Abstract
Aims
This study examines how family-related factors influence the management of children and adolescents with type 1 diabetes (T1DM). We investigate the relationship between family patterns, parental work schedules and metabolic control.
Materials and methods
We analysed data from a nationwide diabetes survey (DPV) focusing on HbA1c, severe hypoglycaemia, diabetic ketoacidosis, hospital admissions and inpatient treatment duration. We used linear regression and negative binomial regression models. Our study includes 15,340 children under the age of 18 with data on family structure and parental division of labour.
Results
Children from two-parent households have better HbA1c outcomes than children from single-parent, blended or no-parent households (p < .0001). Higher HbA1C levels are associated with children living with an unemployed father, as opposed to those with full-time working parents or with a full-time working father and a part-time working mother (p < .001).
Conclusions
These findings emphasise the importance of carefully considering family structure and working time models in the management of paediatric T1DM. Our results highlight risk factors within the family environment and emphasise the need for family-focused counselling of high-risk patients or severe cases in clinical practice.
Keywords: T1DM, family structure, parents’ working time models, German Diabetes Prospective Follow-up Registry (DPV), family psychosomatics, adherence
Introduction
Type 1 diabetes mellitus (T1DM) as a chronic metabolic disease with an autoimmune aetiology and an increasing incidence poses a challenge for the continuous monitoring of patients’ health status to ensure a positive long-term prognosis. In this paper, we study the interrelation of family forms and division of labour between caregivers and the corresponding metabolic adjustment for T1DM-affected children and adolescents. Socioeconomic status and family functionality play an essential role in the effectiveness of T1DM-treatment [1]. Thus, psychosocial factors should be considered right from the start and during the entire treatment of T1DM, as they are known prognostic factors for the maintenance of physical health [2].
From a perspective of family sociology, family arrangements and lifestyles have changed profoundly over the last decades; modern families are gaining more structural complexity due to frequent separations, divorces and re-marriages. Hence, the proportion of new family constellations like one biological parent with their partner, one-parent families with single mothers, and rainbow-families has increased [3]. Baechle et al. [4] described ‘non-classical’ family structures being associated with substantially poorer outcomes for T1DM-related treatment parameters. Furthermore, adolescents living with one parent and having a low socioeconomic status (SES) are more susceptible to a reduced 'health-related quality of life' [5]. An Italian study ascertained a correlation between living with one parent and worse glycaemic control in children with T1DM [6].
As well as contemporary family models, women’s emancipation and double career projects in couples have also made a difference to family functioning. The ‘traditional breadwinner-homemaker arrangement’ has become rarer [7]. It is known from Norway that living in families with both biological parents and a high SES (including education and employment) is associated with better T1DM parameters [8].
Based on these findings, this study analyses the interrelation between family structure and working time models of both parents or workload of a single parent. We hypothesize that the psychosocial risk of living in a one-parent household results in higher HbA1c parameters, higher rates of severe hypoglycaemia (SH), diabetic ketoacidosis (DKA) and longer inpatient treatment. Conversely, a full-time working father in a two-parents household is expected to predict a lower HbA1c, lower rates of hospital admission, SH and DKA.
Materials and methods
Data and participants
The current study is based on data from the multicentre German, Austrian, Swiss, and Luxembourgian Diabetes Prospective Follow-up Registry (i.e., Diabetes-Patienten-Verlaufsdokumentation [DPV]) [9]. This study, ‘Family structure and adherence of type I diabetes mellitus’, received approval from the Ethics Committee in October 2020 (Vote #185/20, department of medicine Justus-Liebig University Gießen). As of August 2023, 490 diabetes centres (hospitals and outpatient centres) and 601 200 patients with diabetes are included in the DPV registry. The data are pseudonymized and transmitted for central plausibility checks and analyses to the Institute of Epidemiology and Medical Biometry of Ulm University (Ulm, Germany). After reporting inconsistent data back to participating centres for validation and correction, the data are then completely anonymised prior to analysis.
Out of the DPV registry, patients who first presented with diabetes before 2000 or after 2018 were excluded (Fig. 1). We included patients with a clinical diagnosis of T1DM between six months and 18 years at the time of diagnosis with information on family structure, parents’ employment characteristics and HbA1c. We included the data of 15,340 patients from 301 diabetes centres in our study sample (286 German centres, 14 Austrian centres, 1 Luxembourgian centre). The data pool was aggregated for a 2-year period prior and following the last psychosocial data collection.
Fig. 1.
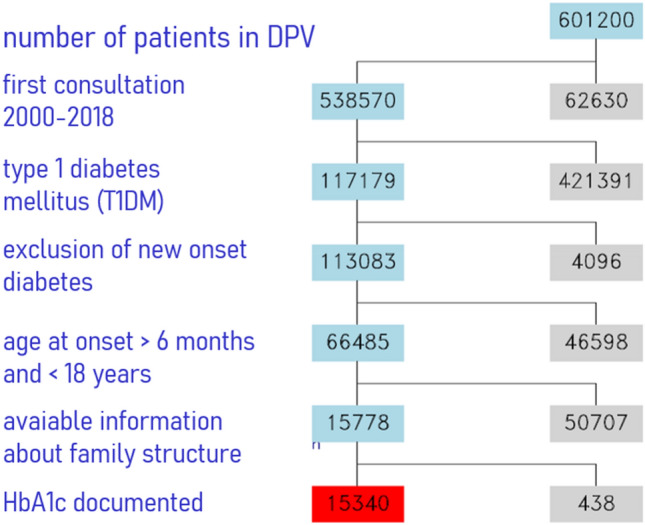
Selection of the Study Population in DPV (Diabetes Patient Follow-up Registry) (grey: excluded, blue: retained, red: final collective of patients) (color figure online)
Variables
Demographic data included age, age at diabetes onset, duration of diabetes, sex, and migratory background (patient or at least one parent born outside Germany, Austria, Switzerland, or Luxembourg).
Clinical parameters were evaluated 2 years prior and following the last psychosocial entry. They included body-mass index standard deviation score (BMI SDS), the daily dose of insulin (IE/kg), the use of continuous or flash glucose monitoring, the type of insulin therapy (multiple daily injections or insulin pump treatment) and HbA1c (percentage; mmol/mol). Further outcome measures included rates of severe hypoglycaemia (with or without coma) and diabetic ketoacidosis, rates of diabetes-associated hospital admissions (i.e., hospitalization), and inpatient days in hospital each year due to diabetes. BMI SDS was calculated using the national KiGGS (German Health Interview and Examination Survey for Children and Adolescents) reference data in Germany [10]. HbA1c values were measured locally and standardized mathematically to the Diabetes Control and Complications Trial (DCCT) reference range (4.05–6.05%) using the multiple of the mean (MOM) method [11]. Severe hypoglycaemia (SH)—an event requiring external assistance by another person; hypoglycaemic coma (SHC)—severe hypoglycaemia associated with seizure or loss of consciousness; and an event of diabetic ketoacidosis (DKA)—a pH of less than 7.3 or serum bicarbonate of less than 15 mmol/L or both were defined according to the guidelines of International Society for Pediatric and Adolescent Diabetes (ISPAD) [12, 13]. Rates of severe hypoglycaemia, DKA, and hospital admission rates were presented as events per 100 patient years. Length of inpatient treatment was given as the number of inpatient days per patient and year.
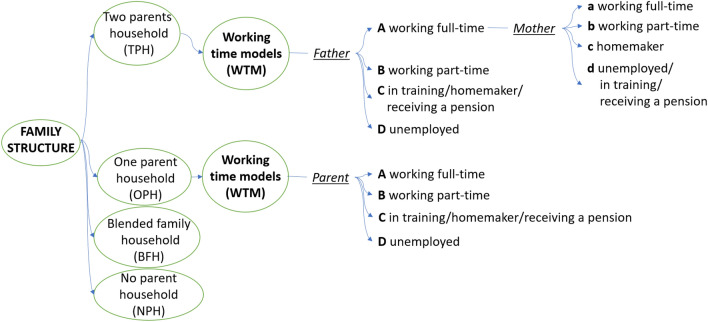
The database includes information on family structure and working time models (WTM) of the children’s caretakers and was stratified according to these categories. We defined living with both biological parents as a two-parents household (TPH) and living with one biological parent as a one-parent household (OPH). Blended family households (BFH) were defined as living with one biological parent and his/her partner in the household. Other living conditions (e.g., living with other relatives, adoptive parents, boarding school, foster care homes of the child welfare service, etc.) were categorized as living without a biological parent (no parent household, NPH). Working time models differentiate into working full-time (A), part-time (B), homemaker, being in training, receiving a pension (C) and being unemployed (D). The categorical system focuses on the father regarding two parent households and the mother in terms of one-parent households (Fig. 2).
Fig. 2.
Categorial system applied for the variables family structure and working time models
Statistical analysis
The data evaluation and statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC, USA). Linear regression models were applied to calculate and compare HbA1c (% or mmol/mol) between family structures and working time models. Rates of SH, SHC, DKA, and hospital admission rates and length of inpatient treatment were analysed via negative binomial regression using individual time under risk as offset. All models were adjusted for age group (< 10, 10 to 15, ≥ 15 years), sex, diabetes duration group (< 2, 2 to 5, ≥ 5 years) and migratory background (yes, no). Results are presented as least-square means with 95% confidence intervals. The Tukey–Kramer method was used for multiple testing in unbalanced data. Goodness-of-fit was assessed via R-Squared. Models were tested for each dependent variable using analysis of variance.
Results
The results are presented by the categories family structure and working time models. The categories will be referred to as depicted in Fig. 2.
Out of the entire cohort 52.0% were males and migratory background was present in 22.6% of the patients. The median age at diagnosis of T1DM was 8.1 years (Q1–Q3: 4.7–11.4) and 12.8 years (9.3–15.4) during follow-up. More than 70% (10,790) of subjects lived in a two-parents household, 17.8% (2726) in a one-parent household, 8.5% (1296) lived in blended families and 3.4% (528) without a biological parent. All F-tests for the analyses of variance were significant (p < 0.01) in all models tested.
Family structure
Patients living in a two-parents household had significantly lower HbA1c levels compared to other living conditions (7.70; 60.70 vs. 8.06; 64.63 for OPHs; 8.07; 64.66 for BFHs; 8.21; 66.22 for NPHs) as well as lower rates of DKA (1.79) than subjects living in one-parent households (3.41), blended family-households (4.00), and no parent households (7.19) (see Table 1).
Table 1.
Family structure, working time models, and quality of diabetes care
| Family structure | Form of social status | Categories | Frequency | HbA1c1 | Severe hypoglycaemia (SH)1 | Rate of SH with coma (SHC)1 | Diabetic ketoacidosis (DKA)1 | Length of hospital stay1 | Hospital admission rate1 |
|---|---|---|---|---|---|---|---|---|---|
| (mmol/mol)/(%) | (per 100 patient years) | (per 100 patient years) | (per 100 patient years) | (days per patient year) | (per 100 patient years) | ||||
| Two-parents household (TPH)2 | 10,790 | 60.70*** (60.43; 60.96) | 14.86 | 3.15** | 1.79*** | 12.82*** | 57.46*** | ||
| 7.70*** (7.68; 7.73) | (13.99; 15.78) | (2.88; 3.43) | (1.62; 1.99) | (12.51; 13.12) | (56.31; 58.65) | ||||
| Working time models (WTM)3 | Aa Father working full-time—mother working full-time | 1848 | 59.85*** (59.24; 60.46) | 13.26 | 2.75 | 1.64 | 15.41*** | 60.58** | |
| 7.63*** (7.57; 7.68) | (11.35; 15.49) | (2.20; 3.44) | (1.26; 2.13) | (14.56; 16.32) | (57.70; 63.60) | ||||
| Ab Father working full-time—mother working part-time | 4446 | 59.71*** (59.32; 60.10) | 12.95 | 2.48* | 1.53* | 11.97*** | 53.66*** | ||
| 7.61*** (7.58; 7.65) | (11.74; 14.29) | (2.14; 2.88) | (1.28; 1.82) | (11.53; 12.42) | (51.95; 55.42) | ||||
| Ac Father working full-time—mother homemaker | 2339 | 60.60** (60.06; 61.14) | 15.88 | 3.09 | 1.86 | 12.28*** | 55.64*** | ||
| 7.69** (7.65; 7.74) | (13.91; 18.14) | (2.54; 3.76) | (1.48; 2.36) | (11.66; 12.93) | (53.21; 58.18) | ||||
| Ad father working full-time—mother is unemployed/in training/receiving a pension | 240 | 60.91 (59.34; 62.38) | 10.13 | 2.9 | 1.46 | 14.84 | 66.29* | ||
| 7.72 (7.57; 7.88) | (6.43; 15.94) | (1.52; 5.52) | (0.68; 3.14) | (12.78; 17.24) | (58.10; 75.64) | ||||
| B Father working part-time | 291 | 60.87 (59.34; 62.38) | 17.76 | 2.98 | 1.79 | 12.14* | 62.22 | ||
| 7.72 (7.58; 7.86) | (12.33; 25.57) | (1.75; 5.08) | (0.98; 3.24) | (10.60; 13.91) | (55.29; 70.03) | ||||
| C Father in training/homemaker/receiving a pension | 302 | 62.53 (61.04; 64.03) | 17.16 | 4.32 | 2.41 | 14.73*** | 75.76*** | ||
| 7.87 (7.74; 8.01) | (11.95; 24.64) | (2.67; 6.99) | (1.39; 4.21) | (12.90; 16.82) | (67.69; 84.79) | ||||
| D Father is unemployed | 369 | 63.53** (62.17; 64.89) | 21.09 | 5.12* | 2.96* | 16.59*** | 72.16*** | ||
| 7.96** (7.84; 8.09) | (15.36; 28.97 | (3.34; 7.85) | (1.84; 4.74) | (14.66; 18.77) | (64.97; 80.15) | ||||
| One-parent household (OPH)2 | 2726 | 64.63*** (64.10; 65.16) | 15.88 | 3.68* | 3.41*** | 16.07*** | 76.02*** | ||
| 8.06*** (8.02; 8.11) | (14.11; 17.87) | (3.11; 4.35) | (2.91; 3.99) | (15.38; 16.79) | (73.25; 78.89) | ||||
| Working time models (WTM)3 | A Parent working full-time | 935 | 64.73 (63.76; 65.70) | 16.21 | 3.12 | 3.26 | 16.19 | 72.77* | |
| 8.07 (7.98; 8.16) | (13.23; 19.86) | (2.33; 4.18) | (2.49; 4.28) | (15.05; 17.42) | (68.36; 77.45) | ||||
| B Parent working part-time | 931 | 64.78 (63.82; 65.74) | 16.67 | 3.96 | 3.34 | 14.63* | 72.25* | ||
| 8.08 (7.99; 8.17) | (13.70; 20.29) | (3.04; 5.16) | (2.56; 4.35) | (13.60; 15.74) | (67.91; 76.86) | ||||
| C Parent in training/homemaker/receiving a pension | 428 | 66.02 (64.57; 67.47) | 14.93 | 4.56 | 3.91 | 16.36 | 84.50* | ||
| 8.19 (8.06; 8.32) | (11.04; 20.20) | (3.09; 6.71) | (2.69; 5.69) | (14.70; 18.20) | (77.31; 92.36) | ||||
| D Parent unemployed | 243 | 67.10 (65.21; 68.99) | 13.17 | 2.9 | 3.39 | 18.36* | 84.53 | ||
| 8.29 (8.12; 8.46) | (8.70; 19.93) | (1.61; 5.21) | (2.00; 5.76) | (15.96; 21.12) | (75.07; 95.19) | ||||
| Blended Family household (BFH)2 | 1296 | 64.65*** (63.89; 65.42) | 16.04 | 4.36* | 3.97*** | 16.10*** | 80.69*** | ||
| 8.07*** (8.00; 8.13) | (13.52; 19.02) | (3.47; 5.48) | (3.20; 4.93) | (15.12; 17.15) | (76.53; 85.08) | ||||
| No parent household (NPH)2 | 528 | 66.22*** (65.02; 67,43) | 20.641 | 6.29** | 7.19*** | 19.01*** | 106.29*** | ||
| 8.21*** (8.10; 8.32) | (15.72; 26.51) | (4.51; 8.79) | (5.42; 9.54) | (17.26; 20.94) | (98.23; 115.01) | ||||
1Data expressed as mean (lower vs. upper bound of 95%-confidence interval); least square means adjusted for age, duration of diabetes, sex and a background of migration
2Significant results compare different family structures
3Significant results compare different working time models
***significant for p < .0001
**significant for p < .01
*significant for p < .05
The rates of hypoglycaemic coma were higher for children living in no-parent households (6.29), compared with children living in two-parent households (3.15), single-parent households (3.68) and blended family households (4.36). Rates of severe hypoglycaemia did not differ significantly between these categories. Length of inpatient treatment and hospital admission rates were lowest for adolescents living in a two-parent household (12.82; 57.46 vs. 16.07; 76.02 for OPHs; 16.10; 80.69 for BFHs; 19.01; 106.29 for NPHs). Table 2 compares the effects between two-parent and single-parent households, with overall poorer outcomes for children living in two-parent households.
Table 2.
Effect of family structure
| (A) Two-parents household (TPH) | (B) One-parent household (OPH) | adj p* | |
|---|---|---|---|
| HbA1ca (%) | 7.7 | 8.06 | < 0.00011 |
| (7.68; 7.73) | (8.02; 8.11) | ||
| HbA1ca (mmol/L) | 60.69 | 64.63 | < 0.00011 |
| (60.43; 60.96) | (64.10; 65.16) | ||
| Rate of severe hypoglycaemiaa | 14.86 | 15.88 | 0.09352 |
| (per 100 patient-years) | (13.99; 15.78) | (14.11; 17.87) | |
| Rate of hypoglycaemia with comaa | 3.15 | 3.68 | 0.00052 |
| (per 100 patient-years) | (2.88; 3.43) | (3.11; 4.35) | |
| Rate of episodes with DKAa | 1.79 | 3.41 | < 0.00012 |
| (per 100 patient-years) | (1.62; 1.99) | (2.91; 3.99) | |
| Length of hospital staya | 12.82 | 16.07 | < 0.00012 |
| (days per patient-year) | (12.51; 13.12) | (15.38; 16.79) | |
| Hospital admission ratea | 57.46 | 76.02 | < 0.00012 |
| (per 100 patient-years) | (56.31; 58.65) | (73.25; 78.89) |
aData expressed as mean (95% confidence interval)
1linear regression model
2negative binomial regression model; least square means adjusted for age, duration of diabetes, sex, and a background of migration
Goodness-of-fit analysis showed 14% of the variability concerning the HbA1C parameters can be explained by family structure (R2 = 0.14, F(3, 15,330) = 92.38, p < 0.0001) (see Table 4).
Table 4.
Goodness of fit family models by dependent variables
| Family structure | Working time models (WTM)—Two-parents household (TPH) | Working time models (WTM)—One-parent household (OPH) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | F | df | p | R2 | F | df | p | R2 | F | df | p | |
| HbA1c | 0.141 | 92.38 | 3 | < 0.00011 | 0.132 | 7.01 | 6 | < 0.00011 | 0.103 | 2.2 | 3 | 0.08581 |
| 155,330 | 9842 | 2527 | ||||||||||
| Severe hypoglycaemia (SH) | 0.006 | 2.07 | 3 | < 0.010192 | 0.007 | 2.84 | 6 | 0.00922 | 0.009 | 0.4 | 3 | 0.75322 |
| 15,329 | 9841 | 2527 | ||||||||||
| Rate of SH with coma (SHC) | 0.007 | 7.05 | 3 | < 0.00012 | 0.007 | 2.34 | 6 | 0.02902 | 0.01 | 1.11 | 3 | 0.34452 |
| 15,329 | 9841 | 2527 | ||||||||||
| Diabetic ketoacidosis (DKA) | 0.019 | 43.08 | 3 | < 0.00012 | 0.008 | 150 | 6 | 0.17232 | 0.018 | 0.22 | 3 | 0.88412 |
| 15,329 | 9841 | 2527 | ||||||||||
| Length of hospital stay | 0.026 | 48.99 | 3 | < 0.00012 | 0.021 | 13.44 | 6 | < 0.00012 | 0.021 | 3.22 | 3 | 0.02202 |
| 10,731 | 6609 | 1916 | ||||||||||
| Hospital admission rate | 0.067 | 138.69 | 3 | < 0.00012 | 0.052 | 11.9 | 6 | < 0.00012 | 0.062 | 4.25 | 3 | 0.00532 |
| 15,329 | 9841 | 2527 | ||||||||||
Goodness of fit via R2 and Type III Tests of Fixed Effects 1linear regression model
2negative binomial regression model
Working time models: two-parents household (TPH, WTM, Aa-D)
The adjusted outcomes indicated significantly higher HbA1c parameters in children of parents with working time models of category C (7.88; 62.58) and D (7.97; 63.55) versus categories Aa (7.62; 59.82) and Ab (7.61; 59.71) as well as category C vs category Ac (7.70; 60.60). Lower rates of hypoglycaemic coma were recorded in children from couples of category Ab compared to D (2.48 vs. 5.12). Rates of severe hypoglycaemia and DKA did not differ significantly between working time models (Table 1). In group comparison, the length of inpatient treatment differed between categories with longer inpatient treatment for C (14.73), D (16.60), Aa (15.41) and shorter treatment for Ab (11.97), Ac (12.28) and B (12.14). Higher hospital admission rates were reported for categories C (75.76) and D (72.16) as well as for Aa versus Ab (60.58 vs. 53.66) and Ab vs Ad (53.66 vs. 66.29). Table 3 contrasts the findings for the categories Ab and D regarding the working time models. Living with a full-time working father and a part-time working mother came with overall lower outcomes compared to living with an unemployed father.
Table 3.
Effect of working time models (TPH)
| (Ab) Father working full-time—mother working part-time | (D) Father is unemployed | adj p* | |
|---|---|---|---|
| HbA1ca (%) | 7.611 | 7.96 | < 0.00011 |
| (7.58; 7.65) | (7.84; 8.09) | ||
| HbA1ca (mmol/L) | 59.71 | 63.53 | < 0.00011 |
| (59.32; 60.10) | (62.17; 64.89) | ||
| Rate of severe hypoglycaemiaa | 12.95 | 21.09 | 0.06211 |
| (per 100 patient-years) | (11.74; 14.29) | (15.36; 28.97) | |
| Rate of hypoglycaemia with comaa | 2.48 | 5.12 | 0.02802 |
| (per 100 patient-years) | (2.14; 2.88) | (3.34; 7.85) | |
| Rate of episodes with DKAa | 1.53 | 2.96 | 0.01002 |
| (per 100 patient-years) | (1.28; 1.82) | (1.84; 4.74) | |
| Length of hospital staya | 11.97 | 16.59 | < 0.00012 |
| (days per patient-year) | (11.53; 12.42) | (14.66; 18.77) | |
| Hospital admission ratea | 53.66 | 72.16 | < 0.00012 |
| (per 100 patient-years) | (51.95; 55.42) | (64.97; 80.15) |
aData expressed as mean (95% confidence interval)
1linear regression model
2negative binomial regression model; least square means adjusted for age, duration of diabetes, sex, and a background of migration
Goodness-of-fit analysis indicated 13% of the variability concerning the HbA1C parameters can be explained by working time models in two-parent households (R2 = 0.13, F(6, 9842) = 7.01, p < 0.0001) (see Table 4).
Working time models: one-parent household (OS, OPH, A-D)
According to goodness-of-fit analysis, there was no additional significant information gained regarding the working time models in one-parent households for HbA1c levels, DKA, severe hypoglycaemia and hypoglycaemic coma. Hospital admission rates were higher for category C (84.50) compared to A (72.77) and B (72.25). Length of inpatient treatment differed between category B (14.63) and D (18.36) (see Table 4).
Discussion
Our study shows significant influences of family structure and working time models in two-parent households on children’s diabetes management. Better metabolic adjustment is seen in children from two-parent households compared to other living arrangements. In detail, the following points should be emphasised.
The data in Tables 1 and 2 depict significant differences in the key parameters of metabolic control comparing children in various family constellations. Living in a one-parent household is a clear risk factor for poor metabolic control in T1DM. These children have a significantly higher risk of metabolic derailment and therefore a higher risk of long-term complications. Conversely, living with both biological parents is a protective factor for diabetes control during course of disease.
“Modern” parents have more freedom and develop a variety of options for how they want to live with their children. This presents new challenges and can lead to insecurity and symptom burden in children [7]. Growing up without both biological parents increases the probability for children to have chronic diseases and problems with emotions or behaviour [14]. Children living with divorced parents appear to experience more overall stress due to the various aspects of separation [15]. Families with high levels of general stress should be identified as they are at risk of poorer health for both parents and children [16]. Consequently, the quality of life in children with T1DM is negatively correlated with family conflict, particularly conflict related to diabetes management [17]. These negative interactions with metabolic control are confirmed by our results—children from one-parent families, blended families and children living without biological parents have worse metabolic outcomes compared to children and adolescents from two-parent households. Similar results were reported by Baechle et al. [4]. About 15% of the variance in HbA1c parameters can be explained by family structure and working time models. This underlines the effect of these psychosocial influences on diabetes adherence when all other possible influences are taken into account.
The data in Table 3 show differences in metabolic control comparing working time models. Paternal unemployment is a significant risk factor for poor diabetes outcomes in the child. There is a higher risk of life-threatening metabolic derailment and long-term consequences. Working parents are therefore a protective factor for metabolic control.
Parental care and involvement are necessary for positive living with T1DM [17, 18]. The risk of unemployment and low SES—which we found to be negatively associated with diabetes outcomes—predict mood disorders in other studies, and in general, parental emotional stress has a negative effect on children's metabolic control [19–21].
Family support may be helpful in preventing complications of T1DM [22]. Other studies have found an association between poorer diabetes control and a higher percentage of one-parent families, which could be due to either low SES or inadequate ability to provide adequate care for children [23].
Although family structure and work schedules play an important role in diabetes management, the pathway to family-oriented interventional improvement is largely unclear. The effect of family therapy is well documented at the level of individual cases [24]. At the level of meta-analyses, the effect of these approaches remains disappointing [25, 26].
The results of the study suggest aspects that could be considered in the management of chronic diseases in general and may provide guidance for other populations. Diabetes is a good paradigm for any chronic disease. The need for 24-h care and the possibility of an objective measure of treatment success, such as HbA1c, make diabetes suitable for such studies. In addition, the DPV registry is an enormously large database, including more than half of the children with T1DM in Germany [27]. Thus, this study may provide evidence on the influence of family structure on the overall care of chronically ill children. Possible new research approaches could include studying bronchial asthma and inflammatory bowel disease in relation to family structure.
Strengths and limitations of the study
An obvious strength of the study is the large sample size. To date, most studies included less participants and were conducted in a single country or culture [8, 22, 23, 28]. This study combines data from three different countries, the data collection is well established, and the database has proven to be a matrix of intensive publication activity.
The interpretation of R2 can be limited so we complemented analyses of variance for all tested models. Nevertheless, we did not include further validation techniques like residual analysis to obtain an even more thorough understanding of the model’s performance.
The study is limited to the categorical system given in the data mask. Overall, the chosen classification reflects a family-related aspect of social status and can be associated with parental education, working hours, occupational status and household income [14]. The influence of parental income and education should be investigated in future research. The data were limited by the information provided and we had to exclude patients who did not provide information on family structure. In addition, future studies should include more diverse modern family forms (e.g., different forms of blended or rainbow families) in their research. This could be helpful in describing coping potential and identifying factors of clinical importance for better treatment options.
Conclusion
The results of this study highlight the importance of carefully considering family structure, parental employment status and work schedules in the management of paediatric T1DM. Our findings highlight risk factors within the family environment and the need for family-focused counselling for high-risk patients or severe cases in clinical practice.
Different family models may influence the quality of diabetes management at home.
Living with both biological parents may be a protective factor.
Single-parent families are particularly at risk for their children with T1DM.
Family issues should be part of diabetes care guidelines.
The social status of the father, a high level of employment and therefore sufficient financial resources seem to facilitate diabetes management.
Children's metabolic control parameters are more favourable in mothers who work part-time or full-time than in mothers who stay at home in two-parent households.
Therefore, single parents, unemployed parents and special family forms need more professional psychosocial support [8, 22, 23, 28].
Acknowledgements
All DPV centres contributed to the data pool. We would like to thank Esther Bollow and Anna Wagner, medical data management, for help with data analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL. The German Centre for Diabetes Research (Deutsches Zentrum für Diabetesforschung), German Diabetes Association, German Robert Koch Institute.
Declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
The ethics committee of the Justus-Liebig-University, department of medicine ('185/20) agreed with the study.
Informed consent
Informed consent was secured.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pauline Schlarb and Janina M. Büttner have contributed equally to this work.
References
- 1.Due-Christensen M, Zoffmann V, Willaing I, Hopkins D, Forbes A. The process of adaptation following a new diagnosis of type 1 diabetes in adulthood: a meta-synthesis. Qual Health Res. 2018;28:245–258. doi: 10.1177/1049732317745100. [DOI] [PubMed] [Google Scholar]
- 2.Köhle K, Herzog W, Joraschky P, Kruse J, Langewitz W, Söllner W, editors. Psychosomatische Medizin: Theoretische Modelle und klinische Praxis. 8. München: Urban & Fischer; 2017. [Google Scholar]
- 3.Statistisches Bundesamt (Destatis) (2018) Alleinerziehende: Tabellenband zur Pressekonferenz am 02.08.2018 in Berlin - Ergebnisse des Mikrozensus -. https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Haushalte-Familien/Publikationen/Downloads-Haushalte/alleinerziehende-tabellenband-5122124179004.pdf?__blob=publicationFile. Accessed 19 May 2021
- 4.Baechle C, Stahl-Pehe A, Castillo K, Selinski S, Holl RW, Rosenbauer J. Association of family structure with type 1 diabetes management and outcomes in adolescents: a population-based cross-sectional survey. Pediatr Diabetes. 2021;22:482–494. doi: 10.1111/pedi.13166. [DOI] [PubMed] [Google Scholar]
- 5.Hanberger L, Ludvigsson J, Nordfeldt S. Health-related quality of life in intensively treated young patients with type 1 diabetes. Pediatr Diabetes. 2009;10:374–381. doi: 10.1111/j.1399-5448.2008.00496.x. [DOI] [PubMed] [Google Scholar]
- 6.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155:668–72.e1-3. doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brosig B. Patient Familie - damals und heute. Psychoanal Familientherapie Z Paar- Fam Sozial. 2012;13:17–32. [Google Scholar]
- 8.Haugstvedt A, Wentzel-Larsen T, Rokne B, Graue M. Psychosocial family factors and glycemic control among children aged 1–15 years with type 1 diabetes: a population-based survey. BMC Pediatr. 2011 doi: 10.1186/1471-2431-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofer SE, Schwandt A, Holl RW. Standardized documentation in pediatric diabetology: experience from Austria and Germany. J Diabetes Sci Technol. 2016;10:1042–1049. doi: 10.1177/1932296816658057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhauser H, Schienkiewitz A, Rosario AS, Dortschy R, Kurth B-M. Referenzperzentile für anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS) Robert Koch-Institut; 2013. [Google Scholar]
- 11.Rosenbauer J, Dost A, Karges B, et al. Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care. 2012;35:80–86. doi: 10.2337/dc11-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham MB, Jones TW, Naranjo D, et al. ISPAD Clinical Practice Consensus Guidelines 2018: assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):178–192. doi: 10.1111/pedi.12698. [DOI] [PubMed] [Google Scholar]
- 13.Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155–177. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 14.Rattay P, von der Lippe E, Lampert T. Health of children and adolescents in single-parent, step-, and nuclear families: results of the KiGGS study: first follow-up (KiGGS Wave 1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2014;57:860–868. doi: 10.1007/s00103-014-1988-2. [DOI] [PubMed] [Google Scholar]
- 15.de Lange M, Dronkers J, Wolbers MHJ. Single-parent family forms and children’s educational performance in a comparative perspective: effects of school’s share of single-parent families. Sch Eff Sch Improv. 2014;25:329–350. doi: 10.1080/09243453.2013.809773. [DOI] [Google Scholar]
- 16.Helgeson VS, Becker D, Escobar O, Siminerio L. Families with children with diabetes: implications of parent stress for parent and child health. J Pediatr Psychol. 2012;37:467–478. doi: 10.1093/jpepsy/jsr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumahorbo H, Karjatin A. The influence of family factors on the quality of life of children with diabetes mellitus type 1 in West Java, Indonesia: a cross-sectional analytic study. GJHS. 2019;11:34. doi: 10.5539/gjhs.v11n10p34. [DOI] [Google Scholar]
- 18.Graue M, Wentzel-Larsen T, Hanestad BR, Søvik O. Health-related quality of life and metabolic control in adolescents with diabetes: the role of parental care, control, and involvement. J Pediatr Nurs. 2005;20:373–382. doi: 10.1016/j.pedn.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Bassi G, Mancinelli E, Di Riso D, Salcuni S. Parental stress, anxiety and depression symptoms associated with self-efficacy in paediatric type 1 diabetes: a literature review. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph18010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buffel V, van de Straat V, Bracke P. Employment status and mental health care use in times of economic contraction: a repeated cross-sectional study in Europe, using a three-level model. Int J Equity Health. 2015;14:29. doi: 10.1186/s12939-015-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TJ, von dem Knesebeck O. Perceived job insecurity, unemployment and depressive symptoms: a systematic review and meta-analysis of prospective observational studies. Int Arch Occup Environ Health. 2016;89:561–73. doi: 10.1007/s00420-015-1107-1. [DOI] [PubMed] [Google Scholar]
- 22.AlHaidar AM, AlShehri NA, AlHussaini MA. Family support and its association with glycemic control in adolescents with type 1 diabetes mellitus in Riyadh, Saudi Arabia. J Diabetes Res. 2020 doi: 10.1155/2020/5151604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson BJ, McKay SV. Barriers to glycemic control in youth with type 1 diabetes and type 2 diabetes. Pediatr Diabetes. 2011;12:197–205. doi: 10.1111/j.1399-5448.2010.00667.x. [DOI] [PubMed] [Google Scholar]
- 24.Günther MP, Winker P, Böttcher C, Brosig B. Family system dynamics and type 1 diabetic glycemic variability: a vector-auto-regressive model. Fam Syst Health. 2013;31:194–204. doi: 10.1037/a0032314. [DOI] [PubMed] [Google Scholar]
- 25.Kodama S, Morikawa S, Horikawa C, et al. Effect of family-oriented diabetes programs on glycemic control: a meta-analysis. Fam Pract. 2019;36:387–394. doi: 10.1093/fampra/cmy112. [DOI] [PubMed] [Google Scholar]
- 26.Law E, Fisher E, Eccleston C, Palermo TM. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev. 2019;3:CD009660. doi: 10.1002/14651858.CD009660.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demirel F, Tepe D, Esen I, Buber N, Boztepe H. Individual and familial factors associated with metabolic control in children with type 1 diabetes. Pediatr Int. 2013;55:710–713. doi: 10.1111/ped.12183. [DOI] [PubMed] [Google Scholar]
- 28.Gesuita R, Maffeis C, Bonfanti R, et al. Socioeconomic inequalities increase the probability of ketoacidosis at diagnosis of type 1 diabetes: a 2014–2016 Nationwide Study of 2,679 Italian Children. Front Pediatr. 2020;8:575020. doi: 10.3389/fped.2020.575020. [DOI] [PMC free article] [PubMed] [Google Scholar]