Abstract
Black carrots are a type of carrot that is naturally dark purple or black in color. They are a good source of antioxidants, vitamins, and minerals, and have been shown to have several health benefits, including reducing the risk of cancer, heart disease, and diabetes. This review article discusses the bioactive compounds present in black carrot, including anthocyanins, phenolic acids, carotenoids, and organic acids and sugars. It also compares the bioactive compounds and antioxidant capacity of black carrot with other carrot varieties. Furthermore, it discusses various postharvest processing methods, both conventional and novel, such as encapsulation, drying, and microbial decontamination, highlighting their effects on preserving and stabilizing the bioactive compounds. The review also emphasizes the incorporation of black carrot into different food products, including dairy items, beverages, and baked goods, and their impact on nutritional enhancement. The article provides knowledge on utilizing black carrot for improved nutritional and functional outcomes.
Keywords: Carrot, Phytochemical, Anthocyanin, Health promoting, Non-thermal processing, Value added products
Introduction
Black carrot (Daucus carota L.), a member of the Apiaceae family native to Turkey, is also cultivated and consumed in eastern nations such as India, Afghanistan, and Pakistan (Büyükdinç et al., 2019; Kamiloglu et al., 2018). Black carrots are a good source of fibres, sugars, vitamins and minerals, and they have a characteristic bluish-purple colour due to anthocyanins (water soluble), which are the main pigments present in them (Algarra et al., 2014; Pandey and Grover, 2020).
The type of compounds a carrot contains is typically determined by its colour. Carotenoid and flavonoid content of black carrot is higher than that of orange and red carrots (Chhetri et al., 2022; Ahmad et al., 2019). Both the Codex alimentarius and the US Food and Drug Administration have classified anthocyanin pigments as natural colourants. These pigments are the most common group responsible for the red, blue, and purple hues in vegetables and fruits. Black carrots are frequently regarded as a potential source of natural food colour and a promising replacement for synthetic colours due to the presence of anthocyanins in them (Shamshad et al., 2023).
As more people have become aware of the importance of these vegetables in a healthy diet, there has been an increase in the production of anthocyanin-containing vegetables (Büyükdinç et al., 2019). Black carrots are an excellent source of phytochemicals, including ascorbic acid, carotenoids, polyacetylenes and phenolic compounds (Chhetri et al., 2022; Ahmad et al., 2019). The high concentration of anthocyanins and other phenolic acids in black carrots is crucial for lowering the risk of disease (Garba and Kaur, 2014).
So far, black carrots have been associated with over 40 different phenolic acids, with 5-O-caffeoylquinic acid being the main one. Moreover, many hydroxybenzoic acid derivatives, ferulic acid and caffeic acid derivatives and a quercetin glycoside, have been found in black carrots. Anthocyanins recovered from cell suspension cultures of black carrots include two non-acylated anthocyanins (cyanidin-3-xylosyl (glucosyl) galactoside and cyanidin-3-xylosylgalactoside), along with three mono-acylated anthocyanins [cyanidin-3-xylosyl (coumaroyl-glucosyl) galactoside, cyanidin 3-xylosyl (sinapoyl-glucosyl) galactoside and cyanidin-3-xylosyl(feruloyl-glucosyl) galactoside] (Koley et al., 2019).
In a study conducted by Smeriglio et al. (2018) 25 polyphenols were identified in black carrot crude extract (anthocyanins 78.06%, phenolic acids 17.89%, and other flavonoids 4.06%), with polyglycosylated cyanidins as the main constituents. Black carrots are considered functional food due to their therapeutic health-promoting qualities, including antidiabetic, anti-inflammatory, anti-cancerous, anti-cardiovascular and antioxidant properties (Chhetri et al., 2022; Kamiloglu et al., 2017; Sevimli-Gur et al., 2013).
Carrots have a moisture content of 85–90%, which is lost due to transpiration after harvest. Transpiration alters the skin texture and affects the appearance of the product, causing wrinkling (Caron et al., 2003). Processing black carrots using various technologies can help in increasing shelf life, tackling seasonality, improve nutritional value and produce convenient products (Kamiloglu et al., 2018). Drying technologies like spray drying and freeze drying have successfully encapsulated black carrot extracts and formed stable powder (Murali et al., 2015). Thermal treatments like pasteurization of black carrots have a negative effect by reducing anthocyanin content (Türkyilmaz et al., 2012).
Emerging techniques like high pressure processing (Agcam et al., 2021), microwave (Barroso et al., 2023), ultrasound (Kaur et al., 2023a), and irradiation (Ghidouche et al., 2013) have effectively reduced microbial load, assisted in extraction of bioactive compounds, improved drying rates, and increased recovery of bioactive volatile compounds non thermally from black carrots (Hasheminya and Dehghannya, 2022; Kumar et al., 2019). Further value addition of the produce can improve shelf-life, help tackle perishability and seasonality (Dhiman et al., 2021b). Black carrot has been utilized to develop various value-added products like bread (Pekmez and Yılmaz, 2018), jam and marmalade (Kamiloglu et al., 2015), kanji (Kaur et al., 2023b), shalgam juice (Tanguler et al., 2014a), cakes (Song et al., 2018), chips (Nath et al., 2022; Yılmaz and Ersus Bilek, 2018), noodles (Singh et al., 2018a) and dairy products (Pandey et al., 2021). These products may help consumers receive the therapeutic benefits of black carrots in different forms.
Considering the above, present review aims to summarize the bioactive composition of black carrots along with their medicinal value. The current review also discusses the technologies used for processing black carrots for the purpose of drying, extraction, encapsulation or preservation and formulation of various convenient and value-added products. This information will promote the cultivation and further processing of black carrots while broadening its research aspect.
Overview of nutritional and bioactive composition of black carrot: functions and health benefits
Nutritional composition
Black carrot is an excellent source of dietary fibres (2.35–2.61 g/100 g) and sugars (11.07%), along with various vitamins and minerals (Büyükdinç et al., 2022), as tabulated (Table 1). It is a rich source of multivitamins such as niacin, thiamine, riboflavin and ascorbic acid, as well as minerals like calcium, phosphorus, salt, potassium, magnesium, iron, and zinc. Carotenoids in black carrot are present in a highly reduced form (Pandey and Grover, 2020). The major pigment found in black carrot is anthocyanin (350 mg/100 g), which is also responsible for its deep purplish colour. Compared to red carrots, black carrots are four times richer in anthocyanins and flavonols, exhibiting high antioxidant activity (Singh et al., 2018b). Thus, black carrots are a great source of polyphenols that possess a high capacity to scavenge free radicals. Researchers from various countries have begun to focus on the potential health benefits of black carrots due to their rich nutritional profile, particularly their higher phenolic content, especially anthocyanins, and the growing demand for them as a natural colourant (Koley et al., 2019).
Table 1.
Nutritional value of black carrot
| Nutrients | Content (per 100 g) |
|---|---|
| Energy (kcal) | 41–43 |
| Moisture (g) | 88 |
| Protein (g) | 1 |
| Fat (g) | 0.14 |
| Ash (g) | 0.26–0.92 |
| Fibre, total dietary (g) | 2.35–2.61 |
| Water soluble (g) | 0.84–0.97 |
| Water insoluble (g) | 1.51–1.65 |
| Total sugar (%) | 11.07 |
| Glucose (g) | 1.85 |
| Fructose (g) | 0.14 |
| Reducing sugar (%) | 0.77 |
| Minerals (mg) | |
| Potassium | 240–320 |
| Sodium | 40–86 |
| Calcium | 34–80 |
| Phosphorous | 21–53 |
| Magnesium | 9–12 |
| Iron | 0.30–2.2 |
| Zinc | 0.2–0.24 |
| Copper | 0.02–0.17 |
| Manganese | 0.143 |
| Vitamins (mg) | |
| Niacin | 0.2 |
| Thiamine | 0.04 |
| Riboflavin | 0.02 |
| Pigments (mg) | |
| Anthocyanin | 350 |
| Carotenes | 5.33 |
Phytochemistry: bioactive compounds
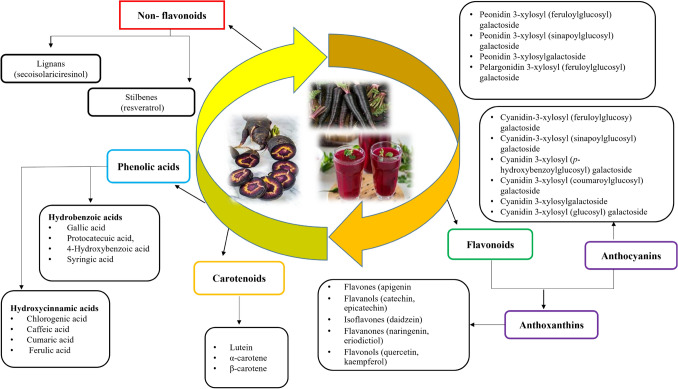
Phytochemicals are the bioactive compounds enriched in nutrients that contribute to the health-related benefits. Phenolic compounds, polyacetylene, carotenoid and ascorbic acid are the four major phytochemicals present in carrots (Ahmad et al., 2019). The bioactive compounds present in black carrots, along with their chemical structures, are presented in Figs. 1 and 2. The predominant polyphenols present in black carrots are anthocyanin and phenolic acids (Kamiloglu et al., 2018). Singh et al. (2018b) found that black carrots are rich in flavonoid and phenolic compounds and are the best source of anthocyanins, which are responsible for colorant properties, nutraceutical values and anti-oxidative potential.
Fig. 1.
Cyclic representation of various bioactive compounds found in black carrot [Adapted from: (Akhtar et al., 2017; Blando et al., 2021; Keskin et al., 2021; Koley et al., 2019; Polat et al., 2022; Smeriglio et al., 2018)]
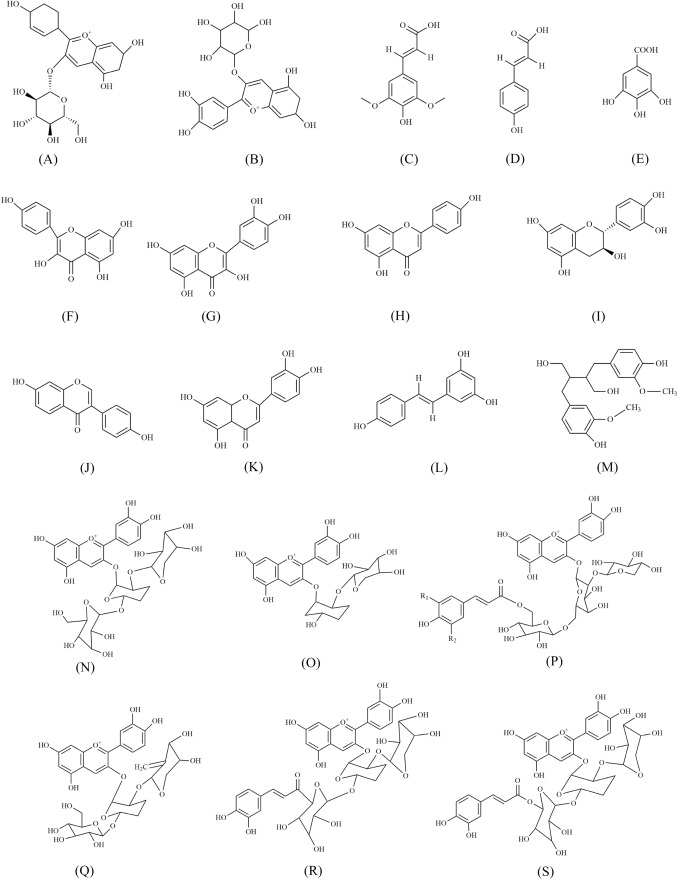
Fig. 2.
Chemical structures of major bioactive constituents of black carrot A Chlorogenic acid, B Cyanidin 3-glucoside, C Sinapic acid, D p-coumaric acid, E Gallic acid, F Kamepferol, G Quercetin, H Apigenin, I Catechin, J Daidzein, K Naringenin, L Stilbenes (resveratrol), M Lignans (Secoisolariciresinol), N Cyanidin 3-xylosylglucosylgalactoside, O Cyanidin 3-xylosylgalactoside, P R1 = R2 = OCH3: Sinapic acid derivative of Cyanidin 3-xylosylglucosylgalatoside; R1 = H; R2 = OCH3: Ferulic acid derivative of Cyanidin 3-xylosylglucosylgalactoside; R1 = R2 = H: Coumaric acid derivative of Cyanidin 3-xylosylglucosylgalactoside, (Q) Peonidin 3-xylosylglucosylgalactoside, (R) Ferulic acid derivative of Pelargonidin 3-xylosylgalactoside and (S) Ferulic acid derivative of Peonidin 3-xylosylglicosylgalactoside. [Redrawn from: (Akhtar et al., 2017; Pandey and Grover, 2020)]
Legal requirements and the rise in consumers preference for natural foods have led to an increase in the use of plant-based hues like anthocyanins instead of chemical additives, and especially pigments (Suzme et al., 2014). Due to their ability to promote health by lowering the risk of atherosclerosis, cancer, diabetes, and neuro-degenerative illnesses, interest in anthocyanins has increased. Consuming a lot of anthocyanin-rich foods has been linked to potential health benefits for conditions like cancer, ageing, neurological diseases, inflammation, diabetes, and bacterial infections (Pala et al., 2017).
Phenolic compounds
Phenolic compounds constitute a large group of secondary plant metabolites, including tannins, lignans, stilbenoids, curcuminoids, phenolic acids and flavonoids (Singh et al., 2018b). The genotype of black coloured carrots is a rich source of free phenolics (ranging from 31.9 to 290.0 mg GAE/100 g Fresh weight). The majority of phenols were found in soluble or free form in the black carrot genotype. Bound phenolics are extracted by further alkaline hydrolysis of the extract residue and are primarily found in ester form, associated with cell-wall components that provide structural stability. Due to their resistance to upper gastrointestinal digestion and subsequent release in the colon, these bound phenolics have special health implications. The majority of the bound phenolics from these materials may be released through colonic digestion by bacteria at this level, where they may have a good impact on one's health.
Chlorogenic acids, which are hydroxycinnamic acid derivatives produced by the esterification of cinnamic acids like caffeic and ferulic, are the primary phenolic compounds present in carrots (Chhetri et al., 2022). In addition, many hydroxybenzoic acid derivatives, ferulic acid and caffeic acid derivatives, a quercetin glycoside, and a few others were found in black carrots (Kamiloglu et al., 2018). Kumar et al. (2019) reported that the high amount of phenolic compounds are found in black carrot can be used as a functional food to prevent or treat a variety of lifestyle disorders.
Non-flavonoids with one functional carboxylic acid group are phenolic acids. Based on their chemical composition, they can be divided into two subgroups: hydroxycinnamic and hydroxybenzoic acids. Although the fundamental structure of both phenolic acids is the same, there is a difference in the number and position of hydroxyl groups on the aromatic ring of phenols. Gallic acid is a prominent example of a hydroxybenzoic acid, having a C6–C1 structure, whereas hydroxycinnamic acids are aromatic compounds with a three-carbon side chain (C6–C3). Common examples of hydroxycinnamic acids include caffeine, ferulic, p-coumaric, and sinapic acids. The main phenolic acid in black carrots was 5-O-caffeoylquinic acid, an ester of caffeic and quinic acid. Several studies have shown that compared to other coloured root vegetables, black carrot roots exhibited a much higher level of phenolic content (Kamiloglu et al., 2018). In a study, Singh et al. (2018b) reported the predominant compound (5-O-caffeoylquinic acid) along with other forty phenolic acids in black carrot. In addition, many hydroxybenzoic acid derivatives, ferulic acid, p-coumaric acid, caffeic acid derivatives, one quercetin glycoside, polyacetylenes, falcarindiol, falcarnidiol-3-acetate and anthocyanins (cyanidin, delphinidin, peonidin, pelargonidin, petunidin and malvidin) (Fig. 2) have been reported in black carrots (Kumar et al., 2019; Akhtar et al., 2017).
In terms of chlorogenic acid content, black carrots exhibited the highest level among genotypes, yielding approximately 7.5 mg of CGA g−1 DW. Chlorogenic acid accounted for 30% of all phenolics in black carrot. Among the phenolic components, black carrots contain a larger amount of (CGA) chlorogenic acid (from 57 to 72.5%), depending on the variety (Blando et al., 2021). The highest antioxidant and reducing capacities were found in black carrot (TEAC: 76.6 ± 10.6 μmol TE g−1 DW; ORAC: 159.9 ± 3.3 μmol TE g−1 DW), both of which were attributed to high polyphenol content (FCR value: 16.6 ± 1.1 μmg GAE g−1 DW; TEAC: 76.6 ± 10.6 μmol TE g−1 DW (Blando et al., 2021).
Anthocyanin
Anthocyanins belong to the flavonoid family. Black carrot is a unique agricultural product because of its colour and strong anthocyanin concentration, which exhibits extremely high stability while maintaining its red colour over a broad pH range. Structurally, they are based on the 2-phenylbenzopyrylium, polyhydroxy or polymethoxy derivatives (flavylium ion) (Carrillo and Kamiloglu, 2020; Zadernowski et al., 2010). More than half of the anthocyanins found in black carrots have an acylated structure, with cyanidin-3-xylosyl-feruloyl-glucosyl-galactoside being the most prevalent anthocyanin (Kamiloglu et al., 2018). Black carrots contain aproximately 1750 mg kg−1 anthocyanin (Talih et al., 2017). In black carrots, the monoacylated anthocyanin boost anti-oxidant activity by maintaining the colour of foods at their natural pH (Büyükdinç et al., 2022). Although author have reported a range from 1.5 to 243 mg cyanidin-3-glucoside (C3G)/100 g FW, and the total monomeric anthocyanin content in the black carrot cultivar varied from 7.4 to 83.4 mg C3G/100 g FW. A coumaryl acid derivative of cyanidin 3-xylosyl (glucosyl) galactoside, accounted for 11% of the total anthocyanins; all values were comparable to the percentage peaks area reported for cv. Antonina and cv. Deep Purple. The major anthocyanin, a ferulic acid derivative of cyanidin 3-xylosyl accounted for 56% of total anthocyanin (Blando et al., 2021; Chhetri et al., 2022).
In black carrot, four primary anthocyanins were identified. Cyanidin 3-feruloyl-xylosyl-glucosyl-galactoside (13.5%) and cyanidin 3-sinapoyl-xylosyl-glucosyl-galactoside (27.5%) were the acylated anthocyanins that made up 41% of the anthocyanins. Approximately 55–99% of actylated anthocyanin were found in different cultivars of black carrot, with 25% and 55% of acylated anthocyanins present in purple haze and antonina varieties, respectively. Antioxidant properties of black carrot extracts were found to be significantly higher than those of previously used orange carrots. Compared to cherry and blood orange, anthocyanins in black carrot are more heat stable due to the fact that that are acylated anthocyanins. These acylated anthocyanins are extremely stable due to their intramolecular co-pigmentation capabilities. In in vitro assays, anthocyanins from black carrot extract have demonstrated high antioxidant activity. In black carrots, anthocyanins alone account for around half of the overall phenolic content (Akhtar et al., 2017; Algarra et al., 2014; Pandey and Grover, 2020). "Black Wonder" and "Pusa Asita" exhibited anthocyanin contents of 0.62 and 11.46 mg/100 g, respectively, on the 45th day of sowing. After 108 days of cultivation, the anthocyanin content of "Pusa Asita" and "Black Wonder" increased to 519.39 and 3.2946 mg/100 g, respectively (Kaur and Sogi, 2020). A high level of anthocyanin was found in black carrots with a solid colour compared to balck carrot with a yellow interior (Yoo et al., 2020). In a study by Yoo et al (2020), it was found that anthocyanins present in black carrots were highly correlated with their antioxidant activity.
Carotenoid
Carotenoid, a lipophilic molecule that is the primary bioactive component of most carrots, is present in black carrots, but only in very small amounts. Small amounts of carotene and lutein were identified in black carrots with black cortex and pith (Pandey and Grover, 2020). Similar amounts of carotene and lutein were found in black carrots with black surface but yellow interior. α-Carotene was present in negligible amounts in black carrots, while β-carotene was found in traces (Yoo et al., 2020).
All carrot genotypes (mainly orange carrots) had high levels of carotenoids (Black carrot: 0.14 ± 0.024 mg g−1 DW; Polignano carrot: 0.33 ± 0.038 mg g−1 DW; Orange carrot: 1.29 ± 0.09 mg g−1 DW), with orange carrots having the highest levels of α, and β -carotene and black carrots having the highest levels of lutein. Purple-yellow carrots were observed to have ten times less α and β-carotene concentration than black and orange carrots. However, contradictory findings have also been reported, with purple carrots having two times the amount of α and β -carotene or a similar amount, compared to orange carrots. Overall carrot carotenoid concentration may vary substantially depending on environmental and hereditary factors (Blando et al., 2021). The carotenoid concentration of the juices from black and yellow carrots likewise showed a significant difference (Purkiewicz et al., 2020).
Therapeutic value
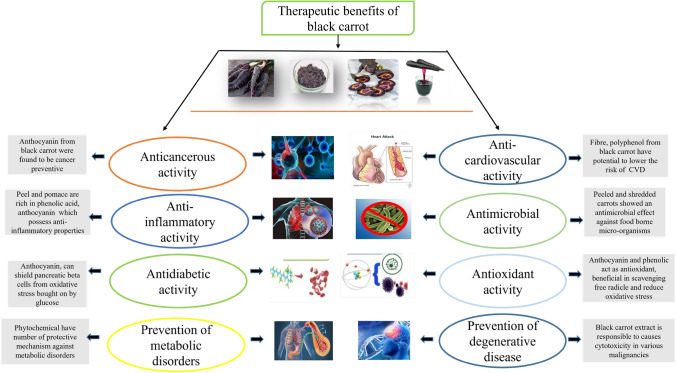
Plants contain a variety of secondary metabolites called polyphenols, including lignin, tannins, phenolic acids, flavonoids, and countless derivatives. These are currently used in numerous food or pharmaceutical applications and are crucial to a variety of plant activities (Frond et al., 2019). Due to their high antioxidant activity, polyphenols, which are naturally occurring substances present in black carrot (Fig. 3), have received extensive research as helpful substances for health (Gu et al., 2020). Phytochemicals found in black carrots play important role in metabolic conditions caused by oxidative stress (Blando et al., 2021). The fundamental components that influence the health benefits of black carrots are their bioavailability and pharmacological mechanism of action (Akhtar et al., 2017; Pandey and Grover, 2020). Black carrots contained the highest level of total phenolics and flavonoids among different carrots, which resulted in the highest level of antioxidant activity (76–83%) (Singh et al., 2018b). Blood cholesterol and glucose levels were decreased by the black carrot bioactive components. The cholesterol level in the liver was found to be decreased by inhibiting activity of 3-hydroxy-3-methylglutaryl-coenzyme A (Ahmad et al., 2019; Pereira-Caro et al., 2021). Black carrot can be utilised as a natural and safe antihyperglycemic nutraceutical. (Koley et al., 2019).
Fig. 3.
Pictorial representation of various therapeutic benefits provided by consumption of black carrots. [Adapted from: (Akhtar et al., 2017; Hmad et al., 2019; Kamiloglu et al., 2016; Pereira-Caro et al., 2021; Scarano et al., 2018; Sevimli-Gur et al., 2013; Singh et al., 2018b)]
Anti-oxidant activity
Black carrots are good source of flavonoid, which are natural compounds with antioxidant properties that help to protect cells from damage caused by free radicals (Sevimli-Gur et al., 2013). Free radicles are responsible for causing degenerative, neurological and CVD diseases. In a study by Algarra et al (2014), from in vitro test it was concluded that black carrots are rich source of anthocyanin, which have high antioxidant properties. In comparison to orange carrots, black carrots have more antioxidant properties, which were found to be beneficial in scavenging the free radicles and reduce oxidative stress (Pandey and Grover, 2020). Black carrots are a good source of acylated anthocyanin (Koley et al., 2019), which have ability to scavenge the free radicals and neutralize them by donating an electron. This stabilizes and preventing them from causing further damage and serves as a stand-in for an ameliorating effect against many physiological risks in people (Akhtar et al., 2017). The antioxidant activity of black carrots varies depending on the variety. For example, the varieties “Pusa Asita” and “Black Wonder” have antioxidant activity ranging from 28.69–91.08% and of 4.55–15.62%, respectively (Kaur and Sogi, 2020).
Cardiovascular protective and preventing metabolic disorder
Bioactive compounds present in black carrots have been reported to aid in the prevention of cardiovascular diseases, including reducing the binding activity of bile acids, lowering body mass index, decreasing triglyceride levels and regulating blood pressure (Ahmad et al., 2019; Pereira-Caro et al., 2021). A higher intake of anthocyanins has been associated with a reduced risk of all-cause mortality, primarily due to a lower risk of cardiovascular mortality. Increased anthocyanin intake is linked to lower incidence of cardiovascular disease and better cardiovascular health indicators in several studies (Bartosz et al., 2022).
The consumption of black carrots, which are a potential source of fibre and polyphenols, has been linked to a lower risk of cardiovascular disease. The bioactive components they contain have the ability to bind molecules like glucose and cholesterol, reducing their availability in the body. Dietary fibre aids in lowering blood levels of low-density lipoprotein and short chain fatty acids produced by colonic bacteria. Anthocyanins, phenolic acids, and carotenoids present in black carrots have been found to improve insulin resistance, dyslipidemia, glucose tolerance, and hypertension in order to treat metabolic disorders (Akhtar et al., 2017).
Anti-inflammatory activity
Peel and pomace of black carrot are rich in polyphenols, which possess anti-inflammatory properties, helping in the reduction of inflammation in endothelial cells. While, phase II enzymes in the small intestine and liver can further convert anthocyanins into methyl, glucuronide, or sulphate conjugates, which may also have an endothelium-protecting effect. Given that the bioactivity of polyphenols is dependent on their bioavailability, gastrointestinal digestion and transepithelial intestinal absorption were also considered during the research on the anti-inflammatory effects of polyphenols. In an in vitro investigation, it was found that the cellular inflammation response system in activated endothelium cells is affected by the polyphenols transported from black carrot and its by-products (Kamiloglu et al., 2016). Polyphenol-rich black carrot and its by-products, such as peel and pomace, were capable of inhibiting the secretion of pro-inflammatory markers such as interleukin-8, monocyte chemoattractant protein-1, vascular endothelial growth factor, and intercellular adhesion molecule-1. This ultimately led to a reduction in inflammation (Akhtar et al., 2017).
Anti-diabetic activity
The phenolic compounds of black carrots are beneficial in lowering the risk of diabetes (Ahmad et al., 2019; Pereira-Caro et al., 2021). Carotenoids, along with acylated and non-acylated anthocyanins, are the compounds that contribute to the prevention of diabetes. Limited insulin release or decreased insulin uptake at the tissue level are known causes of elevated blood glucose concentrations. Anthocyanins have the ability to protect pancreatic β cells from the oxidative stress brought on by glucose. Scientists now believe that anthocyanin can prevent the development of diabetes by decreasing cholesterol levels. It has been discovered that carrot-derived anthocyanins and anthocyanidin-based substances are very effective in increasing insulin release from pancreatic β cells while simultaneously inhibiting COX-2 enzymes. In addition to reducing the elevated postprandial glucose level, these substances have also been demonstrated to possess a significant α glucosidase inhibitory activity (Akhtar et al., 2017). A higher consumption of anthocyanins is associated with improved obesity prevention and a lower risk of type 2 diabetes (Bartosz et al., 2022).
Anti-cancerous activity and preventing degenerative disease
Antioxidant are compounds that neutralize free radicles, which are unstable molecules capable of damaging DNA and other cellular components, potentially leading to cancer (Chhetri et al., 2022). In various studies, it was concluded that anthocyanins from black carrots exhibited cancer-preventative properties (Akhtar et al., 2017; Koley et al., 2019; Netzel et al., 2007), by modifying metabolic activity and carcinogen elimination. For instance, the growth of the colorectal cancer (Caco-2) cells was inhibited by black carrots phenolic compounds in a dose-dependent manner. Anthocyanins found in black carrots have a strong antioxidant impact that inhibits the colorectal cancer cells from proliferating. Inhibition of the growth of cancer-causing HT-29 and HL-60 cells by the use of lyophilized powdered having aqueous black carrot anthocyanins at concentration of 0–2.0 mg mL−1 was found to be significant in inhibiting 80% of cancerous cell. Cancerous cells of human colon, breast adenocarcinomas, musculus neuroblastoma and human prostate adenocarcinoma were inhibited by the ethanolic extract of black carrots rich in anthocyanins. Other bio-active compounds present in black carrot like polyacetylene and β- Carotene are effective to treat leukaemia. Kumar et al. (2019) investigated that black carrot extract is also used against the treatment of brain cancer without causing any side effects. Black carrot extract can be used alone or in combination with anticancer medications to treat cancer types that are resistant to chemotherapy (Ahmad et al., 2019; Pereira-Caro et al., 2021).
Conventional and emerging technologies utilized for processing black carrot and its by-products
Drying
Drying is a traditional method of food preservation that employs both heat and mass transfer to remove a set amount of moisture from food products. Many drying techniques, including convective hot air, freeze, spray, and vacuum drying are employed in food processing (Liu et al., 2022). The effectiveness and success of operating any drying unit, where heat is transferred through conduction, convection, and radiation, is determined by energy conservation and production of high quality dried products (Ibrahim et al., 2023; Moses et al., 2014).
Various drying techniques have been implemented for the encapsulating and preparation of powder from black carrots (Table 2). Polat et al. (2022) examined five distinct drying techniques (freeze drying, microwave drying, convective drying, vacuum convective drying, and conductive hydro drying) for their effects on the colour, anthocyanin content, colourless phenolic content, and volatiles of black carrot pomace. It was found that freeze drying achieved the highest retention of total phenols and other aromatic compounds in the black carrot pomace compared to other drying techniques. This might be due to the fact that freeze drying minimize the degradation and loss of bioactive compounds during drying process, as it involves moisture removal at low temperatures and pressure. This help retain the quality and stability of bioactive compounds, that can be degraded or lost at high temperatures during drying in other methods. In another study by Keskin et al. (2021), freeze drying was found to retain a high amount of bioactive compounds and their bioavailability. This is because freeze drying reduce the particle size and increase the surface area of the dried material. This can improve the solubility and absorption of bioactive compounds, leading to a higher retention of their health benefit. Furthermore, sensory analysis demonstrated that the freeze-drying approach was more acceptable among other drying methods and preserved the aroma to a greater extent. Similarly, Elik (2021) found high retention of colour quality, total phenolics and total anthocyanin in freeze drying.
Table 2.
Conventional and emerging technologies utilized for processing black carrot and its by-products
| S. No | Technology | Objective | Processing condition | Potential findings | Comparative outcomes | References |
|---|---|---|---|---|---|---|
| 1 | Spray drying (SD) and Freeze drying (FD) | Encapsulation of black carrot juice |
Inlet temperatures of SD: 150, 175, 200 and 225 °C Outlet temperatures of SD: 76, 86, 98 and11°C, at 2 °C FD temperature: ˗53 °C FD Vacuum: 0.22–0.11 mbar |
Maltodextrin 20 DE as a carrier material had shown the highest antioxidant activity and maximum anthocyanin retention At 150 °C of SD, the best spray-dried product was obtained FD led to the formation of the most acceptable product with maximum water solubility index (up to 96.93%), encapsulation efficiency (up to 98.51%), colour change (upto 46.40%), anthocyanin content (up to 1650 mg/100 g dry matter) and antioxidant activity (upto 329.4 μmol Trolox/g dry matter) |
Murali et al. (2015) | |
| 2 | Spray drying (SD) | Encapsulation of black carrot extract in liposomes |
Inlet temperature: 160 °C Outlet temperature: 90 °C Feed rate: 2.5 cm3 min−1 Aspirator capacity: 100% |
Encapsulation efficiencies of 86.6 ± 16.1%, 82.2 ± 9.7%, and 46.9 ± 6.3% were obtained for the liposomes with 0.1%, 0.2%, or 0.4% extract, respectively Physical and chemical stability was increased Liposomes had uniformly wrinkled surfaces (SEM images) |
Guldiken et al. (2019) | |
| 3 | Spray drying | Microencapsulation of anthocyanin pigment |
Inlet temperature: 160,180,200 Outlet temperature: 107 ± 2,118 ± 2,13 ± 21 Solid feed: 20% |
Glucodry 210 as a carrier material had showed highest anthocyanin retention (upto 630 mg/ 100 g dry matter of powder) Higher anthocyanin losses at higher inlet/outlet temperature |
Ersus and Yurdagel (2007) | |
| 4 | Spray drying | Encapsulation of anthocyanin for analysing its antioxidant potential and encapsulation efficiency | Temperature: 150 °C |
The powder was found to have anthocyanin: 2766.61 mg/100 g Antioxidant capacity: 290.56 µmol Trolox/g Encapsulation efficiency: 77.12% Particle size: 52.36 µm Whey protein isolate with NBRE-15 was found to be best carrier material |
Kar et al. (2019) | |
| 5 | Freeze drying | To analyse the phenolic, colour profile and volatile compounds in black carrot |
Vacuum pressure: 0.045 mbar (4.5 Pa) Temperature: − 84 °C Drying time: 60 h |
L* value: 36.98 ± 0.90 a* value: 13.52 ± 0.49 b*value: − 0.82 ± 0.22 Total volatile compound: 21,536 ± 22.4 µg kg−1 Total phenolic compound: 503.0 ± 4.33 mg kg−1 Total anthocyanin: 131.6 ± 0.97 mg/100 g |
Freeze drying had highest number of volatiles with high retention of aromatic compounds and higher overall acceptability | Keskin et al. (2021) |
| 6 | Intermittent microwave drying (IMWD) |
Applied power: 250 W Microwave on and off time: 15 s, 29 s Specific applied power: 1.0 W g−1 Drying time: 1.1 h |
L* value: 32.96 ± 0.25 a* value: 11.49 ± 0.10 b*value: 0.42 ± 0.03 Total volatile compound: 14,948 ± 18.6 µg kg−1 Total phenolic compound: 822.6 ± 2.92 mg kg−1 Total anthocyanin: 112.2 ± 2.78 mg/100 g |
|||
| 7 | Hot air convective drying (HACD) |
Temperature: 60 °C Time: 4.3 h |
L* value: 30.89 ± 0.24 a* value: 7.96 ± 0.18 b*value: 0.87 ± 0.04 Total volatile compound: 15,988 ± 8.9 µg kg−1 Total phenolic compound: 713.4 ± 5.12 mg kg−1 Total anthocyanin: 131.6 ± 0.97 mg/100 g |
|||
| 8 | Freeze drying | To analyse the bioactive compounds and colour profile of black carrot pomace | Temperature: − 18 °C |
L* value: 26.29 ± 0.26 a* value: 22.61 ± 0.09 b*value: 4.04 ± 0.09 Total phenolic content: 21.39 ± 0.21 mg GAE g−1 dw Total anthocyanin content: 11.26 ± 0.15 mg g−1 dw |
HARF led to reduction in drying time by 39% in comparison to conventional drying HARF showed better phenolic content, antioxidant capacity, anthocyanin content and color in comparison to conventional drying |
Elik (2021) |
| 9 | Hot air- assisted radio frequency drying technology (HARF) |
Power: 10 kW Frequency: 27.12 MHz Air velocity: 1.5 m s−1 Temperature: 45 ± 1 °C Relative humidity: 13.5 ± 0.5% |
Moisture: 84.3 ± 0.3%, wb (wet basis) L* value: 25.82 ± 0.03 a* value: 19.76 ± 0.07 b*value: 1.69 ± 0.05 Total phenolic content: 16.34 ± 0.32 mg GAE g−1 dw Total anthocyanin content: 3.91 ± 0.55 mg g−1 dw |
|||
| 10 | Conventional hot air drying |
Air velocity: 1.5 m s−1 Temperature: 45 ± 1 °C |
L* value: 23.15 ± 0.17 a* value: 18.27 ± 0.17 b*value: 1.67 ± 0.08 Total phenolic content: 11.17 ± 0.36 mg GAE g−1 dw Total anthocyanin content: 2.84 ± 0.05 mg g−1 dw |
|||
| 11 | Microwave drying | To analyse the characteristic of black carrot pulp |
Powers: 180, 540, and 900W Time: 510 to 2430 s |
Moisture diffusivity values ranges from 2.822 × 10–7- 8.532 × 10–6 m2s−1 Bulk density: 298.61 ± 6.12 kg m−3 Tapped density: 404.08 ± 11.21 kg m−3 Flowability: 29.05 ± 0.65 Cohesiveness: 1.35 ± 0.06 Thickness: 3-7 mm Wettability: 2.25 ± 0.35–7.00 ± 0.90 s Solubility: 3.50 ± 0.70–8.00 ± 0.00 s With the increase in power there is decrease in drying time and moisture content |
Talih et al. (2017) | |
| 12 | Freeze drying | For the analysis of bioactive compounds, volatiles and colour profile in black carrot |
Temperature: -66 °C Pressure: -5 m Torr Time: 1200 min) |
Anthocyanin coloured phenolics (upto 101.8 ± 0.24 mg/100 g DW) Colourless phenolics (upto 81.6 ± 0.4 mg kg−1 DW) Total terpenes (upto 26,203 ± 76.8 μg kg−1) Total alcohol (upto 4757 ± 41.2 μg kg−1) Total acid (upto 882 ± 6.1 μg kg−1) Total aldehydes (upto 2549 ± 76.3 μg kg−1) Total esters (upto 365 ± 27.0 μg kg−1) Total ketones (upto 115 ± 0.2 μg kg−1) Total phenols (upto 279 ± 20.7 μg kg−1) L*value (Brightness) (upto 29.72 ± 1.13) a*value (redness) (upto 32.73 ± 0.76) b*value (yellowness) (upto 10.60 ± 0.53) |
Drying led to reduction in volatile compounds, anthocyanins and colourless phenolics Out of all drying techniques, freeze drying was able to achieved highest retention for total phenols and other aromatic compounds |
Polat et al. (2022) |
| 13 | Microwave drying MWD) |
Power: 100W Air speed: 1-2 m s−1 Time: 180 min |
Anthocyanin coloured phenolics (upto 158.9 ± 1.29 mg/100 g DW) Colourless phenolics (upto 89.2 ± 1.64 mg kg−1 DW) Total terpenes (upto 14,340 ± 24.5 μg kg−1) Total alcohol (upto 3473 ± 32.3 μg kg−1) Total acid (upto1125 ± 10.2 μg kg−1) Total aldehydes (upto 4454 ± 14.5 μg kg−1) Total esters (upto 84.5 ± 6.8 μg kg−1) Total ketones (upto 125 ± 1.1 μg kg−1) Total phenols (upto 94.1 ± 1.6 μg kg−1) L*value (Brightness) (upto 17.06 ± 1.31) a*value (redness) (upto 10.77 ± 1.40) b*value (yellowness) (upto 2.82 ± 0.40) |
|||
| 14 | Convective drying (CD) |
Temperature: 65 °C Time: 510 min |
Anthocyanin coloured phenolics (upto 96.2 ± 0.44 mg/100 g DW) Colourless phenolics (upto 56.4 ± 0.81 mg kg−1 DW) Total terpenes (upto 14,270 ± 31.6 μg kg−1) Total alcohol (upto 3862 ± 41.2 μg kg−1) Total acid (upto 1150 ± 8.7 μg kg−1) Total aldehydes (upto 3944 ± 22.8 μg kg−1) Total esters (upto 91.9 ± 2.2 μg kg−1) Total ketones (upto 112 ± 1.4 μg kg−1) Total phenols (upto 95.3 ± 5.3 μg kg−1) L*value (Brightness) (upto 17.18 ± 1.64) a*value (redness) (upto 9.26 ± 2.05) b*value (yellowness) (upto 2.45 ± 0.62) |
|||
| 15 | Vacuum convective drying (VCD) |
Vacuum pressure: − 40 kPa Temperature: 65 °C Time: 410 min |
Anthocyanin coloured phenolics (upto 156.30 ± 0.63 mg/100 g DW) Colourless phenolics (upto 97.7 ± 0.06 mg kg−1 DW) Total terpenes (upto 13,797 ± 11.2 μg kg−1) Total alcohol (upto 3383 ± 42.7 μg kg−1) Total acid (upto 1103 ± 9.9 μg kg−1) Total aldehydes (upto 3927 ± 12.2 μg kg−1) Total esters (upto 57.7 ± 1.0 μg kg−1) Total ketones (upto 88.0 ± 1.4 μg kg−1) Total phenols (upto 89.6 ± 7.5 μg kg−1) L*value (Brightness) (upto 16.94 ± 2.10) a*value (redness) (upto 10.37 ± 0.80) b*value (yellowness) (upto 2.68 ± 0.21) |
|||
| 16 | Conductive hydro drying (CHD) |
Temperature: 95 °C Time: 150 min |
Anthocyanin coloured phenolics (upto 165.6 ± 1.08 mg/100 g DW) Colourless phenolics (upto 103.0 ± 1.88 mg kg−1 DW) Total terpenes (upto 14,096 ± 44.1 μg kg−1) Total alcohol (upto 3834 ± 26.4 μg kg−1) Total acid (upto 1158 ± 7.3 μg kg−1) Total aldehydes (upto 4412 ± 23.8 μg kg−1) Total esters (upto 64.8 ± 5.3 μg kg−1) Total ketones (upto 45.3 ± 1.3 μg kg−1) Total phenols (upto 45.1 ± 1.0 μg kg−1) L*value (Brightness) (upto 18.34 ± 0.66) a*value (redness) (upto 13.88 ± 0.32) b*value (yellowness) (upto 3.54 ± 0.14) |
|||
| 17 | Pasteurization and Clarification | To analyse the effect on the anthocyanin and percent polymeric colour of black carrot juice |
Temperature: 90 °C, 15 min Bentonite: 0.715 g L−1 |
Decrease in percent polymeric colour Pasteurization led to a 3–16% reduction in anthocyanin Bentonite treatment led to 20% increase in monomeric anthocyanin content Reduction in percent polymeric colour: 22.32% Anthocyanin content (HPLC): 526 ± 2.83 mg L−1 |
Türkyilmaz et al. (2012) | |
| 18 | Microwave | Extraction of bioactive phytoceuticals and antioxidant activity |
Microwave power: 348.07 W Extraction time: 9.8 min Ethanol concentration: 19.8% |
Total phenolic: 264.9 ± 10.02 mg GAE/100 mL Antioxidant capacity (AOC): 13.14 ± 1.0 µmol Colour density: 68.63 ± 5.40 units Total anthocyanin content: 753.4 ± 31.6 mg L−1 TFC: 1662.22 ± 47.3 mg QE L−1 |
Microwave assisted extraction was found to be most suitable | Kumar et al. (2019) |
| 19 | Ultrasound |
Ethanol concentration: 30% Time: 30 min |
Total phenolic compound: 194.5 ± 12.24 mg GAE/100 mL Total flavonoid compound: 1076.66 ± 36.66 mg QE L−1 Total anthocyanin content: 607 ± 55.69 mg L−1 |
|||
| 20 | Conventional heating |
Temperature: 4 °C Ethanol concentration: 30% Time: 300 min |
Total phenolic compound: 108.7 ± 5.63 mg GAE/100 mL Total flavonoid compound: 683.33 ± 34.80 mg QE L−1 Total anthocyanin content: 323.4 ± 21.7 mg L−1 |
|||
| 21 | Microwave | Recovery of maximum anthocyanin from black carrot |
Microwave power: 500W Temperature: 60 °C Time:9 min Centrifugation: 4000 rpm (5 min) |
Anthocyanin content (upto 95.81 mg mL−1) | Microwave showed better anthocyanin retention in comparison to ultrasonication | Pala et al. (2017) |
| 22 | Ultrasonication |
Centrifugation: 4000 rpm (5 min) Temperature at 40 °C Time: 20 min |
Anthocyanin content (upto 91.64 mg L−1) | |||
| 23 | Microwave | Effect of dehydration on different parameters of black carrot at different time intervals |
Microwave power: 2450 MHz Time: 1, 2 and 3 min Temperature: 50, 60, and 70 °C |
Reduction in dehydration time by 45.7%—62.1% Activation energy dropped from 36.31 to 14.24 kJ mol−1 Effective diffusivity dropped from 9.9 ± 10–11 to 3.4 ± 10–10 m s−2 |
Haq et al. (2018) | |
| 24 | Ultrasonication | Extraction of pectin from black carrot pomace |
Power: 660W Time: 1800s Frequency: 37 kHz |
Degree of esterification in pectin (upto 34.3 ± 1.8%) Total phenolic content (upto 1285.3 ± 45.1 mg GAE L−1) Total monomeric anthocyanin (upto 297.9 ± 82.4 mg L−1)) Water holding capacity (upto 0.5 ± 0.01 g g−1) Particle size (upto 1092.0 ± 76.2 nm) Yield (upto 0.08 kg pectin/kg pomace) |
Conventional heating showed better anthocyanin retention, phenolic content and higher degree of esterification in comparison to ultrasonication and microwave | Misra and Yadav (2020) |
| 25 | Microwave |
Power: 550W Time: 300 s Temperature: 110 °C/5 min Centrifuged: 4000 rpm/15 °C (15 min) |
Degree of esterification in pectin (upto 38.3 ± 4.4%) Total phenolic content (upto 1692 ± 79.4 mg GAE L−1) Total monomeric anthocyanin (upto 456.8 ± 38.2 mg L−1)) Water holding capacity (upto 0.7 ± 0.1 g g−1) Particle size (upto 1170.3 ± 61.6 nm) Yield (upto 0.17 kg pectin kg pomace−1) |
|||
| 26 | Conventional heating |
Power: 800W Time: 5400 s Stirring: 110 °C/250 rpm (90 min) Centrifugation: 4000 rpm/15 °C (30 min) |
Increased degree of esterification in pectin (upto 45.2 ± 5.0%) Total phenolic content (upto 1832 ± 26.5 mg GAE L−1) Total monomeric anthocyanin (upto 1213.7 ± 96 mg L−1)) Water holding capacity (upto 0.6 ± 0.02 g g−1) Particle size (upto 1373.3 ± 91.4 nm) Yield (upto 0.22 kg pectin kg pomace−1) |
|||
| 27 | Blanching (Bl) /KMS/citric acid (CA) /calcium chloride (CC) | Effect of different pre-treatments on anthocyanin, flavonoids and ascorbic acid content of black carrot |
Blanching Temperature: 98 °C Time: 3 min Cooling: 2 min |
High anthocyanin (Bl): 231.67 ± 2.88 mg/100 g at 60 °C Highest anthocyanin (KMS): 661.84 ± 10.58 mg/100 g at 40 °C Highest anthocyanin (CA): 661.84 ± 10.58 mg/100 g at 40 °C Highest anthocyanin (CC): 542.02 ± 1.54 mg/100 g at 60 °C Highest flavonoid (Bl): 54.1 ± 12.26 mg/100 g at 40 °C Highest flavonoid (KMS): 219.14 ± 1.53 mg/100 g at 50 °C Highest flavonoid (CA): 44.16 ± 4.37 mg/100 g at 50 °C Highest flavonoid (CC): 116.29 ± 4.86 mg/100 g at 60 °C Highest ascorbic acid (Bl): 14.21 ± 0.04 mg/100 g at 50 °C Highest ascorbic acid (KMS): 10.98 ± 0.1 mg/100 g at 40 °C Highest ascorbic acid (CA): 10.36 ± 0.02 mg/100 g at 40 °C Highest ascorbic acid (CC): 12.16 ± 0.06 mg/100 g at 40 °C |
Combination of calcium chloride with drying temperature of 60 °C and 3 min blanching time resulted in optimum anthocyanin retention | Garba and Kaur, 2014) |
| 27 | Thermo-sonication | Extraction of anthocyanin from black carrot pomace |
Temperature: 50 °C Energy density: 183.1 J g−1 Power: 102.4 W Frequency: 24 kHz |
Results in maximum (C3XGGC) anthocyanin extraction upto 34.2 mg L−1 Anthocyanin concentration were enhanced by 20% |
Agcam et al. (2017) | |
| 28 | High-pressure processing (HPP) | Extraction of bioactive compound and effect on antioxidant activity in black carrot pomace |
Pressure: 266.6 MPa, Thermal treatment: 78.3 °C Time: 13.6 min |
Total phenolic compound: 333.9 mg L−1 Total monomeric anthocyanin: 105.7 mg L−1 Antioxidant activity: 89.3% Other bioactive compounds (upto 41.9 ± 20.0%) Three times increase in antioxidant activity |
Agcam et al. (2021) | |
| 29 | Nanofiltration (NF) & Forward Osmosis (FO) | Focusing on concentrating the acylated anthocyanin in the processing of black carrot juice |
Polymeric membranes (molecular weights): 150–2000 Da Pressure: 2–12 bar |
NF membrane (600–800 Da) shows the best performance Acylated anthocyanins (upto 83.2%) 98% rejection of anthocyanin, 65% rejection of total soluble solid (NF) Most of ions and solutes were rejected in FO, allow flow of pure water only |
Roda-Serrat et al. (2021) | |
| 30 | Irradiation | Evaluation of stability of natural food colour from black carrot |
Irradiation: 1400 ± 30 Lux Storage temperature: 25 °C Time: 6 months |
Anthocyanin is less sable at pH 7 Anthocyanin is more stable at pH 3 50% less absorbance at pH 5 |
Ghidouche et al. (2013) | |
| 31 | Ultraviolet radiation | Effect of ultraviolet-C, and ultraviolet-B light on antioxidant property, total phenolic and microbial population in the black carrot juice |
Time: 0, 5, 15, 30 and 60 min Temperature: 25 ± 1 °C Power: 15 W Wavelength (UV-C): 254 nm Wavelength (UV-B): 365 nm Dose (UV-C): 2.16 J m−2 Dose (UV-B): 1.50 J m−2 |
UV-B treated juice shows better retention of total phenolic (up to 536.08 mg L−1) Increase in flavonoids content (18.86 to 29.59 mg L−1) Increase in anthocyanin content (UV-C) (up to 57.42 ± 3.58 mg L−1) Increase in total antioxidant property UV-C treatment leds to reduction in total aerobic microbes upto 0.5 log10(cfu mL −1), yeast and mould from 3.10 ± 0.20 log10(cfu mL −1) to 1.54 ± 0.18 log10(cfu mL −1) and lactic acid bacteria up to 104 cfu mL−1 |
Türkmen and Takci (2018) | |
| 32 | Ultraviolet radiation | Effect on the concentration of anthocyanin in black carrot | Wavelength: 315–400 nm | Reduction in total anthocyanin from 17.99%—19.81% | Espinosa-Acosta et al. (2018) | |
| 33 | Ultrasonication | Intensification of bioactive compounds and microbiological quality in non-thermally processed black carrot juice |
Time: 0, 4, 8 and 12 min Frequency: 24 kHz Power: 200W |
Ascorbic acid (upto 19.34 ± 0.01 mg/100 mL) Total phenolic content (upto 64.22 ± 0.16 mg GAE/100 mL) Total monomeric anthocyanin (upto 14.58 ± 0.17 mg/100 mL) Total soluble solid (upto 9.22 ± 0.05 ºBrix) Viscosity (upto 1.85 ± 0. 02 mPa.s) Turbidity (upto 0.615 ± 0.001 absorbance at 660 nm) Reduction in microbial population (upto 2.05 ± 0.00log CFU mL−1) |
Hasheminya and Dehghannya (2022) | |
| 34 | Ultrasonication | Fortification of fresh cut apples by vacuum impregnation with black carrot phenolics and calcium |
Ultrasound power: 96 – 198 W Frequency: 35 kHz |
The best result showed at 130W power Calcium content (13.8%), Total phenolics (upto 659.4 ± 13.6 mg/100 g db) Total flavonoids (upto 324.3 ± 14.9 mg/100 g db) Total anthocyanins (upto 23.3 ± 1.1 mg/100 g db) Antioxidant capacities (upto 101.0 ± 3.9 mg AA/100 g) |
Yılmaz and Ersus Bilek (2018) | |
| 35 | Ultrasonication | To improve the stability, quality retention, and antibacterial effectiveness of anthocyanins during hot-air convective drying |
Frequency: 37 kHz Blanching: 98 °C/3 min |
Glass Transition Tg (upto 6.05 ± 0.09 °C) L*value: 4.71 ± 0.32 a*value: 2.97 ± 0.39 b*value: 0.64 ± 0.13 Anthocyanin content (upto 74.22 mg L−1 at 50 °C) Moisture content (upto 60.47 ± 1.45%) Aspergillus niger (inhibition upto 26.0 ± 0.16) E. coli (inhibition upto 14 ± 0.18) |
Sucheta et al. (2019) |
A cost-effective way to preserve natural colourants is by microencapsulating them in a coating material using a spray dryer (Ersus and Yurdagel, 2007). Black carrot juice encapsulation has been done using both spray and freeze drying. Freeze drying produce encapsulated anthocyanins with better physical properties, such as particle size, morphology, and colour, compared to spray drying. (Murali et al., 2015; Guldiken et al., 2019). Garba and Kaur. (2014) reported that anthocyanin was retained most effectively for the control sample at drying air temperatures of 60 °C in conjunction with calcium chloride and 3 min blanching pre-treatments, this might be due to the reason that blanching involves briefly immersing the food material in boiling water or steam, which can inactivate the enzymes and remove the air space from tissues and improve the stability of anthocyanin. While, calcium chloride salt help to stabilize the cell wall and reduce the loss of anthocyanin during drying. These treatments, in combination, improve the retention of anthocyanin during drying at a temperature of 60 °C. Sabarez, (2021) found that conductive hydro drying method is preferable to the other drying methods because of its quicker drying time and resulted in retention of 23,655 ± 76.2 μg kg−1 of volatile compounds (terpenes, alcohol, aldehyde, esters, ketones and phenols). Conductive hyrdo drying uses the high thermal conductivity of water to transfer heat to food material, accelerating the drying process and water also help to maintain a humid environment, thereby retaining volatile compounds. According to reports, thermal processing reduced the amount of phenolics in black carrot pomace, and these reductions were more pronounced in higher drying temperature. As compared to the other drying technologies except freeze drying, hot air convective drying also results in significant retention of volatile compounds, phenolic compounds, anthocyanin. (Keskin et al., 2021). However, conventional food frying, due to exposure of food ingredients to high temperatures or prolonged drying times, is energy-intensive, time-consuming, and harmful to product quality (Sabarez, 2021). To prevent deterioration in quality, innovative drying technologies that intensify gentle processing (i.e., low temperatures) with greater energy efficiency are being developed. In conclusion, apart from encapsulation, freeze drying can also be effective in bio accessibility of bioactive and volatile compounds. More research is needed to optimization and explore the potential of hot air assisted radio frequency drying technology for better retention of bioactive and volatile compounds during drying.
Microwave technology
Microwaves (MW) are electromagnetic radiations with frequency ranging from 300 MHz to 300 GHz. The most common applications of microwave in the context of the food matrix are pasteurisation, sterilisation, extraction, drying, and thawing, owing to their non-ionizing and dielectric attributes (Guzik et al., 2022; Moses et al., 2014). Several authors have investigated the utilization of MW technology, mainly for drying and extraction of bioactive and volatile compounds from black carrot (Table 2). In a study, it was found that high number of phenolic compounds enriched pectin having large particle size and high-water holding capacity was extracted through microwave assisted extraction (Misra and Yadav, 2020). This might be due to the vibration of the polar solvent molecules by microwaves, leading to the formation of hot spots and localized heating, which helps break down cell walls and release bioactive compounds, facilitating their extraction by solvent. Misra and Yadav (2020) concluded that utilizing microwave in combination with conventional heating results in the extraction of high amount of pectin instead of using microwave as a sole technology. This is because MW heating can selectively heat and rupture the cell walls of black carrots, facilitating the release of pectin and simultaneously conventional heating can help to distribute the heat more evenly throughout the sample, reducing localized spots that may enhance the efficiency of pectin extraction. Furthermore, MW induced temperature and pressure changes results in the rupture of the cell wall of the food matrix, facilitating the release of bioactive compounds into the extraction solvent (Barroso et al., 2023). Talih et al. (2017) utilized MW technology to dry black carrot pulp and found that sample thickness and microwave power significantly affected moisture content and drying time. In a study by (Haq et al., 2018), it was found that longer microwave treatment on black carrot shreds reduced dehydration time and overall activation energy, leading to increase of overall moisture dispersion and hence improve drying rates. Kumar et al. (2019) found that the utilization of microwave radiations resulted in extraction of higher amount of phenolic compounds exhibiting high antioxidant activity from the black carrot pomace. This is because microwave energy can penetrate the food matrix more effectively, reducing the diffusion resistance and increasing the mass transfer rate of the bioactive compounds. In another study by Keskin et al. (2021), it was found that microwave assisted drying of fresh black carrot and their powder led to preservation of bioactive and volatile compounds and better colour retention with reduced drying time and lower energy consumption. Microwave drying resulted in 30% reduction of drying time than convective drying. This is because microwave energy is selectively absorbed by water molecules as water molecules have a dipole nature and can rotate rapidly in response to the electromagnetic waves, resulting in faster heating and drying compared to convective heating (Polat et al., 2022). While MW technology has already been used at the laboratory scale for bioactive compound extraction, future research can focus on scaling up the technology to the industrial level to improve the efficiency of extraction and reduce production cost.
Sonication
A sequence of sound waves known as ultrasound has frequencies higher than the human hearing limit (20 kHz). Depending on its intended use, ultrasound can be divided into low frequency (high energy, high intensity) at frequencies between 20 and 100 kHz and high frequency (low energy, low intensity) at frequencies > 100 kHz (MHz range) (Sabarez, 2021). Ultrasound is an advanced technique that has been thoroughly examined in the food industry to enhance operations and performance (Kayaardı et al., 2023). Ultrasound technique is used for the extraction of pectin from black carrot pomace, and in comparison, to microwave and conventional heating ultrasounds take more heating time. This is because ultrasounds relies on molecular friction and vibration to generate heat and is typically used for small or localized heating, resulting in pectin with low degree of esterification and galacturonic (Misra and Yadav, 2020). In another study, utilizing ultrasounds for anthocyanin extraction at 10 °C lower temperature than that of microwave assisted extraction led to retention of higher anthocyanin content. At low temperature, the rate of reaction slows down, which can help to minimize the degradation and loss of bioactive compound and help to retain the quality and stability of extracted compounds (Pala et al., 2017). Sucheta et al. (2019) reported ultrasounds treatment to be effective for retaining 41% anthocyanins in black carrots. This might be due to reduced heat exposure, and the high frequency sound waves generated during ultrasound treatment can disrupt the cell wall and membrane of food matrix, leading to an increased release of bioactive compounds and preservation of other quality characteristics. Protection against anthocyanins degradation may have also been facilitated (Linares and Rojas, 2022).
According to Yılmaz and Ersus Bilek. (2018), to improve the infusion of calcium lactate and black carrot phenolics into ready to eat apple sections, ultrasonography is used during the vacuum impregnation process. At 130W, it has shown the best results (Table 2). Additionally, it was observed that it enhances bioactive compound extraction and significantly reduces the microbial population. This happens due to the high frequency sound waves generated by ultrasound, which creates pressure waves that causes the formation of microscopic bubbles and when the bubbles collapse, they create high temperature and pressure, damaging the cell membrane of microbes, leading to death or inactivation (Hasheminya and Dehghannya, 2022).
However, this method alone cannot provide higher retention of anthocyanins. Thermo-sonication, which combines temperature and ultrasonic energy density, maximizing the extraction of anthocyanins from black carrot pomace. Temperature improved the solubility of anthocyanin in the solvents, while ultrasound enhanced the mass transfer and break down the cell wall, leading to increased release of anthocyanin (Agcam et al., 2017). Thus, ultrasound is a promising technology for post-harvest processing of black carrots. There are several potential areas of research for the use of ultrasound, including product with high quality attributes, preservation, extraction of bioactive compounds, development of novel black carrot products with unique properties. The use of ultrasound as a non-thermal processing method to improve certain fruit juice quality attributes might be considered an emerging technology in the beverage industry.
Ultraviolet radiation
Ultraviolet radiation is a type of electromagnetic radiation that has a shorter wavelength and higher energy than visible light. It is used in the food industry for a variety of purposes, including sterilization, disinfection, and preservation. To ensure microbiological safety in the food business, ultraviolet treatments are gaining popularity as promising eco-friendly decontamination technologies. The process of decontamination by ultraviolet light is based on the emission of radiation in the ultraviolet region (100–400 nm), more specifically the UV-C region (200–280 nm), which has been shown to be germicidal. This process involves the transfer of electromagnetic energy from a light source to an organism's cellular material. The mechanism of action of UV radiation in the food processing is based on the ability of UV light to disrupt the DNA and RNA of microorganism, thereby preventing replication and killing them (Kayaardı et al., 2023). In a study, except for UV-B for 5 min, antioxidant levels were seen to rise during all UV treatments. UV-B has been shown to increase the activity of enzymes that are involved in the synthesis of antioxidants and ultimately rising the antioxidants level. The degree of browning in the juice increased as UV treatment time and dose increased. Juice L*, a*, b*, and chroma values remained in good condition after UV light treatments, as it can stimulate the production of pigments like anthocyanin, carotenoids, which are responsible for colour development. UV-C has a shorter wavelength and higher energy than UV-B radiation, allowing it to penetrate deeper into microbial cell and causing more damage to their DNA and RNA, ultimately leading to the death of microbial cells (Türkmen and Takci, 2018).
Investigations into thermal and UV durability demonstrate that incorporating tetraethyl orthosilicate to anthocyanins improves their stability and it can form a protective layer around anthocyanin molecules, preventing their degradation and increasing stability (Espinosa-Acosta et al., 2018). Overall, the use of UV radiation in black carrot processing may offer several benefits for improving the quality and health benefits of black carrot products. However, the specific condition and parameters of UV treatment should be carefully evaluated to ensure optimal results and the safety of the final product.
Irradiation and high-pressure processing
Irradiation and high-pressure processing are novel processing technologies for non-thermal pasteurization of food (Dhiman et al., 2021a). In addition to pasteurisation, food irradiation is a known and well-established food processing technology. For the production of agricultural goods that are shelf-stable at room temperature, irradiation treatment is particularly crucial (Zhong et al., 2023). When ionizing radiations, such as gamma rays or electron beam penetrates a microbe, they break chemical bonds in the microbes DNA and other cellular component, ultimately causing cell death. As a result, it serves as a crucial resource for ensuring food security and safety (Mastro, 2011).
The black carrot colour exhibited a very good fit for second-order type kinetics when exposed to irradiation under normal conditions, this suggests that degradation of black carrot can be well describe by this model. This means that the rate of degradation is proportional to the square of the concentration of the active spices, which in turn is related to the radiation dose (Ghidouche et al., 2013). Consequently, it can be concluded that this technique is very effecting for microbial destruction.
High pressure processing (HPP) uses high hydrostatic pressure ranging from 100 to 1000 MPa to inactivate microorganisms, enzymes, and other potential spoilage factors in food products (Fam et al., 2021; Nabi et al., 2021). In a study conducted by Agcam et al. (2021), HPP treatment (Table 2) was used to extract bioactive compounds from black carrot. Higher retention of bioactive compounds was achieved by using combined pressure and temperature treatment at 266.6 MPa, 78.3 °C for 13.6 min, resulting in high yield of total monomeric anthocyanin contents, total phenolic compounds and antioxidant activity (Agcam et al., 2021). Thus, in order to retain nutrients and bioactive components while also reducing the microbial load and extending the shelf life of the product, HPP is a crucial method for the processing of black carrot and its products. To further improve the preservation of natural food colour, future studies must take into account the combined application of irradiation and other novel technologies.
Membrane processing
Nano filtration and forward osmosis are membrane-based separation processes that can be used in various application, offering advantages such as high selectivity, low energy consumption, and reduced waste generation. Additionally, a number of membrane-based non-thermal methods for the concentration of liquid foods were developed, with forward osmosis emerging as the most effective, driven by consumer demand for convenience foods of the highest quality with natural flavour and taste, free from preservatives and other chemicals (Rastogi, 2016).
Nano filtration has demonstrated its effective for the retention of anthocyanins compared to other filtration technologies, which is attributed to its smaller pore size, allowing the retention of larger molecules such as anthocyanin. A polyamide thin film composites nano filtration membrane with cut-off of 600–800 Da performed the best. Anthocyanin rejection was 98% and total soluble solid rejection was 65% using this membrane. In the case of forward osmosis membranes, they allow only pure water to flow through, rejecting the majority of solutes and ions. The proper selection of draw solutions is crucial for a successful product since reverse salt flux from the draw to the feed is very frequent (Roda-Serrat et al., 2021).
Food preparation and preservation techniques should maintain the fresh-like qualities of food while ensuring its safety and nutritional content, and an appropriate and feasible shelf life. Hence, it is essential that more research be done on the expansion capacity of these techniques.
Applications in value-added food product and fortification
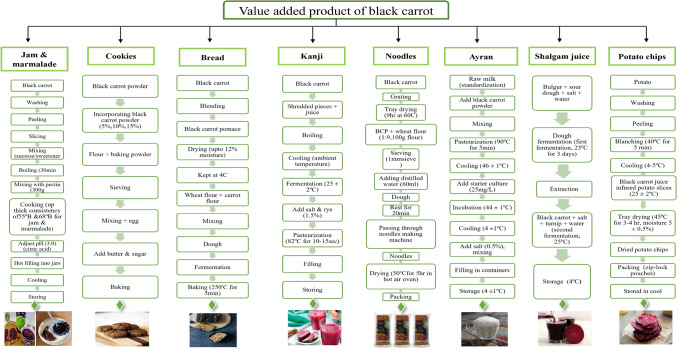
As discussed in the sections above, black carrot is a rich source of bioactive compounds and is associated with therapeutic benefits. Utilization of different conventional and novel processing technologies for encapsulation, extraction, microbial decontamination and drying has further widened the scope of black carrot preservation and stabilization. The commercialization and industrialization of the black carrot in the form of various products, taking into account its health and nutritional advantages, is crucial for meeting the public's nutrient needs, notably as a low-cost source of vitamin A (Haq and Prasad, 2015). The convenience and consumption of black carrot can be further increased by utilizing it into the formation of various value added and fortified foods. In addition to tackling seasonality and perishability of commodity, value addition will also contribute towards improving its economic value and market opportunities (Dhiman et al., 2021a). When added to foods, black carrot not only strengthens their nutritional and bioactive content but also their visual appeal. Black carrot has been used to develop beverages, dairy based, bakery and snack-based products (Fig. 4), which is discussed in sections below.
Fig. 4.
Process flowcharts for developing various value-added products from black carrot [Adapted from: (Baria et al., 2021; Gölge et al., 2022; Kahve et al., 2022; Karaman and Ozca, 2021; Manzoor et al., 2021; Özdemir, 2021; Say et al., 2018; Sharma et al., 2021; Singh et al., 2018a; Tanguler et al., 2014a, b)]
Black carrot-based dairy products
Due to their rich flavour and nutritional value, dairy products like ice cream, yoghurt, and buttermilk are widely consumed. Enriching these products with polyphenolic compounds can further enhance their nutritional and health promoting value. Various researchers have utilized black carrot as a source of colour, fibre and polyphenols in dairy products like yoghurt, ice-cream and buttermilk (Baria et al., 2021; Karaman and Ozca, 2021; Pandey et al., 2021; Say et al., 2018). In terms of sensory acceptance, it was found that addition of 7.5% black carrot concentrate to buttermilk, yoghurt and ice cream was the most acceptable compared to 2.5, 5 and 10% concentrate. The addition of black carrot concentrate significantly improved the polyphenolic content, antioxidant activity and mineral content of dairy products. These products exhibited increased flavonoid content by 14–34 times and anthocyanin content of 24–113 mg/100 g, thereby enhancing their nutraceutical properties (Pandey et al., 2021). A similar enhancement in total phenolics, total anthocyanins and antioxidant activity of yoghurt upon the addition of black carrot concentrate was observed by Baria et al. (2021). Additionally, the colour properties of yoghurt were also improved (a* increased, L* and b* decreased), resembling the colour of strawberries. These changes were attributed to higher degree of acylation.
In a study by Karaman and Ozca (2021), it was found that addition of black carrot fibre improved the textural properties of probiotic yoghurts and boosted their overall phenolic content and antioxidant activity. The formation of gel during storage was found to be increased by the addition of black carrot fibre to probiotic yoghurt. The improved textural properties were attributed to water holding capacity of fibre, which binds water in the yoghurt, thereby increasing consistency and firmness values. Fortification of Ayran (traditionally fermented yoghurt-based drink) with black carrot powder was done successfully by Say et al. (2018), resulting in an increased antioxidant activity of Ayran from 4.7 to 22%.
Black carrot-based snack products
Infusing potato chips with black carrot extract led to an improvement in its bioactive composition (anthocyanin content increased from 22 to 40 mg kg−1), along with colour attributes (increased L*, a* and b* values). Anthocyanin rich extract was able to inhibit the growth of Salmonella and Shigella spp. in potato chips samples (Nath et al., 2022). Similarly, Rojas et al. (2021) utilized black carrot extract to infuse it in apple chips and it resulted in improved colour properties, increased anthocyanin content and antioxidant activity. The incorporation of 10% black carrot powder for the development of noodles led to an increased anthocyanin content (15 mg/100 g), antioxidant activity (35% inhibition) and flavonoid content (30 mg/100 g). Additionally, water absorption capacity was also improved, which can be attributed to the fibres present in black powder (Singh et al., 2018a).
Black carrot-based beverages, jam and marmalade
“Shalgam” and “Kanji” are traditional fermented beverages made from black carrots. Shalgam is mostly produced in Turkey's Mediterranean region and Kanji is a cuisine of Indian and Pakistan (Büyükdinç et al., 2019; Özdemir, 2021). Chemical characterization of black carrot showed lactic acid to be the predominant acid. Presence of anthocyanins like cyanidin-3-arabinoside, cyanidin-3-galactoside and cyanidin-3-glucoside was confirmed using LC/MS/MS. In addition, shalgam juice inhibited growth of Caco-2 cells lines more significantly than black carrot (Ekinci et al., 2016). The size of black carrots affects the phenolic composition and anthocyanin content of shalgam. Cutting black carrot into smaller sizes increased the surface area, thereby increasing the bioactive extraction (Tanguler et al., 2014b). Kahve et al. (2022) identified and characterized endogenous yeast isolated from black carrots. They recommended Pichia kudriavzevii 3-3Y1, 3-3S9 and 3-3S2 as the most suitable strains for use as starter cultures in shalgam production, as these strains dominated the fermentation process. On the other hand, Pediococcus acidilactici was the bacterial strain with highest growth potential in Kanji production. This probiotic drink was reported to have a high content of phenols (40.8 mg mL−1), flavonoids (38.14 mg mL−1), ascorbic acid (110 mg/100 mL) and antioxidant activity (79.96%) (Sharma et al., 2021). Kanji has also been reported to control Shigella boydii and Salmonella enterica, showcasing its unique functionalities (Manzoor et al., 2021). In another study, Kanji mix was successfully prepared by Kaur et al., (2023b), incorporating freeze dried lactic acid bacterial culture into refractance window dried black carrot powder. Black carrot-based jams and marmalades have also been explored as an excellent source of polyphenolic compounds by Kamiloglu et al. (2014). Processing into jam and marmalade decreased phenolic acids, total phenolics and antioxidant capacity, attributed to disruption of cell structure during processing, making carrots prone to non-enzymatic oxidation. However, the percent recovery of bioaccessible phenolic acids and total phenols, along with antioxidant capacity, increased in jam and marmalade processing (Kamiloglu et al., 2015).
Bakery products
Black carrot has been utilized to develop various bakery products like muffins, cakes, cookies, pastries (Gölge et al., 2022). In a study conducted by Song et al. (2016), black carrot flour was used to prepare sponge cake. It was found that replacing 6% of wheat flour had the lowest baking losses compared to replacements of 2, 4 and 8%. On increasing the proportion of black carrot flour, the L*, a* and b* values decreased. In terms of sensory acceptance, antioxidant activity and rheological properties; 6% replacement was most acceptable.
In another study, black carrot pomace powder was used to enhance the nutritional properties of cake. Enrichment of cake flour led to an increase in antioxidant activity, phenolic acids, total phenols and total anthocyanins (Kamiloglu et al., 2017). Muffins prepared by incorporating black carrot dietary fibre had good overall acceptability. Addition of black carrot fibre increased batter viscoelasticity and paste viscosity. This was attributed to the water binding properties of the fibre, which reduced the movement of particles and thus making it viscous. The prepared muffins had decreased water activity, firmness, specific volume and increased total dietary fibre content (Singh et al., 2016). Similarly, Pekmez and Yılmaz (2018) fortified flatbread with black carrot fibre, which led to an increased antioxidant content and provided more attractive appearance.
In conclusion, this review provides a thorough examination of the bioactive value, postharvest processing opportunities, and value addition potential of black carrot. The rich assortment of bioactive compounds in black carrot, such as anthocyanins, polyphenols, and carotenoids, underscores its significance as a functional food with potential health benefits. The extensive range of postharvest processing methods discussed, including encapsulation, drying, irradiation, and high-pressure processing, presents diverse avenues for preserving and enhancing the bioactive content of black carrot while maintaining its nutritional and sensory attributes. The integration of black carrot extracts into various food products, such as dairy items, bakery goods, and beverages, demonstrates its versatility as an ingredient for value-added formulations. These additions not only augment nutritional content but also improve color, flavor, and overall product quality. The insights derived from this review highlight the potential to bridge the gap between traditional dietary staples and modern functional foods, catering to evolving consumer preferences for health-conscious choices.
Despite the substantial progress made in understanding black carrot's bioactive potential and processing capabilities, challenges and opportunities persist. Further research is warranted to optimize processing techniques, develop innovative product formulations, and explore novel applications. A multidisciplinary approach encompassing food science, nutrition, and technology will be pivotal in unlocking the full potential of black carrot as a valuable ingredient in the food industry. Overall, this review contributes to a deeper appreciation of black carrot's significance as a source of bioactive compounds and its role in enhancing the health-promoting attributes of various food products.
Author contributions
PT: Data curation, writing-original draft, preparation, methodology, visualization, writing-review and editing. A: Writing- review and editing. RS: Writing- review and editing. AD: Conceptualization, supervision, data curation, writing-original draft, preparation, methodology, writing- review and editing. SK: Writing-original draft, supervision, data curation, preparation, methodology, Writing- review and editing.
Declarations
Conflict of interest
No conflict of interest is declared by author and co-authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Atul Dhiman, Email: dhimanatul16@yspuniversity.ac.in.
Satish Kumar, Email: satish1666@yspuniversity.ac.in.
References
- Agcam E, Akyıldız A, Balasubramaniam VM. Optimization of anthocyanins extraction from black carrot pomace with thermosonication. Food Chem. 2017;237:461–470. doi: 10.1016/j.foodchem.2017.05.098. [DOI] [PubMed] [Google Scholar]
- Agcam E, Akyıldız A, Kamat S, Balasubramaniam VM. Bioactive compounds extraction from the black carrot pomace with assistance of high pressure processing: an optimization study. Waste Biomass Valoriz. 2021;12:5959–5977. doi: 10.1007/s12649-021-01431-z. [DOI] [Google Scholar]
- Ahmad T, Cawood M, Iqbal Q, Ariño A, Batool A, Sabir Tariq RM, Azam M, Akhtar S. Phytochemicals in Daucus carota and their health benefits—review article. Foods. 2019;8:1–22. doi: 10.3390/foods8090424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar S, Rauf A, Imran M, Qamar M, Riaz M, Mubarak MS. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: a review. Trends Food Sci. Technol. 2017;66:36–47. doi: 10.1016/j.tifs.2017.05.004. [DOI] [Google Scholar]
- Algarra M, Fernandes A, Mateus N, de Freitas V, da Silva JCGE, Casado J. Anthocyanin profile and antioxidant capacity of black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J. Food Compos. Anal. 2014;33:71–76. doi: 10.1016/j.jfca.2013.11.005. [DOI] [Google Scholar]
- Baria B, Kumar A, Narender S, Panjagari R. Colouring properties and stability of black carrot anthocyanins in yoghurt. J. Food Sci. Technol. 2021;58:3953–3962. doi: 10.1007/s13197-020-04858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso TLCT, Castro LEN, Barbero G, Palma M, Carrera C, Rostagno MA, Forster-Carneiro T. Optimization of a microwave-assisted extraction method for the recovery of the anthocyanins from jabuticaba by-products. Agronomy. 2023;13:556. doi: 10.3390/agronomy13020556. [DOI] [Google Scholar]
- Bartosz G, Baran S, Grzesik-Pietrasiewicz M, Sadowska-Bartosz I. Antioxidant capacity and hydrogen peroxide formation by black and orange carrots. Agric. Food Sci. 2022;31:71–77. [Google Scholar]
- Blando F, Marchello S, Maiorano G, Durante M, Signore A, Laus MN, Soccio M, Mita G. Bioactive compounds and antioxidant capacity in anthocyanin-rich carrots: a comparison between the black carrot and the apulian landrace “polignano” carrot. Plants. 2021;10:1–15. doi: 10.3390/plants10030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büyükdinç DT, Kantoğlu YT, Karatas A, Ipek A, Ellialtioglu S. Determination of effective mutagen dose for carrot (Daucus carota ssp. sativus var. atrorubens alef and D. carota) callus cultures. Int. J. Sci. Technol. Res. 2019;2:15–23. [Google Scholar]
- Büyükdinç DT, Kantoğlu KY, Kuşvuran Ş, İpek A, Karataş A, Ellialtıoğlu Ş. Selection of salt tolerant lines at cell level using gamma ray with callus and suspension culture techniques in black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) Damla. Appl. Radiat. Isot. 2022;190:110523. doi: 10.1016/j.apradiso.2022.110523. [DOI] [PubMed] [Google Scholar]
- Caron VC, Jacomino AP, Kluge RA. Storage of ‘Brasília’ carrot treated with waxes. Hortic. Bras. 2003;21:597–600. doi: 10.1590/S0102-05362003000400003. [DOI] [Google Scholar]
- Carrillo C, Kamiloglu S. Co-ingestion of black carrot and strawberry. Effects on anthocyanin stability, bioaccessibility and uptake. Foods. 2020;9:1595. doi: 10.3390/foods9111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhetri L, Rizwan M, Munkombwe S, Dorji N, Mutum E Utilization and characteristics of black carrot (Daucus carrot L.): Potential health benefits and effect of processing Laxmi. Pharma Innov. J. 2022;11:30–41. [Google Scholar]
- Del Mastro NL. Role of irradiation treatment in the food industry. Int. J. Nucl. Gov. Econ. Ecol. 2011;3:266. [Google Scholar]
- Dhiman A, Suhag R, Chauhan DS, Thakur D, Chhikara S, Prabhakar PK. Status of beetroot processing and processed products: thermal and emerging technologies intervention. Trends Food Sci Technol. 2021;114:443–458. doi: 10.1016/j.tifs.2021.05.042. [DOI] [Google Scholar]
- Dhiman A, Suhag R, Thakur D, Gupta V, Pramod K. Current status of loquat (Eriobotrya Japonica Lindl.): bioactive. Food Rev. Int. 2021;38:1–31. [Google Scholar]
- Ekinci FY, Baser GM, Özcan E, Güçlü Ö, Korachi M, Sofu A, Blumberg JB, Chen CYO. Characterization of chemical, biological, and antiproliferative properties of fermented black carrot juice, shalgam. Eur. Food Res. Technol. 2016;242:1355–1368. doi: 10.1007/s00217-016-2639-7. [DOI] [Google Scholar]
- Elik A. Hot air-assisted radio frequency drying of black carrot pomace: kinetics and product quality. Innov. Food Sci. Emerg. Technol. 2021;73:102800. doi: 10.1016/j.ifset.2021.102800. [DOI] [Google Scholar]
- Ersus S, Yurdagel U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J. Food Eng. 2007;80:805–812. doi: 10.1016/j.jfoodeng.2006.07.009. [DOI] [Google Scholar]
- Espinosa-Acosta G, Ramos-Jacques AL, Molina GA, Esparza R, Hernandez-Martinez AR, Sánchez-González I, Estevez M. Stability analysis of anthocyanins using alcoholic extracts from black carrot (Daucus Carota ssp. Sativus Var. Atrorubens Alef.) Molecules. 2018;23:2744. doi: 10.3390/molecules23112744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SN, Khosravi-Darani K, Massoud R, Massoud A. High-pressure processing in food. Biointerface Res. Appl. Chem. 2021;11:11553–11561. [Google Scholar]
- Frond AD, Iuhas CI, Stirbu I, Leopold L, Socaci S, Andreea S, Ayvaz H, Andreea S, Diaconeasa Z, Carmen S. Phytochemical characterization of five edible purple-reddish vegetables: anthocyanins, flavonoids, and phenolic acid derivatives. Molecules. 2019;24:1536. doi: 10.3390/molecules24081536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garba U, Kaur S. Effect of drying and pretreatment on anthocyanins, flavenoids and ascorbic acid content of black. J. Global Biosci. 2014;3:772–777. [Google Scholar]
- Ghidouche S, Rey B, Michel M, Galaffu N. A Rapid tool for the stability assessment of natural food colours. Food Chem. 2013;139:978–985. doi: 10.1016/j.foodchem.2012.12.064. [DOI] [PubMed] [Google Scholar]
- Gölge E, Ova G, Kemahlioğlu ÖK, Demİrağ MK. Effect of black carrot (Daucus carota L.) pomace in cake and cookie formulations as a functional ingredient on sensory analysis. Food Health. 2022;8:103–110. doi: 10.3153/FH22010. [DOI] [Google Scholar]
- Gu C, Suleria HAR, Dunshea FR, Howell K. Dietary lipids influence bioaccessibility of polyphenols from black carrots and affect microbial diversity under simulated gastrointestinal digestion. Antioxidants. 2020;9:1–17. doi: 10.3390/antiox9080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldiken B, Linke A, Capanoglu E, Boyacioglu D, Kohlus R, Weiss J, Gibis M. Formation and characterization of spray dried coated and uncoated liposomes with encapsulated black carrot extract. J. Food Eng. 2019;246:42–50. doi: 10.1016/j.jfoodeng.2018.10.025. [DOI] [Google Scholar]
- Guzik P, Kulawik P, Zając M, Migdał W. Microwave applications in the food industry: an overview of recent developments. Crit. Rev. Food Sci. Nutr. 2022;62:7989–8008. doi: 10.1080/10408398.2021.1922871. [DOI] [PubMed] [Google Scholar]
- Haq R, Kumar P, Prasad K. Effect of microwave treatment on dehydration kinetics and moisture diffusivity of Asiatic Himalayan black carrot. J. Saudi Soc. Agric. Sci. 2018;17:463–470. [Google Scholar]
- Haq R, Prasad K. Nutritional and processing aspects of carrot (Daucus carota)—a review. South Asian J. Food Technol. Environ. 2015;01:1–14. doi: 10.46370/sajfte.2015.v01i01.01. [DOI] [Google Scholar]
- Hasheminya S-M, Dehghannya J. Non-thermal processing of black carrot juice using ultrasound: Intensification of bioactive compounds and microbiological quality. Int. J. Food Sci. Technol. 2022;57:5848–5858. doi: 10.1111/ijfs.15901. [DOI] [Google Scholar]
- Ibrahim A, Cattaneo TMP, Amer A, Helyes L. Drying technology evolution and global concerns related to food security and sustainability. In: Food Preservation and Packaging-Recent Process and Technological Advancements. pp. 1-48 (2023).
- Kahve HI, Akbulut M, Coklar H. Identification and technological characterization of endogenous yeast isolated from fermented black carrot juice, shalgam. LWT Food Sci. Technol. 2022;154:112823. doi: 10.1016/j.lwt.2021.112823. [DOI] [Google Scholar]
- Kamiloglu S, Ayfer A, Ozcelik B, Van Camp J, Capanoglu E. Influence of different processing and storage conditions on in vitro bioaccessibility of polyphenols in black carrot jams and marmalades. Food Chem. 2014;186:74–82. doi: 10.1016/j.foodchem.2014.12.046. [DOI] [PubMed] [Google Scholar]
- Kamiloglu S, Van Camp J, Capanoglu E. Black carrot polyphenols: effect of processing, storage and digestion—an overview. Phytochem. Rev. 2018;17:379–395. doi: 10.1007/s11101-017-9539-8. [DOI] [Google Scholar]
- Kamiloglu S, Grootaert C, Capanoglu E, Ozkan C, Smagghe G, Raes K, Van Camp J. Anti-inflammatory potential of black carrot (Daucus carota L.) polyphenols in a co-culture model of intestinal Caco-2 and endothelial EA.hy926 cells. Mol. Nutr. Food Res. 2016;61:1–34. doi: 10.1002/mnfr.201600455. [DOI] [PubMed] [Google Scholar]
- Kamiloglu S, Ozkan G, Isik H, Horoz O, Van Camp J, Capanoglu E. Black carrot pomace as a source of polyphenols for enhancing the nutritional value of cake: an in vitro digestion study with a standardized static model. LWT. 2017;77:475–481. doi: 10.1016/j.lwt.2016.12.002. [DOI] [Google Scholar]
- Kamiloglu S, Pasli AA, Ozcelik B, Van Camp J, Capanoglu E. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: effect of processing, storage conditions and in vitro gastrointestinal digestion. J. Funct. Foods. 2015;13:1–10. doi: 10.1016/j.jff.2014.12.021. [DOI] [Google Scholar]
- Kar A, Mahato DK, Patel AS, Bal L. The encapsulation efficiency and physicochemical characteristics of anthocyanin from black carrot (Daucus Carota ssp. Sativus) as affected by encapsulating materials. Curr. Agric. Res. J. 2019;7:26–36. doi: 10.12944/CARJ.7.1.04. [DOI] [Google Scholar]
- Karaman S, Ozca T. Determination of gelation properties and bio-therapeutic potential of black carrot fibre-enriched functional yoghurt produced using pectin and gum Arabic as prebiotic. Int. J. Dairy Technol. 2021;74:505–517. doi: 10.1111/1471-0307.12776. [DOI] [Google Scholar]
- Kaur G, Sharma N, Singh A, Kapoor S, Khatkar SK. Ultrasound-assisted microemulsions of anthocyanins extracted from black carrot pomace and its utilisation as functional component in kulfi: implication on in vitro release, bio-functional components and rheological characteristics. Int. J. Food Sci. Technol. 2023;58:2744–2753. doi: 10.1111/ijfs.16102. [DOI] [Google Scholar]
- Kaur A, Sogi DS. Influence of development stages on the physicochemical properties of black carrots (Daucus carota L.) Cogent Food Agric. 2020;6:1841358. doi: 10.1080/23311932.2020.1841358. [DOI] [Google Scholar]
- Kaur P, Zalpouri R, Modi R, Sahota PP, Dhillon TS, Kaur A. Development and standardization of processing technique for ready-to-use lab fermented Kanji mix using refractance window dried black carrot powder. Sci. Rep. 2023;13:185. doi: 10.1038/s41598-023-27450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaardı S, Uyarcan M, Atmaca I, Yıldız D, Benzer Gürel D. Effect of non-thermal ultraviolet and ultrasound technologies on disinfection of meat preparation equipment in catering industry. Food Sci. Technol. Int. 2023 doi: 10.1177/10820132221151097. [DOI] [PubMed] [Google Scholar]
- Keskin M, Guclu G, Sekerli YE, Soysal Y, Selli S, Kelebek H. Comparative assessment of volatile and phenolic profiles of fresh black carrot (Daucus carota L.) and powders prepared by three drying methods. Scientia Hortic. 2021;287:110256. doi: 10.1016/j.scienta.2021.110256. [DOI] [Google Scholar]
- Koley TK, Srivastava S, Tripath YB, Banerjee K, Oulkar D, Goon A, Tripathi A, Singh B. High-resolution LCMS profiling of phenolic compounds of high Indian black carrot and evaluation of its effect on antioxidant defense and glucose metabolism in animal model. Agric. Res. 2019;8:481–489. doi: 10.1007/s40003-018-0389-4. [DOI] [Google Scholar]
- Kumar M, Dahuja A, Sachdev A, Kaur C, Varghese E, Saha S, Sairam KVSS. Valorisation of black carrot pomace: microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box-Behnken design. J. Food Sci. Technol. 2019;56:995–1007. doi: 10.1007/s13197-018-03566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares G, Rojas ML. Ultrasound-assisted extraction of natural pigments from food processing by-products: a review. Front. Nutr. 2022;9:1–17. doi: 10.3389/fnut.2022.891462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang Z, Hu L. High efficient freeze-drying technology in food industry. Crit. Rev. Food Sci. Nutr. 2022;62:3370–3388. doi: 10.1080/10408398.2020.1865261. [DOI] [PubMed] [Google Scholar]
- Manzoor M, Sharma V, Singh D, Sohal JS, Aseri GK, Khare N, Vij S, Saroop J, Sharma D. Functional Pediococcus acidilactici BC1 for the revitalization of ethnic black carrot kanji of indian subcontinent. Biocatal. Agric. Biotechnol. 2021;31:101291. doi: 10.1016/j.bcab.2021.101921. [DOI] [Google Scholar]
- Misra NN, Yadav SK. Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: kinetics, characterization and process economics. Food Hydrocolloids. 2020;102:105592. doi: 10.1016/j.foodhyd.2019.105592. [DOI] [Google Scholar]
- Moses JA, Alagusundaram K, Tiwari BK. Novel drying techniques for the food industry. Food Eng. Rev. 2014;6:43–55. doi: 10.1007/s12393-014-9078-7. [DOI] [Google Scholar]
- Murali S, Kar A, Mohapatra D, Kalia P. Encapsulation of black carrot juice using spray and freeze drying. Food Sci. Technol. Int. 2015;21:604–612. doi: 10.1177/1082013214557843. [DOI] [PubMed] [Google Scholar]
- Nabi BG, Mukhtar K, Arshad RN, Radicetti E, Tedeschi P, Shahbaz MU, Walayat N, Nawaz A, Inam-Ur-raheem M, Aadil RM. High-pressure processing for sustainable food supply. Sustainability. 2021;13:13908. doi: 10.3390/su132413908. [DOI] [Google Scholar]
- Nath P, Dukare A, Kumar S, Kale S, Kannaujia P. Black carrot (Daucus carota subsp. sativus) anthocyanin- infused potato chips: effect on bioactive composition, color attributes, cooking quality, and microbial stability. J. Food Proces. Preserv. 2022;46:e16180. doi: 10.1111/jfpp.16180. [DOI] [Google Scholar]
- Netzel M, Netzel G, Kammerer DR, Schieber A, Carle R, Simons L, Bitsch I, Bitsch R, Konczak I. Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins. Innov. Food Sci. Emerg. Technol. 2007;8:365–372. doi: 10.1016/j.ifset.2007.03.011. [DOI] [Google Scholar]
- Özdemir SS. Şalgam ve kanji: kültürlerarasi bir ürün olarak fermente siyah havuç içecekleri. Motif Akademi Halkbilimi Dergisi. 2021;14:1074–1109. [Google Scholar]
- Pala C, Sevimli-Gur C, Yesil-Celiktas O. Green extraction processes focusing on maximization of black carrot anthocyanins along with cytotoxic activities. Food Anal. Methods. 2017;10:529–538. doi: 10.1007/s12161-016-0599-y. [DOI] [Google Scholar]
- Pandey P, Grover K. Characterization of black carrot (Daucus carota L.) polyphenols; role in health promotion and disease prevention: an overview. J. Pharmacogn. Phytochem. 2020;9:2784–2792. doi: 10.22271/phyto.2020.v9.i5am.12764. [DOI] [Google Scholar]
- Pandey P, Grover K, Dhillon TS, Kaur A, Javed M. Evaluation of polyphenols enriched dairy products developed by incorporating black carrot (Daucus carota L.) concentrate. Heliyon. 2021;7:5. doi: 10.1016/j.heliyon.2021.e06880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekmez H, Yılmaz BB. Quality and antioxidant properties of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) fiber fortified flat bread (Gaziantep Pita) J. Agric. Sci. Technol. B. 2018;8:522–529. [Google Scholar]
- Pereira-Caro G, Ordóñez-Díaz JL, de Santiago E, Moreno-Ortega A, Cáceres-Jiménez S, Sánchez-Parra M, Roldán-Guerra FJ, Ortiz-Somovilla V, Moreno-Rojas JM. Antioxidant activity and bio-accessibility of polyphenols in black carrot (Daucus carota L. ssp. sativus var. atrorubens Alef.) and two derived products during simulated gastrointestinal digestion and colonic fermentation. Foods. 2021;10:457. doi: 10.3390/foods10020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat S, Guclu G, Kelebek H, Keskin M, Selli S. Comparative elucidation of colour, volatile and phenolic profiles of black carrot (Daucus carota L.) pomace and powders prepared by five different drying methods. Food Chem. 2022;369:130941. doi: 10.1016/j.foodchem.2021.130941. [DOI] [PubMed] [Google Scholar]
- Purkiewicz A, Ciborska J, Tańska M, Narwojsz A, Starowicz M, Przybyłowicz KE, Sawicki T. The impact of the method extraction and different carrot variety on the carotenoid profile, total phenolic content and antioxidant properties of juices. Plants. 2020;9:1–13. doi: 10.3390/plants9121759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi NK. Opportunities and challenges in application of forward osmosis in food processing. Crit. Rev. Food Sci. Nutr. 2016;56:266–291. doi: 10.1080/10408398.2012.724734. [DOI] [PubMed] [Google Scholar]
- Roda-Serrat MC, Schytt-Nielsen JF, Braekevelt S, Dzhanzefova T, Joernsgaard B, Norddahl B, Errico M. Processing of black carrot juice by nanofiltration and forward osmosis. Chemi. Eng. Trans. 2021;87:547–552. [Google Scholar]
- Rodrigues JPB, Liberal Â, Petropoulos SA, Ferreira ICFR, Oliveira MBPP, Fernandes Â, Barros L. Agri-food surplus, waste and loss as sustainable biobased ingredients: a review. Molecules. 2022;27:5200. doi: 10.3390/molecules27165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas ML, Augusto PED, Cárcel JA. Combining ethanol pre-treatment and ultrasound-assisted drying to enhance apple chips by fortification with black carrot anthocyanin. J. Sci. Food Agric. 2021;101:2078–2089. doi: 10.1002/jsfa.10830. [DOI] [PubMed] [Google Scholar]
- Sabarez H. Advanced drying technologies of relevance in the food industry. In: Innovative Food Processing Technologies: A Comprehensive Review. pp. 64-81 (2021).
- Say D, Saydam İB, Güzeler N. Some properties of ayran fortified with black carrot powder. J. Adv. Vetbio Sci. Tech. 2018;3:54–60. doi: 10.31797/vetbio.499749. [DOI] [Google Scholar]
- Scarano A, Gerardi C, Amico LD, Accogli R, Santino A. Phytochemical analysis and antioxidant properties in colored tiggiano carrots. Agriculture. 2018;1-8:102. doi: 10.3390/agriculture8070102. [DOI] [Google Scholar]
- Sevimli-Gur C, Cetin B, Akay S, Gulce-Iz S, Yesil-Celiktas O. Extracts from black carrot tissue culture as potent anticancer agents. Plant Foods Hum Nutr. 2013;68:293–298. doi: 10.1007/s11130-013-0371-z. [DOI] [PubMed] [Google Scholar]
- Shamshad A, Butt MS, Nayik GA, Obaid S, Ansari MJ, Karabagias IK, Sarwar N, Ramniwas S. Effect of storage on physicochemical attributes of ice cream enriched with microencapsulated anthocyanins from black carrot. Food Sci. Nutr. 2023;11:3976–3988. doi: 10.1002/fsn3.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Sahota PP, Kaur S. Physicochemical and microbiological evaluation of antioxidant-rich traditional black carrot beverage: Kanji. Bull. Natl. Res. Centre. 2021;45:143. doi: 10.1186/s42269-021-00594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Kaur S, Rasane P. Evaluation of the nutritional and quality characteristics of black carrot fortified instant noodles. Curr. Nutr. Food Sci. 2018;14:1–8. [Google Scholar]
- Singh JP, Kaur A, Singh N. Development of eggless gluten-free rice muffins utilizing black carrot dietary fibre concentrate and xanthan gum. J. Food Sci. Technol. 2016;53:1269–1278. doi: 10.1007/s13197-015-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BK, Koley TK, Maurya A, Singh PM, Singh B. Phytochemical and antioxidative potential of orange, red, yellow, rainbow and black coloured tropical carrots (Daucus carota subsp. sativus Schubl. and Martens) Physiol. Mol. Biol. Plants. 2018;24:899–907. doi: 10.1007/s12298-018-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeriglio A, Denaro M, Barreca D, Dangelo V, Germanò MP, Trombetta D. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.) Fitoterapia. 2018;124:49–57. doi: 10.1016/j.fitote.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Song KY, Hyeonbin O, Zhang Y, Joung KY, Choi DW, Kim YS. Effect of black carrot (Daucus carota L.) flour on quality properties and retarding retrogradation by shelf-life of Sulgidduck (rice cake) Progr. Nutr. 2018;19:442–449. [Google Scholar]
- Song KY, Hyeonbin O, Zhang Y, Kim YS. Quality characteristics and antioxidant properties of sponge cakes containing black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) flour. Progr. Nutr. 2016;18:176–183. [Google Scholar]
- Chaturvedi K, Yadav SK. Ultrasonication assisted salt-spices impregnation in black carrots to attain anthocyanins stability, quality retention and antimicrobial efficacy on hot-air convective drying. Ultrason. Sonochem. 2019;58:104661. doi: 10.1016/j.ultsonch.2019.104661. [DOI] [PubMed] [Google Scholar]
- Suzme S, Boyacioglu D, Toydemir G, Capanoglu E. Effect of industrial juice concentrate processing on phenolic profile and antioxidant capacity of black carrots. Int. J. Food Sci. Technol. 2014;49:819–829. doi: 10.1111/ijfs.12370. [DOI] [Google Scholar]
- Talih M, Çal G, Dirim SN. Determination of the drying characteristics of black carrot pulp during drying in a microwave oven. J. Food Phys. 2017;30:22–32. [Google Scholar]
- Tanguler H, Gunes G, Erten H. Influence of addition of different amounts of black carrot (Daucus carota) on shalgam quality. J. Food Agric. Environ. 2014;12:60–65. [Google Scholar]
- Tanguler H, Utus D, Erten H. Effect of black carrot size usage on the quality of shalgam (salgam): a traditional Turkish lactic acid fermented beverage. Indian J. Tradit. Knowl. 2014;13:647–653. [Google Scholar]
- Türkmen FU, Takci HAM. Ultraviolet-C and ultraviolet-B lights effect on black carrot (Daucus carota ssp. sativus) juice. J. Food Meas. Charact. 12: 1038-1046 (2018).
- Türkyilmaz M, Yemi O, Özkan M. Clarification and pasteurisation effects on monomeric anthocyanins and percent polymeric colour of black carrot (Daucus carota L.) juice. Food Chem. 2012;134:1052–1058. doi: 10.1016/j.foodchem.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Yılmaz FM, Ersus Bilek S. Ultrasound-assisted vacuum impregnation on the fortification of fresh-cut apple with calcium and black carrot phenolics. Ultrasonics Sonochem. 2018;48:509–516. doi: 10.1016/j.ultsonch.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Yoo KS, Bang H, Pike L, Patil BS, Lee EJ. Comparing carotene, anthocyanins, and terpenoid concentrations in selected carrot lines of different colors. Hortic. Environ. Biotechnol. 2020;61:385–393. doi: 10.1007/s13580-019-00225-6. [DOI] [Google Scholar]
- Zadernowski R, Piłat B, Czaplicki S, Ogrodowska D. Characteristics of the black carrot (Daucus carota ssp. sativus var. atrorubens alef.) Pol. J. Nat. Sci. 2010;25:438–443. doi: 10.2478/v10020-010-0040-8. [DOI] [Google Scholar]
- Zhong Y, Dong S, Cui Y, Dong X, Xu H, Li M. Recent advances in postharvest irradiation preservation technology of edible Fungi: a review. Foods. 2023;12:103. doi: 10.3390/foods12010103. [DOI] [PMC free article] [PubMed] [Google Scholar]