Abstract
Tuberculosis in cattle remains a major zoonotic and economic problem in many countries. The standard diagnostic assay for bovine tuberculosis, the intradermal tuberculin test, has low accuracy. Therefore, alternative immunodiagnostic methods, such as serological assays, are needed for detection of infected animals. Development of an accurate serodiagnostic test requires a detailed understanding of the humoral immune responses during bovine tuberculosis and, in particular, identification of the key antigens of Mycobacterium bovis involved in antibody production. In this study, we characterized antibody responses in cattle experimentally infected with M. bovis. Sequential serum samples were collected every 3 to 4 weeks for up to 27 months postinfection. Circulating immunoglobulin G antibody levels were measured by an enzyme-linked immunosorbent assay using 12 highly purified recombinant proteins of M. bovis. Six proteins, ESAT-6, 14-kDa protein, MPT63, MPT70, MPT51, and MPT32, were identified as major seroreactive antigens in bovine tuberculosis. A remarkable animal-to-animal variation of antigen recognition by serum antibodies was observed. Kinetic analyses of the antibody production to individual antigens during infection revealed that the heterogeneous antigen recognition profile changed markedly in a given infected animal as disease progressed.
Mycobacterium bovis is a major bacterial pathogen in cattle and farmed cervids as well as in captive and wild animals (31, 35), causing serious economic problems worldwide. Also, M. bovis can cause disease in humans (15, 35). Human tuberculosis (TB) due to M. bovis contributes to the bovine TB cycle in Africa (6), a threat that is exacerbated by the present pandemic of AIDS (8, 15). The success of eradication programs for bovine TB adopted by many countries (11, 31) requires effective control measures.
The existing diagnostic methods for TB in living cattle are inadequate. The standard assay is the intradermal tuberculin test that measures delayed-type hypersensitivity reactions to purified protein derivative (PPD) (32). However, this test has low diagnostic accuracy (34), and it affects the immune status of animals subjected to repeated testing (10). Therefore, alternative immunodiagnostic methods are needed for early detection of infected cattle. Serological assays could represent a useful approach because they are generally simple, rapid, and inexpensive.
The outcome of numerous attempts to develop a sensitive serodiagnostic assay specific for bovine TB has been unsatisfactory. Antibody responses in cattle have been investigated in studies using unfractionated, highly cross-reactive antigen preparations, such as PPD, whole-culture filtrates, and sonicates of M. bovis (7, 16, 17). More recently, several protein antigens purified from the culture filtrates have been serologically characterized in bovine TB (12, 14). Some of these antigens, MPB70 (13, 21, 25), MPB64 (13), MPB83 (20, 22), and P27 (3), displayed immunological specificity to M. bovis. However, immunoassays based on a single antigen usually provided detection of serological responses in only a minority of infected cattle (4, 40). Thus, the serodiagnostic value of earlier assays has been limited due to a lack of specificity and/or sensitivity. Development of improved serodiagnostic assays requires a detailed understanding of the humoral immune mechanisms induced by infection with M. bovis in cattle and identification of the key antigens involved in the antibody responses during bovine TB.
In this study, we characterized serum immunoglobulin G (IgG) antibody responses during experimental bovine TB against a panel of 12 highly purified recombinant proteins of M. tuberculosis (5) that are also produced by M. bovis. In addition to MPT70, a major secreted protein of M. bovis (18, 19), five other antigens, ESAT-6, 14-kDa protein, MPT63, MPT51, and MPT32, were identified as potent antigenic targets for the humoral immune response in bovine TB. Analyses of the kinetic antibody responses revealed variable patterns of multiple antigens recognized by sera from different animals, with marked changes in antigen predominance profiles in the same host during disease.
MATERIALS AND METHODS
Experimental infection.
Ten Friesian-cross castrated males, approximately 6 months of age, were obtained from cattle herds with no history of M. bovis infection for at least 5 years. All animals were housed in strict isolation. In one experiment, two animals, 193 and 198, were infected by intranasal instillation of 107 CFU of a strain of M. bovis, T/92/1378, isolated from a field case of bovine TB (33). Twenty-eight weeks later two other animals, 30 and 31, were placed into the same enclosed air space where animals 193 and 198 were housed. Housing conditions allowed direct contact of animals 30 and 31 with the intranasally infected group. Exposure to experimentally infected cattle resulted in development of contact M. bovis infection in animals 30 and 31. In two additional experiments, six animals were intranasally infected with 106 CFU of M. bovis T/92/1378. Cell-mediated immune responses were monitored weekly in all animals by assaying proliferation and gamma interferon (IFN-γ) production by peripheral blood lymphocytes in response to stimulation with PPD in vitro. Serum samples were collected from each animal preinoculation and every 3 to 4 weeks for 8 to 27 months postinfection and were stored frozen at −20°C. All infected cattle had macroscopic tuberculous lesions at postmortem examinations performed as described previously (33) and were culture positive for M. bovis.
Recombinant antigens.
Twelve genes encoding culture filtrate proteins of M. tuberculosis (Table 1) were expressed in Escherichia coli as NH2-terminally polyhistidine-tagged fusion proteins as previously described (27, 28). Recombinant antigens were purified to near homogeneity by using a three-step chromatographic protocol (5).
TABLE 1.
Recombinant protein antigens of M. bovis used in this study
Enzyme-linked immunosorbent assay (ELISA).
Polystyrene 96-well microtiter plates were coated overnight at 4°C with purified protein at 1 μg/ml or with unfractionated culture filtrate of M. bovis at 3 μg/ml in 0.1 M carbonate-bicarbonate buffer (pH 9.6). Prior to use, antigen-coated plates were washed extensively with 0.1 M phosphate-buffered saline (pH 7.4) containing 0.05% Tween 20 (PBS-T). Serum samples were diluted 1:100 in PBS-T and added in duplicate to wells coated with each protein. Plates were incubated for 1 h at room temperature and then washed extensively with PBS-T. Bound antibodies were detected by incubation with mouse monoclonal anti-bovine IgG-alkaline phosphatase conjugate (Sigma) at a dilution 1:2,000 in PBS-T for 1 h at room temperature. After plates were extensively washed with PBS-T, 100 μl of substrate solution (p-nitrophenylphosphate; Bio-Rad) was added to each well. Plates were incubated for another 20 min at room temperature, and color development was stopped by addition of 100 μl of 0.4 M NaOH per well. Optical density at 405 nm (OD405) was measured with an automatic microplate reader (Spectra Shell; Tecan).
Data analysis.
For each antigen, serum antibody levels were evaluated by using a cutoff value based on optical density index (ODI), a ratio between OD405 obtained for a test serum sample collected postinfection and OD405 obtained for a serum sample collected from the same animal at an initial, preinfection time point. An ODI of ≥2 was taken as indicative of the antibody response; an ODI of ≥3 indicated high levels of serum antibodies. An animal was considered reactive to an antigen if at least one of the sequential serum samples yielded an ODI of ≥2 with that antigen.
RESULTS
Antibody responses to intranasal M. bovis infection.
Evaluation by ELISA of sequential serum specimens collected in the first experiment for a period of 27 months postinfection revealed antibody responses to various antigens. Of the 12 proteins used in the study, 9 and 10 were recognized by animals 193 and 198, respectively (Table 2). Among those seroreactive antigens, four (ESAT-6, 14-kDa protein, MPT70, and MPT51) in animal 193 and five (the same four plus MPT63) in animal 198 elicited high-level antibody responses (ODI ≥ 3). Lower antibody titers (ODI ≥ 2) were found against 19-kDa protein in animal 193, against MTC28 antigen in animal 198, and against MPT64, Ag85B, 38 kDa, and MPT32 in both animals (Table 2). No serum antibody against KatG was detected in either animal.
TABLE 2.
Reactivity of recombinant antigens of M. bovis with serum IgG antibodies from cattle with experimental tuberculosisa
| Antigen | Responseb
|
||||||
|---|---|---|---|---|---|---|---|
| Intranasal M. bovis infection
|
Contact infection
|
||||||
| 12 | 23 | 33 | 193 | 198 | 30 | 31 | |
| ESAT-6 | ++ | − | − | ++ | ++ | ++ | + |
| 14 kDa | ++ | + | − | ++ | ++ | ++ | − |
| MPT63 | ++ | + | ++ | − | ++ | + | − |
| 19 kDa | + | − | − | + | − | ++ | + |
| MPT70 | ++ | ++ | + | ++ | ++ | ++ | − |
| MPT64 | ++ | + | − | + | + | + | − |
| MPT51 | + | − | − | ++ | ++ | ++ | − |
| MTC28 | − | − | − | − | + | − | − |
| Ag85B | − | − | − | + | + | − | − |
| 38 kDa | + | − | − | + | + | − | − |
| MPT32 | ++ | + | − | + | + | ++ | ++ |
| KatG | − | − | − | − | − | − | − |
Cattle were infected by intranasal instillation with 106 CFU of M. bovis (animals 12, 23, and 33) or with 107 CFU of M. bovis (animals 193 and 198) or by sharing close quarters with intranasally infected animals 193 and 198 (contact infection; animals 30 and 31). For each animal, serum samples were collected preinfection (week 0) and at multiple times postinfection (see legends to Fig. 1 to 3 for details). Levels of circulating IgG antibodies were measured by ELISA using 12 recombinant proteins of M. bovis. Antibody responses to each antigen were expressed as ODI by dividing OD450 readings obtained with each postinfection serum sample by OD450 readings obtained with the preinfection serum of the same animal. Data are shown for 7 responders among 10 animals tested.
−, ODI < 2 (no antibody response); +, ODI ≥ 2 (antibody response); ++, ODI ≥ 3 (high-level antibody response) with at least one serum sample.
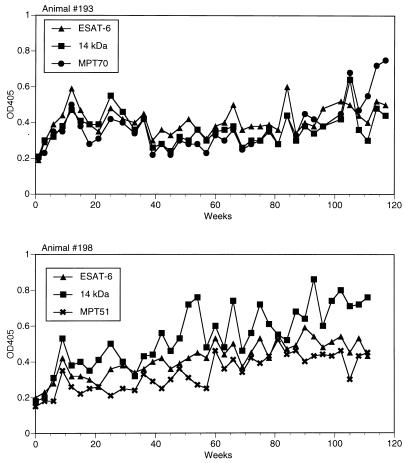
Figure 1 shows the time course of antibody responses against the three most seroreactive antigens in animal 193 (ESAT-6, 14-kDa protein, and MPT70) and in animal 198 (ESAT-6, 14-kDa protein, and MPT51). Serum antibodies to some of these antigens were detected as early as 6 weeks postinfection. An initial sharp peak of the humoral immune response involving all seroreactive antigens was observed at week 12 in animal 193 and at week 9 in animal 198. This peak was followed by a significant decline of antibody levels for most antigens to preinfection values. As infection progressed, antibody responses showed multipeak kinetics in both animals.
FIG. 1.
Development of humoral immune responses to intranasal M. bovis infection in animals 193 and 198 (experiment 1). Cattle were infected by intranasal instillation with 107 CFU of M. bovis. Serum samples were collected preinfection (week 0) and every 3 to 4 weeks for a total of 117 weeks postinfection. Levels of circulating IgG antibodies (presented as OD405 values) were measured in sera by ELISA using the 12 recombinant proteins listed in Table 1. Kinetic antibody responses against the three most seroreactive antigens in these animals are shown. Preinfection OD405 values were 0.181 (ESAT-6), 0.199 (14-kDa protein), and 0.187 (MPT70) for animal 193 and 0.161 (ESAT-6), 0.190 (14-kDa protein), and 0.158 (MPT51) for animal 198. Standard error values were within 15% of mean OD405.
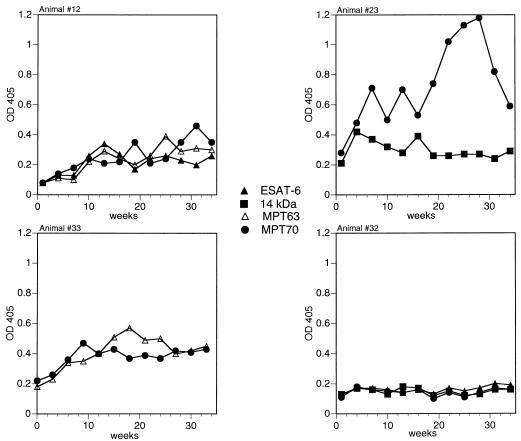
In the second and third experiments, circulating antibodies to several antigens were detected in sequential sera collected over a period of 34 weeks postinfection from three infected animals, 12, 23, and 33 (Fig. 2). Animal 12 recognized nine proteins (Table 2), with strong antibody responses against six (ESAT-6, 14-kDa protein, MPT63, MPT70, MPT64, and MPT32). Figure 2 shows kinetic data on antibody production to the three most seroreactive antigens in this animal. Sera from cattle 23 and 33 were reactive with five and two proteins, respectively (Table 2). Each of these animals produced high-level antibodies against only one antigen, MPT70 for animal 23 and MPT63 for animal 33 (Fig. 2). Time-course of antibody responses to individual antigens in animals 12, 23, and 33 involved multipeak changes, similar to those observed in animals 193 and 198. Three proteins, MTC28, Ag85B, and KatG, showed no seroreactivity in these animals.
FIG. 2.
Development of humoral immune responses to intranasal M. bovis infection in animals 12, 23, 33, and 32 (experiments 2 and 3). Cattle were infected intranasally with 106 CFU of M. bovis. Serum samples were collected preinfection and every 3 weeks for a total of 34 weeks postinfection. IgG antibodies were measured as described in the legend to Fig. 1. Kinetic antibody responses against two or three most seroreactive antigens in selected animals (12, 23, and 33) are shown. ELISA readings obtained for sera from an antibody nonresponder (animal 32) and three of the most seroreactive antigens in our panel are also presented. Preinfection OD405 values were 0.086 (ESAT-6), 0.089 (MPT63), and 0.088 (MPT70) for animal 12, 0.220 (14-kDa protein) and 0.269 (MPT70) for animal 23, 0.191 (MPT63) and 0.211 (MPT70) for animal 33, and 0.156 (ESAT-6), 0.150 (14-kDa protein), and 0.144 (MPT70) for animal 32. Standard error values were within 15% of mean OD405.
No or very modest elevation in serum reactivity to some antigens (ODI < 2) was found in three additional animals (32, 37, and 83) in the second and third experiments. Data for one such animal, 32, are included in Fig. 2. Sera were further analyzed by using unfractionated culture filtrate proteins to determine whether animals 32, 37, and 83 produced antibodies against antigens that were not tested in the present study. Only animal 32 had serum antibodies against culture filtrate proteins (data not shown). However, all three animals were infected, for they tested positive in assays measuring cell-mediated immune responses (proliferation and IFN-γ production by peripheral blood lymphocytes in response to stimulation with PPD in vitro) (data not shown).
Antibody responses to contact M. bovis infection.
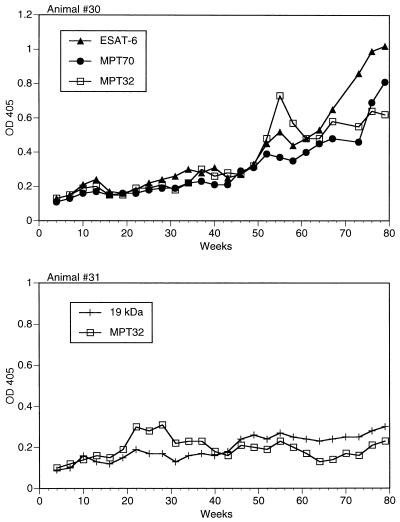
Two animals, 30 and 31, developed TB following exposure to intranasally infected cattle (see Materials and Methods). ELISA results obtained with sequential serum samples from these animals are presented in Fig. 3. In contrast to intranasally infected cattle, neither animal infected by contact developed significant antibody responses for 5 to 8 months postexposure. However, after the first year of infection, animal 30 developed a vigorous humoral immune response involving eight antigens (Table 2), six of which (ESAT-6, 14- and 19-kDa proteins, MPT70, MPT51, and MPT32) elicited high-level antibody production. In contrast, animal 31 generated poor antibody responses. In this animal, MPT32 was the only antigen that gave an ODI of ≥3 in ELISA with serum samples collected between weeks 22 and 28 postexposure (Fig. 3). Low-level antibody responses against ESAT-6 and 19-kDa protein were also detected at later stages of infection in animal 31. Neither animal recognized MTC28, Ag85B, 38-kDa protein, or KatG, the same four antigens that showed very low, if any, seroreactivity in intranasally infected cattle (Table 2).
FIG. 3.
Development of humoral immune responses during contact M. bovis infection. Airborne infection with M. bovis of animals 30 and 31 was achieved by keeping these cattle in close quarters with animals 193 and 198 infected intranasally 28 weeks earlier as described in the legend to Fig. 1. Serum samples were collected preexposure and every 3 weeks for a total of 79 weeks postexposure. IgG antibodies were measured as described in the legend to Fig. 1. Kinetic antibody responses against the two or three most seroreactive antigens for these animals are shown. Preinfection OD405 values were 0.123 (ESAT-6), 0.141 (MPT70), and 0.157 (MPT32) for animal 30 and 0.123 (19-kDa protein) and 0.131 (MPT32) for animal 31. Standard error values were within 15% of mean OD405.
Animal-to-animal heterogeneity of antigen recognition.
Characterization of the humoral immune response in cattle infected with M. bovis, intranasally or by exposure to experimental bovine TB, identified six antigens (ESAT-6, 14-kDa protein, MPT63, MPT70, MPT51, and MPT32) that were strongly recognized by serum IgG antibodies (Table 2). Detailed analysis of antigen recognition by individual sera revealed a remarkable variation of antibody responses in different animals. None of the seven responders recognized all six proteins as the most seroreactive antigens. Indeed, no single antigen or set of antigens was recognized by all antibody responders. The number of antigens involved in high-level antibody responses also varied markedly, from one (in animals 23, 31, and 33) to six (in animals 12 and 30). No association was found between the magnitude of the immune response and the number of antigens recognized. For example, the highest level of antibody to MPT70 in this study was detected in sera from animal 23 that strongly recognized only this antigen (Fig. 2).
Animal-to-animal differences could also be seen in the kinetics of antibody production. As shown in Fig. 1, the multiple spikes in the levels of antibodies to various antigens in animal 193 appeared synchronized, while in animal 198, after an initial peak, changes in antibody responses to different antigens, such as the 14-kDa protein and MPT51, often seemed reciprocal. In addition, even though essentially the same antigens were recognized by both animals, the individual levels of serum antibodies against some of these antigens varied from animal to animal and from antigen to antigen. For instance, the 14-kDa protein was the most prominent B-cell antigenic target in animal 198 but not in animal 193, whose sera recognized equally well two other antigens, ESAT-6 and MPT70 (Fig. 1). Similar examples are provided by antibody responses in other animals. Three antigens, MPT63, MPT64, and MPT70, were equally reactive with sera of animal 12, only MPT70 was strongly seroreactive in animal 23, and MPT63 was predominantly recognized by animal 33 (Fig. 2). Likewise, in contact infection, preferential recognition of ESAT-6, MPT70, or MPT32 could be seen at various time points in animal 30, whereas MPT32 was the only antigen recognized by high-level antibodies in animal 31 (Fig. 3).
Stage-dependent variation of antigen recognition during infection.
Kinetic analysis of antibody responses to individual antigens in animal 30 revealed changes in the antigen immunodominance pattern during infection (Fig. 3). MPT32 was recognized predominantly by serum samples collected at weeks 55 to 58 postinfection. A few weeks later, however, the anti-MPT32 antibody response declined, while antibody production to ESAT-6 increased. As a result, after week 61, ESAT-6 elicited the highest levels of specific IgG antibody in this animal. A similar but less striking phenomenon occurred in other infected cattle. A switch in preferential antigen recognition was observed in animals 31 (from MPT32 to 19-kDa protein) (Fig. 3) and 33 (from MPT70 to MPT63) (Fig. 2). A multiantigen shift (ESAT-6 to MPT70 to MPT63 to MPT70) was seen in animal 12 (Fig. 2).
DISCUSSION
This analysis of kinetic antibody responses in cattle experimentally infected with M. bovis demonstrates that the humoral immune response during bovine TB involves multiple protein antigens and is characterized by a marked temporal and animal-to-animal variation in antigen recognition. We have identified the largest number of serologically reactive antigens to date. Six of eleven seroreactive antigens, ESAT-6, 14-kDa protein, MPT63, MPT70, MPT51, and MPT32, were capable of eliciting high-level antibody production in most responders. Except for MPB70, a major secreted protein of M. bovis (13, 18, 21), these antigens have not previously been serologically characterized in cattle. It is worth noting that seroreactive antigens identified in the present work elicited high-level antibodies during intranasal and contact infection with M. bovis. Although the onset of the immune response is delayed in contact versus intranasal infection, the set of most seroreactive antigens is essentially maintained, indicating that our experimental findings should apply to naturally acquired bovine TB.
This work, along with our serological analyses of human TB (26), indicates that important differences exist between human and bovine serological recognition. Some antigens, such as 19-kDa protein, 38-kDa protein, and MTC28, which were among those preferentially involved in human antibody responses, showed little or no seroreactivity in experimentally infected cattle. Other antigens—MPT63, MPT51, and MPT32—elicited only weak antibody production, and only in a few cases, in human disease. ESAT-6 and 14-kDa protein were the only antigens displaying potent seroreactivity in both human and bovine TB. Whether differences in antigen immunodominance are due to differential expression of the homologous genes by M. tuberculosis and M. bovis (39) or to the host-determined immune recognition, processing, and presentation of mycobacterial antigens remains to be determined.
This study demonstrates that the humoral immune response to M. bovis infection in cattle is characterized by highly heterogeneous antigen recognition, as also seen in human TB (26). This phenomenon was previously suggested in serological analyses using complex antigen preparations (4, 17) but never established. Using purified proteins, here we show that the number and pattern of antigens recognized and the magnitude of antibody responses to individual antigens vary markedly from animal to animal. Furthermore, the kinetic analysis of antibody responses to individual antigens reveals a remarkable shift in antigen immunodominance pattern during disease in the same host. This stage-associated variation of antigen recognition in the immune response could be a consequence of differential production of mycobacterial proteins in the course of infection.
Experimental infection of cattle with M. bovis represents a valuable animal model to investigate TB in humans. The long-term kinetic studies of serum antibody production in controlled bovine experiments should help clarify the humoral immune response in human TB. For example, the finding of a substantial degree of diversity in recognition of multiple antigens by serum antibodies in cattle experimentally infected with a single strain of M. bovis indicates that the development of heterogeneous antibody responses in TB is not due merely to antigenic variation among strains of the pathogen. As previously suggested (26), the immunogenetic background of the infected host is most likely to be a key determinant of variable multiantigen recognition in TB.
In conclusion, the findings presented above indicate that the humoral immune response in experimental bovine TB involves multiple antigens that are differentially recognized in different animals and at different stages of disease in the same host. The extensive variation of antigen recognition established in this study implies that improved serodiagnostic test for bovine TB can be developed only by rational design of carefully selected multiantigen cocktails covering a broad spectrum of antibody specificities. Several challenges must be met to develop a multiantigen cocktail for the accurate diagnosis of bovine TB. First, to obtain high diagnostic specificity, ideal candidates for inclusion in a cocktail should be antigens that are serologically specific for the M. tuberculosis complex. In the case of antigens sharing B-cell epitopes with nontuberculous mycobacteria (MPT32 has this property [27]), peptides representing immunodominant, M. tuberculosis-specific epitopes could be used in lieu of full-length proteins. Second, should optimal serologic activity of certain antigens be affected by posttranslational modification of protein (e.g., MPT32 is a glycoprotein [9]), antigen production could utilize recombinant techniques in fast-growing, nonpathogenic mycobacteria rather than E. coli. Development of a multiantigen-based serodiagnostic approach for bovine TB is in progress.
ACKNOWLEDGMENTS
We thank the members of the Bacteriology and Pathology Departments of the Veterinary Science Division who were involved with the experimental model of infection. We also thank Karl Drlica for comments on the manuscript.
R.C. was the recipient of an AIDS training fellowship from the Istituto Superiore di Sanitá, Rome, Italy. We gratefully acknowledge support from NIH grant AI-36896 (M.L.G.).
REFERENCES
- 1.Andersen Å B, Hansen E B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989;57:2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashbridge K R A, Booth R J, Watson J D, Lathigra R B. Nucleotide sequence of the 19kDa antigen gene from Mycobacterium tuberculosis. Nucleic Acids Res. 1989;17:1249. doi: 10.1093/nar/17.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bigi F, Espitia C, Alito A, Zumarraga M, Romano M I, Cravero S, Cataldi A. A novel 27 kDa lipoprotein antigen from Mycobacterium bovis. Microbiology. 1997;143:3599–3605. doi: 10.1099/00221287-143-11-3599. [DOI] [PubMed] [Google Scholar]
- 4.Cataldi A, Romano M I, Bigi F. A Western blot characterization of Mycobacterium bovis antigens recognized by cattle sera. Res Microbiol. 1994;145:689–698. doi: 10.1016/0923-2508(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 5.Colangeli R, Heijbel A, Williams A, Manca C, Chan J, Lyashchenko K, Gennaro M L. Three-step purification of lipopolysaccharide-free, polyhistidine-tagged recombinant antigens of Mycobacterium tuberculosis. J Chromatogr. 1998;714:223–235. doi: 10.1016/s0378-4347(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 6.Daborn C J, Grange J M, Kazwala R R. The bovine tuberculosis cycle—an African perspective. J Appl Bacteriol. 1996;81:27S–32S. doi: 10.1111/j.1365-2672.1996.tb04595.x. [DOI] [PubMed] [Google Scholar]
- 7.Daniel T M, Debanne S M. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am Rev Respir Dis. 1987;135:1137–1151. doi: 10.1164/arrd.1987.135.5.1137. [DOI] [PubMed] [Google Scholar]
- 8.Dankner W M, Waecker N J, Essey M A, Moser K, Thompson M, Davis C E. Mycobacterium bovis infections in San Diego: a clinicoepidemiologic study of 73 patients and a historical review of a forgotten pathogen. Medicine. 1993;72:11–37. [PubMed] [Google Scholar]
- 9.Dobos K M, Swiderek K, Khoo K H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty M L, Monaghan M L, Bassett H F, Quinn P J. Effect of a recent injection of purified protein derivative on diagnostic tests for tuberculosis in cattle infected with Mycobacterium bovis. Res Vet Sci. 1995;58:217–221. doi: 10.1016/0034-5288(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 11.Essey M A, Koller M A. Status of bovine tuberculosis in North America. Vet Microbiol. 1994;40:15–22. doi: 10.1016/0378-1135(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 12.Fifis T, Corner L A, Rothel J S, Wood P R. Cellular and humoral immune responses of cattle to purified Mycobacterium bovis antigens. Scand J Immunol. 1994;39:267–274. doi: 10.1111/j.1365-3083.1994.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 13.Fifis T, Costopoulos C, Corner L A, Wood P R. Serological reactivity to Mycobacterium bovis protein antigens in cattle. Vet Microbiol. 1992;30:343–354. doi: 10.1016/0378-1135(92)90021-k. [DOI] [PubMed] [Google Scholar]
- 14.Fifis T, Rothel J S, Wood P R. Soluble Mycobacterium bovis protein antigens: studies on their purification and immunological evaluation. Vet Microbiol. 1994;40:65–81. doi: 10.1016/0378-1135(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 15.Grange J M, Daborn C, Cosivi O. HIV-related tuberculosis due to Mycobacterium bovis. Eur Respir J. 1994;7:1564–1566. doi: 10.1183/09031936.94.07091564. [DOI] [PubMed] [Google Scholar]
- 16.Hammam H, Refai M, Bisping W, Kirpal G. Studies on the efficiency of absorbed bovine PPD in tuberculin and serological tests. J Vet Med. 1989;36:175–179. doi: 10.1111/j.1439-0450.1989.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 17.Hanna J, Neill S D, O’Brien J J. ELISA tests for antibodies in experimental bovine tuberculosis. Vet Microbiol. 1992;31:243–249. doi: 10.1016/0378-1135(92)90082-5. [DOI] [PubMed] [Google Scholar]
- 18.Harboe M, Nagai S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am Rev Respir Dis. 1984;129:444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- 19.Harboe M, Nagai S, Patarroyo M E, Torres M, Ramirez C, Cruz N. Properties of MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harboe M, Nagai S, Wiker H G, Sletten K, Haga S. Homology between the MPB70 and MPB83 proteins of Mycobacterium bovis BCG. Scand J Immunol. 1995;42:46–51. doi: 10.1111/j.1365-3083.1995.tb03624.x. [DOI] [PubMed] [Google Scholar]
- 21.Harboe M, Wiker H G, Duncan J R, Garcia M M, Dukes T W, Brooks B W, Turcotte C, Nagai S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J Clin Microbiol. 1990;28:913–921. doi: 10.1128/jcm.28.5.913-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewinson R G, Michell S L, Russell W P, McAdam R A, Jacobs W R. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand J Immunol. 1996;43:490–499. doi: 10.1046/j.1365-3083.1996.d01-78.x. [DOI] [PubMed] [Google Scholar]
- 23.Heym B, Zhang Y, Pulet S, Young D, Cole S T. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J Bacteriol. 1993;175:4255–4259. doi: 10.1128/jb.175.13.4255-4259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lightbody K A, Skuce R A, Neill S D, Pollock J M. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet Rec. 1998;142:295–300. doi: 10.1136/vr.142.12.295. [DOI] [PubMed] [Google Scholar]
- 26.Lyashchenko K, Colangeli R, Houde M, Jahdali H A, Menzies D, Gennaro M L. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66:3936–3940. doi: 10.1128/iai.66.8.3936-3940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manca C, Lyashchenko K, Colangeli R, Gennaro M L. MTC28, a novel 28-kilodalton proline-rich secreted antigen specific for the Mycobacterium tuberculosis complex. Infect Immun. 1997;65:4951–4957. doi: 10.1128/iai.65.12.4951-4957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manca C, Lyashchenko K, Wiker H G, Usai D, Colangeli R, Gennaro M L. Molecular cloning, purification, and serological characterization of MPT63, a novel secreted antigen of Mycobacterium tuberculosis. Infect Immun. 1997;65:16–23. doi: 10.1128/iai.65.1.16-23.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto S, Matsuo T, Ohara N, Hotokezaka H, Naito M, Minami J, Yamada T. Cloning and sequencing of a unique antigen MPT70 from Mycobacterium tuberculosis H37Rv and expression in BCG using E. coli-mycobacteria shuttle vector. Scand J Immunol. 1995;41:281–287. doi: 10.1111/j.1365-3083.1995.tb03565.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular α-antigen. J Bacteriol. 1988;170:3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moda G, Daborn C J, Grange J M, Cosivi O. The zoonotic importance of Mycobacterium bovis. Tubercle Lung Dis. 1996;77:103–108. doi: 10.1016/s0962-8479(96)90022-2. [DOI] [PubMed] [Google Scholar]
- 32.Monaghan M L, Doherty M L, Collins J D, Kazda J F, Quinn P J. The tuberculin test. Vet Microbiol. 1994;40:111–124. doi: 10.1016/0378-1135(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 33.Neill S D, Hanna J, O’Brien J J, McCraken R. Excretion of Mycobacterium bovis by experimentally infected cattle. Vet Rec. 1988;123:340–343. doi: 10.1136/vr.123.13.340. [DOI] [PubMed] [Google Scholar]
- 34.O’Reilly L M. Tuberculin skin tests: sensitivity and specificity. In: Thoen C O, Steele J H, editors. Mycobacterium bovis infection in animals and humans. Ames, Iowa: Iowa State University Press; 1995. pp. 85–91. [Google Scholar]
- 35.O’Reilly, L. M., and C. J. Darborn. 1995. The epidemiology of Mycobacterium bovis infection in animals and man: a review. Tubercle Lung Dis. 76(Suppl. 1):1–46. [DOI] [PubMed]
- 36.Ohara N, Kitaura H, Hotokezaka H, Nishiyama T, Wada N, Matsumoto S, Matsuo T, Naito M, Yamada T. Characterization of the gene encoding the MPB51, one of the major secreted protein antigens of Mycobacterium bovis BCG, and identification of the secreted protein closely related to the fibronectin binding 85 complex. Scand J Immunol. 1995;41:433–442. doi: 10.1111/j.1365-3083.1995.tb03589.x. [DOI] [PubMed] [Google Scholar]
- 37.Sørensen A L, Nagai S, Houen G, Andersen P, Andersen Å B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbon A, Hartskeerl R A, Schuitema A, Kolk A H J, Young D B, Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992;174:1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiker H G, Nagai S, Hewinson R G, Russell W P, Harboe M. Heterogeneous expression of the related MPB70 and MPB83 proteins distinguish various substrains of Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv. Scand J Immunol. 1996;43:374–380. doi: 10.1046/j.1365-3083.1996.d01-61.x. [DOI] [PubMed] [Google Scholar]
- 40.Wood P R, Corner L A, Rothel J S, Ripper J L, Fifis T, McCormick B S, Francis B, Melville L, Small K, Witte K D, Tolson J, Ryan T J, deLisle G W, Cox J C, Jones S L. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet Microbiol. 1992;31:71–79. doi: 10.1016/0378-1135(92)90142-g. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi R, Matsuo K, Yamazaki A, Abe C, Nagai S, Teresaka K, Yamada T. Cloning and characterization of the gene for immunogenic protein MPB64 of Mycobacterium bovis BCG. Infect Immun. 1989;57:283–288. doi: 10.1128/iai.57.1.283-288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]