Abstract
This study identified the aroma profile of salmon by-product for high utilization of by-products, including hydrolysates of head, frame, and skin were treated with reducing sugars and thermal processing. Electronic nose (E-nose) and gas chromatography-mass spectrometry (GC–MS) coupled with gas chromatography–olfactometry (GC–O) were used to analyzed the aroma profile. A total of 140 and 90 volatile compounds were detected through E-nose and GC–MS respectively, and the main volatile compounds were aldehydes. A total of 23 odor active compounds were recognized using GC–O, and 3-methyl-butanal, heptanal, benzaldehyde, octanal, furfural, and methoxy-phenyl-oxime were identified as the aroma of salmon. Using multivariate analysis, the pattern between the pretreated samples and aroma profiles was confirmed, and there were clear separations among the samples. The results of this study provide the aroma profile of salmon by-products and are expected salmon by-products to be used as a potential food source.
Keywords: Salmon, By-product, E-nose, GC–MS, GC–O
Introduction
Salmon is one of the representative fish species with different breeding and spawning regions (Heu et al., 2015), and contains polyunsaturated fatty acids (PUFA) and high-quality amino acids, making it an excellent food not only in taste but also in nutrition (Heu et al., 2015; Nilsuwan et al., 2021). The annual production of salmon is about 400,000 tons (Arnesen and Gildberg, 2007), and a large number of fish by-products from salmon are also generated as the usage increases. Fish by-products can’t be used in the processing of fish or crustaceans, and about 35% of the world's seafood production is said to be generated as by-products and discarded (Ahn et al., 2021). Consumption patterns in modern society are increasing the production of processed foods and meal kits, which are expected to continue to generate high levels of fish by-products(Ahn et al., 2021). Especially, salmon by-products contain large amounts of protein in the head, skin, and frames, so interest in using them as a source of food ingredients is increasing (Cha et al., 2020; Peinado et al., 2016).
One of the useful ways to utilize fish by-products is to convert them into fish protein hydrolysates through enzymatic hydrolysis (Gao et al., 2020). Protein hydrolysate improves nutritional value, and functional properties, and has potential biological activity (Zhao et al., 2016). Enzymatic hydrolysate is reported to affect flavor development, such as producing flavor enhancers from fish by-products (Li et al., 2021), and be able to produce flavorings, such as meat with thermal processing (Guo et al., 2010; Zhao et al., 2016). However, because negative substances can be formed in the process of hydrolyzing by-products (Zhao et al, 2016), low-value by-product hydrolysate is said to be effective in improving aroma by thermal processing with other additives such as xylose that is reducing sugar or by combining them with various effective methods (Gao et al., 2020). Previous studies investigated the characteristics of volatile compounds according to parts of fish by-products such as skin, intestines, and gills, including carp fish (Wang et al., 2018), and flavor properties using halibut by-products according to processing methods such as hydrolysis and Maillard reaction (Jeon et al., 2016).
Analysis techniques such as E-nose, GC–MS, and GC–O are widely applied to determine the volatile compounds of food (Boo et al., 2020; Di Rosa et al., 2017; Dong et al., 2019). E-nose is used for patterning food through sensors that detect volatile compounds in food (Di Rosa et al., 2017; Dong et al., 2019). GC–MS is commonly used to identify different substances in liquids or volatile samples, and headspace-solid phase microextraction (HS-SPME) is a technique used to extract and separate volatile compounds quickly and simply (Boo et al., 2020; Song and Liu, 2018; Arthur and Pawliszyn, 1990). Recently, through these analysis techniques, many experiments on fish have been conducted, such as analyzing changes in volatile compounds caused by fish sauce, fish parts, and fish freezing processes (Fukami et al., 2002; Miyasaki et al., 2011; Wang et al, 2018).
With the trend of modern society, various studies on the use of by-products are increasing, but research on aroma profiles according to pre-treatment and parts is insufficient. Therefore, to increase the utilization of salmon by-products, this study aims to check changes in volatile compounds through hydrolysis and thermal processing in by-products such as salmon head, frame, and skin. In the future, the data is expected to be basic data for salmon by-products or the aroma profile of salmon.
Materials and methods
Materials
The salmon by-products used in this study were collected and used from a domestic salmon processing facility (Busan, Korea), and frozen (frozen instruments, C053AF, LG Electronics, Seoul, Korea) after washing three times. It was sufficiently thawed at the refrigerated temperature for 24 h before use in the experiment. After thawing, head, frame, and skin were separated. It was washed three or more times in water, cut into a 5 cm × 5 cm, and hydrolyzed and thermal processing. The samples were indicated as follows: HXG, head_thermal_xylose + glucose; HG, head_thermal_glucose; HX, head_thermal_xylose; HC, head_thermal_control; HH, head_hydrolysate; FXG, frame_thermal_xylose + glucose; FG, frame_thermal_glucose; FX, frame_thermal_xylose; FC, frame_thermal_control; FH, frame_hydrolysate; SXG, skin_thermal_xylose + glucose; SG, skin_thermal_glucose; SX, skin_thermal_xylose; SC, skin_thermal_control; and SH, skin_hydrolysate, respectively.
Hydrolysis and thermal processing of samples
Salmon by-products were quantified by 500 g for each part, and enzymatic hydrolysis was performed by putting them in distilled water with a pH 8.0 to which protein hydrolysis enzyme (alcalase) was added at a concentration of 2.4 AU/kg. Protein hydrolysis was stirred with a propeller at 55 °C for 1 h, and the hydrolysis was stopped by heating at 85 °C for 20 min to deactivate the enzyme. The prepared protein hydrolysate derived from salmon by-products was cooled at room temperature and filtered using an experimental sieve (size 500 μm/35, line thickness 315 μm, 885705, chung gye sangong, Seoul, Korea), and the filtered hydrolysate was refrigerated at 4 °C before use in the experiment.
A hydrolysate was used for thermal processing. Except for the control group, a total of four pre-treatment groups were prepared with a simple thermal processing group, 1% (w/v) xylose treatment group, 1% (w/v) glucose treatment group, and 0.5% (w/v) xylose + 0.5% (w/v) glucose treatment group and thermal processing at 95 °C for 1 h using waterbath.
Electronic nose analysis for volatile compounds
An electronic nose system (HERACLES Neo, Alpha MOS, Toulouse, France) was used to analyze the volatile compounds of the pre-treated salmon by-products, and an MXT-5 column (Alpha MOS) was used as the analysis column. In the electronic nose analysis, 5 g of the previously treated sample was stirred at 60 °C for 30 min with 100 mL of purified water at 300 rpm, and then filtered. The 4 mL of the filtrate was taken, put in a headspace vial (22.5 × 75 mm, PTEE/silicone septum, aluminum cap) for electronic nose analysis, and stirred at 500 rpm with 50 °C for 20 min to saturate the volatile compounds inside the vial. Volatile compounds were collected through an automatic sample collector attached to the E-nose, and the 2,000 μL of the collected volatile compounds were taken using a syringe and injected into the gas chromatography injection port mounted on the E-nose. The analysis conditions were 1 mL/min of hydrogen gas flow rate, acquisition time was 227 s, trap absorption temperature was 40 °C, and trap desorption temperature was 250 °C. The oven temperature was maintained at 40 °C for 5 s, and then increased to 270 °C at a rate of 4 °C/s for 30 s at 270 °C. The retention index based on carbon atoms was based on Kovat's index library, and the separated peak components were identified using the AcroChemBase (Alpha MOS) of Electronic Co. The electronic nose analysis system was based on triple repetitions per sample, and the volatile compounds pattern was confirmed using multivariate analysis (Boo et al., 2020).
GC–MS coupled with GC–O for volatile odor compounds
Headspace analysis was used to capture volatile compounds of pre-treated salmon by-products, and SPME (Supelco Inc., Bellefonte, PA, USA) coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) of 50/30 μm was used. The 2 g of sample was put in the vial, sealed with an aluminum cap, equilibrium at 60 °C for 20 min, SPME fiber was injected into a vial, and volatile compounds were absorbed in SPME fiber at 60 °C for 30 min. volatile compounds collected in SPME were analyzed through GC/MS (Agilent 7890A & 5975C, Santa Clara, CA, USA) with HP-5MS column (30 m × 0.25 mm i.d × 0.25 um film thickness). The analysis conditions of GC–MS increased at a rate of 5 °C/min to 200 °C after maintaining the oven temperature of 40 °C for 5 min, and the temperature of the injector was 220 °C. Flow gas was poured in helium at 1 mL/min, and split ratio was 1:10. Identification of volatile compounds separated by total ionization chromatogram was performed using mass spectrum library (NIST version 12) and literature. Each volatile compound in the sample was calculated as μg/g using pentadecane as an internal standard.
The odor active compounds were analyzed using GC–O [olfactometry detection port (ODP), Gerstel Co., Linthicum, MD, USA]. combined with GC–MS. Identification of odor active compounds of salmon by-products was performed through solvent dissolution time of 0–5 min and general detection time of odor active compounds for 5–25 min. The intensity of the odor active compounds recognized through GC–O was expressed in numerical values from 1 to 4, and the higher the value, the stronger the intensity of the odor active compounds (Boo et al., 2020).
Statistical analysis
To identify the patterns of salmon by-products and aroma profiles, XLSTAT software ver. 9.2 (Addinsoft, New York, NY, USA) was used to analyze principal component analysis (PCA) and cluster analysis (CA) for the chemometric analyses.
Results and discussion
E-nose analysis for volatile compounds
The volatile compounds of salmon by-products analyzed using the electronic nose system were shown in Table 1. In pre-treatment salmon by-product samples, 7 acids and esters, 26 alcohols, 17 aldehydes, 31 heterocyclic compounds, 31 hydrocarbons, 16 ketones, and 12 sulfur containing compounds were detected, resulting in a total of 140 volatile compounds. Among 15 samples, the peak area of aldehydes was the highest. In HXG, HG, HX, FXG, FG, FX, FC, SXG, and SH, the peak area of sulfur containing compounds was the lowest, and in HC and FH, the peak area of ketones was the lowest. In HH and SC, the lowest peak area of acids and esters was detected, and in samples SG and SX, the lowest peak area of heterocyclic compounds were detected. Most volatile compounds (acid and ester, alcohol, aldehydes, and hydrocarbons) showed the highest peak area in sample FX, while ketones and sulfur containing compounds showed the highest peak area in sample SX. The heterocyclic compounds had the highest peak area detected in sample HH. According to the E-nose analysis results of this study, the difference between the parts was confirmed as the peak area higher than skin was detected in the samples of the head and frame based on the aldehydes, which showed the highest peak area in all samples. In addition, the peak area of aldehydes with the same pattern was identified in the order of thermal_xylose, thermal_xylose + glucose, thermal_control, thermal_glucose, and hydrolysate, and the difference according to the processing method was also confirmed.
Table 1.
Volatile compounds within by-product of salmon using E-nose
| (Peak area × 103) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | RT(RI) | Sensory description | HXG | HG | HX | HC | HH | FXG | FG |
| Acids and Esters (7) | |||||||||
| Acetic acid | 23.78(613) | Sour | ND | ND | ND | 0.19 ± 0.02 | ND | 0.28 ± 0.04 | 0.21 ± 0.02 |
| Propanoic acid | 36.21(739) | Pungent | ND | ND | 0.05 ± 0.05 | ND | ND | ND | ND |
| 2-Methylpropanoic acid | 40.40(774) | Butter | ND | ND | 0.10 ± 0.09 | ND | ND | ND | ND |
| Butanoic acid | 45.21(817) | Butter | ND | ND | ND | ND | ND | ND | ND |
| 3-Methylbutanoic acid | 49.64(860) | Fruity, Sweet | ND | ND | ND | 0.29 ± 0.03 | 0.40 ± 0.01 | ND | ND |
| Hexanoic acid | 62.00(996) | Sweet | ND | ND | 2.93 ± 0.34 | ND | ND | 3.89 ± 0.15 | ND |
| Decanoic acid | 82.16(1331) | Citrus, Sour | ND | ND | ND | 1.95 ± 0.10 | ND | ND | ND |
| Alcohols(26) | |||||||||
| Ethanol | 15.29(439) | Sweet | ND | ND | ND | ND | ND | 3.35 ± 0.09 | 1.31 ± 0.05 |
| 2-Propanaol | 17.63(491) | Acetone | ND | ND | 12.60 ± 0.35 | ND | ND | ND | ND |
| 2-Butanol | 23.77(613) | Sweet | ND | ND | ND | ND | 0.24 ± 0.02 | 4.12 ± 0.07 | ND |
| 2-Pentanol | 29.91(681) | Green | ND | ND | ND | ND | ND | ND | ND |
| 3-Pentanol | 32.05(704) | Sweet | ND | ND | ND | ND | ND | ND | 1.14 ± 0.02 |
| 3-Methylbutanol | 36.02(737) | Pungent | ND | ND | ND | ND | ND | 0.20 ± 0.03 | ND |
| 2-Methyl-1-butanol | 36.09(738) | Butter, Oil | 0.11 ± 0.04 | 0.08 ± 0.01 | ND | ND | ND | ND | ND |
| 1,2-Butanediol | 38.51(758) | Butter | ND | ND | ND | ND | ND | ND | ND |
| Pentanol | 38.63(759) | Oil | 0.67 ± 0.10 | ND | ND | ND | ND | ND | ND |
| 2-Penten-1-ol | 40.13(772) | Green | ND | ND | ND | ND | ND | ND | ND |
| 1-Hexen-3-ol | 40.30(773) | Rum | ND | ND | ND | ND | ND | 0.16 ± 0.02 | ND |
| 2,3-Dimethyl-1-pentanol | 45.29(818) | – | ND | ND | ND | ND | ND | ND | ND |
| 2-Hexenol | 49.59(859) | Butter(cooked) | ND | ND | ND | ND | ND | ND | 0.16 ± 0.01 |
| 2-Hexen-1-ol | 49.71(860) | Green | ND | ND | ND | ND | ND | ND | ND |
| Hexanol | 50.92(872) | Toasty, Sweet | ND | ND | ND | ND | ND | 0.07 ± 0.02 | ND |
| 3-Hexenol | 51.06(873) | Fresh, Oily | ND | ND | 0.04 ± 0.03 | ND | ND | ND | ND |
| 2-Heptanol | 52.24(885) | Fresh, Green | ND | ND | 0.08 ± 0.07 | ND | ND | 2.88 ± 0.02 | 3.91 ± 0.07 |
| Heptanol | 59.35(965) | Fatty, Nutty | ND | ND | ND | ND | 0.40 ± 0.02 | ND | ND |
| 3-Octanol | 62.02(996) | Citrus, Nutty | ND | ND | ND | 2.92 ± 0.12 | ND | ND | ND |
| 2-Octanol | 67.42(1073) | Oily, Burnt | ND | ND | ND | ND | ND | ND | ND |
| Octanol | 67.43(1074) | Burnt, Oil | 2.34 ± 0.05 | 2.64 ± 0.36 | ND | ND | ND | 7.48 ± 0.30 | ND |
| 8-Cymenol | 73.83(1176) | Citrus, Sweet | 2.07 ± 0.29 | ND | 2.07 ± 0.19 | ND | ND | ND | 1.59 ± 0.14 |
| 4-Terpineol | 73.86(1177) | Terpenic | ND | ND | ND | ND | ND | ND | ND |
| 3-Decanol | 75.15(1198) | Oily | ND | ND | ND | ND | ND | ND | ND |
| Undecanol | 83.87(1366) | Citrus, Mandarin | ND | ND | ND | ND | ND | ND | ND |
| 2-Pentadecanol | 99.48(1713) | Floral | ND | ND | ND | ND | ND | 0.38 ± 0.03 | ND |
| Aldehydes (17) | |||||||||
| Acetaldehyde | 15.26(438) | Fresh, Pungent | 2.66 ± 0.16 | 1.12 ± 0.05 | 4.51 ± 0.25 | 0.95 ± 0.05 | 0.89 ± 0.07 | 0.04 ± 0.03 | ND |
| Propenal | 16.55(467) | Almond | ND | ND | 1.85 ± 0.09 | 2.84 ± 0.23 | 2.08 ± 0.54 | ND | ND |
| Propanal | 17.60(490) | Nutty | ND | 8.39 ± 0.38 | ND | 5.61 ± 0.30 | 5.56 ± 0.01 | ND | 4.57 ± 0.10 |
| 2-Methylpropanal | 18.77(516) | Burnt | 0.29 ± 0.15 | ND | ND | 0.21 ± 0.08 | ND | 0.20 ± 0.10 | ND |
| 2-Butenal | 27.28(652) | Green | 31.81 ± 1.03 | 6.96 ± 0.45 | 61.45 ± 1.32 | 6.27 ± 0.41 | 3.90 ± 0.14 | 36.01 ± 0.29 | 6.71 ± 0.22 |
| Pentanal | 32.07(704) | Almond, Nutty | 1.26 ± 0.06 | 1.40 ± 0.12 | ND | ND | ND | ND | ND |
| 2-Methylpentanal | 38.57(759) | Earthy, Fruity | ND | ND | ND | ND | ND | ND | 0.34 ± 0.03 |
| 3-Hexenal | 42.02(788) | Fruity | ND | ND | ND | ND | ND | 0.15 ± 0.04 | ND |
| Hexanal | 43.70(802) | Fishy | ND | ND | ND | 5.26 ± 0.35 | 6.05 ± 0.12 | 3.52 ± 0.05 | 4.34 ± 0.11 |
| 2-Hexenal | 49.58(859) | Almond, Fruity | ND | ND | ND | ND | ND | 0.11 ± 0.01 | ND |
| 2-Methylhexanal | 52.29(885) | Green | ND | ND | ND | ND | 0.09 ± 0.01 | ND | ND |
| Heptanal | 54.13(903) | Citrus, Fishy | ND | 0.80 ± 0.05 | ND | 0.97 ± 0.07 | ND | ND | ND |
| 4-Heptenal | 54.17(904) | Oil, Fishy | 0.83 ± 0.05 | ND | 0.81 ± 0.02 | ND | ND | ND | ND |
| Benzaldehyde | 59.47(966) | Almond, Oil | ND | ND | ND | 0.29 ± 0.02 | ND | 0.19 ± 0.01 | 0.19 ± 0.02 |
| 6-Decenal | 75.20(1199) | Green | ND | 2.65 ± 0.48 | ND | 2.31 ± 0.29 | ND | ND | ND |
| Tridecanal | 91.25(1526) | Sweet | 1.38 ± 0.13 | 1.73 ± 0.45 | ND | ND | ND | ND | ND |
| Pentadecanal | 99.71(1718) | Fresh | ND | ND | 0.39 ± 0.13 | 0.42 ± 0.10 | ND | ND | ND |
| Heterocyclic compounds (31) | |||||||||
| Trimethylamine | 14.05(411) | Fishy | ND | ND | ND | ND | ND | ND | ND |
| tert-Butylmethylether | 20.41(552) | Minty | ND | ND | ND | ND | ND | ND | 2.70 ± 0.09 |
| 2-Methylfuran | 22.63(601) | Chocolate | ND | ND | ND | ND | ND | ND | 2.55 ± 0.06 |
| Ethyl acetate | 23.76(613) | Sweet | ND | ND | ND | ND | ND | ND | ND |
| Butanamine | 24.35(620) | Fishy | ND | ND | ND | ND | 0.16 ± 0.03 | ND | ND |
| 3-Methylfuran | 24.44(621) | – | ND | ND | ND | 0.12 ± 0.03 | ND | ND | ND |
| Pyrazine | 36.10(738) | Bitter, Hazelnut | ND | ND | ND | ND | ND | ND | ND |
| Ethyl isobutyrate | 37.96(754) | Sweet | ND | ND | ND | ND | ND | ND | ND |
| Pyridine | 38.00(754) | Amine, Burnt | ND | 0.40 ± 0.01 | ND | 0.40 ± 0.01 | ND | ND | ND |
| Pyrrole | 38.04(754) | Coffee, Nutty | ND | ND | ND | ND | 0.61 ± 0.01 | ND | ND |
| Methyl crotonate | 38.11(755) | Fruity, Green | ND | ND | 1.05 ± 0.15 | ND | ND | 0.72 ± 0.05 | ND |
| Ethyl butyrate | 42.11(789) | Sweet | ND | ND | ND | ND | ND | ND | ND |
| Butyl acetate | 45.27(818) | Bitter, Green | ND | ND | 0.09 ± 0.03 | ND | ND | ND | ND |
| Furfural | 47.69(841) | Almond | 0.38 ± 0.02 | ND | ND | ND | ND | ND | ND |
| 2-Furanmethanol | 49.61(859) | Bread, Coffee | 0.21 ± 0.01 | 0.28 ± 0.01 | ND | ND | ND | ND | ND |
| 2-Butylfuran | 53.09(893) | Spicy, Sweet | ND | ND | ND | ND | ND | ND | 0.07 ± 0.03 |
| 2-Furanone | 54.11(903) | Butter | ND | ND | ND | ND | ND | ND | ND |
| Dihydro-2-furanone | 54.14(903) | Oily | 0.69 ± 0.02 | ND | ND | ND | ND | ND | ND |
| Butyl propanoate | 54.95(913) | Sweet | ND | ND | ND | ND | ND | ND | ND |
| 4-Pentanolide | 59.28(964) | Cocoa | ND | ND | ND | ND | ND | ND | ND |
| Butyl butanoate | 61.97(996) | Fresh, Green | ND | ND | ND | ND | ND | ND | ND |
| Ethyl hexanoate | 62.03(997) | Sweet | 2.68 ± 0.02 | 2.49 ± 0.09 | ND | ND | ND | ND | ND |
| 2-Acetylpyridine | 64.67(1034) | Corn, Fatty | ND | ND | ND | ND | 1.40 ± 0.09 | ND | ND |
| Hexyl butyrate | 74.63(1189) | Sweet | ND | ND | ND | ND | 2.46 ± 0.03 | ND | ND |
| Methyl decanoate | 82.06(1329) | Oil | ND | 2.26 ± 0.43 | ND | ND | ND | 1.87 ± 0.18 | ND |
| Triacetin | 82.23(1332) | Fruity | ND | ND | ND | ND | 2.21 ± 0.08 | ND | ND |
| Myristicin | 91.23(1526) | Spicy, Balsamic | ND | ND | ND | ND | ND | ND | ND |
| Molinate | 91.30(1527) | Aromatic | ND | ND | ND | ND | ND | 1.25 ± 0.20 | ND |
| Methyl dodecanoate | 91.39(1529) | Creamy, Oil | ND | ND | 1.34 ± 0.01 | 1.02 ± 0.21 | 1.53 ± 0.12 | ND | 0.87 ± 0.15 |
| Methyl tetradecanoate | 100.31(1732) | Coconut | ND | ND | ND | ND | ND | ND | 0.24 ± 0.10 |
| Ambroxide | 104.94(1837) | Sweet | ND | ND | ND | 0.93 ± 0.28 | ND | ND | ND |
| Hydrocarbons (31) | |||||||||
| 2-Methylbutane | 16.51(466) | – | 2.61 ± 0.15 | 2.51 ± 0.12 | ND | ND | ND | ND | ND |
| Acetonitrile | 20.37(551) | Sweet | 8.24 ± 0.36 | ND | 16.70 ± 0.32 | ND | ND | 11.33 ± 0.24 | ND |
| 3-Methylpentane | 21.95(586) | – | 1.04 ± 0.11 | 1.70 ± 0.04 | ND | ND | ND | ND | ND |
| Hexane | 22.59(600) | Alkane | 3.12 ± 0.12 | 2.53 ± 0.03 | 4.33 ± 0.15 | ND | ND | ND | ND |
| 1,2-Dichloroethene | 23.75(613) | Sweet | 0.73 ± 0.10 | 0.65 ± 0.03 | 0.73 ± 0.07 | 1.20 ± 0.12 | 1.00 ± 0.06 | ND | ND |
| Trichloroethane | 28.19(662) | Sweet | ND | ND | ND | ND | ND | ND | ND |
| Benzene | 28.21(663) | – | 7.24 ± 0.20 | ND | ND | ND | ND | 7.99 ± 0.03 | 1.41 ± 0.06 |
| 1,1-Dichloropropene | 28.27(663) | – | ND | ND | ND | ND | 0.84 ± 0.02 | ND | ND |
| Isooctane | 29.92(681) | Gasoline | ND | ND | ND | ND | ND | 0.57 ± 0.03 | ND |
| 3-Ethylpentane | 29.93(682) | – | 0.83 ± 0.03 | ND | ND | ND | ND | ND | 0.17 ± 0.01 |
| Heptane | 29.96(682) | Alkane | ND | 0.65 ± 0.02 | ND | ND | ND | ND | 1.57 ± 0.07 |
| Trichloroethylene | 32.11(704) | Sweet | ND | ND | ND | ND | 5.50 ± 0.25 | ND | ND |
| Dibromomethane | 32.25(706) | – | ND | ND | 1.00 ± 0.03 | 1.78 ± 0.12 | ND | ND | ND |
| Chloropentane | 37.95(754) | Sweet | ND | ND | ND | ND | ND | ND | ND |
| Toluene | 42.05(788) | Pungent | 0.09 ± 0.01 | ND | ND | 0.15 ± 0.02 | 0.05 ± 0.04 | ND | ND |
| Octane | 43.61(802) | Sweet | 3.80 ± 0.11 | 4.41 ± 0.33 | 3.16 ± 0.05 | ND | ND | ND | ND |
| 2-Octene | 45.12(816) | – | ND | ND | ND | 0.08 ± 0.01 | 0.05 ± 0.05 | ND | ND |
| 4-Methyloctane | 51.00(873) | – | ND | 0.06 ± 0.01 | ND | ND | ND | ND | ND |
| Ethylbenzene | 51.04(873) | Sweet | 0.04 ± 0.04 | ND | ND | ND | ND | ND | ND |
| 1,1,2,2,-Tetrachloroethane | 55.92(924) | Pungent | ND | ND | ND | ND | ND | 0.06 ± 0.01 | ND |
| α-Pinene | 56.01(926) | Fresh, Terpenic | 0.07 ± 0.00 | ND | 0.07 ± 0.01 | ND | 0.07 ± 0.01 | ND | ND |
| 2-Mehtylnonane | 59.41(966) | – | ND | ND | 0.18 ± 0.05 | ND | ND | ND | ND |
| 1,2,4-Trimethylbenzene | 62.01(996) | Herbaceous | ND | ND | ND | ND | ND | ND | 3.06 ± 0.28 |
| Myrcene | 62.07(997) | Etheral | ND | ND | ND | ND | ND | ND | ND |
| Decane | 62.11(998) | Sweet | ND | ND | ND | ND | 4.28 ± 0.24 | ND | ND |
| Limonene | 64.58(1033) | Citrus | ND | ND | ND | ND | ND | 1.18 ± 0.06 | ND |
| β-Phellandrene | 64.59(1033) | Minty, Fruity | ND | ND | ND | ND | ND | ND | 1.09 ± 0.07 |
| p-Cymene | 64.63(1033) | Citrus, Fresh | 1.42 ± 0.20 | 1.42 ± 0.19 | ND | 1.27 ± 0.14 | ND | ND | ND |
| p-Methylacetophenone | 74.52(1188) | Cherry | ND | ND | ND | ND | ND | 1.91 ± 0.37 | ND |
| Tridecane | 81.07(1309) | Alkane, Citrus | 1.77 ± 0.05 | ND | ND | ND | ND | ND | ND |
| 6-Methyl-tridecane | 82.09(1329) | – | ND | ND | ND | ND | ND | ND | 1.82 ± 0.02 |
| Ketones (16) | |||||||||
| 2-Propanone | 17.59(490) | Fruity, Sweet | 10.57 ± 0.42 | ND | ND | ND | ND | 8.50 ± 0.25 | ND |
| 2,3-Butandione | 22.05(588) | Butter, Creamy | ND | ND | 1.45 ± 0.15 | ND | ND | ND | ND |
| 3-Buten-2-one | 22.07(589) | Pungent | ND | ND | ND | ND | ND | ND | ND |
| 1-Penten-3-one | 30.02(683) | Fishy, Onion | ND | ND | ND | 0.50 ± 0.05 | ND | ND | ND |
| 2,3-Pentanedione | 31.53(699) | Almond, Burnt | 1.54 ± 0.03 | 1.53 ± 0.10 | 1.34 ± 0.06 | 1.71 ± 0.15 | ND | 1.36 ± 0.04 | ND |
| 1-Hexen-3-one | 40.35(774) | Vegetable | 0.18 ± 0.00 | 0.16 ± 0.01 | ND | ND | ND | ND | ND |
| Cyclopentanone | 40.44(775) | Minty | ND | ND | ND | ND | ND | ND | ND |
| 3-Hexanone | 42.21(790) | Fresh, Sweet | ND | ND | 0.17 ± 0.01 | ND | ND | ND | ND |
| 3-Heptanone | 51.05(873) | Green, Sweet | 0.07 ± 0.01 | ND | ND | ND | ND | ND | ND |
| 2-Heptanone | 53.12(893) | Woody, Fruity | ND | ND | ND | ND | ND | 0.04 ± 0.04 | ND |
| 2-Octanone | 62.04(997) | Fruity | ND | ND | ND | ND | ND | ND | ND |
| 3-Octen-2-one | 64.69(1034) | Butter, Nutty | ND | ND | 1.23 ± 0.11 | ND | ND | ND | ND |
| 1-Nonen-3-one | 67.53(1075) | – | ND | ND | 2.32 ± 0.13 | ND | ND | ND | ND |
| 3-Decanone | 74.50(1187) | Citrus, Floral | ND | ND | ND | ND | ND | ND | ND |
| γ-Nonalactone | 83.93(1367) | Coconut, Oil | ND | ND | 1.68 ± 0.20 | ND | ND | ND | ND |
| δ-Dodecalactone | 99.83(1721) | Fresh, Oil | 0.47 ± 0.12 | ND | ND | ND | ND | ND | ND |
| Sulfur-containing compounds (12) | |||||||||
| Methanethiol | 15.35(440) | Fishy | ND | ND | ND | ND | ND | ND | ND |
| 2-Methyl-2-propanethiol | 22.56(600) | Sulfurous | ND | ND | ND | 2.41 ± 0.14 | 2.00 ± 0.06 | ND | ND |
| Propanethiol | 24.93(626) | Onion, Sweet | ND | ND | 0.17 ± 0.05 | ND | ND | ND | ND |
| Thiophene | 30.43(687) | Sulfurous | ND | ND | ND | ND | 0.24 ± 0.01 | ND | ND |
| 2-Methylthiophene | 40.53(775) | Alliaceous | ND | ND | ND | ND | ND | ND | 0.10 ± 0.02 |
| Pentanethiol | 45.11(816) | Sulfurous | 0.11 ± 0.01 | 0.08 ± 0.02 | ND | ND | ND | 0.08 ± 0.02 | ND |
| Dimethyl sulfoxide | 47.81(842) | Oil | ND | ND | 0.73 ± 0.01 | ND | ND | 0.45 ± 0.01 | ND |
| 2-Methyl-3-furanthiol | 49.73(860) | Nutty | ND | ND | 0.15 ± 0.01 | ND | ND | ND | ND |
| Methional | 54.29(905) | Creamy | ND | ND | ND | ND | 0.93 ± 0.02 | ND | ND |
| 1-Hexanethiol | 55.98(925) | Oily | ND | ND | ND | ND | ND | ND | ND |
| Dimethyl trisulfide | 59.31(964) | Fishy, Sulfurous | 0.22 ± 0.01 | 0.26 ± 0.00 | ND | ND | ND | ND | ND |
| Decanethiol | 82.19(1331) | – | ND | ND | ND | ND | ND | ND | ND |
| Compounds | RT(RI) | Sensory description | FX | FC | FH | SXG | SG | SX | SC | SH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acids and esters (7) | |||||||||||
| Acetic acid | 23.78(613) | Sour | 0.24 ± 0.10 | 0.24 ± 0.06 | ND | ND | ND | ND | ND | ND | |
| Propanoic acid | 36.21(739) | Pungent | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Methylpropanoic acid | 40.40(774) | Butter | 0.25 ± 0.02 | ND | ND | ND | ND | ND | ND | ND | |
| Butanoic acid | 45.21(817) | Butter | ND | ND | ND | ND | 0.08 ± 0.01 | ND | 0.07 ± 0.02 | ND | |
| 3-Methylbutanoic acid | 49.64(860) | Fruity, Sweet | ND | ND | 0.38 ± 0.00 | ND | 0.06 ± 0.01 | ND | ND | ND | |
| Hexanoic acid | 62.00(996) | Sweet | 4.56 ± 0.04 | ND | ND | ND | ND | ND | ND | ND | |
| Decanoic acid | 82.16(1331) | Citrus, Sour | ND | ND | ND | ND | ND | ND | 2.23 ± 0.09 | ND | |
| Alcohols (26) | |||||||||||
| Ethanol | 15.29(439) | Sweet | ND | ND | ND | ND | ND | ND | 1.85 ± 0.16 | 1.74 ± 0.02 | |
| 2-Propanaol | 17.63(491) | Acetone | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Butanol | 23.77(613) | Sweet | 6.09 ± 0.22 | ND | ND | ND | ND | 1.49 ± 0.02 | 0.23 ± 0.04 | 0.24 ± 0.01 | |
| 2-Pentanol | 29.91(681) | Green | ND | ND | ND | ND | ND | ND | ND | 0.42 ± 0.02 | |
| 3-Pentanol | 32.05(704) | Sweet | 0.99 ± 0.07 | ND | ND | ND | 1.51 ± 0.02 | ND | ND | ND | |
| 3-Methylbutanol | 36.02(737) | Pungent | 0.11 ± 0.00 | ND | ND | ND | ND | ND | ND | ND | |
| 2-Methyl-1-butanol | 36.09(738) | Butter, Oil | ND | ND | 0.11 ± 0.02 | ND | ND | 0.15 ± 0.02 | ND | 0.13 ± 0.01 | |
| 1,2-Butanediol | 38.51(758) | Butter | ND | ND | ND | ND | ND | ND | 0.18 ± 0.02 | ND | |
| Pentanol | 38.63(759) | Oil | ND | ND | 0.59 ± 0.04 | ND | ND | ND | ND | ND | |
| 2-Penten-1-ol | 40.13(772) | Green | ND | ND | ND | ND | 0.19 ± 0.00 | ND | ND | ND | |
| 1-Hexen-3-ol | 40.30(773) | Rum | ND | ND | ND | 0.10 ± 0.03 | ND | ND | ND | ND | |
| 2,3-Dimethyl-1-pentanol | 45.29(818) | – | ND | ND | 0.07 ± 0.01 | ND | ND | ND | ND | ND | |
| 2-Hexenol | 49.59(859) | Butter(cooked) | 0.08 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | |
| 2-Hexen-1-ol | 49.71(860) | Green | ND | ND | ND | ND | 0.17 ± 0.01 | 0.07 ± 0.01 | 0.18 ± 0.00 | ND | |
| Hexanol | 50.92(872) | Toasty, Sweet | 0.06 ± 0.01 | ND | ND | ND | ND | 0.06 ± 0.07 | ND | ND | |
| 3-Hexenol | 51.06(873) | Fresh, Oily | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Heptanol | 52.24(885) | Fresh, Green | 2.41 ± 0.08 | 5.99 ± 0.09 | 4.45 ± 0.11 | 0.06 ± 0.06 | 0.79 ± 0.03 | ND | ND | ND | |
| Heptanol | 59.35(965) | Fatty, Nutty | ND | ND | 0.36 ± 0.09 | ND | ND | ND | ND | ND | |
| 3-Octanol | 62.02(996) | Citrus, Nutty | ND | ND | ND | ND | 2.11 ± 0.33 | ND | ND | ND | |
| 2-Octanol | 67.42(1073) | Oily, Burnt | ND | ND | ND | 2.33 ± 0.11 | ND | ND | ND | ND | |
| Octanol | 67.43(1074) | Burnt, Oil | 6.21 ± 0.04 | ND | ND | ND | 2.94 ± 0.15 | 3.17 ± 0.30 | 3.49 ± 0.03 | 3.58 ± 0.20 | |
| 8-Cymenol | 73.83(1176) | Citrus, Sweet | 1.62 ± 0.15 | ND | 2.57 ± 0.74 | ND | ND | 3.07 ± 0.48 | ND | ND | |
| 4-Terpineol | 73.86(1177) | Terpenic | ND | ND | ND | 2.31 ± 0.13 | 2.81 ± 0.17 | ND | ND | ND | |
| 3-Decanol | 75.15(1198) | Oily | ND | ND | ND | ND | ND | ND | 3.18 ± 0.12 | 3.31 ± 0.18 | |
| Undecanol | 83.87(1366) | Citrus, Mandarin | 1.44 ± 0.08 | ND | ND | ND | ND | ND | ND | ND | |
| 2-Pentadecanol | 99.48(1713) | Floral | 0.37 ± 0.04 | 0.35 ± 0.01 | ND | ND | ND | ND | ND | ND | |
| Aldehydes (17) | |||||||||||
| Acetaldehyde | 15.26(438) | Fresh, Pungent | 5.37 ± 0.32 | 1.28 ± 0.06 | 1.30 ± 0.03 | 2.00 ± 0.07 | ND | 2.64 ± 0.11 | 0.74 ± 0.08 | 0.76 ± 0.03 | |
| Propenal | 16.55(467) | Almond | ND | ND | 1.57 ± 0.05 | ND | ND | ND | ND | ND | |
| Propanal | 17.60(490) | Nutty | ND | 5.30 ± 0.22 | 4.98 ± 0.15 | ND | ND | ND | 4.46 ± 0.20 | 4.77 ± 0.03 | |
| 2-Methylpropanal | 18.77(516) | Burnt | 0.17 ± 0.01 | 0.23 ± 0.12 | 0.24 ± 0.09 | ND | 0.16 ± 0.02 | 0.31 ± 0.05 | 0.27 ± 0.08 | 0.23 ± 0.08 | |
| 2-Butenal | 27.28(652) | Green | 70.14 ± 0.82 | 6.91 ± 0.23 | 5.58 ± 0.13 | 26.08 ± 0.54 | 6.69 ± 0.25 | 50.89 ± 1.00 | 5.23 ± 0.10 | 3.69 ± 0.02 | |
| Pentanal | 32.07(704) | Almond, Nutty | ND | ND | ND | 1.06 ± 0.03 | ND | 0.98 ± 0.06 | 1.77 ± 0.08 | ND | |
| 2-Methylpentanal | 38.57(759) | Earthy, Fruity | ND | ND | ND | ND | ND | ND | ND | ND | |
| 3-Hexenal | 42.02(788) | Fruity | ND | ND | ND | ND | ND | ND | ND | ND | |
| Hexanal | 43.70(802) | Fishy | 2.82 ± 0.06 | 6.10 ± 0.12 | 4.99 ± 0.13 | 2.50 ± 0.04 | 3.02 ± 0.09 | ND | 3.63 ± 0.09 | 3.74 ± 0.05 | |
| 2-Hexenal | 49.58(859) | Almond, Fruity | ND | ND | ND | 0.11 ± 0.00 | ND | ND | 0.05 ± 0.04 | ND | |
| 2-Methylhexanal | 52.29(885) | Green | ND | ND | ND | ND | ND | ND | ND | ND | |
| Heptanal | 54.13(903) | Citrus, Fishy | ND | ND | ND | ND | ND | ND | ND | 0.85 ± 0.08 | |
| 4-Heptenal | 54.17(904) | Oil, Fishy | ND | ND | ND | ND | ND | ND | ND | ND | |
| Benzaldehyde | 59.47(966) | Almond, Oil | 0.16 ± 0.02 | ND | ND | ND | ND | 0.20 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.04 | |
| 6-Decenal | 75.20(1199) | Green | ND | ND | ND | ND | ND | ND | ND | ND | |
| Tridecanal | 91.25(1526) | Sweet | ND | ND | ND | ND | 1.39 ± 0.06 | ND | ND | ND | |
| Pentadecanal | 99.71(1718) | Fresh | ND | ND | 0.23 ± 0.05 | 0.42 ± 0.14 | ND | 0.53 ± 0.06 | 0.57 ± 0.06 | 0.58 ± 0.26 | |
| Heterocyclic compounds (31) | |||||||||||
| Trimethylamine | 14.05(411) | Fishy | 0.08 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | |
| tert-Butylmethylether | 20.41(552) | Minty | ND | ND | 2.02 ± 0.14 | ND | ND | ND | ND | ND | |
| 2-Methylfuran | 22.63(601) | Chocolate | ND | 3.11 ± 0.21 | 2.47 ± 0.14 | ND | ND | ND | ND | ND | |
| Ethyl acetate | 23.76(613) | Sweet | ND | ND | ND | 0.64 ± 0.08 | ND | ND | ND | ND | |
| Butanamine | 24.35(620) | Fishy | ND | ND | ND | ND | ND | ND | ND | ND | |
| 3-Methylfuran | 24.44(621) | – | ND | 0.17 ± 0.11 | 0.16 ± 0.06 | ND | ND | 0.11 ± 0.02 | 0.13 ± 0.06 | 0.16 ± 0.02 | |
| Pyrazine | 36.10(738) | Bitter, Hazelnut | ND | 0.21 ± 0.04 | ND | ND | 0.13 ± 0.01 | ND | 0.13 ± 0.01 | ND | |
| Ethyl isobutyrate | 37.96(754) | Sweet | ND | ND | ND | 0.39 ± 0.02 | 0.17 ± 0.08 | ND | ND | 0.36 ± 0.01 | |
| Pyridine | 38.00(754) | Amine, Burnt | ND | ND | ND | ND | ND | ND | ND | ND | |
| Pyrrole | 38.04(754) | Coffee, Nutty | ND | 0.39 ± 0.01 | ND | ND | ND | ND | ND | ND | |
| Methyl crotonate | 38.11(755) | Fruity, Green | ND | ND | ND | ND | ND | ND | ND | ND | |
| Ethyl butyrate | 42.11(789) | Sweet | ND | ND | ND | ND | ND | 0.08 ± 0.01 | ND | ND | |
| Butyl acetate | 45.27(818) | Bitter, Green | ND | ND | ND | 0.07 ± 0.02 | ND | 0.09 ± 0.03 | ND | ND | |
| Furfural | 47.69(841) | Almond | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Furanmethanol | 49.61(859) | Bread, Coffee | ND | 0.22 ± 0.01 | 0.04 ± 0.04 | ND | ND | ND | ND | ND | |
| 2-Butylfuran | 53.09(893) | Spicy, Sweet | ND | 0.10 ± 0.01 | 0.12 ± 0.06 | ND | 0.08 ± 0.01 | ND | ND | 0.18 ± 0.04 | |
| 2-Furanone | 54.11(903) | Butter | ND | ND | ND | ND | ND | ND | 0.98 ± 0.02 | ND | |
| Dihydro-2-furanone | 54.14(903) | Oily | ND | ND | ND | ND | ND | ND | ND | ND | |
| Butyl propanoate | 54.95(913) | Sweet | ND | ND | ND | ND | ND | 0.73 ± 0.03 | ND | ND | |
| 4-Pentanolide | 59.28(964) | Cocoa | ND | 0.23 ± 0.03 | ND | ND | ND | ND | ND | ND | |
| Butyl butanoate | 61.97(996) | Fresh, Green | ND | ND | ND | ND | ND | ND | 2.57 ± 0.34 | ND | |
| Ethyl hexanoate | 62.03(997) | Sweet | ND | ND | ND | 2.13 ± 0.07 | ND | ND | ND | ND | |
| 2-Acetylpyridine | 64.67(1034) | Corn, Fatty | ND | ND | 1.21 ± 0.15 | ND | ND | ND | ND | ND | |
| Hexyl butyrate | 74.63(1189) | Sweet | ND | ND | ND | ND | ND | ND | ND | ND | |
| Methyl decanoate | 82.06(1329) | Oil | ND | ND | ND | ND | ND | ND | ND | ND | |
| Triacetin | 82.23(1332) | Fruity | ND | ND | ND | ND | ND | ND | ND | ND | |
| Myristicin | 91.23(1526) | Spicy, Balsamic | 1.01 ± 0.25 | 1.71 ± 0.35 | 1.26 ± 0.08 | 1.38 ± 0.26 | ND | 1.78 ± 0.45 | ND | ND | |
| Molinate | 91.30(1527) | Aromatic | ND | ND | ND | ND | ND | ND | ND | ND | |
| Methyl dodecanoate | 91.39(1529) | Creamy, Oil | ND | ND | ND | ND | ND | ND | ND | ND | |
| Methyl tetradecanoate | 100.31(1732) | Coconut | ND | ND | ND | ND | ND | ND | ND | ND | |
| Ambroxide | 104.94(1837) | Sweet | ND | ND | ND | ND | ND | ND | ND | ND | |
| Hydrocarbons (31) | |||||||||||
| 2-Methylbutane | 16.51(466) | – | ND | ND | ND | 1.60 ± 0.06 | ND | 3.27 ± 0.03 | ND | ND | |
| Acetonitrile | 20.37(551) | Sweet | 21.79 ± 0.64 | 2.66 ± 0.19 | ND | ND | ND | ND | ND | ND | |
| 3-Methylpentane | 21.95(586) | – | 1.79 ± 0.09 | ND | ND | 0.94 ± 0.09 | ND | ND | ND | ND | |
| Hexane | 22.59(600) | Alkane | ND | ND | ND | 2.12 ± 0.07 | 1.83 ± 0.14 | 2.91 ± 0.04 | 2.07 ± 0.19 | 1.60 ± 0.04 | |
| 1,2-Dichloroethene | 23.75(613) | Sweet | ND | ND | ND | ND | 1.53 ± 0.06 | ND | 1.15 ± 0.14 | 1.02 ± 0.05 | |
| Trichloroethane | 28.19(662) | Sweet | ND | ND | ND | ND | ND | ND | 1.13 ± 0.05 | 1.02 ± 0.02 | |
| Benzene | 28.21(663) | – | 15.29 ± 0.22 | 1.40 ± 0.06 | ND | ND | ND | ND | ND | ND | |
| 1,1-Dichloropropene | 28.27(663) | – | ND | ND | 1.27 ± 0.05 | ND | 1.57 ± 0.06 | ND | ND | ND | |
| Isooctane | 29.92(681) | Gasoline | ND | ND | ND | ND | ND | ND | ND | ND | |
| 3-Ethylpentane | 29.93(682) | – | ND | ND | ND | ND | ND | ND | ND | ND | |
| Heptane | 29.96(682) | Alkane | ND | 1.55 ± 0.15 | ND | ND | ND | ND | ND | ND | |
| Trichloroethylene | 32.11(704) | Sweet | ND | ND | ND | ND | ND | ND | ND | ND | |
| Dibromomethane | 32.25(706) | – | ND | ND | 3.79 ± 0.20 | ND | ND | ND | ND | ND | |
| Chloropentane | 37.95(754) | Sweet | 1.26 ± 0.04 | ND | ND | ND | ND | ND | ND | ND | |
| Toluene | 42.05(788) | Pungent | ND | ND | ND | ND | ND | ND | ND | ND | |
| Octane | 43.61(802) | Sweet | ND | ND | ND | ND | ND | 2.53 ± 0.05 | ND | ND | |
| 2-Octene | 45.12(816) | – | ND | ND | ND | ND | ND | ND | ND | 0.08 ± 0.01 | |
| 4-Methyloctane | 51.00(873) | – | ND | ND | ND | ND | ND | ND | ND | ND | |
| Ethylbenzene | 51.04(873) | Sweet | ND | 0.08 ± 0.01 | ND | ND | ND | ND | ND | ND | |
| 1,1,2,2,-Tetrachloroethane | 55.92(924) | Pungent | ND | ND | ND | ND | ND | ND | ND | ND | |
| α-Pinene | 56.01(926) | Fresh, Terpenic | ND | 0.04 ± 0.03 | ND | ND | ND | ND | ND | 0.09 ± 0.00 | |
| 2-Mehtylnonane | 59.41(966) | – | ND | ND | ND | ND | ND | ND | ND | ND | |
| 1,2,4-Trimethylbenzene | 62.01(996) | Herbaceous | ND | ND | ND | ND | ND | ND | ND | ND | |
| Myrcene | 62.07(997) | Etheral | ND | ND | 6.12 ± 0.54 | ND | ND | ND | ND | ND | |
| Decane | 62.11(998) | Sweet | ND | ND | ND | ND | ND | ND | ND | 3.68 ± 0.45 | |
| Limonene | 64.58(1033) | Citrus | 1.16 ± 0.07 | 1.22 ± 0.10 | ND | ND | ND | ND | ND | ND | |
| β-Phellandrene | 64.59(1033) | Minty, Fruity | ND | ND | ND | ND | ND | ND | ND | ND | |
| p-Cymene | 64.63(1033) | Citrus, Fresh | ND | ND | ND | ND | ND | ND | ND | ND | |
| p-Methylacetophenone | 74.52(1188) | Cherry | ND | ND | ND | ND | ND | ND | ND | ND | |
| Tridecane | 81.07(1309) | Alkane, Citrus | ND | ND | ND | ND | ND | ND | ND | ND | |
| 6-Methyl-tridecane | 82.09(1329) | – | ND | ND | ND | ND | ND | ND | ND | ND | |
| Ketones (16) | |||||||||||
| 2-Propanone | 17.59(490) | Fruity, Sweet | 12.33 ± 0.53 | ND | ND | 7.17 ± 0.14 | ND | 8.63 ± 0.15 | ND | ND | |
| 2,3-Butandione | 22.05(588) | Butter, Creamy | ND | ND | ND | ND | ND | ND | ND | 0.69 ± 0.03 | |
| 3-Buten-2-one | 22.07(589) | Pungent | ND | ND | ND | ND | 0.63 ± 0.04 | ND | ND | ND | |
| 1-Penten-3-one | 30.02(683) | Fishy, Onion | 0.45 ± 0.07 | 0.51 ± 0.05 | 0.27 ± 0.03 | 0.22 ± 0.01 | 0.47 ± 0.03 | 0.59 ± 0.02 | 0.44 ± 0.04 | ND | |
| 2,3-Pentanedione | 31.53(699) | Almond, Burnt | 1.23 ± 0.05 | 1.96 ± 0.09 | ND | 0.86 ± 0.02 | 1.03 ± 0.05 | 1.08 ± 0.03 | 1.15 ± 0.05 | ND | |
| 1-Hexen-3-one | 40.35(774) | Vegetable | ND | ND | ND | ND | ND | ND | ND | 0.18 ± 0.01 | |
| Cyclopentanone | 40.44(775) | Minty | 0.09 ± 0.07 | ND | ND | ND | ND | ND | ND | ND | |
| 3-Hexanone | 42.21(790) | Fresh, Sweet | ND | ND | ND | ND | ND | 0.14 ± 0.12 | 0.20 ± 0.02 | ND | |
| 3-Heptanone | 51.05(873) | Green, Sweet | ND | ND | ND | ND | ND | ND | 0.10 ± 0.02 | ND | |
| 2-Heptanone | 53.12(893) | Woody, Fruity | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Octanone | 62.04(997) | Fruity | ND | 5.82 ± 0.42 | ND | ND | ND | 2.77 ± 0.09 | ND | ND | |
| 3-Octen-2-one | 64.69(1034) | Butter, Nutty | ND | ND | ND | 1.68 ± 0.10 | 1.71 ± 0.10 | 1.88 ± 0.08 | 1.80 ± 0.03 | 2.22 ± 0.07 | |
| 1-Nonen-3-one | 67.53(1075) | – | ND | ND | ND | ND | ND | ND | ND | ND | |
| 3-Decanone | 74.50(1187) | Citrus, Floral | ND | 3.18 ± 0.40 | ND | ND | ND | ND | ND | ND | |
| γ-Nonalactone | 83.93(1367) | Coconut, Oil | ND | ND | ND | ND | ND | ND | ND | ND | |
| δ-Dodecalactone | 99.83(1721) | Fresh, Oil | ND | ND | ND | ND | ND | ND | ND | ND | |
| Sulfur-containing compounds (12) | |||||||||||
| Methanethiol | 15.35(440) | Fishy | ND | ND | ND | ND | 0.70 ± 0.02 | ND | ND | ND | |
| 2-Methyl-2-propanethiol | 22.56(600) | Sulfurous | ND | ND | ND | ND | ND | ND | ND | ND | |
| Propanethiol | 24.93(626) | Onion, Sweet | ND | ND | ND | ND | 0.49 ± 0.08 | 0.60 ± 0.02 | |||
| Thiophene | 30.43(687) | Sulfurous | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Methylthiophene | 40.53(775) | Alliaceous | ND | 0.14 ± 0.12 | 0.12 ± 0.01 | ND | ND | ND | ND | ND | |
| Pentanethiol | 45.11(816) | Sulfurous | ND | 0.06 ± 0.06 | ND | ND | ND | ND | ND | ND | |
| Dimethyl sulfoxide | 47.81(842) | Oil | 0.94 ± 0.05 | ND | ND | 0.36 ± 0.02 | ND | 0.96 ± 0.01 | ND | ND | |
| 2-Methyl-3-furanthiol | 49.73(860) | Nutty | ND | ND | ND | ND | ND | 0.11 ± 0.01 | ND | 0.27 ± 0.00 | |
| Methional | 54.29(905) | Creamy | ND | ND | ND | ND | ND | ND | ND | ND | |
| 1-Hexanethiol | 55.98(925) | Oily | 0.08 ± 0.01 | ND | ND | 0.06 ± 0.00 | ND | 0.07 ± 0.00 | ND | ND | |
| Dimethyl trisulfide | 59.31(964) | Fishy, Sulfurous | ND | ND | ND | 0.18 ± 0.01 | 0.19 ± 0.03 | ND | ND | ND | |
| Decanethiol | 82.19(1331) | – | ND | ND | 2.69 ± 0.67 | 1.80 ± 0.14 | ND | 2.17 ± 0.65 | ND | ND | |
RT retention time (s), RI retention indice, ND: not detected, HXG head_thermal_xylose + glucose, HG head_thermal_glucose, HX head_thermal_xylose, HC head_thermal_control, HH head_hydrolysate, FXG frame_thermal_xylose + glucose, FG frame_thermal_glucose, FX frame_thermal_xylose, FC frame_thermal_control, FH frame_hydrolysate, SXG skin_thermal_xylose + glucose, SG skin_thermal_glucose, SX skin_thermal_xylose, SC skin_thermal_control, SH skin_hydrolysate, respectively
According to a study by Iglesias et al. (2010) salmon, trout, and anchovies have the highest carbonyl compounds content (Iglesias et al., 2010). The results of this study also showed that the content of aldehydes, which is carbonyl compounds, was the highest, and ketones also had a relatively high content. Aldehyde is mainly produced from the decomposition of fatty acids and is a major cause of the aroma of meat products due to low thresholds. Heptanal and hexanal were found to be the main odor active aldehydes of pufferfish and were identified also as important volatile compounds in other fish (Li et al., 2023). Heptanal is known for its characteristic fish odor in unsmoked fish. A study by Jónsdóttir et al. (2008) described the overall characteristic aroma of salmon as the aroma of boiled potatoes, and the combination of 4-heptenal and heptanal contributed to the aroma of boiled potatoes with these characteristic smoked salmon (Jónsdóttir et al., 2008). In this study, 4-heptenal and heptanal were mainly detected in the head part. Hexanal is reported to have a sweet and green odor description, and fatty properties, contributing to the sweet and fat smell of smoked salmon (Jónsdóttir et al., 2008). In addition, it was reported that hexanal mainly has grassy and tallowy odor (Li et al., 2023), and it was confirmed in all samples except HXG, HG, HX, and SX in this study. 2-Butenal is produced as a result of hydroperoxide decomposition due to the oxidation of ω-3 polyunsaturated fatty acids (PUFA). This is known as crotonaldehyde, it was an α, β-unsaturated aldehyde with a four-carbon. This α, β-unsaturated aldehyde is detected in vegetable or fish oil (Papastergiadis et al., 2014). 2-Butenal was detected in all samples, and the content was found to be particularly high in xylose-treated samples. Alcohol is also produced from the decomposition of fatty acids and has herbaceous, woody, and fatty smell (Li et al, 2023). 2-Methyl-1-butanol is reported as an alcohol produced mainly by microbial activity in Jónsdóttir et al. (2008), and contributes to the spoilage smell in cold smoked salmon. 2-Methyl-1-butanol representing off-flavor was a small amount detected in HXG, HG, FH, SX, and SH. 3-Methyl-1-butanol which was short-chain alcohol and a spoilage-related compound (Jónsdóttir et al., 2008), was also a small amount detected in FXG and FX at the frame.
Multivariate analysis of volatile compound using E-nose
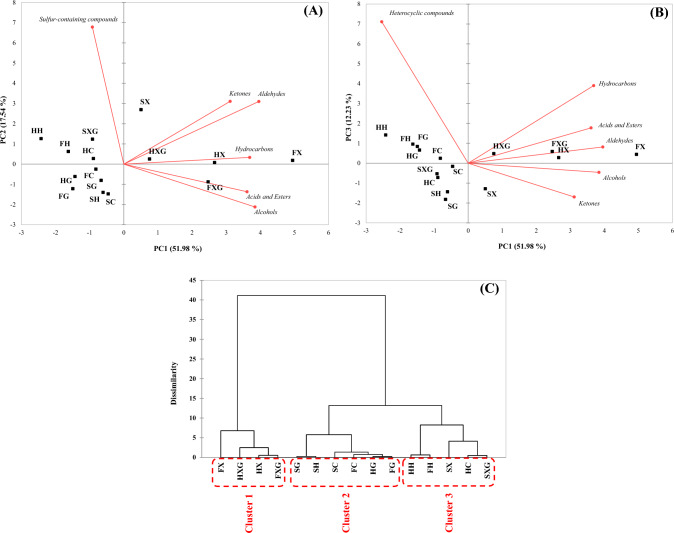
The multivariate analyses of salmon by-products analyzed using E-noses were shown through PCA and CA, and are shown in Fig. 1(A), (B), and (C), respectively. Figure 1 shows the patterns of 15 salmon samples based on the volatile compounds analyzed using E-nose. Figure 1(A) was separated based on PC1-2, and the variance was 51.98% and 17.54%, respectively, and the total variance of 69.52% was confirmed. HXG, HX, FXG, FX, and SX were located in a positive to PC1, and were located in the first quadrant under the influence of ketones, aldehydes, and hydrocarbons except for the FXG. FXG was located in the fourth quadrant, which is negative for PC2, under the influence of acids and esters, and alcohols. HC, HH, FH, and SXG were located in two quadrants which were negative to PC1 and positive to PC2, and the remaining samples were located in three quadrants which were negative to PC1 and PC2. Figure 1(B) was separated based on PC1-3, and the variance was 51.98% and 12.23%, respectively, and a total variance of 64.21% was confirmed. HXG, HX, FXG, FX, and SX were located in a positive to PC1, and were located in the first quadrant under the influence of hydrocarbons, acids and esters, and aldehydes except for SX. The sample SX was located in the fourth quadrant, which is a negative to PC2, under the influence of ketones and alcohols. HG, HH, FG, FC, and FH were located in two quadrants, which were negative to PC1 and positive to PC2, and the remaining samples were located in three quadrants, which were negative to PC1 and PC2.
Fig. 1.
PCA bi-plot and dendrogram for volatile compounds pattern of hydrolysate of salmon by-product treated reducing sugars and thermal processing based on the part using E-nose. (A) PC1-2, (B) PC1-3, and (C) dendrogram. HXG head_thermal_xylose + glucose, HG head_thermal_glucose, HX head_thermal_xylose, HC head_thermal_control, HH head_hydrolysate, FXG frame_thermal_xylose + glucose, FG frame_thermal_glucose, FX frame_thermal_xylose, FC frame_thermal_control, FH frame_hydrolysate, SXG skin_thermal_xylose + glucose, SG skin_thermal_glucose, SX skin_thermal_xylose, SC skin_thermal_control, SH skin_hydrolysate, respectively
The dissimilarity between samples was confirmed using cluster analysis as an dendrogram, and it is shown in Fig. 1(C). A total of 3 clusters were identified. Cluster 1 included HXG, HX, FXG, and FX, and Cluster 2 included HG, FG, FC, SG, SC, and SH. And Cluster 3 included HC, HH, FH, SXG, and SX. Cluster 1 and cluster 2, 3 had relatively the highest dissimilarity. As a result of multivariate analysis of the E-nose, it was confirmed that HXG, HX, FXG, FX, and SX showed high separation from other samples, which were affected by various volatile compounds. The samples showed a high degree of separation under the influence of xylose, and it is judged that the volatile compounds were affected by the same pre-treatment conditions rather than the by-product part.
The E-nose is a device that mimics the human sense of smell and has been in the spotlight in the field of sensors in the past 20 years. It has been used in various fields such as food, cosmetics, and the environment, and has improved the quality of products through monitoring using an E-nose in the product manufacturing process. E-nose generally have the advantage of being inexpensive and rapid analysis. It also provides consistent data compared to panel testing, preventing data from being biased (Tan and Xu, 2020; Wilson and Baietto, 2009). In Xu et al. (2021) the quality of rice was evaluated based on the change in aroma profile according to the aging process, and the pattern was shown through PCA (Xu et al., 2021). Tian et al. (2011) monitored the freshness of hairtail and pork using an E-nose and showed patterns using principal component analysis (Tian et al., 2011). This study provides aroma profiles for each part of salmon according to various pre-treatments, and it is expected that E-nose will continue to be applied to various fields in the future, including the aforementioned studies. These results provide difference in volatile compounds according to the part and pre-treatment of salmon and are expected to be used as a database of volatile compounds of salmon by-products in the food industry.
GC–MS analysis for volatile compounds
The volatile compounds of salmon by-products analyzed using GC–MS are shown in Table. 2. In salmon by-product samples, 3 acids and esters, 17 alcohols, 14 aldehydes, 24 heterocyclic compounds, 24 hydrocarbons, 6 ketones, and 2 sulfur containing compounds were detected, resulting in a total of 90 volatile compounds. HXG, HX, HC, HH, SXG, SG, SX, and SH were measured to have the highest alcohol content, and HXG, HX, SXG, and SX were measured to have the lowest ketones content. HC, HH, SXG, and SH had the lowest content of acids and esters. HG, FXG, FG, FX, FC, FH, and SC were measured to have the highest aldehydes content. Among them, HG and FG were measured to have the lowest ketones content, while FXG, FX, and FH were measured to have the lowest acids and esters content. FC was measured to have the lowest hydrocarbon content, and SC was measured to have the lowest sulfur containing compounds content. Acids and esters, alcohols, heterocyclic compounds, and hydrocarbons were measured to have the highest content in SH, and hydrocarbons were also measured to have the highest content in SG. In FH, HC, and SC, the contents of aldehydes, ketones, and sulfur containing compounds were measured to be the highest, respectively. As a result of GC–MS, aldehydes were identified as the main volatile compounds of salmon, similar to the E-nose, and the volatile compounds were detected in a relatively high content in the sample frame, confirming the difference between the parts. However, unlike the E-nose result, the change in the volatile compounds of the sample did not show the same pattern based on pre-treatment.
Table 2.
Volatile compounds by-product of salmon using GC–MS
| Compounds | RT | RI | HXG | HG | HX | HC | HH | FXG | (Peak area × 103) | |
|---|---|---|---|---|---|---|---|---|---|---|
| FG | I.D. | |||||||||
| Acids and esters (3) | ||||||||||
| 2-Amino-4-methylbenzoic acid | 11.31 | 907 | ND | ND | ND | ND | ND | ND | ND | MS |
| Octyl acetate | 19.07 | 1153 | ND | ND | ND | ND | 0.01 ± 0.01 | ND | ND | MS/RI |
| Butyl butylate | 25.22 | 1376 | 0.14 ± 0.03 | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.02 | 0.06 ± 0.01 | MS |
| Alcohols (17) | ||||||||||
| 2-Ethyl-hexanol | 15.41 | 1033 | 0.85 ± 0.01 | 0.22 ± 0.08 | 0.71 ± 0.01 | 0.85 ± 0.14 | 1.03 ± 0.01 | 0.39 ± 0.08 | 0.42 ± 0.04 | MS |
| Octanol | 16.71 | 1074 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.08 ± 0.02 | 0.02 ± 0.01 | MS |
| Xylitol pentacetate | 16.99 | 1083 | ND | ND | 0.01 ± 0.01 | ND | ND | ND | ND | MS |
| Menthol | 19.79 | 1177 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 | ND | 0.02 ± 0.01 | MS/RI |
| Nonadecanol | 20.05 | 1185 | ND | ND | ND | ND | ND | ND | ND | MS |
| Hexadecanol | 20.11 | 1187 | ND | ND | ND | ND | 0.03 ± 0.01 | ND | ND | MS/RI |
| Octadecanol | 20.55 | 1201 | ND | ND | ND | ND | 0.03 ± 0.01 | ND | ND | MS |
| Tetradecanol | 20.61 | 1204 | ND | ND | ND | ND | ND | 0.01 ± 0.01 | ND | MS |
| 2-Propylheptanol | 20.85 | 1213 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2-Isopropyl-5-methyl-1-heptanol | 20.88 | 1214 | ND | ND | ND | ND | ND | ND | ND | MS |
| Octadecanol | 21.26 | 1228 | ND | ND | ND | ND | 0.02 ± 0.01 | ND | ND | MS |
| 2-Dodecanol | 21.29 | 1229 | ND | ND | ND | ND | ND | ND | ND | MS |
| β-Acorenol | 26.34 | 1420 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2,4-Di-tert-butylphenol | 28.53 | 1509 | 0.11 ± 0.01 | 0.07 ± 0.01 | 0.11 ± 0.01 | 0.08 ± 0.02 | 0.12 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.02 | MS |
| 2,4-Di-tert-butyl-6-methylphenol | 28.61 | 1512 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2,6-bis(1,1-dimethylethyl)-4-methyl-Phenol | 28.62 | 1513 | ND | ND | ND | ND | 0.01 ± 0.01 | ND | ND | MS |
| Cubenol | 30.81 | 1606 | 0.01 ± 0.01 | ND | ND | ND | ND | ND | ND | MS |
| Aldehydes (14) | ||||||||||
| Pentanal | 3.20 | < 800 | ND | ND | ND | ND | ND | ND | ND | MS/RI |
| 3-Methyl butanal | 4.47 | < 800 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 | ND | ND | 0.04 ± 0.03 | ND | MS |
| Hexanal | 8.10 | 813 | 0.06 ± 0.01 | 0.13 ± 0.01 | 0.06 ± 0.01 | 0.16 ± 0.08 | 0.14 ± 0.12 | 0.05 ± 0.01 | 0.13 ± 0.01 | MS/RI |
| Heptanal | 11.26 | 906 | ND | ND | ND | 0.08 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.61 ± 0.04 | MS/RI |
| Benzaldehyde | 13.27 | 968 | 0.21 ± 0.01 | 0.18 ± 0.02 | 0.38 ± 0.05 | 0.27 ± 0.08 | 0.37 ± 0.05 | 0.24 ± 0.06 | 0.23 ± 0.04 | MS/RI |
| Octanal | 14.54 | 1004 | ND | ND | ND | ND | ND | ND | 0.33 ± 0.06 | MS/RI |
| 2,4-Heptadienal | 14.87 | 1015 | ND | ND | ND | ND | ND | ND | ND | MS/RI |
| Phenylacetaldehyde | 15.93 | 1050 | 0.08 ± 0.01 | ND | 0.16 ± 0.07 | 0.04 ± 0.01 | 0.08 ± 0.01 | ND | 0.05 ± 0.01 | MS |
| 2-Undecenal | 16.32 | 1062 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2-Octenal | 16.35 | 1063 | ND | ND | ND | ND | 0.06 ± 0.01 | ND | ND | MS/RI |
| Nonanal | 17.75 | 1106 | 0.13 ± 0.01 | 0.12 ± 0.11 | 0.09 ± 0.02 | 0.30 ± 0.06 | 0.31 ± 0.05 | 0.53 ± 0.24 | 1.50 ± 0.15 | MS/RI |
| Decanal | 20.69 | 1207 | 0.02 ± 0.01 | 0.02 ± 0.01 | ND | 0.03 ± 0.01 | 0.03 ± 0.01 | ND | 0.02 ± 0.01 | MS/RI |
| 2,4-Decadienal | 23.71 | 1317 | ND | ND | ND | ND | ND | ND | ND | MS |
| Tetradecanal | 26.04 | 1407 | ND | ND | ND | ND | ND | ND | ND | MS |
| Heterocyclics (24) | ||||||||||
| 2-Ethylfuran | 5.44 | < 800 | ND | ND | ND | ND | ND | ND | ND | MS |
| Furfural | 9.07 | 844 | ND | ND | ND | ND | ND | ND | ND | MS/RI |
| 4-(1-Methyl-4-piperidinyl)-1,2-benzenediol | 10.96 | 897 | ND | ND | ND | ND | ND | ND | 0.02 ± 0.01 | MS |
| Methoxy-phenyl-oxime | 11.05 | 899 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | ND | 0.10 ± 0.01 | ND | ND | MS |
| 1-Methylbutyl 4-ethylbenzoate | 11.34 | 908 | ND | ND | ND | ND | ND | ND | ND | MS |
| 3-Acetyldibenzofuran | 13.11 | 963 | 0.08 ± 0.01 | ND | ND | 0.09 ± 0.01 | ND | ND | 0.06 ± 0.01 | MS |
| 2-Pentylfuran | 14.26 | 995 | ND | ND | ND | ND | 0.04 ± 0.02 | ND | ND | MS |
| 2-Formylpyrrole | 14.75 | 1011 | 0.04 ± 0.04 | ND | 0.14 ± 0.11 | ND | ND | 0.08 ± 0.04 | ND | MS |
| 2-Ethyl-3,5-dimethylpyrazine | 16.96 | 1082 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2-Hydrazino-4,6-dimethylpyrimidine | 17.95 | 1113 | ND | ND | ND | ND | ND | 0.20 ± 0.01 | ND | MS |
| Auramine | 18.00 | 1115 | ND | ND | ND | 0.16 ± 0.01 | ND | ND | ND | MS |
| Ergotamine | 18.06 | 1118 | ND | ND | ND | ND | 0.02 ± 0.01 | ND | ND | MS |
| Corydine | 18.92 | 1147 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2,2-Dimethyl-1,4,4-triphenylbut-3-en-1-one oxime | 19.02 | 1151 | ND | ND | 0.04 ± 0.01 | ND | ND | ND | ND | MS |
| 4-Methoxyformanilide | 19.89 | 1180 | ND | ND | ND | ND | ND | ND | ND | MS |
| Tetrahydro-melosmine | 21.91 | 1252 | ND | ND | ND | 0.02 ± 0.01 | ND | ND | ND | MS |
| 3,6-dimethoxy-9-(2-phenylethynyl)-fluoren-9-ol | 21.93 | 1253 | ND | ND | ND | ND | ND | ND | ND | MS |
| 4-(p-Chlorophenyl)-2,6-diphenylpyridine | 21.95 | 1254 | ND | ND | ND | 0.03 ± 0.01 | ND | ND | ND | MS |
| Tetrahydrofuran | 24.66 | 1355 | 0.12 ± 0.02 | 0.07 ± 0.04 | 0.11 ± 0.02 | 0.13 ± 0.04 | 0.17 ± 0.02 | 0.12 ± 0.02 | 0.14 ± 0.03 | MS |
| 2,6-Dibromo-3,5-difluoro-4-piperidinylpyridine | 25.30 | 1379 | ND | ND | ND | ND | ND | 0.02 ± 0.01 | ND | MS |
| 1,1-Diphenyl-3-methylbuta-1,2-dien | 27.61 | 1472 | 0.06 ± 0.01 | ND | ND | ND | ND | ND | ND | MS |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 30.55 | 1594 | 0.04 ± 0.01 | 0.04 ± 0.01 | ND | ND | ND | ND | ND | MS |
| Cystine | 32.06 | 1661 | 0.17 ± 0.01 | ND | ND | 0.09 ± 0.01 | ND | 0.17 ± 0.04 | 0.11 ± 0.03 | MS |
| Trioctyl phosphate | 32.35 | 1674 | ND | ND | ND | ND | ND | ND | ND | MS |
| Hydrocabons (24) | ||||||||||
| 3-Ethoxy propene | 3.39 | < 800 | ND | ND | 0.06 ± 0.01 | ND | ND | ND | ND | MS |
| 4-D1-Heptane | 3.62 | < 800 | ND | ND | ND | ND | ND | ND | ND | MS/RI |
| Ethoxypropene | 3.74 | < 800 | ND | ND | ND | ND | ND | ND | ND | MS |
| Nonane | 3.81 | < 800 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2-Methyl-2,4-hexadiene | 5.30 | < 800 | ND | ND | ND | ND | ND | ND | ND | MS |
| 4-Methyl cyclohexene | 5.37 | < 800 | ND | ND | ND | ND | ND | ND | ND | MS |
| 4,4-Dimethylcyclopentene | 5.40 | < 800 | ND | ND | ND | ND | ND | ND | ND | MS |
| Benzaldehyde | 13.27 | 968 | 0.21 ± 0.01 | 0.18 ± 0.02 | 0.38 ± 0.05 | 0.27 ± 0.08 | 0.37 ± 0.05 | 0.24 ± 0.06 | 0.23 ± 0.04 | MS/RI |
| 1-Ethynyl-2-methylbicyclohexane | 13.39 | 971 | ND | ND | ND | 0.32 ± 0.01 | ND | ND | ND | MS |
| p-Cymene | 15.31 | 1030 | ND | ND | 0.07 ± 0.01 | ND | 0.10 ± 0.01 | ND | ND | MS/RI |
| Propyl benzene | 15.91 | 1049 | ND | 0.03 ± 0.01 | ND | ND | ND | ND | ND | MS |
| 5,5-Dimethyl-1,3-hexadiene | 19.56 | 1169 | ND | ND | ND | ND | ND | ND | 0.01 ± 0.01 | MS |
| Borneol | 19.62 | 1171 | 0.03 ± 0.01 | ND | ND | 0.03 ± 0.01 | 0.05 ± 0.01 | ND | ND | MS/RI |
| Pentyl-2-propylcyclopentane | 20.18 | 1189 | ND | ND | ND | ND | ND | ND | ND | MS |
| δ-3-Carene | 20.31 | 1193 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.21 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | MS |
| Camphene | 20.32 | 1194 | 0.14 ± 0.01 | ND | 0.10 ± 0.01 | ND | ND | ND | ND | MS |
| 4-methyl-2-Heptene | 20.95 | 1216 | ND | ND | ND | ND | ND | ND | ND | MS |
| Cyclooctane | 20.97 | 1217 | ND | ND | ND | ND | ND | ND | ND | MS |
| 3-Octene | 20.98 | 1218 | ND | ND | ND | ND | 0.02 ± 0.01 | ND | ND | MS |
| 9-Methyl-3-undecene | 22.23 | 1264 | ND | ND | ND | ND | ND | ND | ND | MS |
| 3-Ethyl-5-(2-ethylbutyl)octadecane | 25.77 | 1396 | ND | ND | ND | ND | ND | ND | ND | MS |
| Cedrene | 30.84 | 1607 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | ND | 0.03 ± 0.01 | ND | ND | MS |
| Nonadecane | 32.66 | 1687 | ND | ND | ND | ND | ND | ND | ND | MS |
| Hexacosane | 32.81 | 1693 | ND | ND | ND | ND | ND | ND | ND | MS |
| Ketones (6) | ||||||||||
| 2,2-Diethoxyacetophenone | 13.82 | 983 | ND | ND | ND | 0.70 ± 0.01 | ND | 0.33 ± 0.01 | ND | MS |
| 2-Nonanone | 17.38 | 1094 | ND | ND | ND | ND | ND | ND | ND | MS/RI |
| Geranylacetone | 27.13 | 1453 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2,5-Di-tert-butyl-1,4-benzoquinone | 27.55 | 1469 | ND | ND | ND | ND | ND | 0.01 ± 0.01 | ND | MS |
| 2,6-Di-tert-butylbenzoquinone | 27.56 | 1470 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.04 ± 0.01 | 0.11 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.01 | MS |
| 2,5-dibenzylcyclopentan-1-one | 27.59 | 1471 | 0.02 ± 0.01 | ND | 0.02 ± 0.01 | ND | ND | ND | ND | MS |
| Sulfur-containing compounds (2) | ||||||||||
| Ethyl-1,3-dithioisoindoline | 8.23 | 818 | ND | ND | ND | ND | ND | ND | ND | MS |
| 1-Octanesulfonyl chloride | 21.00 | 1219 | ND | ND | ND | ND | ND | ND | ND | MS |
| Compounds | RT | RI | FC | FH | SXG | SG | SX | SC | SH | FX | I.D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acids and esters (3) | |||||||||||
| 2-Amino-4-methylbenzoic acid | 11.31 | 907 | ND | ND | 0.05 ± 0.01 | ND | ND | ND | ND | ND | MS |
| Octyl acetate | 19.07 | 1153 | ND | ND | ND | ND | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | ND | MS/RI |
| Butyl butylate | 25.22 | 1376 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.30 ± 0.17 | 0.33 ± 0.01 | 0.05 ± 0.01 | MS |
| Alcohols (17) | |||||||||||
| 2-Ethyl-hexanol | 15.41 | 1033 | 0.30 ± 0.22 | 0.71 ± 0.01 | 1.13 ± 0.12 | 1.35 ± 0.30 | 1.34 ± 0.10 | 1.87 ± 0.33 | 2.25 ± 0.05 | 0.58 ± 0.05 | MS |
| Octanol | 16.71 | 1074 | 0.02 ± 0.01 | 0.06 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.12 ± 0.08 | 0.15 ± 0.01 | 0.03 ± 0.01 | MS |
| Xylitol pentacetate | 16.99 | 1083 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Menthol | 19.79 | 1177 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.08 ± 0.01 | 0.14 ± 0.06 | 0.14 ± 0.01 | 0.03 ± 0.01 | MS/RI |
| Nonadecanol | 20.05 | 1185 | ND | ND | ND | ND | ND | 0.03 ± 0.01 | ND | ND | MS |
| Hexadecanol | 20.11 | 1187 | ND | 0.03 ± 0.01 | ND | 0.02 ± 0.01 | ND | 0.09 ± 0.01 | ND | ND | MS/RI |
| Octadecanol | 20.55 | 1201 | ND | ND | ND | ND | 0.05 ± 0.01 | 0.10 ± 0.04 | 0.05 ± 0.04 | ND | MS |
| Tetradecanol | 20.61 | 1204 | ND | ND | ND | ND | ND | 0.04 ± 0.01 | ND | ND | MS |
| 2-Propylheptanol | 20.85 | 1213 | ND | ND | ND | 0.02 ± 0.01 | ND | ND | ND | ND | MS |
| 2-Isopropyl-5-methyl-1-heptanol | 20.88 | 1214 | ND | ND | 0.02 ± 0.01 | ND | ND | ND | ND | ND | MS |
| Octadecanol | 21.26 | 1228 | ND | ND | 0.01 ± 0.01 | ND | ND | ND | ND | ND | MS |
| 2-Dodecanol | 21.29 | 1229 | ND | ND | ND | ND | ND | 0.07 ± 0.04 | 0.07 ± 0.01 | ND | MS |
| β-Acorenol | 26.34 | 1420 | ND | ND | ND | ND | ND | 0.04 ± 0.01 | 0.02 ± 0.01 | ND | MS |
| 2,4-Di-tert-butylphenol | 28.53 | 1509 | 0.07 ± 0.03 | 0.10 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.01 | 0.16 ± 0.01 | 0.31 ± 0.15 | 0.28 ± 0.01 | 0.08 ± 0.01 | MS |
| 2,4-Di-tert-butyl-6-methylphenol | 28.61 | 1512 | ND | ND | ND | ND | 0.02 ± 0.01 | ND | ND | ND | MS |
| 2,6-bis(1,1-dimethylethyl)-4-methyl-Phenol | 28.62 | 1513 | ND | ND | ND | 0.04 ± 0.01 | ND | 0.02 ± 0.01 | 0.03 ± 0.01 | ND | MS |
| Cubenol | 30.81 | 1606 | ND | ND | ND | 0.03 ± 0.01 | ND | ND | ND | ND | MS |
| Aldehydes (14) | |||||||||||
| Pentanal | 3.20 | < 800 | ND | ND | ND | ND | ND | ND | ND | 0.05 ± 0.01 | MS/RI |
| 3-Methyl butanal | 4.47 | < 800 | ND | ND | 0.01 ± 0.01 | ND | 0.03 ± 0.01 | ND | ND | 0.08 ± 0.04 | MS |
| Hexanal | 8.10 | 813 | 0.01 ± 0.01 | 0.18 ± 0.10 | 0.05 ± 0.02 | 0.05 ± 0.03 | 0.04 ± 0.01 | 0.13 ± 0.04 | 0.13 ± 0.01 | 0.05 ± 0.05 | MS/RI |
| Heptanal | 11.26 | 906 | 0.28 ± 0.06 | 0.54 ± 0.08 | ND | ND | 0.04 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.12 ± 0.02 | MS/RI |
| Benzaldehyde | 13.27 | 968 | 0.17 ± 0.10 | 0.32 ± 0.05 | 0.13 ± 0.05 | 0.12 ± 0.01 | 0.25 ± 0.02 | 0.24 ± 0.13 | 0.20 ± 0.01 | 1.02 ± 0.04 | MS/RI |
| Octanal | 14.54 | 1004 | 0.53 ± 0.38 | 0.91 ± 0.11 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.02 | ND | ND | 0.25 ± 0.01 | MS/RI |
| 2,4-Heptadienal | 14.87 | 1015 | ND | 0.16 ± 0.01 | ND | ND | ND | ND | ND | ND | MS/RI |
| Phenylacetaldehyde | 15.93 | 1050 | 0.05 ± 0.01 | 0.16 ± 0.03 | 0.06 ± 0.03 | 0.04 ± 0.01 | 0.13 ± 0.02 | 0.14 ± 0.09 | 0.11 ± 0.01 | 0.18 ± 0.01 | MS |
| 2-Undecenal | 16.32 | 1062 | 0.03 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2-Octenal | 16.35 | 1063 | ND | 0.08 ± 0.02 | ND | ND | ND | 0.05 ± 0.01 | 0.04 ± 0.01 | ND | MS/RI |
| Nonanal | 17.75 | 1106 | 1.17 ± 0.75 | 4.47 ± 0.71 | 0.13 ± 0.04 | 0.31 ± 0.02 | 0.24 ± 0.01 | 0.71 ± 0.28 | 0.58 ± 0.01 | 0.81 ± 0.07 | MS/RI |
| Decanal | 20.69 | 1207 | 0.09 ± 0.01 | 0.07 ± 0.01 | ND | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.13 ± 0.05 | 0.09 ± 0.01 | 0.02 ± 0.01 | MS/RI |
| 2,4-Decadienal | 23.71 | 1317 | ND | 0.04 ± 0.02 | ND | ND | ND | ND | ND | ND | MS |
| Tetradecanal | 26.04 | 1407 | ND | ND | ND | 0.02 ± 0.01 | ND | 0.06 ± 0.01 | 0.02 ± 0.01 | ND | MS |
| Heterocyclics (24) | |||||||||||
| 2-Ethylfuran | 5.44 | < 800 | ND | ND | ND | ND | ND | ND | 0.04 ± 0.01 | ND | MS |
| Furfural | 9.07 | 844 | ND | ND | ND | ND | 0.04 ± 0.01 | ND | ND | 0.06 ± 0.03 | MS/RI |
| 4-(1-Methyl-4-piperidinyl)-1,2-benzenediol | 10.96 | 897 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Methoxy-phenyl-oxime | 11.05 | 899 | ND | ND | 0.03 ± 0.01 | 0.12 ± 0.03 | 0.02 ± 0.01 | ND | 0.10 ± 0.01 | ND | MS |
| 1-Methylbutyl 4-ethylbenzoate | 11.34 | 908 | ND | ND | ND | ND | ND | ND | 0.08 ± 0.01 | ND | MS |
| 3-Acetyldibenzofuran | 13.11 | 963 | ND | ND | ND | ND | ND | 0.13 ± 0.01 | 0.11 ± 0.01 | ND | MS |
| 2-Pentylfuran | 14.26 | 995 | ND | ND | ND | ND | ND | 0.11 ± 0.01 | 0.10 ± 0.01 | ND | MS |
| 2-Formylpyrrole | 14.75 | 1011 | ND | ND | ND | ND | 0.32 ± 0.06 | ND | ND | 0.30 ± 0.02 | MS |
| 2-Ethyl-3,5-dimethylpyrazine | 16.96 | 1082 | ND | ND | ND | ND | ND | ND | ND | 0.05 ± 0.01 | MS |
| 2-Hydrazino-4,6-dimethylpyrimidine | 17.95 | 1113 | 0.21 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | MS |
| Auramine | 18.00 | 1115 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Ergotamine | 18.06 | 1118 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Corydine | 18.92 | 1147 | 0.03 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | MS |
| 2,2-Dimethyl-1,4,4-triphenylbut-3-en-1-one oxime | 19.02 | 1151 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 4-Methoxyformanilide | 19.89 | 1180 | ND | ND | ND | ND | ND | ND | ND | 0.03 ± 0.01 | MS |
| Tetrahydro-melosmine | 21.91 | 1252 | 0.01 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | MS |
| 3,6-dimethoxy-9-(2-phenylethynyl)-fluoren-9-ol | 21.93 | 1253 | ND | 0.02 ± 0.01 | ND | ND | ND | ND | ND | ND | MS |
| 4-(p-Chlorophenyl)-2,6-diphenylpyridine | 21.95 | 1254 | ND | ND | 0.04 ± 0.01 | 0.02 ± 0.01 | ND | 0.07 ± 0.01 | ND | ND | MS |
| Tetrahydrofuran | 24.66 | 1355 | 0.12 ± 0.05 | 0.22 ± 0.01 | 0.18 ± 0.02 | 0.22 ± 0.01 | 0.25 ± 0.01 | 0.59 ± 0.28 | 0.39 ± 0.02 | 0.15 ± 0.02 | MS |
| 2,6-Dibromo-3,5-difluoro-4-piperidinylpyridine | 25.30 | 1379 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 1,1-Diphenyl-3-methylbuta-1,2-dien | 27.61 | 1472 | ND | ND | 0.04 ± 0.03 | ND | ND | ND | ND | ND | MS |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 30.55 | 1594 | 0.02 ± 0.01 | ND | 0.04 ± 0.01 | ND | 0.05 ± 0.01 | ND | 0.09 ± 0.01 | ND | MS |
| Cystine | 32.06 | 1661 | 0.12 ± 0.01 | 0.08 ± 0.01 | 0.14 ± 0.03 | ND | 0.13 ± 0.01 | ND | 0.14 ± 0.01 | ND | MS |
| Trioctyl phosphate | 32.35 | 1674 | ND | ND | ND | 0.02 ± 0.01 | ND | ND | ND | ND | MS |
| Hydrocabons (24) | |||||||||||
| 3-Ethoxy propene | 3.39 | < 800 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 4-D1-Heptane | 3.62 | < 800 | ND | ND | ND | ND | ND | ND | ND | 0.01 ± 0.01 | MS/RI |
| Ethoxypropene | 3.74 | < 800 | ND | ND | ND | ND | 0.05 ± 0.01 | ND | ND | ND | MS |
| Nonane | 3.81 | < 800 | ND | ND | ND | ND | 0.01 ± 0.01 | ND | ND | ND | MS |
| 2-Methyl-2,4-hexadiene | 5.30 | < 800 | ND | ND | ND | ND | ND | ND | 0.07 ± 0.01 | ND | MS |
| 4-Methyl cyclohexene | 5.37 | < 800 | ND | ND | ND | ND | ND | ND | 0.02 ± 0.01 | ND | MS |
| 4,4-Dimethylcyclopentene | 5.40 | < 800 | ND | ND | ND | ND | ND | ND | 0.04 ± 0.01 | ND | MS |
| Benzaldehyde | 13.27 | 968 | 0.17 ± 0.10 | 0.32 ± 0.05 | 0.13 ± 0.05 | 0.12 ± 0.01 | 0.25 ± 0.02 | 0.24 ± 0.13 | 0.20 ± 0.01 | 1.02 ± 0.04 | MS/RI |
| 1-Ethynyl-2-methylbicyclohexane | 13.39 | 971 | 0.04 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | MS |
| p-Cymene | 15.31 | 1030 | ND | ND | ND | 0.09 ± 0.01 | ND | 0.09 ± 0.01 | ND | ND | MS/RI |
| Propyl benzene | 15.91 | 1049 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 5,5-Dimethyl-1,3-hexadiene | 19.56 | 1169 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| Borneol | 19.62 | 1171 | ND | 0.05 ± 0.01 | 0.04 ± 0.02 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.15 ± 0.05 | 0.16 ± 0.02 | ND | MS/RI |
| Pentyl-2-propylcyclopentane | 20.18 | 1189 | ND | ND | ND | ND | ND | ND | 0.05 ± 0.01 | ND | MS |
| δ-3-Carene | 20.31 | 1193 | ND | ND | ND | ND | 0.29 ± 0.01 | ND | ND | 0.09 ± 0.01 | MS |
| Camphene | 20.32 | 1194 | ND | ND | ND | 0.23 ± 0.01 | ND | ND | ND | ND | MS |
| 4-methyl-2-Heptene | 20.95 | 1216 | ND | ND | ND | ND | ND | 0.02 ± 0.01 | ND | ND | MS |
| Cyclooctane | 20.97 | 1217 | ND | ND | 0.04 ± 0.01 | 0.02 ± 0.01 | ND | ND | ND | ND | MS |
| 3-Octene | 20.98 | 1218 | ND | ND | ND | 0.05 ± 0.02 | ND | 0.10 ± 0.01 | ND | MS | |
| 9-Methyl-3-undecene | 22.23 | 1264 | ND | 0.04 ± 0.02 | ND | ND | ND | ND | ND | ND | MS |
| 3-Ethyl-5-(2-ethylbutyl)octadecane | 25.77 | 1396 | ND | ND | ND | 0.02 ± 0.01 | ND | ND | ND | ND | MS |
| Cedrene | 30.84 | 1607 | ND | 0.01 ± 0.01 | 0.02 ± 0.01 | ND | 0.04 ± 0.01 | 0.09 ± 0.05 | 0.09 ± 0.01 | ND | MS |
| Nonadecane | 32.66 | 1687 | ND | ND | ND | 0.04 ± 0.01 | ND | ND | ND | ND | MS |
| Hexacosane | 32.81 | 1693 | ND | ND | ND | 0.05 ± 0.01 | ND | ND | ND | ND | MS |
| Ketones (6) | |||||||||||
| 2,2-Diethoxyacetophenone | 13.82 | 983 | ND | ND | ND | ND | ND | ND | ND | 0.65 ± 0.01 | MS |
| 2-Nonanone | 17.38 | 1094 | ND | 0.04 ± 0.01 | ND | ND | ND | 0.06 ± 0.01 | 0.04 ± 0.01 | ND | MS/RI |
| Geranylacetone | 27.13 | 1453 | ND | ND | ND | ND | ND | 0.04 ± 0.01 | 0.02 ± 0.01 | ND | MS |
| 2,5-Di-tert-butyl-1,4-benzoquinone | 27.55 | 1469 | ND | ND | ND | ND | ND | ND | ND | ND | MS |
| 2,6-Di-tert-butylbenzoquinone | 27.56 | 1470 | 0.05 ± 0.03 | 0.07 ± 0.01 | 0.02 ± 0.01 | 0.21 ± 0.01 | 0.04 ± 0.02 | 0.35 ± 0.18 | 0.31 ± 0.01 | 0.02 ± 0.02 | MS |
| 2,5-dibenzylcyclopentan-1-one | 27.59 | 1471 | ND | ND | ND | ND | 0.05 ± 0.01 | ND | ND | ND | MS |
| Sulfur-containing compounds (2) | |||||||||||
| Ethyl-1,3-dithioisoindoline | 8.23 | 818 | 0.13 ± 0.01 | ND | ND | ND | ND | 0.13 ± 0.01 | ND | ND | MS |
| 1-Octanesulfonyl chloride | 21.00 | 1219 | ND | ND | ND | ND | ND | 0.06 ± 0.01 | ND | ND | MS |
RT retention time (min), RI retention indice, ND not detected, ID identification, HXG head_thermal_xylose + glucose, HG head_thermal_glucose, HX head_thermal_xylose, HC head_thermal_control, HH head_hydrolysate, FXG frame_thermal_xylose + glucose, FG frame_thermal_glucose, FX frame_thermal_xylose, FC frame_thermal_control, FH frame_hydrolysate, SXG skin_thermal_xylose + glucose, SG skin_thermal_glucose, SX skin_thermal_xylose, SC skin_thermal_control, SH skin_hydrolysate, respectively
According to Wang et al. (2018), most of the major volatile compounds detected in fish belonged to aldehydes (Wang et al., 2018), and in this study, the content of aldehydes was also high in most samples. Among the aldehydes, pentanal, hexanal, heptanal, octanal, nonanal, and benzaldehyde are considered the major volatile compounds found in fish (Wang et al., 2018), and hexanal, benzaldehyde, and nonanal were found in all samples in this study. 3-Methyl butanal, used as an indicator of spoilage in smoked salmon products, was detected in HXG, HG, and HX in the head of salmon, and in the case of frame and skin, in FXG and SXG and SX (Jónsdóttir et al., 2008). These results were confirmed to have appeared in most samples treated with reducing sugar. In general, alcohols, which do not contribute significantly to the spoilage smell of fish compared to aldehydes and ketones, were measured in higher content in samples in the head and skin than other volatile compounds (Jónsdóttir et al., 2008). Among the samples in the frame, FH was measured to contain high aldehydes because nonanal, which is considered the major volatile compound of fish, was detected to be very high (Wang et al., 2018). In this study, total 9 compounds were detected, including furan, pyrrole, pyrazine, and pyridine, which are considered thermal processing products. The heterocyclic compounds detected in all samples were tetrahydrofuran, especially content was measured higher in samples of the skin (Jónsdóttir et al., 2008; Li et al., 2023).
GC–O analysis for volatile active compounds
The odor active compounds of 15 types of salmon by-products analyzed using GC–MS are shown in Table 3. A total of 23 odor active compounds were detected in salmon by-product samples, including 1 acid, 3 alcohols, 9 aldehydes, 5 heterocyclic compounds, 3 hydrocarbons, and 2 ketones. The odor descriptor group of the recognized odor active compounds were expressed as fishy & salty, fishy & savory, savory & nutty, and sweet and other odor. Of the 23 odor active compounds identified, 6 were recognized as the aroma of salmon. Among the odor active compounds, 3-methyl-butanal, heptanal, benzaldehyde, octanal, furfural, and methoxy-phenyl-oxime were recognized as the aroma of salmon. 2-Ethyl-hexanol, 2-amino-4-methylbenzoic acid, 2-undecenal, 1-ethyl-2-methylbicyclohexane, 2,2-diethoxyacetophenone, and 2-nonanone were described as other odors, such as feed, grass, and sour. Octanol detected in 12 samples was recognized as a savory aroma, and was not detected in HXG, HG, and HC. Heptanal recognized as the aroma of salmon was measured to have a high odor intensity, and most of them were detected in samples in the frame. Phenylacetaldehyde was mostly detected in samples at the head and frame. In this study, odor active compounds of the aldehydes and heterocyclic compounds were recognized more than other odor active compounds. As a result of GC–O, the recognition of odor active compounds was confirmed to be high in the order of frame, head, and skin, and the recognition of odor active compounds recognized as the aroma of fish was also confirmed to be the highest in the frame. And as in the GC–MS results, the change in the recognition of the odor active compounds of the sample did not show the same pattern depending on the pre-treatment.
Table 3.
Relative intensities of odor active compounds in by-product of salmon using GC–olfactometry
| Compounds | RT | HXG | HG | HX | HC | HH | FXG | FG | FX | FC | FH | SXG | SG | SX | SC | SH | Odor description |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acid | |||||||||||||||||
| 2-Amino-4-methylbenzoic acid | 11.31 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 2 | NR | NR | NR | NR | Other odors |
| Alcohols (3) | |||||||||||||||||
| 2-Ethyl-hexanol | 15.41 | NR | NR | NR | NR | NR | 1 | 1 | NR | 1 | NR | NR | NR | NR | NR | NR | Other odors |
| Octanol | 16.71 | NR | NR | 1 | NR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Savory, nutty |
| Menthol | 19.79 | 2 | 2 | 2 | 1 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | Sweet |
| Aldehydes (9) | |||||||||||||||||
| 3-Methyl-Butanal | 4.47 | NR | NR | NR | NR | NR | 1 | NR | 1 | NR | NR | NR | NR | NR | NR | NR | Fishy, salty |
| Hexanal | 8.10 | NR | 2 | NR | NR | NR | 1 | 1 | 1 | 1 | 1 | NR | 1 | NR | 1 | 1 | Sweet |
| Heptanal | 11.26 | NR | NR | NR | NR | 1 | 2 | 2 | 2 | 2 | 2 | NR | NR | NR | 2 | 1 | Fishy, salty |
| Benzaldehyde | 13.27 | NR | NR | NR | NR | NR | 1 | 2 | 1 | 1 | 1 | NR | 1 | NR | 1 | NR | Fishy, salty |
| Octanal | 14.54 | NR | NR | NR | NR | NR | NR | 1 | 1 | 1 | 1 | NR | NR | NR | NR | NR | Savory, fishy |
| Phenylacetaldehyde | 15.93 | 2 | NR | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | NR | NR | NR | NR | 1 | Sweet |
| 2-Undecenal | 16.32 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | NR | NR | NR | NR | NR | NR | Other odors |
| Nonanal | 17.75 | NR | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | NR | NR | NR | NR | Savory, nutty |
| Decanal | 20.69 | NR | NR | NR | 1 | 1 | NR | 1 | NR | 1 | NR | NR | NR | NR | NR | NR | Savory, nutty |
| Heterocyclics (5) | |||||||||||||||||
| Furfural | 9.07 | NR | NR | NR | NR | NR | NR | NR | 1 | NR | NR | NR | NR | 1 | NR | NR | Savory, fishy |
| Methoxy-phenyl-oxime | 11.05 | 2 | 1 | 2 | NR | 1 | NR | NR | NR | NR | NR | NR | 2 | 2 | NR | 2 | Savory, fishy |
| 1-Methylbutyl 4-ethylbenzoate | 11.34 | NR | NR | NR | 1 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 1 | Savory, nutty |
| 2-Pentylfuran | 14.26 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 1 | 1 | Savory, nutty |
| 2-Ethyl-3,5-dimethylpyrazin | 16.96 | NR | NR | NR | NR | NR | NR | NR | 1 | NR | NR | NR | NR | NR | NR | NR | Savory, nutty |
| Hydrocabons (3) | |||||||||||||||||
| 1-Ethynyl-2-methylbicyclohexane | 13.39 | NR | NR | NR | 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | Other odors |
| δ-3-Carene | 20.31 | NR | NR | NR | 1 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | Savory, nutty |
| Camphene | 20.32 | NR | NR | 2 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | savory, nutty |
| Ketones (2) | |||||||||||||||||
| 2,2-Diethoxyacetophenone | 13.82 | NR | NR | NR | NR | 1 | 1 | NR | 2 | NR | NR | NR | NR | NR | NR | NR | Other odors |
| 2-Nonanone | 17.38 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 1 | NR | Other odors |
RT retention time (min), NR not recognized, HXG head_thermal_xylose + glucose, HG head_thermal_glucose, HX head_thermal_xylose, HC head_thermal_control, HH head_hydrolysate, FXG frame_thermal_xylose + glucose, FG frame_thermal_glucose, FX frame_thermal_xylose, FC frame_thermal_control, FH frame_hydrolysate, SXG skin_thermal_xylose + glucose, SG skin_thermal_glucose, SX skin_thermal_xylose, SC skin_thermal_control, SH skin_hydrolysate, respectively
Heptanal, benzaldehyde, and methoxy-phenyl-oxime were found in 7 of the 15 samples, all of which were recognized as the aroma of salmon. According to research by Jónsdóttir et al. (2008), heptanal is considered to be a volatile compound that represents the aroma of fresh salmon (Jónsdóttir et al., 2008). 3-Methylbutanal represents the aroma of smoked salmon and was detected in FXG and FX (Wierda et al., 2006). Hexanal, known as the aroma of fresh salmon, was detected in 9 of the 15 samples and was recognized as a sweet aroma (Wierda et al., 2006). The octanal detected in most samples of the frame is known to be the aroma of fresh salmon, and in this study, it was also recognized as the aroma of fish and savory salmon (Wierda et al., 2006). Nonanal, known as the aroma of fresh salmon, was detected in 10 of 15 samples, and was recognized as a savory aroma in this study (Wierda et al., 2006). Recognized as the aroma of salmon, hexanal, nonanal, and decanal are known as oxidatively induced volatile compounds (Jónsdóttir et al., 2008). According to Refsgaard et al. (1999) hexanal, heptanal, octanal, and nonanal are volatile compounds found in cod, skate, and mackerel in addition to salmon, and are reported to have a major influence on fish odor (Refsgaard et al., 1999). The substances produced by the heat treatment change aroma of the salmon, such as grass, stir-fry, nut, and bitter, have a stronger influence on the aroma of smoked salmon (Varlet et al., 2006). A furan derivative is produced by thermal, and one of them, furfuryl, was detected only in FX and SX. The volatile compound was recognized as the aroma of fishy & savory, which is judged to be the smell of smoke generated during the processing process (Varlet et al., 2006). 2-Pentylfuran detected only in SC and SH is known for its bread aroma, and in this study, it was recognized as a savory aroma and nut aroma (Mack et al., 2019).
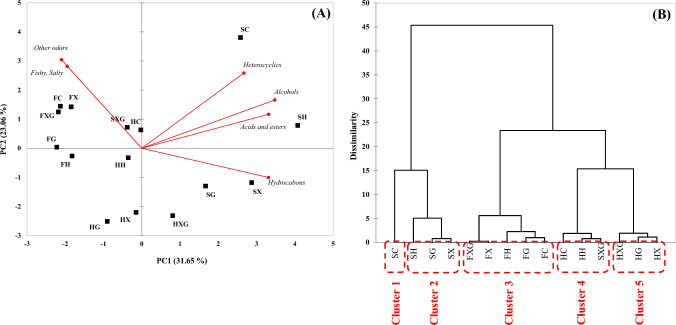
Multivariate analysis of volatile compounds and odor active compounds using GC–MS coupled with GC–O
The multivariate analysis of 15 types of salmon by-products identified through GC–MS/Olfactometry was separated using PCA and CA and the results of the separated pattern were shown in Fig. 2(A) and (B). In Fig. 2(A), 15 types of samples were separated and patterned based on the volatile compounds detected through GC–MS and the odor description of the odor active compounds recognized through GC–O. The total variation of PC1-2 shown in Fig. 2 was 54.71%, and the variation of PC1 and PC2 was 31.65% and 23.06%, respectively. HXG, SG, SX, SC, and SH were positive to PC1, and HG, HX, HH, FXG, FX, FC, FH, and SXG were located in a negative to PC1. SC and SH are positive to PC2 and are located in the first quadrant under the influence of heterocyclics, acids, and esters. HXG, SG, and SX are negative for PC2 and are located in the fourth quadrant under the influence of hydrocarbons. FXG, FX, and FC were located in the second quadrant, which is positive to PC2, under the influence of fishy & salty compared to other samples. HX, HC, HH, and SXG did not show a large degree of separation based on PC1 compared to other samples, and FXG and FH did not show a large degree of separation based on PC2.
Fig. 2.
PCA bi-plot and dendrogram for volatile compounds and odor active compounds pattern of hydrolysate of salmon by-product treated reducing sugars and thermal processing based on the part using E-nose and GC–MS/O. (A) PCA and (B) dendrogram. HXG head_thermal_xylose + glucose, HG head_thermal_glucose, HX head_thermal_xylose, HC head_thermal_control, HH head_hydrolysate, FXG frame_thermal_xylose + glucose, FG frame_thermal_glucose, FX frame_thermal_xylose, FC frame_thermal_control, FH frame_hydrolysate, SXG skin_thermal_xylose + glucose, SG skin_thermal_glucose, SX skin_thermal_xylose, SC skin_thermal_control, SH skin_hydrolysate, respectively
The results of confirming the difference in salmon by-products through CA are shown in Fig. 2(B), and 5 clusters were identified. The SC was identified as cluster 1, and SG, SX, and SH were identified as cluster 2. Cluster 3 was identified as FXG, FG, FX, FC, and FH, while cluster 4 was identified as HC, HH, and SXG. Cluster 5 was identified as HXG, HG, and HX. It was confirmed that cluster 1, 2, and cluster 3, 4, and 5 showed the highest dissimilarity, and relatively low dissimilarity was shown in cluster 1, 2, and cluster 4, and 5. Unlike the results of the electronic nose, as a result of multivariate analysis of GC–MS and GC–O, the difference according to each part was relatively clearly confirmed. In particular, compared to samples of the head and skin, the samples of the frame showed the lowest dispersion, which determined that the frame was least affected by the processing treatment method.
GC–O is an analysis method for detecting odor active compounds in food through an olfactory port and is widely used in various fields of food analysis research (Boo et al., 2020). Varlet et al. (2006) GC–MS and GC–O were used to analyze the volatile compounds of silver salmon and smoked salmon (Varlet et al., 2006), and Wierda et al. (2006) used GC–MS to analyze the volatile compounds of salmon according to the storage period (Wierda et al., 2006). In addition to fish studies, Boo et al. (2020) detected various volatile compounds of Wintering Radishes through GC–MS and GC–O, and samples were separated according to aroma profiles through principal component analysis (Boo et al., 2020), and Yu et al. (2022) detected a total of 27 odor active compounds through GC–O and separated them through multivariate analysis (Yu et al., 2022). This study also used GC–MS and GC–O to identify the volatile compounds and odor active compounds of salmon by-products according to various parts and pre-treatment. It is expected to be used as the primary data for various studies on the sensory characteristics of salmon in the future.
This study compared and analyzed the volatile compounds of salmon by-products through various parts and hydrolysis and thermal processing using E-nose, GC–MS, and GC–O. As a result of the E-nose and GC–MS, an abundance of aldehydes was detected in most samples. The E-nose confirmed the difference between the samples based on both pre-treatment and parts, and in the case of GC–MS, the samples showed the difference only depending on the by-product part. As a result of GC–O, 3-methyl butanal, heptanal, benzaldehyde, octanal, furfural, and methoxy-phenol-oxime were detected as odor active compounds recognized as the aroma of salmon. The recognition of the odor active compounds did not exhibit the same pattern in accordance with the pre-treatment, but it was confirmed to be the highest in the frame, indicating a difference in parts. Multivariate analysis results of GC–MS and GC–O also confirmed differences according to parts. This study analyzed the aroma profile characteristics of salmon by-products parts along with various pre-treatment, and it is expected that these results will be used as basic data or sensory evaluation data for product manufacturing in the fisheries and food industries that utilize salmon by-products.
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Project for Development of Development of companion animal feed additives to improve palatability using byproduct, funded by Ministry of Agriculture, Food and Rural Affairs (322089031HD020).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyangyeon Jeong and Sojeong Yoon have contributed equally to this work.
References
- Ahn S, Lee WK, Jang D, Kang DH. Current status and evaluation of fisheries by-products: Major options to marine bioindustrial application. Ocean and Polar Research. 2021;43:149–164. [Google Scholar]
- Arnesen JA, Gildberg A. Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresource Technology. 2007;98:53–57. doi: 10.1016/j.biortech.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Arthur CL, Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Analytical Chemistry. 1990;62:2145–2148. doi: 10.1021/ac00218a019. [DOI] [Google Scholar]
- Boo CG, Hong SJ, Lee Y, Park S, Shin E. Quality characteristics of wintering radishes produced in Jeju Island using E-nose, E-Tongue, and GC-MSD approach. Journal of the Korean Society of Food Science and Nutrition. 2020;49:1407–1415. doi: 10.3746/jkfn.2020.49.12.1407. [DOI] [Google Scholar]
- Cha JW, Yoon IS, Park SY, Kang SI, Lee JS, Heu MS, Kim JS. Development of fish cake using salmon Oncorhynchus keta frame muscle. Korean Journal of Fisheries and Aquatic Sciences. 2020;53:147–155. [Google Scholar]
- Di Rosa AR, Leone F, Cheli F, Chiofalo V. Fusion of electronic nose, electronic tongue and computer vision for animal source food authentication and quality assessment—A review. Journal of Food Engineering. 2017;210:62–75. doi: 10.1016/j.jfoodeng.2017.04.024. [DOI] [Google Scholar]
- Dong W, Hu R, Long Y, Li H, Zhang Y, Zhu K, Chu Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPMEGC-MS. Food Chemistry. 2019;272:723–731. doi: 10.1016/j.foodchem.2018.08.068. [DOI] [PubMed] [Google Scholar]
- Fukami K, Ishiyama S, Yaguramaki H, Masuzawa T, Nabeta Y, Endo K, Shimoda M. Identification of distinctive volatile compounds in fish sauce. Journal of Agricultural and Food Chemistry. 2002;50(19):5412–5416. doi: 10.1021/jf020405y. [DOI] [PubMed] [Google Scholar]
- Gao P, Xia W, Li X, Liu S. Optimization of the Maillard reaction of xylose with cysteine for modulating aroma compound formation in fermented tilapia fish head hydrolysate using response surface methodology. Food Chemistry. 2020;331:127353. doi: 10.1016/j.foodchem.2020.127353. [DOI] [PubMed] [Google Scholar]
- Guo X, Tian S, Small DM. Generation of meat-like flavourings from enzymatic hydrolysates of proteins from Brassica sp. Food Chemistry. 2010;119:167–172. doi: 10.1016/j.foodchem.2009.05.089. [DOI] [Google Scholar]
- Heu MS, Choi BD, Kim KH, Kang SI, Kim YJ, Kim JS. Comparison on the food quality characteristics of muscles from salmonids according to species, imported country, and separated part. Korean Journal of Fisheries and Aquatic Sciences. 2015;48:16–25. doi: 10.5657/KFAS.2015.0016. [DOI] [Google Scholar]
- Iglesias J, Gallardo JM, Medina I. Determination of carbonyl compounds in fish species samples with solid-phase microextraction with on-fibre derivatization. Food Chemistry. 2010;123:771–778. doi: 10.1016/j.foodchem.2010.05.025. [DOI] [Google Scholar]
- Jeon HJ, Lee SH, Yang SY, Lee KW, Kim YS. Development and characterization of flavor based on Maillard reaction products with flatfish byproducts hydrolysates. Journal of Chitin and Chitosan Science. 2016;21:128–134. doi: 10.17642/jcc.21.2.8. [DOI] [Google Scholar]
- Jónsdóttir R, Ólafsdóttir G, Chanie E, Haugen JE. Volatile compounds suitable for rapid detection as quality indicators of cold smoked salmon (Salmo salar) Food Chemistry. 2008;109:184–195. doi: 10.1016/j.foodchem.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang S, Zhu Y, Shi W, Zhang Y, Liu Y. Effect of different drying methods on the taste and volatile compounds, sensory characteristics of Takifugu obscurus. Food Science and Human Wellness. 2023;12:223–232. doi: 10.1016/j.fshw.2022.07.012. [DOI] [Google Scholar]
- Li Y, Wang X, Xue Y, Ruan S, Zhou A, Huang S, Ma H. The preparation and identification of characteristic flavour compounds of Maillard reaction products of protein hydrolysate from grass carp (Ctenopharyngodon idella) bone. Journal of Food Quality. 2021 doi: 10.1155/2021/8394152. [DOI] [Google Scholar]
- Mack CI, Egert B, Liberto E, Weinert CH, Bub A, Hoffmann I, Bicchi C, Kulling SE, Cordero C. Robust markers of coffee consumption identified among the volatile organic compounds in human urine. Molecular Nutrition & Food Research. 2019;63:1801060. doi: 10.1002/mnfr.201801060. [DOI] [PubMed] [Google Scholar]
- Miyasaki T, Hamaguchi M, Yokoyama S. Change of volatile compounds in fresh fish meat during ice storage. Journal of Food Science. 2011;76:C1319–C1325. doi: 10.1111/j.1750-3841.2011.02388.x. [DOI] [PubMed] [Google Scholar]
- Nilsuwan K, Chantakun K, Chotphruethipong L, Benjakul S. Development of hydrolysis and defatting processes for production of lowered fishy odor hydrolyzed collagen from fatty skin of sockeye salmon (Oncorhynchus nerka) Foods. 2021;10:2257. doi: 10.3390/foods10102257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastergiadis A, Fatouh A, Shrestha K, Van Langenhove H, De Meulenaer B. Investigation of the formation of (E)-2-butenal in oils and foods during frying. Food Research International. 2014;62:43–49. doi: 10.1016/j.foodres.2014.02.046. [DOI] [Google Scholar]
- Peinado I, Miles W, Koutsidis G. Odour characteristics of seafood flavour formulations produced with fish by-products incorporating EPA, DHA and fish oil. Food Chemistry. 2016;212:612–619. doi: 10.1016/j.foodchem.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Refsgaard HH, Haahr AM, Jensen B. Isolation and quantification of volatiles in fish by dynamic headspace sampling and mass spectrometry. Journal of Agricultural and Food Chemistry. 1999;47:1114–1118. doi: 10.1021/jf9807770. [DOI] [PubMed] [Google Scholar]
- Song H, Liu J. GC–O–MS technique and its applications in food flavor analysis. Food Research International. 2018;114:187–198. doi: 10.1016/j.foodres.2018.07.037. [DOI] [PubMed] [Google Scholar]
- Tan J, Xu J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: a review. Artificial Intelligence in Agriculture. 2020;4:104–115. doi: 10.1016/j.aiia.2020.06.003. [DOI] [Google Scholar]
- Tian XY, Cai Q, Zhang YM. Rapid classification of hairtail fish and pork freshness using an electronic nose based on the PCA method. Sensors. 2011;12:260–277. doi: 10.3390/s120100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlet V, Knockaert C, Prost C, Serot T. Comparison of odor-active volatile compounds of fresh and smoked salmon. Journal of Agricultural and Food Chemistry. 2006;54:3391–3401. doi: 10.1021/jf053001p. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang J, Zhu Y, Wang X, Shi W. Volatile components present in different parts of grass carp. Journal of Food Biochemistry. 2018;42:e12668. doi: 10.1111/jfbc.12668. [DOI] [Google Scholar]
- Wierda RL, Fletcher G, Xu L, Dufour JP. Analysis of volatile compounds as spoilage indicators in fresh King salmon (Oncorhynchus tshawytscha) during storage using SPME−GC−MS. Journal of Agricultural and Food Chemistry. 2006;54:8480–8490. doi: 10.1021/jf061377c. [DOI] [PubMed] [Google Scholar]
- Wilson AD, Baietto M. Applications and advances in electronic-nose technologies. Sensors. 2009;9:5099–5148. doi: 10.3390/s90705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu K, Zhang C. Electronic nose for volatile organic compounds analysis in rice aging. Trends in Food Science & Technology. 2021;109:83–93. doi: 10.1016/j.tifs.2021.01.027. [DOI] [Google Scholar]
- Yu M, Li T, Song H. Characterization of key aroma-active compounds in four commercial oyster sauce by SGC/GC× GC–O–MS, AEDA, and OAV. Journal of Food Composition and Analysis. 2022;107:104368. doi: 10.1016/j.jfca.2021.104368. [DOI] [Google Scholar]
- Zhao Q, Shen Q, Guo R, Wu J, Dai ZY. Characterization of flavor properties from fish (Collichthys niveatus) through enzymatic hydrolysis and the Maillard reaction. Journal of Aquatic Food Product Technology. 2016;25:482–495. doi: 10.1080/10498850.2013.873965. [DOI] [Google Scholar]