very challenging as BP is a chronic disease requiring long-term management. Glucocorticoids are the cornerstone of BP treatment. High-dose systemic corticosteroids are used to induce remission, followed by gradual tapering (Table 1) [6]. However, BP is a chronic disease and therefore frequently requires long-term use of systemic corticosteroids, which may cause a variety of adverse effects, such as hypertension, bone fracture, cataract, gastrointestinal discomfort and metabolic conditions (e.g. weight gain, hyperglycemia) [24].
Table 1.
Synopsis of the current treatment options in bullous pemphigoid
| Treatment options | Comments | References | |||
|---|---|---|---|---|---|
| Systemic glucocorticoids | |||||
|
High dose to induce remission Tapering to prevent relapse |
Cornerstone of BP treatment Long-term use may cause adverse effects |
[6] | |||
| High potency topical corticosteroids | |||||
| Clobetasol propionate | High efficacy, difficult in the home-setting of elderly patients | [25] | |||
| Steroid sparing agents | |||||
| Azathioprine |
• When monotherapy with oral steroids is inadequate to achieve disease control • By relapse during steroid tapering • By contraindications to oral steroids or existing comorbidities |
[25–33] | |||
| Methotrexate | |||||
| Mycophenolate mofetil | |||||
| Dapsone | |||||
| Cyclosporin | |||||
| Novel target treatments | |||||
| Agent | Target | Clinical Trials | Comment | Evidence from various trials and studies | References |
| Rituximab | CD20 | NCT04128176 | Open-label, phase III, single-group, ongoing |
• C grade level of evidence for refractory forms of BP, randomized controlled clinical trials are lacking • NCT04128176: evaluates the efficacy and safety of rituximab combined with omalizumab |
[37, 130] |
| Dupilumab | IL-4RA | NCT04206553 | Double-blind, placebo-control, phase III, ongoing |
• Case series of 13 BP patients: 92.3% (12/13) achieved disease clearance or satisfactory response, 53.8% (7/13) achieved total clearance of BP; no adverse events were reported • Case report of a 72-year-old woman with BP, dupilumab treatment not only alleviated pruritus and improved skin lesions but also resulted in the normalization of anti-BP180 antibodies • Case report: combination of omalizumab with dupilumab demonstrated high efficacy in a patient with refractory BP • Two case reports demonstrated that dupilumab is promising for the treatment of highly refractory, rituximab-resistant or immune checkpoint inhibitor-induced BP • Retrospective cohort study: 300 mg of dupilumab every 2 weeks following an initial 600 mg dose led to disease control in 87.0% of patients |
[55, 63, 66–69] |
| Omalizumab | IgE | NCT04128176 | Open-label, noncontrolled, phase III, ongoing |
• Multiple case reports: omalizumab effectively reduced the severity of BP by diminishing itching and blister counts, and enables a reduction in corticosteroid dosage in responsive patients • Case study: 68-year-old man diagnosed with a severe and atypical form of BP, characterized by anti-BP230 IgE and anti-p200 IgG, was effectively treated with omalizumab • Case series: six BP patients with severe symptoms, intense itching, and either insufficient response to systemic corticosteroids or contraindications for them. Omalizumab led to significant improvement in itching, re-epithelialization, and enhancement in BPDAI scores. The treatment was well tolerated, and no adverse effects were reported • Retrospective study: 77% of BP patients who failed multiple lines of treatment achieved complete remission, and complete remission was more common in patients with elevated serum baseline levels of anti-BP180-NC16A IgE |
[117–121, 127] |
| Nomacopan | C5, leukotriene B4 | NCT04035733 | Single-group, phase IIa, nonrandomized controlled trial, terminated, results published | • NCT04035733: 7 of 9 patients demonstrated remarkable reduction of BPDAI and pruritus after approximately 1.5 months. No serious adverse events were reported | [140] |
| NCT05061771 | Randomized, part A partial-blinded, part B double-blinded, placebo-controlled phase III trial, ongoing | • NCT05061771: Is enrolling 148 patients to evaluate the efficacy of nomacopan in BP. Patients will be randomized to receive either nomacopan plus oral corticosteroids or placebo plus oral corticosteroids for a treatment period of 24 weeks. Oral corticosteroids will be tapered over the course of the treatment if the symptoms of disease improve | [141] | ||
| Avdoralimab | C5aR1 | NCT04563923 | Case-control, randomized, open-label, phase II, ongoing | • NCT04563923: Is enrolling 40 patients to evaluate the efficacy of avdoralimab in BP in addition to superpotent topical corticosteroids (complete clinical remission) compared with superpotent topical corticosteroids alone | [142] |
| Sutimlimab | C1s | NCT02502903 | Prospective, double-blind, randomized, placebo-controlled, phase I, terminated, results published | NCT02502903: Four weekly 60 mg/kg infusions of sutimlimab were well-tolerated (mild to moderate adverse effects, such as coryzal symptoms) and led to the absence of C3 deposition at the DEJ in four of five patients. Limitations: Small sample size, short treatment duration, lack of overt disease activity | [143] |
| Mepolizumab | IL-5 | NCT01705795 | Randomized, placebo-controlled, double-blind, phase II, terminated, results published | • NCT01705795: The primary endpoint (defined as the cumulative rate of relapse-free patients after initiation of therapy) was not met, yet mepolizumab markedly lowered blood eosinophil levels. Limitations: small sample size and the short follow-up period | [97] |
| Reslizumab | IL-5 | • Case report: Can rapidly improve bullous skin lesions, allowing tapering of systemic corticosteroid dosage; re-exacerbation of skin lesions was noted upon discontinuation of reslizumab, despite maintenance of cyclosporin | [101] | ||
| Benralizumab | IL-5Ra | NCT04612790 | Randomized, double-blind, parallel-group, placebo-control, phase III, terminated, not published | [104] | |
| Secukinumab | IL-17A |
• Three case reports: successful treatment of BP. Decreases circulating anti-BP180 antibodies • Case report: adjuvant secukinumab therapy in addition to standard therapy with prednisolone, led to a long lasting and complete remission. Limitation: utilization of secukinumab was accompanied by standard therapy for BP, making it difficult to arrive at a definitive assessment of the effectiveness of secukinumab |
[145–147] | ||
| Ixekizumab | IL-17A | NCT03099538 | Open-label, single-arm, phase II, prematurely terminated, results not yet published |
• Case report: Induced clinical remission of BP in a patient with concurrent psoriasis • NCT03099538: Ixekizumab failed to reach the primary endpoint (cessation of blisters) in four BP participants who were enrolled and analyzed |
[149, 150] |
| Tildrakizumab | IL-23 | NCT04465292 | Open-label, single-group, early phase I, ongoing, terminated, results not yet published | • Case study: 71-year-old patient with psoriasis and concurrent BP. Following his second tildrakizumab injection, the patient has remained in remission for both conditions | [156] |
| Ustekinumab | IL-12, IL-23 | NCT04117932 | Open-label, single-group, phase II, ongoing |
• Case report of a patient with BP and psoriasis: Clearing of both diseases after treatment with ustekinumab • Case reports: Culprit for drug-induced BP • NCT04117932: Is evaluating the efficacy and safety profile of ustekinumab as an adjuvant treatment to topical superpotent corticosteroids in BP • Case report: 68-year-old BP patient with concomitant psoriasis with PsA. Under ustekinumab and doxycycline, her conditions have remained well-managed |
[156–161] |
| Bertilimumab | Eotaxin-1 | NCT02226146 | Open-label, single-group, phase II, terminated, results not published | • NCT02226146: Disease severity decreased by 81% 13 weeks after the use of bertilimumab. Molecule was well tolerated. Limitations: lack of a control group, small number of patients (n = 9) and short duration of treatment (4 weeks) | [86] |
| AKST4290 | CCR3 eotaxin receptor | NCT04499235 | Randomized, double-blinded, placebo-control, phase II, terminated, not published | • NCT04499235: AKST4290 400 mg administered twice in patients with BP (together with mometasone furoate) can lead to disease control | [87] |
| Efgartigimod | FcRN | NCT05267600 | Randomized, double-blinded, placebo-controlled, parallel-group, phase II/III, ongoing | • NCT05267600: The agent will be administered via subcutaneous injections in adult participants with moderate to severe BP | [165] |
| Upadacitinib | JAK | • Case report: Upadacitinib, administered at a daily oral dose of 15 mg, along with a gradual reduction of prednisolone over 20 days, led to complete remission of BP within 2 months. A follow-up at 5 months of treatment showed no disease recurrence or flares, and no adverse events were noted | [169] | ||
| Baricitinib | JAK | • Case report: Baricitinib at a daily oral dose of 4 mg resulted in a complete remission of BP and psoriasis in 24 weeks without any adverse effects | [170] | ||
| Tofacitinib | JAK | • Case report: Well-tolerated and effective for the treatment of BP and concurrent psoriasis vulgaris | [171] | ||
BP bullous pemphigoid, BPDAI Bullous Pemphigoid Disease Area Index, IL interleukin, IL-4RA alpha subunit of the interleukin-4 receptor, Ig immunoglobulin, DEJ dermo-epidermal junction, C5 complement 5, C5aR1 Complement 5a receptor 1, C1s complement 1s, IL interleukin, IL-5RA alpha subunit of the interleukin-5 receptor, FcRN neonatal Fc receptor, JAK Janus kinase, PsA psoriatic arthritis
Consequently, one of the key principles in the management of BP is to reduce the patient’s exposure to systemic glucocorticoids. Using high potency topical corticosteroids, such as clobetasol propionate, as a topical regimen may have similar efficacy but is often difficult to realize in the home-setting of elderly patients [25]. Corticosteroid-sparing adjuvant agents are prescribed when monotherapy with glucocorticoids is inadequate to achieve disease control or when there is a relapse during corticosteroid tapering [25, 26]. Moreover, these drugs are considered if there are contraindications to oral corticosteroids or existing comorbidities, such as hypertension, diabetes mellitus, osteoporosis and psychosis [27]. The choice of adjuvant agents depends on the availability, price, practical experience of the treating clinician, and the presence of specific contraindications [27]. For BP, the adjuvant agents may include (Table 1):
azathioprine – depending on thiopurine methyltransferase activity [28];
doxycycline – alone or in combination with daily oral nicotinamide [29];
mycophenolate mofetil or mycophenolic acid [28];
methotrexate – which may eventually be used as a monotherapy also [30, 31];
dapsone [32];
cyclosporin [33];
rituximab.
However, certain patients remain irresponsive to conventional therapies, highlighting a pressing demand for treatments with reduced adverse effects and enhanced efficacy.
The aim of this review was to summarize and discuss the novel targeted BP treatment options that have been developed and tested in clinical trials in recent years, based on the complex pathophysiology of BP.
Rituximab
B lymphocytes play an important role in the pathogenesis of autoimmune bullous diseases. They participate in several cellular functions, including the secretion of autoantibodies, activation of T cells, and production of proinflammatory cytokines [6]. Therefore, they are considered an important therapeutic target.
Rituximab, a chimeric IgG1 monoclonal antibody, targets the CD20 transmembrane receptor expressed on the surface of B lymphocytes and in the pre-plasma cell lineage. It induces a B lymphocyte depletion and prevents the differentiation of B lymphocytes into antibody-secreting plasma cells (Fig. 2) [6]. Moreover, rituximab inhibits CD4+ T lymphocytes and increases the number of FOXP3+ regulatory cells [6]. Rituximab therapy usually results in a major reduction in circulating BP180-specific B lymphocytes, as well as a dramatic reduction of anti-BP180 antibodies, and decreases expression of proinflammatory IL-15 and IL-6, leading to an improvement of BP skin manifestations [6, 34, 35].
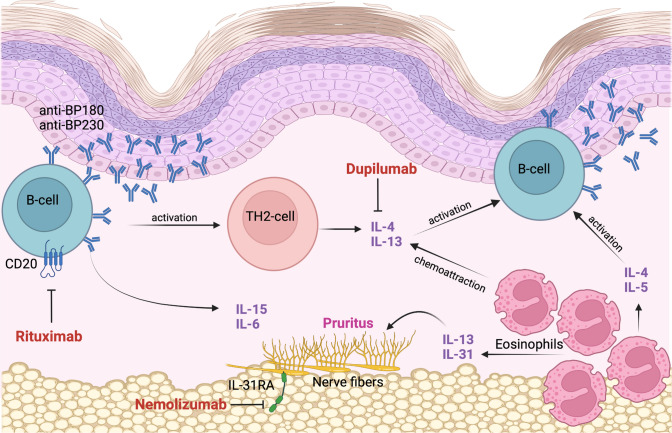
Fig. 2.
Th2 pathways are considered the primary triggers for antibody production in BP. Rituximab targets the CD20 transmembrane receptor expressed on the surface of B-lymphocytes. It induces a B-lymphocyte depletion and prevents the differentiation of B-lymphocytes into antibody-secreting plasma cells. Th2 cytokines IL-4 and IL-13 play an important role in eosinophil chemoattraction. Autoreactive Th2 cells stimulate B-cell autoantibody production and participate in the recruitment and activation of eosinophils. Eosinophils also contribute to the maintenance of Th2-type inflammatory responses by further producing IL-4, IL-5 and IL-13. IL-13 can directly stimulate peripheral nerve fibers and indirectly recruit IL-31-secreting eosinophils to the site of skin lesions, and is therefore involved in BP-associated itch. Dupilumab can inhibit IL-4 and IL-13 signaling. It downregulates eosinophil chemotaxins and inhibits Th2-associated chemokine activity, B-cell proliferation and autoantibody production. Dupilumab may further improve pruritus in BP by directly reducing IL-13 and by indirectly downregulating IL-31 production by eosinophils. Nemolizumab is an anti-IL-31 receptor A antibody that can contribute to a great reduction of pruritus. BP bullous pemphigoid, IL interleukin, CD20 cluster of differentiation 20, Th2 type 2 helper
Rituximab has the ability to significantly reduce the concentration of serum autoantibodies without substantially affecting the overall antibody titers [36]. That is because rituximab primarily acts on plasma cells producing pathogenic autoantibodies and not on CD20 plasma cells that produce antimicrobial antibodies [36]. These autoreactive plasma cells differ fundamentally from the protective antimicrobial plasma cells in terms of their differentiation, migration, and survival characteristics [36]. Rituximab specifically targets short-lived autoreactive plasmablasts expressing CD20, which represent an early stage of differentiation into plasma cells [36]. These plasmablasts have a shorter lifespan and higher turnover rate compared with normal, long-lived plasma cells [36].
The current body of evidence supporting the use of rituximab in refractory BP stands at a C grade level. This evidence is primarily derived from various study types, including prospective and retrospective cohort studies, case-control studies, and case series [37]. Notably, the field lacks robust, well-designed, randomized controlled clinical trials in this context [37]. Consequently, rituximab has gained approval exclusively for pemphigus treatment. However, it is frequently employed off-label for managing severe refractory pemphigoid disease or for BP patients with contraindications or poor tolerance to conventional immunosuppressive agents [37].
Furthermore, it appears that rituximab is less efficacious in treating BP compared with pemphigus. BP patients tend to exhibit a lower frequency of complete response, a higher rate of relapse, and reduced tolerance to rituximab treatment compared with pemphigus patients [38–41]. This variance might stem from the persistence of IgE autoantibodies or CD20-positive plasma cells in BP, which can endure even after rituximab intervention [42]. These lingering elements have been associated with early relapse of blistering skin lesions, posing a challenge to sustained therapeutic outcomes [42]. Additionally, the role of complement in activating innate immunity may further exacerbate the immune response in BP [43–45]. This heightened response suggests that even low levels of anti-BP180 autoantibodies can exert a profound impact, making it challenging to achieve a response after rituximab treatment or to taper patients off low doses of prednisolone.
The initial documentation of rituximab's application in BP dates back to 2007 and was based on the lymphoma protocol, involving a dosage of 375 mg/m2 administered weekly for 4 weeks [46]. The rheumatoid arthritis protocol, which consists of two infusions of 1000 mg spaced 2 weeks apart, has also been utilized. Presently, the optimal dosing regimen for BP remains ambiguous. However, an escalating number of studies are exploring the effectiveness of lower doses (such as two infusions of 500 mg 2 weeks apart or four weekly infusions of 500 mg), or even ultra-low doses (100 mg administered weekly for 4 weeks) of rituximab in the context of BP [39, 47–49].
Dupilumab
Type 2 inflammation is an immune response against parasites, allergens, certain viral or bacterial infections and endogenous molecules [50]. It is predominately mediated by group 2 innate lymphoid cells, type 2 helper (Th2) cells, eosinophils and cytokines, such as IL-4, IL-5 and IL-13 [51, 52].
Th2 pathways are considered the primary triggers for antibody production in BP [53]. BP patients show higher concentrations of IL-4 and IL-13 producing CD4+ and CD8+ T cells in their blister fluids and sera than healthy controls [54], as well as elevated serum levels of IgE and eosinophilia [55]. Th2 cytokines IL-4, IL-13 and IL-31 play an important role in eosinophil chemoattraction, maturation and activity and therefore in the induction of pruritus [56, 57]. They have been found elevated in peripheral blood and skin lesions of BP patients [56–58] and they upregulate Th2 immunological responses that are responsible for the loss of tolerance towards BP180 (Fig. 2) [59]. Autoreactive Th2 cells stimulate B-cell autoantibody production and participate in the recruitment and activation of eosinophils (Fig. 2). Eosinophils also contribute to the maintenance of Th2-type inflammatory responses by further producing IL-4, IL-5 and IL-13 [60]. IL-13 can directly stimulate peripheral nerve fibers and indirectly recruit IL-31-secreting eosinophils to the site of skin lesions and is therefore involved in BP-associated itch (Fig. 2) [61].
Dupilumab, a fully human monoclonal antibody, binds to the alpha subunit of the IL-4 receptor. This subunit is shared by both IL-4 and IL-13, therefore dupilumab inhibits IL-4 and IL-13 signaling [39, 62]. Hence, it downregulates eosinophil chemotaxis, B-cell proliferation and autoantibody production (Fig. 2) [60]. Dupilumab may further improve pruritus in BP by directly reducing IL-13 and by indirectly downregulating IL-31 production by eosinophils [61]. It is approved for the treatment of moderate to severe atopic dermatitis and is currently being studied for many other Th2 inflammatory diseases.
In a case series involving 13 BP patients from five academic centers receiving dupilumab, 92.3% (12/13) of the patients achieved disease clearance or satisfactory response (defined as documented improvement noted by clinicians and the patient's willingness to continue the medication without reaching complete clearance) [63]. Among the patients, 53.8% (7/13) achieved total clearance of BP, and no adverse events were reported during the treatment [63].
In the documented case of a 72-year-old woman with BP, dupilumab treatment not only alleviated pruritus and improved skin lesions but also resulted in the normalization of anti-BP180 antibodies [55]. Additionally, following dupilumab therapy, there was a reduction in the percentages of circulating CD4 T cells producing IL-4, IL-13, IL-17, and IL-31 [55]. Notably, there were no significant changes in the proportions of interferon (IFN)-γ-producing CD4 and CD8 T cells before and after treatment [55]. These findings suggest that the effectiveness of dupilumab may primarily be attributed to its suppression of Th2 cytokines [55].
BP patients who receive dupilumab in combination with conventional therapies achieve faster clinical response and reduction of the cumulative corticosteroid dose, when compared with those treated only with conventional therapies [64, 65]. A case report indicated that combination of omalizumab with dupilumab demonstrated high efficacy in a patient with refractory BP [66]. Two case reports have demonstrated that dupilumab is promising for the treatment of highly refractory, rituximab-resistant or immune checkpoint inhibitor-induced BP [67, 68]. Regarding the studies involving immune checkpoint inhibitors, a notable limitation is the absence of discussion on the role of IL-4 blockade in tumor immunity, giving rise to safety concerns. Consequently, these promising results should be interpreted cautiously, as many questions remain unanswered.
In a recent retrospective cohort study conducted by the National Autoimmune Bullous Diseases Cooperative Group of China, 146 patients diagnosed with BP were administered a dosage of dupilumab 300 mg every 2 weeks, following an initial 600 mg dose [69]. Among these patients, 127 (87.0%) attained disease control within 4 weeks, defined as the absence of new lesions and itching, as well as the healing of existing lesions [69]. The median (interquartile range [IQR]) time for achieving this control was 14 (7–14) days [69]. Additionally, 52 (35.6%) patients experienced complete remission, while 13 (8.9%) BP patients relapsed during the observation period [69]. The most common adverse events were infections and eosinophilia [69]. Anti-BP180 antibody levels >50 relative units/mL, as well as female sex, were associated with better response to treatment [69].
Dupilumab may be administered at the dosing scheme approved for atopic dermatitis: 600 mg subcutaneously initially, followed by 300 mg subcutaneously every other week. However, some BP patients may need a maintenance dose more frequently than every other week, suggesting the need for higher doses [63].
Clinical trials are needed to further confirm the efficacy of dupilumab in patients with BP and its long-term effect. Additionally, more studies are also required to establish the optimal dosage and the treatment intervals for the maximal benefit. Currently, there is an ongoing phase III randomized, double-blind, placebo-controlled trial for the use of dupilumab in BP (NCT04206553) [70].
Eosinophil-Related Molecules
Eosinophil infiltration is a common characteristic in BP skin lesions, and these cells are attracted by various chemokines present in blister fluid, such as IL-5, eotaxin, and galectin-9 [71]. Moreover, more than 50% of untreated BP patients exhibit elevated levels of blood eosinophils, which have been found to be positively correlated with disease severity and itch intensity [72–76]. Recent studies have identified increased levels of the eotaxin receptor CCR3 and its ligand, eotaxin, in BP, suggesting their involvement in the condition [77–79]. Eotaxin-1 and eotaxin-3, significantly upregulated in the serum and blister fluid of BP patients, are strongly linked to eosinophil numbers and activation [75, 80]. Notably, eotaxin-1 has been coined as the ‘aging factor’ due to its age-dependent rise in serum levels and its recent association with aging and neurocognitive decline [75, 80].
BP patients with eosinophilia are typically older and often exhibit more extensive palmoplantar involvement than others [74]. Moreover, the levels of eosinophilic cationic protein (ECP), eosinophil-derived neurotoxin (EDN), and major basic protein (MBP) released by eosinophils are higher in the serum and blister fluid of BP patients compared with healthy controls [81]. ECP and EDN can induce blister formation by disrupting the detachment of keratinocytes from the extracellular matrix [81]. Eosinophils also stimulate the secretion of matrix metalloproteinase (MMP)-9, which facilitates the degradation of BP180 and cleavage of the DEJ [82–84]. Additionally, eosinophils are considered the primary source of IL-31 in BP, a well-known pruritogen and promoting factor for blister formation [85]. Therefore, targeting eosinophils holds promise as a potential treatment strategy for BP.
Bertilimumab is a humanized monoclonal antibody targeting eotaxin-1 (CCL-11). By binding to this chemokine, bertilimumab impairs eosinophil migration to the skin. Currently, it has not received approval for treating any other conditions. However, the FDA has recently granted bertilimumab orphan drug designation for the treatment of BP. In a phase II, open-label clinical trial of patients with newly diagnosed moderate to extensive BP (NCT02226146), disease severity decreased by 81% 13 weeks after the use of bertilimumab. Subjects received only three doses of bertilimumab (on days 0, 14 and 28) [86]. The molecule was also well tolerated, indicating that bertilimumab is a well tolerated and efficacious treatment for BP [86]. Limitations of this trial included lack of a control group, a small number of patients (n = 9) and a short duration of treatment (4 weeks) [86].
AKST4290 is an antagonist of the CCR3 eotaxin receptor on eosinophils. In a phase II, double-blinded clinical trial (NCT04499235), patients with BP were administered 400 mg of AKST4290 twice, in combination with mometasone furoate. The treatment exhibited efficacy, resulting in disease control [87].
Nemolizumab is an anti-IL-31 receptor A antibody that is licensed in Japan to treat atopic dermatitis and awaits worldwide approval for prurigo nodularis, as it strongly reduces pruritus (Fig. 2) [88]. Consequently, it might be considered a potential additional medication for controlling pruritus in BP in the future. However, no clinical trials have been planned thus far [22].
IL-5 is a Th2 cell-induced cytokine detected in blister fluid of patients with BP and is present in the acute phase of BP [56, 57]. It contributes to eosinophilic maturation, activation and chemotactic activity by increasing CCR3 expression [61, 89, 90]. Serum IL-5 is positively correlated with BP disease activity, as it is associated with blood eosinophilia and eosinophil infiltration in the skin [91]. In other words, in the presence of BP antibodies, IL-5 activates eosinophils which may directly contribute to blister formation [91].
Mepolizumab is a humanized monoclonal antibody targeting IL-5 (Fig. 1). It has been utilized in various clinical trials for the treatment of eosinophilic granulomatosis with polyangiitis, eosinophilic asthma, and chronic rhinosinusitis with nasal polyps, showing high efficacy and safety [92–96]. However, in a double-blind, phase II pilot study in patients with BP (NCT01705795), there was no significant difference in the cumulative rates of patients who achieved and maintained disease control between the mepolizumab (with corticosteroids) and placebo (with corticosteroids) groups, and the primary endpoint (defined as the cumulative rate of relapse-free patients after initiation of therapy) was not met [97]. However, mepolizumab markedly lowered blood eosinophil levels [97]. The small sample size and the short follow-up period are the major limitations of this study.
Reslizumab is another humanized monoclonal antibody targeting IL-5 that has been approved for adults with severe eosinophilic asthma [98–100]. A case report of reslizumab in BP reported that this monoclonal antibody can rapidly improve bullous skin lesions, allowing tapering of systemic corticosteroid dosage (Fig. 1) [101]. However, re-exacerbation of skin lesions was noted upon discontinuation of reslizumab, despite maintenance of cyclosporine [101].
Benralizumab is a humanized monoclonal antibody against IL-5Rα, which cause direct apoptosis of eosinophils and basophils (Fig. 1) [102, 103]. A phase III, randomized clinical trial is underway to evaluate the use of benralizumab in the treatment of BP (NCT04612790) [104].
Omalizumab
BP patients frequently demonstrate elevated serum levels of IgE and eosinophilia (Fig. 1) [55]. Depositions of IgE in the basal membrane zone (BMZ) were first described in 1974 and later studies showed that IgE autoantibodies target the NC16A region of BP180, which is the same region targeted by anti-BP180 IgG [105, 106]. In vitro, anti-BP180 IgE can cause a decline in keratinocyte adhesion and hemidesmosomal density, suggesting its contribution in blister formation [107].
The levels of anti-BP180 IgE are associated with disease activity, as patients with high total IgE and high anti-BP180 IgE levels have twice the extension in body surface area compared with patients with only anti-BP180 IgG [108]. Indeed, the deposition of IgE in the BMZ correlates positively to the Bullous Pemphigoid Disease Area Index (BPDAI) score and to disease course [109]. Moreover, there is a positive correlation between disease remission and decrease in total IgE, anti-BP180 IgE, and anti-BP180 IgG levels [110].
Other studies have detected anti-BP230 IgE in patients with BP [111, 112] and their levels have been associated with disease activity and local eosinophil infiltration [113, 114]. Additionally, anti-BP230 IgE is more common among patients who are resistant to topical corticosteroids, being a potential marker for systemic corticosteroid therapy [115].
Omalizumab is a high affinity monoclonal antibody against the Cε3 domain of IgE, blocking its interaction with the specific FcεRI receptor, leading to a reduction of total IgE levels and eosinophilia (Fig. 1) [116]. It is currently approved for use in chronic idiopathic or spontaneous urticaria and asthma.
Multiple case reports have shown that omalizumab effectively reduced the severity of BP by diminishing itching and blister counts [117–119]. Furthermore, it enabled a reduction in corticosteroid dosage in responsive patients, thereby mitigating the long-term complications associated with corticosteroid therapy [117–119]. In a different case study, a 68-year-old man diagnosed with a severe and atypical form of BP, characterized by anti-BP230 IgE and anti-p200 IgG, was effectively treated with omalizumab [120]. In a series involving six BP patients with severe symptoms, intense itching, and either insufficient response to systemic corticosteroids or contraindications for them, omalizumab (administered through subcutaneous injections of 300 mg every 4 weeks) led to significant improvement in itching, re-epithelialization, and enhancement in BPDAI scores [121]. The treatment was well tolerated, and no adverse effects were reported [121].
Total IgE and eosinophils were frequently found to be elevated at the start of therapy, with a rapid reduction of eosinophils after therapy, and a slower, sometimes undetectable, reduction in IgE (probably due to the formation of immunocomplexes between the drug and IgEs that have a slower clearance) [118, 120, 122, 123]. However, other studies demonstrated no correlation between response to omalizumab and eosinophil or IgE levels in blood [119, 121, 124]. Indeed, omalizumab may also show efficacy in patients without detectable serum anti-BP180/BP230 IgE [121, 125, 126]. Responders show a decrease in IgE skin deposition, circulating IgG autoantibody levels, and basophils, suggesting immune-modulatory effects beyond IgE inhibition [121, 125, 126]. After 4 weeks of omalizumab, there is a significant reduction of IgE+ and FceRI+ cells in the dermis in immunohistochemistry stainings [126]. That is to say, only a subset of BP patients responds favorably to omalizumab treatment and lesional IgE and FceRI expression might be useful to distinguish this subset of patients who will benefit from omalizumab therapy [126].
There are limited reports on the effectiveness of omalizumab in the treatment of BP. Certain studies indicate a recurrence of BP within a few weeks of omalizumab therapy, whereas others document complete remission of the disease after several months [6, 125]. These discrepancies may be linked to the fact that most patients were concurrently undergoing other immunosuppressive treatments. The timing of disease recurrence could be influenced by whether these other therapies were fully or partially discontinued [6].
A recent multicenter, retrospective study conducted in France involved 100 patients with BP who were treated with omalizumab after failing one or multiple lines of treatment [127]. The study revealed that 77% of patients achieved complete remission, while 9% experienced partial remission [127]. Complete remission was more common in patients with elevated serum baseline levels of anti-BP180-NC16A IgE [127]. Interestingly, urticarial lesions, total IgE levels, and eosinophil count did not serve as predictors for complete remission [127]. Among those who achieved complete remission, 11.7% maintained it without any additional therapy, 57.1% required ‘minimal therapy’, and 31.2% needed ‘non-minimal therapy’ [127]. The median time to complete remission was 3 months (with a range of 2.2–24.5 months), and the relapse rate stood at 14% during the median follow-up period of 12 months [127]. Adverse events were reported in four patients [127]. It was observed that patients receiving omalizumab dosages exceeding 300mg every 4 weeks exhibited a similar final outcome [127]. However, they achieved significantly faster control of disease activity [10 (5–30) vs. 15 (10–60) days, p < 0.001] and complete remission [2.4 (2.2–8.2) vs. 3.9 (2.3–24.5) months, p < 0.001] compared with those on lower dosages [127].
Omalizumab has been administrated differently in various studies, with some studies based on the asthma and some on the chronic urticaria dosing nomogram, ranging from 375 mg every 2 weeks to 300 mg every 8 weeks [6]. Other case reports were adjusting the dose interval according to disease course [128, 129]. Currently there is an ongoing, open-label, phase III, single-group trial testing the efficacy of rituximab combined with the administration of omalizumab 300 mg every 2 weeks (NCT04128176) [130].
Complement System Inhibitors
Complement activation plays a crucial role in the pathogenesis of BP. In 90% of BP patients, C3 deposits are evident along the DEJ, making the detection of C3 through direct immunofluorescence (DIF) microscopy of perilesional skin a highly valuable diagnostic marker for BP [131]. The activation of complement at the DEJ occurs due to pathogenic autoantibodies binding to BP180, initiating and sustaining the inflammatory process, leading to subepidermal blistering [19]. Furthermore, complement components, including C1, C3, C3d, P, C5, and membrane attack complex (MAC), have been identified in the basal membrane and BP blister fluid [132, 133]. C5a responds to IgG autoantibody deposition, stimulating leukotriene B4, which recruits neutrophils to the dermal-epidermal junction and triggers tissue inflammation [134, 135]. Additionally, C3a and C5a enhance eosinophil recruitment, activation, and extravasation [136, 137]. Studies have shown that animals with complete deficiencies in complement components C3, C4, C5, and C5aR do not develop BP lesions when injected with pathologic autoantibodies [138, 139].
Nomacopan (rVA576) is a C5 and leukotriene B4 inhibitor. In a phase II, nonrandomized, controlled trial (NCT04035733), seven of nine patients demonstrated remarkable reduction of BPDAI and pruritus after approximately 1.5 months [140]. No serious adverse events were reported [140]. An ongoing, randomized, double blind, placebo-controlled, phase III trial aims at enrolling 148 patients to evaluate the efficacy of nomacopan in BP (NCT05061771) [141]. In this study, patients will be randomized to receive either nomacopan plus oral corticosteroids or placebo plus oral corticosteroids for a treatment period of 24 weeks [141]. Oral corticosteroids will be tapered over the course of the treatment if the symptoms of disease improve [141].
Avdoralimab is a specific anti-C5aR1 monoclonal antibody. An open-label, randomized, case-controlled, multicenter, phase II clinical trial (NCT04563923) is enrolling 40 patients to evaluate the efficacy of avdoralimab in BP in addition to superpotent topical corticosteroids (complete clinical remission) compared with superpotent topical corticosteroids alone [142].
Sutimlimab (BIVV009) is a humanized IgG4 monoclonal antibody that inhibits C1s. Its safety and tolerability have been tested in a phase I clinical trial of eight patients with BP (NCT02502903) [143]. The trial indicated that four weekly 60 mg/kg infusions of sutimlimab were well tolerated (mild to moderate adverse effects, such as coryzal symptoms) and led to the absence of C3 deposition at the DEJ in four of five patients [143]. The limitations of this study include its small sample size, the short treatment duration, and the lack of overt disease activity [143].
Interleukin (IL)-17 and IL-23 Inhibitors
In previous in vitro studies, IL-17 enhanced eosinophilia (Fig. 1) [144]. Three recent case reports have demonstrated a successful treatment of BP with secukinumab, a monoclonal anti-IL-17A antibody [145–147]. The administration of secukinumab has been found to decrease circulating anti-BP180 antibodies [146]. In a case report of an 85-year-old female patient with severe BP, adjuvant secukinumab therapy in addition to standard therapy with prednisolone led to a long lasting and complete remission [145]. One constraint of this observation is that the utilization of secukinumab was accompanied by standard therapy for BP, making it difficult to arrive at a definitive assessment of the effectiveness of secukinumab [145]. Additionally, there is a reported instance of BP emergence subsequent to psoriasis treatment involving secukinumab [148].
Ixekizumab, another anti-IL-17A monoclonal antibody has been shown to induce clinical remission of BP in a patient with concurrent psoriasis [149]. However, in an open-label, phase II trial, ixekizumab failed to reach the primary endpoint (cessation of blisters) in four BP participants who were enrolled and analyzed (NCT03099538) [150].
IL-23 is an important cytokine in IgE-mediated responses, which promotes and maintains IL-17 activity and therefore eosinophilia [151, 152]. However, eosinophil count has also been found to be negatively associated with IL-23 levels in BP [153]. IL-23 additionally upregulates the secretion of MMP-9 along the DEJ, leading to blister formation [154]. Additionally, both IL-23 and IL-17 can help identify BP patients at risk of future relapse upon corticosteroid therapy [154].
An early open-label, phase I pilot study evaluated the role of tildrakizumab (an anti-IL-23 antibody) in the treatment of BP (Fig. 1) [NCT04465292] [155]. In a recent case study, a 71-year-old male patient with psoriasis and concurrent BP had been treated with mycophenolate mofetil at a dosage of 500 mg daily and prednisone at a dosage of 10 mg every other day [156]. Notably, the patient's BP had been well-managed, with no eruptions for the past 2 years; however, he was unable to discontinue the use of corticosteroids [156]. Mycophenolate was discontinued and the patient was started on cyclosporine at a daily dose of 250 mg, leading to the resolution of his lesions over several weeks [156]. The prednisone was gradually tapered off. The patient's condition remained stable as cyclosporine was slowly reduced and eventually discontinued, after which he was initiated on tildrakizumab [156]. He experienced a moderate psoriasis flare a few weeks after discontinuing cyclosporine, which was resolved with a brief 3-week cyclosporine taper [156]. Following his second tildrakizumab injection, the patient has remained in remission for both conditions [156]. It is worth noting that he had a mild BP lesion flare 6 months into tildrakizumab treatment, which spontaneously resolved after 1–2 weeks without any intervention [156].
Ustekinumab is an IL-12 and IL-23 inhibitor (targets their common p40 subunit) that is approved for psoriasis, psoriatic arthritis (PsA) and Crohn’s disease. A case report of a patient with BP and psoriasis demonstrated clearing of both diseases after treatment with ustekinumab [157, 158]. Other reports suggest that ustekinumab is a culprit for drug-induced BP (Fig. 1) [159, 160]. A currently ongoing phase II, open-label study is evaluating the efficacy and safety profile of ustekinumab as an adjuvant treatment to topical superpotent steroids in BP (NCT04117932) [161]. In a recent case report, a 68-year-old female with BP and concomitant psoriasis with PsA was receiving prednisolone 15 mg daily, narrow-band UV-B light (NBUVB) therapy, and once weekly doxycycline and niacinamide [156]. Previous attempts with topical corticosteroids for her BP had been ineffective. Ustekinumab at a dose of 45 mg on days 1 and 14 was initiated, followed by once every 3 months [156]. Doxycycline was continued at 100 mg daily, while niacinamide was discontinued due to gastrointestinal problems [156]. Prednisone was gradually tapered, and NBUVB therapy was stopped [156]. The patient remained in remission from BP and had excellent control of her PsA for about 2 years without corticosteroids [156]. However, due to issues with her health insurance, there was a delay in receiving her ustekinumab dose [156]. Consequently, she experienced a flare-up of her BP and PsA 4 weeks after missing her dose, despite continuing doxycycline [156]. Upon resuming ustekinumab, she reported rapid relief of her symptoms without requiring additional treatments [156]. Since then, she has consistently taken ustekinumab and doxycycline, and her conditions have remained well-managed [156].
Neonatal Fc Receptor Antagonists
The neonatal Fc receptor (FcRn) plays a key role in homeostasis and in prolonging the lifespan of IgG [162, 163]. The formation of IgG-FcRn complexes prevents degradation and allows recycling and release of IgG [164]. Targeting the FcRn may offer a novel therapeutic opportunity for many autoimmune diseases, leading to reduced overall IgG and pathological autoantibody levels.
Efgartigimod is an IgG1-derived Fc-fragment that binds to FcRN and enhances the degradation of pathogenic IgG. There is a current international phase III study underway to assess the efficacy, safety, and tolerability of efgartigimod PH20 in BP (NCT05267600) [165]. The agent will be administered via subcutaneous injections in adult participants with moderate to severe BP [165].
Topical Therapy
The inflammasome, an innate immune signaling complex, has an important role in many inflammatory and autoimmune diseases. It has been demonstrated that mRNA levels of the nucleotide-binding domain, leucin-rich family protein 3 (NLRP3) inflammasome components (the NLRP3, pro-caspase-1 and pro-IL-18 axis) were significantly upregulated in peripheral blood mononuclear cells from BP patients, when compared with those from healthy controls, and were also positively correlated with disease activity [166]. AC-203 is a topical formulation of an oral modulator of inflammasome and IL-1β pathways. There is a randomized, open-label, controlled trial designed to test the safety, tolerability, efficacy, and pharmacokinetics of AC-203 ointment in subjects with BP in comparison with a topical corticosteroid representing standard of care (NCT03286582) [167].
Another phase III study (NCT05594472) was designed to assess the disinfectant and healing-promoting effect of ozonated olive oil in the treatment of BP and pemphigus vulgaris in comparison with treatment with topical antibiotics [168].
Janus Kinase Inhibition
Janus kinase (JAK) inhibitors are presently employed in the treatment of conditions such as rheumatoid arthritis and atopic dermatitis, and they hold promise for managing various other inflammatory skin conditions. However, their application in autoimmune bullous diseases has not been extensively explored.
In a recent case report, an 81-year-old woman diagnosed with BP showed inadequate response to prednisone treatment [169]. She was subsequently prescribed upadacitinib, a JAK inhibitor, at a daily dose of 15 mg. Her prednisone dosage was gradually reduced over 20 days. During her 2-month follow-up appointment, she had been off prednisolone for more than a month and was solely taking upadacitinib, achieving complete disease remission [169]. Since starting upadacitinib, the patient did not experience any new blister formations, and her itch had completely resolved [169]. A follow-up evaluation 3 months later (after 5 months of taking upadacitinib) demonstrated the continued effectiveness of the treatment, with no disease recurrence or flares. Importantly, the patient tolerated the medication well, experiencing no adverse events [169].
A recent case study featured an 83-year-old Chinese man who had a severe form of BP along with plaque psoriasis [170]. Considering his underlying health conditions, including stage III hypertension, and the risk of serious adverse effects associated with systemic corticosteroid treatment, he was prescribed oral baricitinib, another JAK inhibitor, at a daily dose of 4 mg [170]. Remarkably, after 12 weeks of treatment, there was a significant improvement in both skin lesions and itching [170]. At the 24-week follow-up, the patient achieved complete remission of both his bullous and psoriatic lesions without experiencing any adverse effects [170].
A different case study highlighted a 33-year-old man diagnosed with BP and concurrent psoriasis vulgaris who was treated with tofacitinib, a JAK inhibitor [171]. The report suggested that tofacitinib could potentially serve as a well-tolerated and effective treatment choice for individuals dealing with both BP and psoriasis [171].
Conclusions
The complex pathophysiology of BP indicates that the antibody-antigen binding activates simultaneously different molecular pathways, rather than a single cascade [172]. Recent studies have shed light on the important role of type 2 immunity (B cells, eosinophil-related molecules, IgE) and innate immunity (complement factors), indicating many new potential therapeutic targets (Table 1). Furthermore, a combination of different targeted therapies may be a promising way to reduce cumulative exposure to systemic corticosteroids [172]. For example, a combination of rituximab/dupilumab might effectively target the T-/B-cell crosstalk that leads to loss of tolerance against BP autoantigens [172]. On the other hand, a combination of anti-complement drugs and eosinophil-targeting therapies might be the most suitable way to block the effector phase of BP inflammation and pruritus [172].
The number of available therapeutic options is rapidly increasing, allowing us to move past the era of high-dose systemic corticosteroids or corticosteroid-sparing agents for the treatment of BP (Table 1). Further research is warranted to identify clinical (e.g. bullous vs. non-bullous phenotypes, pruritus intensity), laboratory (e.g. neutrophil-rich vs. eosinophil-rich infiltrates at histopathology, intensity of complement deposition at DIF), serum (e.g. IgG vs. IgE anti-BP180/BP230 antibodies) and molecular (e.g. cytokine concentration) biomarkers for therapy response and therapy selection [172].
Furthermore, there exists a significant imperative for meticulously planned clinical trials to be executed, aiming to shed more light on the effectiveness of emerging targeted therapies. The forthcoming research objective must revolve around crafting precise targeted treatments, preferably as standalone therapies, or in conjunction with minimal cumulative doses of systemic corticosteroids. This strategy aims to attain rapid remission, minimizing instances of relapse and reducing the extent of immunosuppression. This undertaking is of great importance in order to help patients find a relief from this very disabling ailment.
Declarations
Conflicts of Interest
Aikaterini Patsatsi declares honoraria as an advisor, speaker or investigator for AbbVie, Argenx, Janssen, Pfizer, Pharmaserv Lilly, Sanofi and UCB. Meropi Karakioulaki and Kilian Eyerich declare no conflicts of interest.
Funding
Open access funding provided by HEAL-Link Greece.
Author Contributions
Writing of the manuscript, contribution to the discussion, finalization of the manuscript and approval of the submitted article: MK, KE, AP. Conception of the topic, integrity and accuracy: AP.
Ethics Approval
Not applicable.
Patient Consent to Participate/Publish
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
- 1.van Beek N, et al. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol. 2016;12(3):267–277. doi: 10.1586/1744666X.2016.1123092. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 3.Bagci IS, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16(5):445–455. doi: 10.1016/j.autrev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Kridin K, Shihade W, Bergman R. Mortality in patients with bullous pemphigoid: a retrospective cohort study, systematic review and meta-analysis. Acta Derm Venereol. 2019;99(1):72–77. doi: 10.2340/00015555-2930. [DOI] [PubMed] [Google Scholar]
- 5.Kridin K, et al. Mortality in bullous pemphigoid: a systematic review and meta-analysis of standardized mortality ratios. J Dermatol. 2018;45(9):1094–1100. doi: 10.1111/1346-8138.14503. [DOI] [PubMed] [Google Scholar]
- 6.D'Agostino GM, et al. Bullous pemphygoid and novel therapeutic approaches. Biomedicines. 2022;10(11):2844. doi: 10.3390/biomedicines10112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyamoto D, et al. Bullous pemphigoid. An Bras Dermatol. 2019;94(2):133–146. doi: 10.1590/abd1806-4841.20199007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai ZT, et al. Diabetes mellitus and hyperglycemic complications in bullous pemphigoid. J Am Acad Dermatol. 2020;82(5):1234–1237. doi: 10.1016/j.jaad.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Kibsgaard L, et al. Increased frequency of multiple sclerosis among patients with bullous pemphigoid: a population-based cohort study on comorbidities anchored around the diagnosis of bullous pemphigoid. Br J Dermatol. 2017;176(6):1486–1491. doi: 10.1111/bjd.15405. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, et al. Association of bullous pemphigoid and comorbid health conditions: a case-control study. Arch Dermatol Res. 2021;313(5):327–332. doi: 10.1007/s00403-020-02100-2. [DOI] [PubMed] [Google Scholar]
- 11.Baum, S., et al., Prevalence, Spectrum and Clinical Implications of Malignancies in Patients with Bullous Pemphigoid. Acta Derm Venereol, 2023. 103:adv00888. [DOI] [PMC free article] [PubMed]
- 12.Salemme A, et al. Gliptin-associated bullous pemphigoid shows peculiar features of anti-BP180 and -BP230 humoral response: Results of a multicenter study. J Am Acad Dermatol. 2022;87(1):56–63. doi: 10.1016/j.jaad.2022.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl Peptidase-4 Inhibitor-Associated Bullous Pemphigoid. Front Immunol. 2019;10:1238. doi: 10.3389/fimmu.2019.01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afarideh M, Borucki R, Werth VP. A review of the immunologic pathways involved in bullous pemphigoid and novel therapeutic targets. J Clin Med. 2022;11(10):2856. doi: 10.3390/jcm11102856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasperkiewicz M, et al. COVID-19 pandemic and autoimmune bullous diseases: a cross-sectional study of the International Pemphigus and Pemphigoid Foundation. J Eur Acad Dermatol Venereol. 2021;35(7):e418–e421. doi: 10.1111/jdv.17228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maronese CA, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian Multicentre Study. Front Med (Lausanne) 2022;9:841506. doi: 10.3389/fmed.2022.841506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. 2007;33(1–2):67–77. doi: 10.1007/s12016-007-0030-y. [DOI] [PubMed] [Google Scholar]
- 18.Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol. 2016;11:175–197. doi: 10.1146/annurev-pathol-012615-044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genovese G, et al. New insights into the pathogenesis of bullous pemphigoid: 2019 update. Front Immunol. 2019;10:1506. doi: 10.3389/fimmu.2019.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt E, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136(2):174–178. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- 21.Iwata Y, et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol. 2008;144(1):41–48. doi: 10.1001/archdermatol.2007.9. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, et al. Bullous pemphigoid: The role of type 2 inflammation in its pathogenesis and the prospect of targeted therapy. Front Immunol. 2023;14:1115083. doi: 10.3389/fimmu.2023.1115083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saniklidou AH, et al. IgE autoantibodies and their association with the disease activity and phenotype in bullous pemphigoid: a systematic review. Arch Dermatol Res. 2018;310(1):11–28. doi: 10.1007/s00403-017-1789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice JB, et al. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–2229. doi: 10.1016/j.clinthera.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Joly P, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346(5):321–327. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 26.Feliciani C, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172(4):867–877. doi: 10.1111/bjd.13717. [DOI] [PubMed] [Google Scholar]
- 27.Zeng FAP, et al. Side effects of steroid-sparing agents in patients with bullous pemphigoid and pemphigus: a systematic review. JAAD Int. 2022;9:33–43. doi: 10.1016/j.jdin.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bystryn JC. Comparative effectiveness of azathioprine or mycophenolate mofetil as an adjuvant for the treatment of bullous pemphigoid. Arch Dermatol. 2008;144(7):946. doi: 10.1001/archderm.144.7.946-a. [DOI] [PubMed] [Google Scholar]
- 29.Fivenson DP, et al. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch Dermatol. 1994;130(6):753–758. [PubMed] [Google Scholar]
- 30.Wojtczak M, et al. Can methotrexate be employed as monotherapy for bullous pemphigoid? Analysis of efficiency and tolerance of methotrexate treatment in patients with bullous pemphigoid. J Clin Med. 2023;12(4):1638. doi: 10.3390/jcm12041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du-Thanh A, et al. Combined treatment with low-dose methotrexate and initial short-term superpotent topical steroids in bullous pemphigoid: an open, multicentre, retrospective study. Br J Dermatol. 2011;165(6):1337–1343. doi: 10.1111/j.1365-2133.2011.10531.x. [DOI] [PubMed] [Google Scholar]
- 32.Bouscarat F, et al. Treatment of bullous pemphigoid with dapsone: retrospective study of thirty-six cases. J Am Acad Dermatol. 1996;34(4):683–684. doi: 10.1016/s0190-9622(96)80085-5. [DOI] [PubMed] [Google Scholar]
- 33.Barthelemy H, et al. Cyclosporin in the treatment of bullous pemphigoid: preliminary study. Ann Dermatol Venereol. 1986;113(4):309–313. [PubMed] [Google Scholar]
- 34.Kremer N, et al. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. Am J Clin Dermatol. 2019;20(2):209–216. doi: 10.1007/s40257-018-0401-6. [DOI] [PubMed] [Google Scholar]
- 35.Garrido PM, et al. Emerging treatments for bullous pemphigoid. J Dermatolog Treat. 2022;33(2):649–661. doi: 10.1080/09546634.2020.1782325. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107(10):4658–4663. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas RM, Colon A, Motaparthi K. Rituximab in autoimmune pemphigoid diseases: indications, optimized regimens, and practice gaps. Clin Dermatol. 2020;38(3):384–396. doi: 10.1016/j.clindermatol.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 38.Amber KT, et al. Targeted therapies for autoimmune bullous diseases: current status. Drugs. 2018;78(15):1527–1548. doi: 10.1007/s40265-018-0976-5. [DOI] [PubMed] [Google Scholar]
- 39.Berkani N, et al. B-cell depletion induces a shift in self antigen specific B-cell repertoire and cytokine pattern in patients with bullous pemphigoid. Sci Rep. 2019;9(1):3525. doi: 10.1038/s41598-019-40203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polansky M, et al. Rituximab therapy in patients with bullous pemphigoid: a retrospective study of 20 patients. J Am Acad Dermatol. 2019;81(1):179–186. doi: 10.1016/j.jaad.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 41.Tovanabutra N, Payne AS. Clinical outcome and safety of rituximab therapy for pemphigoid diseases. J Am Acad Dermatol. 2020;82(5):1237–1239. doi: 10.1016/j.jaad.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Hall RP, 3rd, et al. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. J Invest Dermatol. 2013;133(12):2786–2788. doi: 10.1038/jid.2013.236. [DOI] [PubMed] [Google Scholar]
- 43.Papara C, et al. The relevance of complement in pemphigoid diseases: a critical appraisal. Front Immunol. 2022;13:973702. doi: 10.3389/fimmu.2022.973702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards G, et al. Complement activation in autoimmune bullous dermatoses: a comprehensive review. Front Immunol. 2019;10:1477. doi: 10.3389/fimmu.2019.01477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiorean RM, et al. Complement-activating capacity of autoantibodies correlates with disease activity in bullous pemphigoid patients. Front Immunol. 2018;9:2687. doi: 10.3389/fimmu.2018.02687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallet-Faber N, et al. Epidermolysis bullosa acquisita following bullous pemphigoid, successfully treated with the anti-CD20 monoclonal antibody rituximab. Dermatology. 2007;215(3):252–255. doi: 10.1159/000106585. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q, et al. Three cases of refractory bullous pemphigoid in the elderly treated successfully with ultra-low-dose rituximab. J Dermatol. 2023;50(4):561–564. doi: 10.1111/1346-8138.16668. [DOI] [PubMed] [Google Scholar]
- 48.Suarez-Carantona C, et al. Low-dose rituximab for bullous pemphigoid. Protocol and single-center experience. Actas Dermosifiliogr. 2023;114(1):T62–T68. doi: 10.1016/j.ad.2021.10.024. [DOI] [PubMed] [Google Scholar]
- 49.Cho YT, Chu CY, Wang LF. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br J Dermatol. 2015;173(1):302–304. doi: 10.1111/bjd.13633. [DOI] [PubMed] [Google Scholar]
- 50.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135(3):626–635. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337(6093):431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eyerich K, Eyerich S. Immune response patterns in non-communicable inflammatory skin diseases. J Eur Acad Dermatol Venereol. 2018;32(5):692–703. doi: 10.1111/jdv.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang H, Li Q, Wang G. The role of T cells in pemphigus vulgaris and bullous pemphigoid. Autoimmun Rev. 2020;19(11):102661. doi: 10.1016/j.autrev.2020.102661. [DOI] [PubMed] [Google Scholar]
- 54.Tavakolpour S. Dupilumab: a revolutionary emerging drug in atopic dermatitis and its possible role in pemphigus. Dermatol Ther. 2016;29(5):299. doi: 10.1111/dth.12327. [DOI] [PubMed] [Google Scholar]
- 55.Takamura S, Teraki Y. Treatment of bullous pemphigoid with dupilumab: Dupilumab exerts its effect by primarily suppressing T-helper 2 cytokines. J Dermatol. 2022;49(9):845–850. doi: 10.1111/1346-8138.16428. [DOI] [PubMed] [Google Scholar]
- 56.Feliciani C, et al. A Th2-like cytokine response is involved in bullous pemphigoid. The role of IL-4 and IL-5 in the pathogenesis of the disease. Int J Immunopathol Pharmacol. 1999;12(2):55–61. [PubMed] [Google Scholar]
- 57.Giomi B, et al. Th1, Th2 and Th3 cytokines in the pathogenesis of bullous pemphigoid. J Dermatol Sci. 2002;30(2):116–128. doi: 10.1016/s0923-1811(02)00067-1. [DOI] [PubMed] [Google Scholar]
- 58.Pickford WJ, et al. T cell participation in autoreactivity to NC16a epitopes in bullous pemphigoid. Clin Exp Immunol. 2015;180(2):189–200. doi: 10.1111/cei.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Messingham KN, et al. Human eosinophils express the high affinity IgE receptor, FcepsilonRI, in bullous pemphigoid. PLoS ONE. 2014;9(9):e107725. doi: 10.1371/journal.pone.0107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cozzani E, et al. Immunoglobulin E and bullous pemphigoid. Eur J Dermatol. 2018;28(4):440–448. doi: 10.1684/ejd.2018.3366. [DOI] [PubMed] [Google Scholar]
- 61.Gounni Abdelilah S, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. Role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120(2):220–231. doi: 10.1016/j.clim.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 62.Gooderham MJ, et al. Dupilumab: A review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 Suppl 1):S28–S36. doi: 10.1016/j.jaad.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Abdat R, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83(1):46–52. doi: 10.1016/j.jaad.2020.01.089. [DOI] [PubMed] [Google Scholar]
- 64.Yang J, et al. Dupilumab combined with low-dose systemic steroid therapy improves efficacy and safety for bullous pemphigoid. Dermatol Ther. 2022;35(8):e15648. doi: 10.1111/dth.15648. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, et al. Efficacy and safety of dupilumab in moderate-to-severe bullous pemphigoid. Front Immunol. 2021;12:738907. doi: 10.3389/fimmu.2021.738907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seyed Jafari SM, et al. Case Report: Combination of Omalizumab and Dupilumab for Recalcitrant Bullous Pemphigoid. Front Immunol. 2020;11:611549. doi: 10.3389/fimmu.2020.611549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruni M, et al. A case of nivolumab-induced bullous pemphigoid successfully treated with dupilumab. Dermatol Online J. 2022;28(2). [DOI] [PubMed]
- 68.Pop SR, Strock D, Smith RJ. Dupilumab for the treatment of pembrolizumab-induced bullous pemphigoid: a case report. Dermatol Ther. 2022;35(8):e15623. doi: 10.1111/dth.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao L, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol. 2023;159(9):953–960. doi: 10.1001/jamadermatol.2023.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.US National Library of Medicine, ClinicalTrials.gov. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy and Safety of Dupilumab in Adult Patients with Bullous Pemphigoid, NCT04206553. 2019. Available at: https://clinicaltrials.gov/ct2/show/NCT04206553.
- 71.Pruessmann J, et al. Immunomodulator galectin-9 is increased in blood and skin of patients with bullous pemphigoid. Acta Derm Venereol. 2021;101(3):adv00419. doi: 10.2340/00015555-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engmann J, et al. increased activity and apoptosis of eosinophils in blister fluids, skin and peripheral blood of patients with bullous pemphigoid. Acta Derm Venereol. 2017;97(4):464–471. doi: 10.2340/00015555-2581. [DOI] [PubMed] [Google Scholar]
- 73.Gore Karaali M, et al. Tissue eosinophil levels as a marker of disease severity in bullous pemphigoid. Australas J Dermatol. 2021;62(2):e236–e241. doi: 10.1111/ajd.13547. [DOI] [PubMed] [Google Scholar]
- 74.Kridin K. Peripheral eosinophilia in bullous pemphigoid: prevalence and influence on the clinical manifestation. Br J Dermatol. 2018;179(5):1141–1147. doi: 10.1111/bjd.16679. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y, et al. Factors associated with the activity and severity of bullous pemphigoid: a review. Ann Med. 2020;52(3–4):55–62. doi: 10.1080/07853890.2020.1742367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park SH, et al. Circulating eosinophil and neutrophil counts correlate with disease severity in bullous pemphigoid. Ann Dermatol. 2018;30(5):544–549. doi: 10.5021/ad.2018.30.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frezzolini A, et al. Increased expression of eotaxin and its specific receptor CCR3 in bullous pemphigoid. Eur J Dermatol. 2002;12(1):27–31. [PubMed] [Google Scholar]
- 78.Shrikhande M, et al. Increased coexpression of eotaxin and interleukin 5 in bullous pemphigoid. Acta Derm Venereol. 2000;80(4):277–280. doi: 10.1080/000155500750012162. [DOI] [PubMed] [Google Scholar]
- 79.Wakugawa M, et al. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: correlation with tissue eosinophilia. Br J Dermatol. 2000;143(1):112–116. doi: 10.1046/j.1365-2133.2000.03599.x. [DOI] [PubMed] [Google Scholar]
- 80.Gunther C, et al. Up-regulation of CCL11 and CCL26 is associated with activated eosinophils in bullous pemphigoid. Clin Exp Immunol. 2011;166(2):145–153. doi: 10.1111/j.1365-2249.2011.04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amber KT, et al. Mechanisms of pathogenic effects of eosinophil cationic protein and eosinophil-derived neurotoxin on human keratinocytes. Exp Dermatol. 2018;27(12):1322–1327. doi: 10.1111/exd.13782. [DOI] [PubMed] [Google Scholar]
- 82.Kelly EA, et al. Potent synergistic effect of IL-3 and TNF on matrix metalloproteinase 9 generation by human eosinophils. Cytokine. 2012;58(2):199–206. doi: 10.1016/j.cyto.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Z, et al. A critical role for neutrophil elastase in experimental bullous pemphigoid. J Clin Invest. 2000;105(1):113–123. doi: 10.1172/JCI3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okada S, et al. Transmigration of eosinophils through basement membrane components in vitro: synergistic effects of platelet-activating factor and eosinophil-active cytokines. Am J Respir Cell Mol Biol. 1997;16(4):455–463. doi: 10.1165/ajrcmb.16.4.9115757. [DOI] [PubMed] [Google Scholar]
- 85.Rudrich U, et al. Eosinophils are a major source of interleukin-31 in bullous pemphigoid. Acta Derm Venereol. 2018;98(8):766–771. doi: 10.2340/00015555-2951. [DOI] [PubMed] [Google Scholar]
- 86.US National Library of Medicine, ClinicalTrials.gov. Evaluation of Safety, Efficacy and Pharmacodynamic Effect of Bertilimumab in Patients With Bullous Pemphigoid. 2018; Available at: https://clinicaltrials.gov/study/NCT02226146.
- 87.US National Library of Medicine, ClinicalTrials.gov. A Study to Assess the Therapeutic Effect and Safety of Adjunctive AKST4290 in Subjects With Bullous Pemphigoid. 2023. Available at: https://clinicaltrials.gov/study/NCT04499235?a=4.
- 88.Keam SJ. Nemolizumab: FIRST APPROVAL. Drugs. 2022;82(10):1143–1150. doi: 10.1007/s40265-022-01741-z. [DOI] [PubMed] [Google Scholar]
- 89.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277(5334):2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 90.Stirling RG, et al. Interleukin-5 induces CD34(+) eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1403–1409. doi: 10.1164/ajrccm.164.8.2010002. [DOI] [PubMed] [Google Scholar]
- 91.D'Auria L, et al. IL-5 levels in the serum and blister fluid of patients with bullous pemphigoid: correlations with eosinophil cationic protein, RANTES, IgE and disease severity. Arch Dermatol Res. 1998;290(1–2):25–27. doi: 10.1007/s004030050272. [DOI] [PubMed] [Google Scholar]
- 92.Bettiol A, et al. Mepolizumab for eosinophilic granulomatosis with polyangiitis: a European multicenter observational study. Arthritis Rheumatol. 2022;74(2):295–306. doi: 10.1002/art.41943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han JK, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9(10):1141–1153. doi: 10.1016/S2213-2600(21)00097-7. [DOI] [PubMed] [Google Scholar]
- 94.Ortega HG, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 95.Pavord ID, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 96.Wechsler ME, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simon D, et al. Mepolizumab failed to affect bullous pemphigoid: a randomized, placebo-controlled, double-blind phase 2 pilot study. Allergy. 2020;75(3):669–672. doi: 10.1111/all.13950. [DOI] [PubMed] [Google Scholar]
- 98.Castro M, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 99.Deeks ED, Brusselle G. Reslizumab in eosinophilic asthma: a review. Drugs. 2017;77(7):777–784. doi: 10.1007/s40265-017-0740-2. [DOI] [PubMed] [Google Scholar]
- 100.Markham A. Reslizumab: first global approval. Drugs. 2016;76(8):907–911. doi: 10.1007/s40265-016-0583-2. [DOI] [PubMed] [Google Scholar]
- 101.Rhyou HI, Han SH, Nam YH. Successful induction treatment of bullous pemphigoid using reslizumab: a case report. Allergy Asthma Clin Immunol. 2021;17(1):117. doi: 10.1186/s13223-021-00619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bagnasco D, et al. Anti-interleukin 5 (IL-5) and IL-5Ra biological drugs: efficacy, safety, and future perspectives in severe eosinophilic asthma. Front Med (Lausanne) 2017;4:135. doi: 10.3389/fmed.2017.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.FitzGerald JM, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 104.US National Library of Medicine, ClinicalTrials.gov. A Study to Investigate the Use of Benralizumab in Patients With Bullous Pemphigoid. (FJORD). NCT04612790. 2023. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT04612790.
- 105.Dresow SK, et al. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol. 2009;160(2):429–432. doi: 10.1111/j.1365-2133.2008.08858.x. [DOI] [PubMed] [Google Scholar]
- 106.Provost TT, Tomasi Jr. TB. Immunopathology of bullous pemphigoid Basement membrane deposition of IgE, alternate pathway components and fibrin. Clin Exp Immunol. 1974;18(2):193–200. [PMC free article] [PubMed] [Google Scholar]
- 107.Messingham KA, et al. Functional characterization of an IgE-class monoclonal antibody specific for the bullous pemphigoid autoantigen, BP180. Hybridoma (Larchmt) 2012;31(2):111–117. doi: 10.1089/hyb.2011.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kalowska M, et al. Enzyme-linked immunoassay index for anti-NC16a IgG and IgE auto-antibodies correlates with severity and activity of bullous pemphigoid. Acta Derm Venereol. 2016;96(2):191–196. doi: 10.2340/00015555-2101. [DOI] [PubMed] [Google Scholar]
- 109.Kamata A, et al. Basement membrane zone IgE deposition is associated with bullous pemphigoid disease severity and treatment results. Br J Dermatol. 2020;182(5):1221–1227. doi: 10.1111/bjd.18364. [DOI] [PubMed] [Google Scholar]
- 110.van Beek N, et al. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol. 2017;153(1):30–38. doi: 10.1001/jamadermatol.2016.3357. [DOI] [PubMed] [Google Scholar]
- 111.Fania L, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143(3):236–245. doi: 10.1016/j.clim.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 112.Hashimoto T, et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol. 2017;177(1):141–151. doi: 10.1111/bjd.15114. [DOI] [PubMed] [Google Scholar]
- 113.Cozzani E, et al. Anti-230 kDa circulating IgE in bullous pemphigoid: relationship with disease activity. Acta Derm Venereol. 1997;77(3):236. doi: 10.2340/0001555577236. [DOI] [PubMed] [Google Scholar]
- 114.Ishiura N, et al. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2008;49(2):153–161. doi: 10.1016/j.jdermsci.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 115.Shih YC, et al. BP230 IgE autoantibodies in topical-steroid-resistant bullous pemphigoid. J Dermatol. 2021;48(9):1372–1380. doi: 10.1111/1346-8138.15952. [DOI] [PubMed] [Google Scholar]
- 116.Kawakami T, Blank U. From IgE to Omalizumab. J Immunol. 2016;197(11):4187–4192. doi: 10.4049/jimmunol.1601476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balakirski G, et al. Successful treatment of bullous pemphigoid with omalizumab as corticosteroid-sparing agent: report of two cases and review of literature. J Eur Acad Dermatol Venereol. 2016;30(10):1778–1782. doi: 10.1111/jdv.13758. [DOI] [PubMed] [Google Scholar]
- 118.James T, et al. IgE blockade in autoimmunity: Omalizumab induced remission of bullous pemphigoid. Clin Immunol. 2019;198:54–56. doi: 10.1016/j.clim.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 119.Yu KK, et al. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71(3):468–474. doi: 10.1016/j.jaad.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sarrazin M, Jouen F, Duvert-Lehembre S. Refractory bullous pemphigoid with IgE anti-BP230 and IgG anti-p200 antibodies successfully treated with omalizumab. Ann Dermatol Venereol. 2021;148(1):60–62. doi: 10.1016/j.annder.2020.08.053. [DOI] [PubMed] [Google Scholar]
- 121.De D, et al. Omalizumab: an underutilized treatment option in bullous pemphigoid patients with co-morbidities. J Eur Acad Dermatol Venereol. 2021;35(7):e469–e472. doi: 10.1111/jdv.17229. [DOI] [PubMed] [Google Scholar]
- 122.Gonul MZ, et al. Bullous pemphigoid successfully treated with omalizumab. Indian J Dermatol Venereol Leprol. 2016;82(5):577–579. doi: 10.4103/0378-6323.183628. [DOI] [PubMed] [Google Scholar]
- 123.London VA, et al. Successful treatment of bullous pemphigoid with omalizumab. Arch Dermatol. 2012;148(11):1241–1243. doi: 10.1001/archdermatol.2012.1604. [DOI] [PubMed] [Google Scholar]
- 124.Dufour C, et al. Successful management of severe infant bullous pemphigoid with omalizumab. Br J Dermatol. 2012;166(5):1140–1142. doi: 10.1111/j.1365-2133.2011.10748.x. [DOI] [PubMed] [Google Scholar]
- 125.Maglie R, et al. Dramatic exacerbation of bullous pemphigoid following rituximab and successful treatment with omalizumab. Eur J Dermatol. 2019;29(2):213–215. doi: 10.1684/ejd.2019.3499. [DOI] [PubMed] [Google Scholar]
- 126.Seyed Jafari SM, et al. Effects of omalizumab on FcepsilonRI and IgE expression in lesional skin of bullous pemphigoid. Front Immunol. 2019;10:1919. doi: 10.3389/fimmu.2019.01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chebani R, et al. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol. 2023;ljad369. [DOI] [PubMed]
- 128.Garrido PM, et al. Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid efficiently treated with omalizumab. Dermatol Ther. 2020;33(6):e14160. doi: 10.1111/dth.14160. [DOI] [PubMed] [Google Scholar]
- 129.Vico-Alonso C, et al. Omalizumab as an alternative therapeutic tool in the treatment of bullous pemphigoid: a case report. Dermatol Ther. 2019;32(2):e12829. doi: 10.1111/dth.12829. [DOI] [PubMed] [Google Scholar]
- 130.US National Library of Medicine, ClinicalTrials.gov. An Open-Label Study to Evaluate the Efficacy and Safety of Rituximab Combined with Omalizumab in Patients with Bullous Pemphigoid, NCT04128176. 2019. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT04128176.
- 131.Beek NV, Zillikens D, Schmidt E. Bullous Autoimmune Dermatoses-Clinical Features, Diagnostic Evaluation, and Treatment Options. Dtsch Arztebl Int. 2021;118(24):413–420. doi: 10.3238/arztebl.m2021.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iwata H, Kitajima Y. Bullous pemphigoid: role of complement and mechanisms for blister formation within the lamina lucida. Exp Dermatol. 2013;22(6):381–385. doi: 10.1111/exd.12146. [DOI] [PubMed] [Google Scholar]
- 133.Lessey E, et al. Complement and cutaneous autoimmune blistering diseases. Immunol Res. 2008;41(3):223–232. doi: 10.1007/s12026-008-8028-y. [DOI] [PubMed] [Google Scholar]
- 134.Sadik CD, et al. The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin. Semin Immunol. 2018;37:21–29. doi: 10.1016/j.smim.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 135.Sezin T, et al. Dual inhibition of complement factor 5 and leukotriene B4 synergistically suppresses murine pemphigoid disease. JCI Insight. 2019;4(15):e128239. doi: 10.1172/jci.insight.128239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.DiScipio RG, Schraufstatter IU. The role of the complement anaphylatoxins in the recruitment of eosinophils. Int Immunopharmacol. 2007;7(14):1909–1923. doi: 10.1016/j.intimp.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 137.Zeck-Kapp G, et al. Mechanisms of human eosinophil activation by complement protein C5a and platelet-activating factor: similar functional responses are accompanied by different morphologic alterations. Allergy. 1995;50(1):34–47. doi: 10.1111/j.1398-9995.1995.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 138.Karsten CM, et al. Tissue destruction in bullous pemphigoid can be complement independent and may be mitigated by C5aR2. Front Immunol. 2018;9:488. doi: 10.3389/fimmu.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mihai S, et al. Specific inhibition of complement activation significantly ameliorates autoimmune blistering disease in mice. Front Immunol. 2018;9:535. doi: 10.3389/fimmu.2018.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sadik CD, et al. Evaluation of nomacopan for treatment of bullous pemphigoid: a phase 2a nonrandomized controlled trial. JAMA Dermatol. 2022;158(6):641–649. doi: 10.1001/jamadermatol.2022.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.US National Library of Medicine, ClinicalTrials.gov. Nomacopan Therapy in Adult Patients With Bullous Pemphigoid Receiving Adjunct Oral Corticosteroid Therapy (ARREST-BP) (ARREST-BP). 2021. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT05061771.
- 142.US National Library of Medicine, ClinicalTrials.gov. Treatment of Bullous Pemphigoid With Avdoralimab (IPH5401), an Anti-C5aR1 Monoclonal Antibody (IPH). 2020. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT04563923.
- 143.Freire, P.C., et al., Specific Inhibition of the Classical Complement Pathway Prevents C3 Deposition along the Dermal-Epidermal Junction in Bullous Pemphigoid. J Invest Dermatol, 2019. 139(12):2417-2424 e2. [DOI] [PubMed]
- 144.Dias PM, Banerjee G. The role of Th17/IL-17 on eosinophilic inflammation. J Autoimmun. 2013;40:9–20. doi: 10.1016/j.jaut.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 145.Holtsche MM, et al. Adjuvant treatment with secukinumab induced long term remission in a patient with severe bullous pemphigoid. J Dtsch Dermatol Ges. 2020;18(12):1478–1480. doi: 10.1111/ddg.14291. [DOI] [PubMed] [Google Scholar]
- 146.Kamata M, et al. Secukinumab decreased circulating anti-BP180-NC16a autoantibodies in a patient with coexisting psoriasis vulgaris and bullous pemphigoid. J Dermatol. 2019;46(6):e216–e217. doi: 10.1111/1346-8138.14760. [DOI] [PubMed] [Google Scholar]
- 147.Yun JS, et al. Successful secukinumab treatment of active bullous pemphigoid and chronic severe psoriasis: a case report. Australas J Dermatol. 2022;63(2):e155–e158. doi: 10.1111/ajd.13803. [DOI] [PubMed] [Google Scholar]
- 148.Ho PH, Tsai TF. Development of bullous pemphigoid during secukinumab treatment for psoriasis. J Dermatol. 2017;44(9):e220–e221. doi: 10.1111/1346-8138.13909. [DOI] [PubMed] [Google Scholar]
- 149.Lu L, et al. Incidental amelioration of bullous pemphigoid during ixekizumab treatment for psoriasis. J Dermatol. 2022;49(1):e13–e15. doi: 10.1111/1346-8138.16189. [DOI] [PubMed] [Google Scholar]
- 150.US National Library of Medicine, ClinicalTrials.gov. Ixekizumab in the Treatment of Bullous Pemphigoid. 2020. Available at: https://clinicaltrials.gov/study/NCT03099538?tab=results.
- 151.Gaffen SL, et al. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116(5):1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Delli FS, et al. Total IgE, eosinophils, and interleukins 16, 17A, and 23 correlations in severe bullous pemphigoid and treatment implications. Dermatol Ther. 2020;33(6):e13958. doi: 10.1111/dth.13958. [DOI] [PubMed] [Google Scholar]
- 154.Plee J, et al. Integrating longitudinal serum IL-17 and IL-23 follow-up, along with autoantibodies variation, contributes to predict bullous pemphigoid outcome. Sci Rep. 2015;5:18001. doi: 10.1038/srep18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.US National Library of Medicine, ClinicalTrials.gov. The Effects of Tildrakizumab in Treatment of Bullous Pemphigoid. 2020. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT04465292.
- 156.Cole C, Amber KT. Two patients with concomitant bullous pemphigoid and psoriasis successfully treated by IL-23 inhibition. J Eur Acad Dermatol Venereol. 2023;37(11):e1339–e1340. doi: 10.1111/jdv.19317. [DOI] [PubMed] [Google Scholar]
- 157.Majima Y, et al. A successful treatment with ustekinumab in a case of antilaminin-gamma1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168(6):1367–1369. doi: 10.1111/bjd.12163. [DOI] [PubMed] [Google Scholar]
- 158.Loget J, et al. A successful treatment with ustekinumab in a case of relapsing bullous pemphigoid associated with psoriasis. J Eur Acad Dermatol Venereol. 2017;31(5):e228–e230. doi: 10.1111/jdv.14002. [DOI] [PubMed] [Google Scholar]
- 159.Marin M, et al. Bullous pemphigoid induced by ustekinumab: a case report. Eur J Hosp Pharm. 2021;28(1):47–49. doi: 10.1136/ejhpharm-2018-001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Le Guern A, et al. Bullous pemphigoid during ustekinumab therapy. JAAD Case Rep. 2015;1(6):359–360. doi: 10.1016/j.jdcr.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.US National Library of Medicine, ClinicalTrials.gov. Efficacy and Safety of Ustekinumab in Bullous Pemphigoid (PB-USTE). 2019; Available at: https://classic.clinicaltrials.gov/ct2/show/NCT04117932.
- 162.Didona D, et al. Pemphigus Vulgaris: Present and Future Therapeutic Strategies. Dermatol Pract Concept. 2022;12(1):e2022037. doi: 10.5826/dpc.1201a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93(11):5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Goebl NA, et al. Neonatal Fc receptor mediates internalization of Fc in transfected human endothelial cells. Mol Biol Cell. 2008;19(12):5490–5505. doi: 10.1091/mbc.E07-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.US National Library of Medicine, ClinicalTrials.gov. A Phase 2/3 Study of Efgartigimod PH20 SC in Adult Participants With Bullous Pemphigoid (BALLAD). 2023. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT05267600.
- 166.Fang H, et al. Increased expression of NLRP3 inflammasome components and interleukin-18 in patients with bullous pemphigoid. J Dermatol Sci. 2016;83(2):116–123. doi: 10.1016/j.jdermsci.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 167.US National Library of Medicine, ClinicalTrials.gov. A Proof-of-Concept Study of Topical AC-203 in Patients With Bullous Pemphigoid. 2019; Available at: https://clinicaltrials.gov/study/NCT03286582.
- 168.US National Library of Medicine, ClinicalTrials.gov. Ozonated Olive Oil in Treatment of Pemphigus Vulgaris and Bullous Pemphigoid. NCT05594472. 2022 Available at: https://clinicaltrials.gov/study/NCT05594472.
- 169.Nash D, Kirchhof MG. Bullous pemphigoid treated with Janus kinase inhibitor upadacitinib. JAAD Case Rep. 2023;32:81–83. doi: 10.1016/j.jdcr.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xiao Y, Xiang H, Li W. Concurrent bullous pemphigoid and plaque psoriasis successfully treated with Janus kinase inhibitor Baricitinib. Dermatol Ther. 2022;35(10):e15754. doi: 10.1111/dth.15754. [DOI] [PubMed] [Google Scholar]
- 171.Li H, et al. Concurrent bullous pemphigoid and psoriasis vulgaris successfully treated with Janus kinase inhibitor tofacitinib: a case report and review of the literature. Int Immunopharmacol. 2023;122:110591. doi: 10.1016/j.intimp.2023.110591. [DOI] [PubMed] [Google Scholar]
- 172.Maglie R, et al. The cytokine milieu of bullous pemphigoid: Current and novel therapeutic targets. Front Med (Lausanne) 2023;10:1128154. doi: 10.3389/fmed.2023.1128154. [DOI] [PMC free article] [PubMed] [Google Scholar]