Abstract
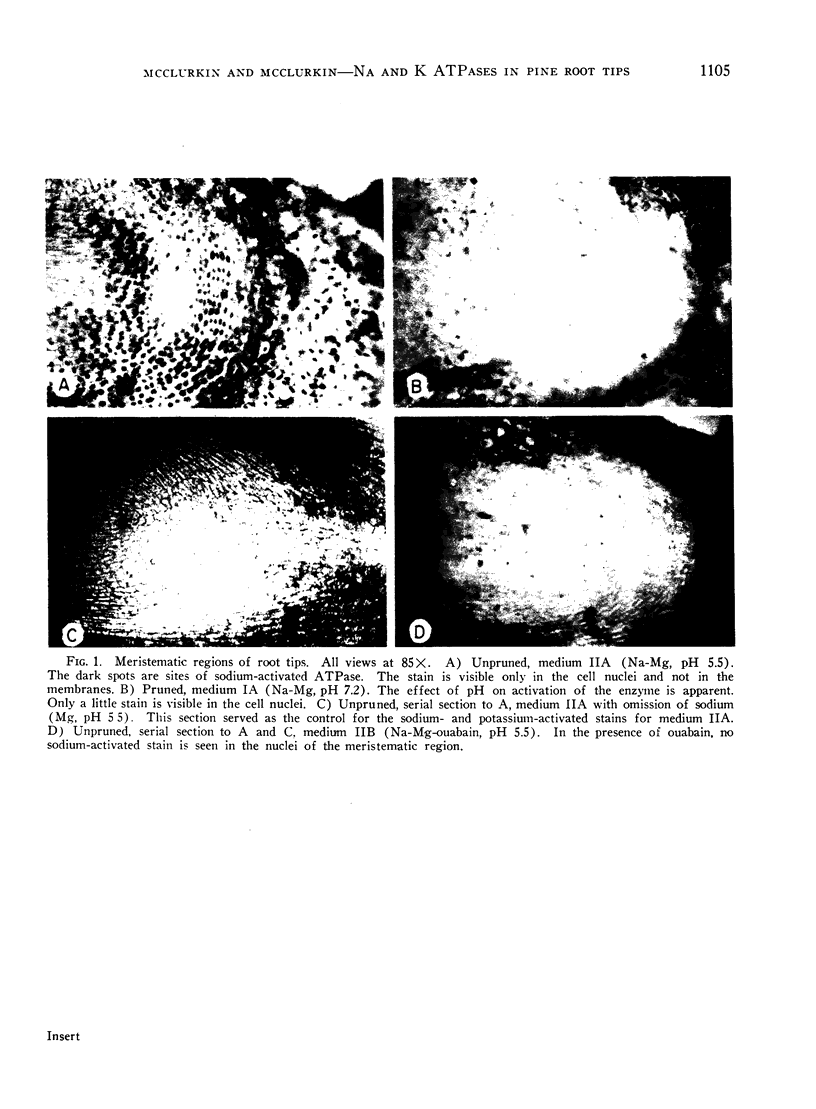
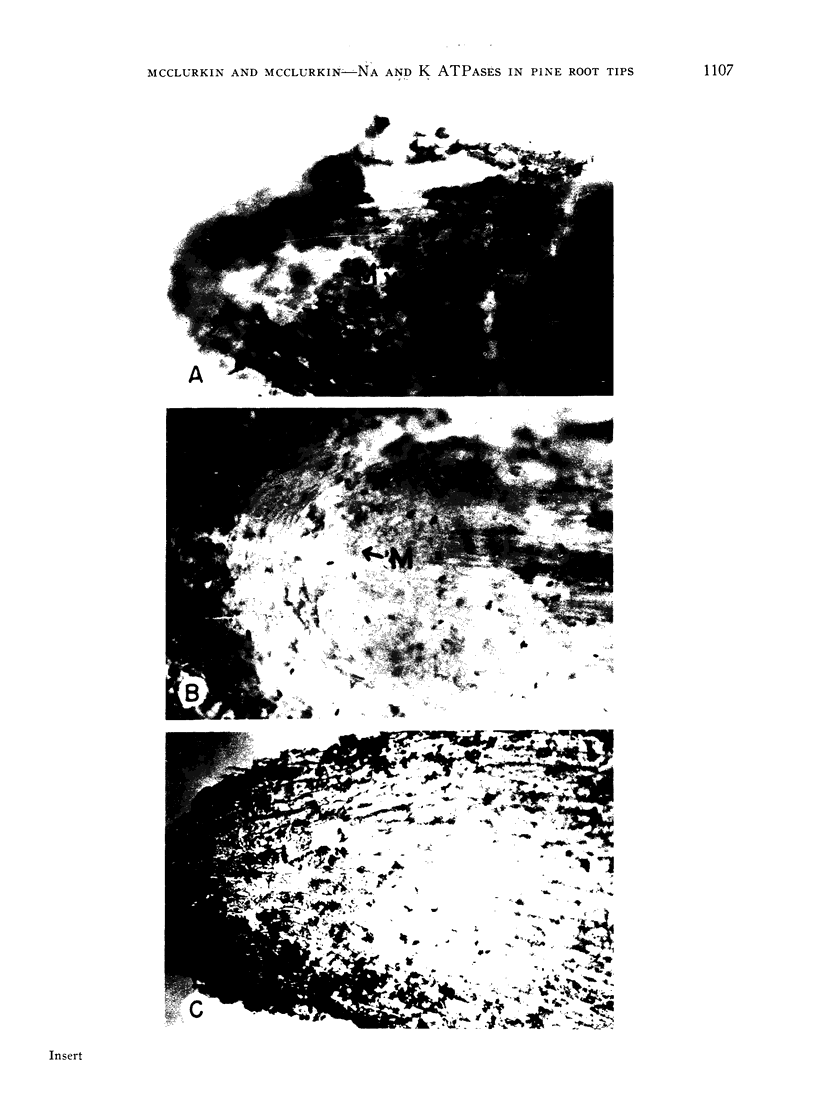
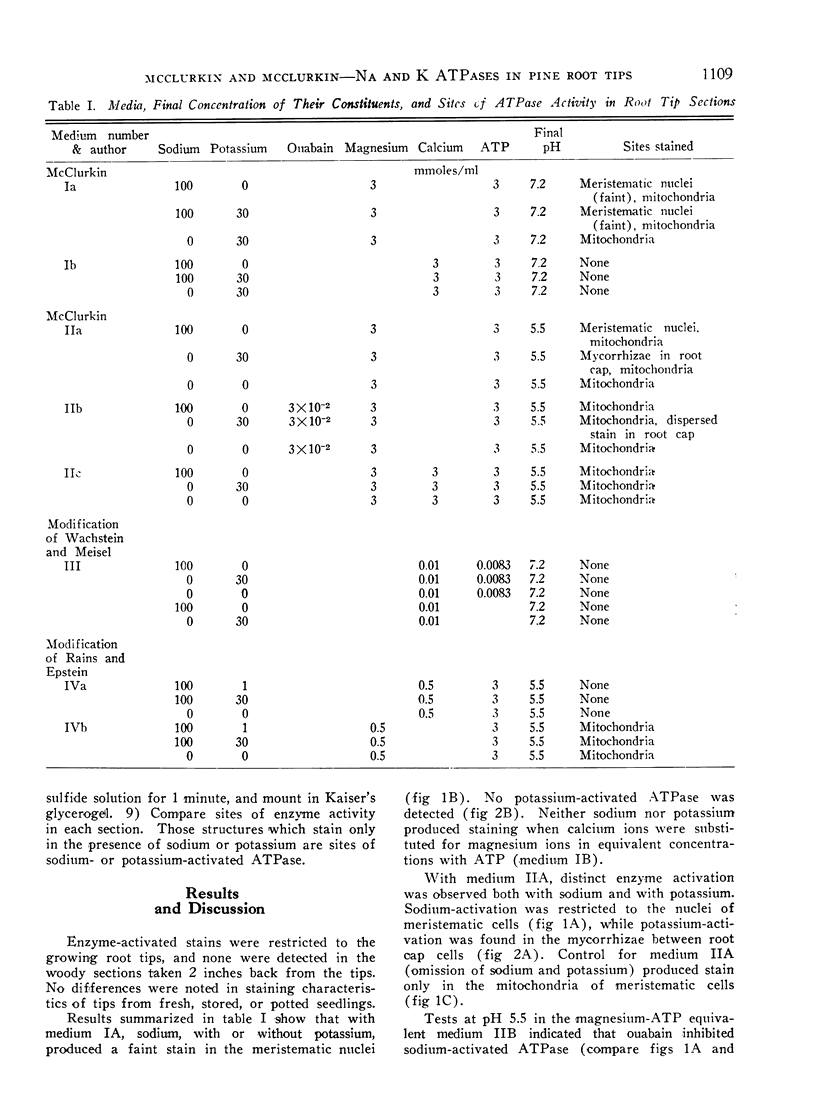
Sodium stimulated ATPase activity in the nuclei of the meristematic cells, while potassium stimulated it in the mycorrhizae between root cap cells. The detection of these 2 mutually exclusive cation-stimulated ATPases, which both require magnesium-ATP in equivalents, which have a similar optimum pH of about 5.5, and which are located in entirely different parts of the root tip, suggests that particular enzyme systems can only be activated by a specific cation and that one cation cannot substitute for the other. Such a feature may explain the capacity of plants to differentiate between ions as closely similar as sodium and potassium. The similarities between the enzyme system described for salt transport in animal tissues and that depicted here cytochemically in pine roots at the most active site of salt uptake in roots indicate this may be a carrier mechanism for salt entry into plant roots. The presence of the potassium-activated enzyme only in the mycorrhizae may relate to the dependence of pine trees on mycorrhizae for growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERG G. G. THE STAINING OF TRIPHOSPHATASES BY THE CHELATE REMOVAL METHOD. J Histochem Cytochem. 1964 May;12:341–358. doi: 10.1177/12.5.341. [DOI] [PubMed] [Google Scholar]
- HENDRICKS S. B. SALT TRANSPORT ACROSS CELL MEMBRANES. Am Sci. 1964 Sep;52:306–333. [PubMed] [Google Scholar]
- MCCLURKIN I. T. A METHOD FOR THE CYTOCHEMICAL DEMONSTRATION OF SODIUM-ACTIVATED ADENOSINE TRIPHOSPHATASE. J Histochem Cytochem. 1964 Sep;12:654–658. doi: 10.1177/12.9.654. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., DRUCKER J., SHIN W. Y., GOLDFISCHER S. Further studies of the apparent adenosinetriphosphatase activity of cell membranes in formol-calcium-fixed tissues. J Histochem Cytochem. 1961 Jul;9:434–451. doi: 10.1177/9.4.434. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., GOLDFISCHER S., ESSNER E. The importance of fixation in a cytochemical method for the Golgi apparatus. J Histochem Cytochem. 1961 Jul;9:459–460. doi: 10.1177/9.4.459. [DOI] [PubMed] [Google Scholar]
- Rains D. W., Epstein E. Transport of Sodium in Plant Tissue. Science. 1965 Jun 18;148(3677):1611–1611. doi: 10.1126/science.148.3677.1611. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WHEELER K. P. The sensitivity of a kidney ATPase to ouabain and to sodium and potassium. Biochim Biophys Acta. 1961 Aug 19;51:622–624. doi: 10.1016/0006-3002(61)90633-3. [DOI] [PubMed] [Google Scholar]