Abstract
To assess the relationship between capillary leakage and inflammatory mediators during sepsis, blood samples were taken on hospital admission, as well as 24 and 72 h later, from 52 children (median age, 3.3 years) with severe meningococcal sepsis, of whom 38 survived and 14 died. Parameters related to cytokines (interleukin 6 [IL-6] IL-8, plasma phospholipase A2, and C-reactive protein [CRP]), to neutrophil degranulation (elastase and lactoferrin), to complement activation (C3a, C3b/c, C4b/c, and C3- and C4-CRP complexes), and to complement regulation (functional and inactivated C1 inhibitor and C4BP) were determined. The degree of capillary leakage was derived from the amount of plasma infused and the severity of disease by assessing the pediatric risk of mortality (PRISM) score. Levels of IL-6, IL-8, C3b/c, C3-CRP complexes, and C4BP on admission, adjusted for the duration of skin lesions, were significantly different in survivors and nonsurvivors (C3b/c levels were on average 2.2 times higher in nonsurvivors, and C3-CRP levels were 1.9 times higher in survivors). Mortality was independently related to the levels of C3b/c and C3-CRP complexes. In agreement with this, levels of complement activation products correlated well with the PRISM score or capillary leakage. Thus, these data show that complement activation in patients with severe meningococcal sepsis is associated with a poor outcome and a more severe disease course. Further studies should reveal whether complement activation may be a target for therapeutical intervention in this disease.
The complement system is involved in many aspects of the inflammatory response in patients with sepsis: among other things, it mediates chemotaxis of neutrophils and opsonization of particles and microorganisms, stimulates the release of granules from leukocytes (WBC), induces vasodilatation, and enhances vascular permeability, either via its influence on the cytokine network and coagulation system or through other means (7). Septic shock with purpura in children is mainly caused by meningococci. It is generally accepted that this condition results from the release of large amounts of lipopolysaccharide (LPS) into the circulation, leading to capillary leakage, severe hypotension, microthrombosis, and, ultimately, to organ failure and death (3, 15). The capillary leakage which occurs during sepsis is considered to be induced by the release and activation of endogenous inflammatory mediators, such as cytokines and activated components of the complement and contact systems. Genetic deficiencies of the complement system, in particular those of the membrane attack complex, lead to a higher susceptibility to and recurrent infections with Neisseria meningitidis (10). However, most children with severe meningococcal disease (SMD) are complement sufficient (1, 17). Thus, excessive complement activation, and not complement deficiency, is responsible for low levels of native complement components in most patients with severe meningococcal sepsis (2, 5, 7, 9, 12, 30). The degree of complement activation in SMD is related to the concentration of LPS in the plasma (4, 5). Patients with mild disease (low or undetectable LPS plasma levels) have a low degree of complement activation, whereas patients with septic shock and purpura are characterized by high circulating levels of LPS as well as excessive complement activation.
This study was designed to assess the extent of complement activation in children with septic shock with purpura and its possible route and regulation in relation to the severity of disease, as reflected by the degree of capillary leakage and by circulating levels of inflammatory mediators.
(This work was presented in part at two Shock Society conferences, the Fourth International Congress on the Immune Consequences of Trauma, Shock, and Sepsis, Munich, Germany, 4 to 8 March 1997 [14a], and the 20th Annual Conference on Shock, Indian Wells, Calif., 15 to 18 June 1997 [14b].)
MATERIALS AND METHODS
Study protocol.
Children between 3 months and 18 years of age with septic shock and petechiae/purpura were enrolled in this study after informed consent was obtained from their parents or legal representatives. They were admitted to the pediatric intensive care unit (PICU) of the Sophia Children’s Hospital between August 1988 and December 1994. Patients were eligible for inclusion when they met both of the following criteria: (i) the presence of petechiae/purpura for less than 12 h and (ii) the presence of shock, defined as sustained hypotension (i.e., systolic blood pressure of <75 mm of Hg for children between 3 and 12 months of age, <80 mm of Hg for children 1 to 5 years old, <85 mm of Hg for children 6 to 12 years old, and <100 mm of Hg for children older than 12 years) requiring intensive care treatment, or evidence of poor end organ perfusion. The last was defined as the presence of at least two of the following criteria: (i) unexplained metabolic acidosis (i.e., a pH of ≤7.3, a base excess of ≤−5 mmol/liter, or plasma lactate levels of >2 mmol/liter), (ii) arterial hypoxia (i.e., an arterial O2 tension [PaO2] of ≤75 mm of Hg, a PaO2/fraction of inspired O2 [FiO2] ratio of <250, or a transcutaneous arterial O2 percent saturation [SaO2] of ≤0.96) in patients without overt cardiopulmonary disease, (iii) acute renal failure (i.e., diuresis of <0.5 ml/kg of body weight/h for at least 1 h despite acute volume supplementation or evidence of adequate intravascular volume [defined by palpability of the liver, a cardiothoracic ratio of >0.4 on chest radiography, and a central venous pressure of >5 mm of Hg]) without preexisting renal disease, or (iv) sudden deterioration of the baseline mental status. The pediatric risk of mortality (PRISM) score (24) was calculated by using the most abnormal value of each variable recorded during the first 4 h after admission to the PICU. The exact amount of volume supplementation (human plasma or fresh frozen plasma) administered during the stay in the ICU to restore the hemodynamic condition of the patient was recorded. All patients received maximal supportive therapy when appropriate: antibiotics, volume administration, inotropic support, and mechanical ventilation.
The study protocol was approved by the Medical Ethics Committee of the University Hospital Rotterdam.
Collection of blood.
Arterial blood samples were collected within 2 h after admission and after 24 and 72 h. Blood for analysis of complement parameters was collected in vials containing 3.8% trisodium citrate, immediately chilled on ice, and centrifuged at 4,000 × g for 10 min. Subsequently, platelet-poor plasma was obtained by centrifugation of the supernatant at 20,000 × g for 30 min at 4°C. Plasma was stored at −80°C until tests were performed.
Assays.
WBC and platelet counts were determined with an automated platelet counter (H1 system; Technicon Instruments, Salem, N.H.). Lactate was measured by enzymatic endpoint determination, and C-reactive protein (CRP) was measured by nephelometric assay (28).
C3a levels were determined by a radioimmunoassay (RIA) as described previously (14) and were expressed as nanomolar concentrations. Levels of C3a in healthy persons are below 5 nM. Activation of C4 was assessed with an enzyme-linked immunosorbent assay (ELISA) as described previously (33). In brief, monoclonal antibody (MAb) anti-C4-1, which recognizes a neoepitope on the C4 activation products C4b, C4bi, and C4c (collectively referred to as C4b/c) and not on native C4, was used as a catching antibody (Ab). Biotinylated polyclonal rabbit anti-human C4 Abs were used as detecting Abs. Results were expressed in nanomolar concentrations. The activation of C3 was assessed with a similar ELISA (33). In brief, MAb anti-C3-9, recognizing a neoepitope on C3b, C3bi, and C3c (collectively referred to as C3b/c) was used as the catching Ab, and biotinylated polyclonal rabbit anti-human C3c Abs were used as detecting Abs. Results were expressed in nanomolar concentrations. Complement-CRP complexes were determined by a novel method (34). In short, purified complement complexes were quantified by differential antibody ELISAs. In these ELISAs MAbs directed against C4d and C3d were used to capture complexes. These antibodies capture C4b-C4bi-C4d and C3b-C3bi-C3d, respectively. CRP-complement complexes were detected by biotinylated MAbs against CRP. Results were expressed as picomolar concentrations of complement fixed to CRP. The levels of both types of complexes in healthy volunteers were below the limit of detection (4 pM).
Functional and proteolytically inactive C1 inhibitor (fC1-Inh and iC1-Inh, respectively), were determined by RIAs (20). The concentrations of fC1-Inh and iC1-Inh in normal pooled plasma were 2.5 and 0.08 μM, respectively; the levels in the patients were expressed as percents of this plasma pool level. Type II secretory phospholipase A2 (sPLA2) was determined by ELISA (35). sPLA2 levels in healthy volunteers are below 5 μg/liter. Lactoferrin and elastase-α1-antitrypsin complexes were determined by RIAs (21). Interleukin 6 (IL-6) and IL-8 were measured by ELISAs obtained from the Department of Immune Reagents (Central Laboratory of The Netherlands Red Cross Blood Transfusion Services, Amsterdam, The Netherlands). These ELISAs were performed according to the manufacturer’s instructions. Normal levels are <10 pg/ml for IL-6 and <20 pg/ml for IL-8. C4BP (C4b binding protein) concentrations were measured by electroimmunodiffusion. Normal levels are 68 to 140% of normal plasma pool level.
Statistical analysis.
Results are expressed as medians (and ranges) unless otherwise specified. The Mann-Whitney U test and the Wilcoxon signed-rank test were used to evaluate between- and within-group differences. The relationships between parameters were tested by assessing Spearman’s rank correlation. Multiple regression analysis, taking account of the duration of skin lesions, was used to compare survivors and nonsurvivors in regard to the logarithmically transformed levels of inflammatory parameters. Logistic regression was performed to evaluate the relation of the various parameters to mortality. This analysis proceeded in two steps. In the first step, parameters were grouped into categories (i.e., cytokine parameters, neutrophil degranulation, complement activation products, and complement regulation). For each category separately, the variables most predictive of mortality were determined by using backward elimination. In the second step, the variables remaining after the first step were combined and the backward-elimination procedure was applied again. The same procedure was used with multiple regression to assess the relation between the various parameters and capillary leakage and lactate. Two-tailed P values of ≤0.05 were considered statistically significant in each test.
RESULTS
Demographics.
Fifty-two patients fulfilling the entry criteria and admitted to the PICU were enrolled in the study. Thirty-two were males (62%), and 20 were females (38%). The median age was 3.3 years (range, 0.4 to 17.9 years). Thirty-eight of the 52 patients (73%) survived; of the 14 (27%) deaths, 12 were from irreversible shock and 2 were from cerebral herniation. Cultures of blood, cerebrospinal fluid, or skin biopsy materials revealed N. meningitidis in 46 (89%) patients and Haemophilus influenzae in 1 patient. Cultures from five patients were sterile. Thirty-three (64%) patients needed mechanical ventilation. Inotropic support was necessary for 48 (92.3%) patients. Due to the lack of sufficient plasma, complement parameters were not determined on admission for seven patients. Forty-nine of the children participated in a randomized, placebo-controlled trial to evaluate the efficacy of a human MAb, HA-1-A (Centoxin; Centocor, Malvern, Pa.), in meningococcal septic shock. On admission, HA-1-A or a placebo was administered after blood was collected. There was no significant difference in mortality between HA-1-A-treated and placebo-treated patients.
Differences between survivors and nonsurvivors.
In Tables 1 and 2 clinical and laboratory parameters related to the severity of disease, and to the extent of cytokine release, neutrophil degranulation, and complement activation, in survivors and nonsurvivors are compared. As expected, clinical and biochemical parameters related to the severity of disease were significantly different in survivors and nonsurvivors. As for parameters related to cytokines, IL-6 and IL-8 levels were substantially elevated and significantly higher in nonsurvivors than in survivors. Levels of the two acute-phase proteins CRP and sPLA2, whose synthesis is induced by cytokines and which therefore reflect the release of cytokines, were elevated in both patient groups, but in contrast to cytokines, the highest levels occurred in survivors. All patients had evidence of increased degranulation of neutrophils: elastase levels were elevated in all patients, although the difference between levels in survivors and those in nonsurvivors did not reach statistical significance (P = 0.08). Also, circulating levels of lactoferrin were increased in all but one child, and the levels in nonsurvivors were significantly higher.
TABLE 1.
Clinical and laboratory parameters related to severity of disease on admission in children with septic shock and purpura
| Parameter | Valuea
|
||
|---|---|---|---|
| Survivors (n = 38) | Nonsurvivors (n = 14) | Normal | |
| PRISM | 10 (1–38) | 20 (8–25)b | |
| Lactate (mmol/liter) | 4.3 (1.1–15.5) | 7.3 (2.9–20.0)b | <2.0 |
| Duration of petechiae (h) | 5.3 (1.0–11.5) | 3.6 (0.5–8.5)b | |
| WBC (109/liter) | 13.2 (1.4–44.4) | 5.8 (1.3–12.6)b | 35,894 |
| Plasma infusion (ml/kg) | 65 (12–307) | 120 (59–182)b | |
Values are medians (range).
P < 0.05 for the difference between groups (Mann-Whitney U test).
TABLE 2.
Parameters related to cytokines, neutrophil degranulation, complement activation, and complement regulation on days 0, 1, and 3 after admission in children with septic shock and purpura
| Parameter | Valuea
|
|||||
|---|---|---|---|---|---|---|
| Day 0
|
Day 1
|
Day 3
|
Normal | |||
| Survivors (n = 38) | Nonsurvivors (n = 14) | Survivors (n = 34) | Nonsurvivors (n = 5) | Survivors (n = 31) | ||
| IL-6 (ng/ml) (49)b | 15.3 (0.036–1,507) | 280.6 (0.087–2,002)c | 0.1 (0.01–186)d | 20.7 (0.7–50.0)c | 0.02 (0.002–7.4)d | <0.001 |
| IL-8 (ng/ml) (49) | 1.1 (0.013–381) | 8.8 (0.023–369)c | 0.03 (0.001–23.7)d | 0.8 (0.15–2.1)c | 0.03 (0.001–0.8)d | <0.002 |
| CRP (mg/liter) (51) | 128 (34–258) | 70 (38–162)c | 255 (145–444)d | 210 (141–378)d | 152 (42–401) | <2 |
| sPLA2 (ng/ml) (48) | 131 (2–1,461) | 97 (9–1,907) | 167 (15–847) | 102 (12–573) | 18 (1–384)d | <5 |
| Elastase (ng/ml) (49) | 512 (181–3,376) | 641 (251–3,132) | 317 (94–572) | 410 (277–699)c | 177 (67–908)d | <100 |
| Lactoferrin (ng/ml) (49) | 2,584 (124–32,285) | 4,275 (1,757–31,717)c | 385 (146–3,518)d | 662 (307–1,896)d | 206 (88–920)d | <400 |
| C3a (nmol/liter) (46) | 12 (2–52) | 30 (17–58)c | 12 (3–49) | 20 (6–30) | 5.8 (<2–67)d | <5 |
| C3b/c (nmol/liter) (49) | 199 (26–826) | 478 (196–1,087)c | 33 (14–604)d | 67 (19–92)d | 25 (9–305)d | <50 |
| C3-CRP (pmol/liter) (31) | 291 (68–695) | 156 (59–286)c | 425 (136–1,221)d | 266 (210–426)c | 374 (79–1,562)d | <4 |
| C4b/c (nmol/liter) (49) | 56 (22–1,340) | 110 (33–562)c | 52 (13–255) | 181 (33–310) | 47 (20–175) | <25 |
| C4-CRP (pmol/l) (30) | 67 (30–164) | 53 (26–77) | 99 (33–842)d | 85 (52–106) | 74 (15–341)d | <4 |
| fC1-Inh (%) (46) | 79 (43–156) | 72 (34–99) | 100 (38–179)d | 92 (76–103) | 132 (52–192)d | 80–120 |
| iC1-Inh (%) (48) | 378 (53–1,141) | 863 (96–2,100) | 168 (56–514)d | 166 (128–476) | 128 (75–181)d | <160 |
| C4BP (%) (50) | 72 (33–108) | 53 (24–89)c | 92 (58–139)d | 99 (94–107)d | 113 (73–224)d | 80–120 |
Values are medians (range).
Number of measurements.
P < 0.05 for the difference between groups (Mann-Whitney U test).
P < 0.05 for the differences within groups (Wilcoxon signed-rank test).
As for the activation of complement, C3a and C3b/c levels were increased in all but four of the patients, whereas C4b/c was elevated in all but one. Levels of either complement activation product were higher in nonsurvivors. All patients had increased levels of complement-CRP complexes (C3 and C4). However, the levels of these complexes were lower in nonsurvivors and the difference between the levels of C3-CRP complexes in survivors and nonsurvivors was significant. fC1-Inh levels varied widely. In survivors, levels were decreased in some patients whereas they were normal or elevated in others. In the nonsurvivors, fC1-Inh levels were decreased or normal but never elevated. However, the differences between survivors and nonsurvivors were not significant. Levels of iC1-Inh were normal in nine of the patients. In the other patients, the levels, though substantially elevated, varied widely. The difference between survivors and nonsurvivors was just short of statistical significance (P = 0.07).
Relation between inflammatory and clinical parameters.
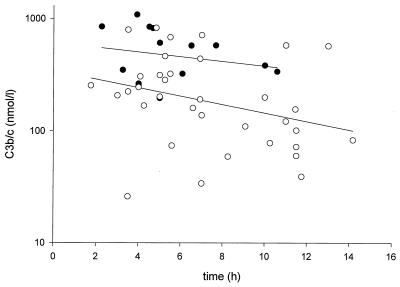
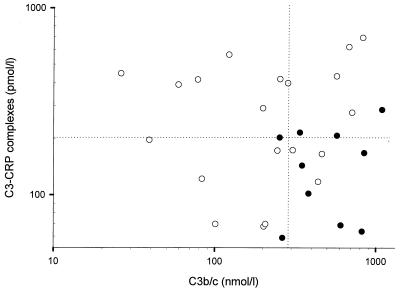
Multiple regression analysis for the relation between levels of inflammatory parameters and survival and duration of skin lesions showed that time-adjusted concentrations of IL-6, IL-8, C3b/c, C3-CRP complexes, C4BP, and WBC were significantly different in survivors and nonsurvivors: IL-8 levels were on average 11.8 times higher in nonsurvivors (P = 0.003) and were also negatively related to the duration of petechiae (P = 0.005). Time-adjusted levels of C3b/c on admission were on average 2.2 times higher (P = 0.004) in nonsurvivors than in survivors (Fig. 1). C3-CRP levels were on average 1.9 times higher in survivors than in nonsurvivors (P = 0.035) and were not related to the duration of petechiae. Logistic regression with backward elimination showed that mortality was independently related to the levels of C3b/c (P = 0.03) and C3-CRP complexes (P = 0.03). Figure 2 shows the dichotomous distribution of these two parameters.
FIG. 1.
C3b/c levels on admission versus the time between the onset of petechiae and the moment of blood sampling in surviving (open circles) and nonsurviving (solid circles) patients. The lines represent least-squares regression lines of the two groups (upper line, survivors; lower line, nonsurvivors). The angles of inclination of the regression lines do not differ significantly. Time-adjusted C3b/c values were on average 2.2 times higher in nonsurvivors (P = 0.004).
FIG. 2.
C3-CRP complex levels versus C3b/c levels on admission in surviving (open circles) and nonsurviving (solid circles) patients show a dichotomous distribution (dotted lines represent medians). Both variables were independently related to survival.
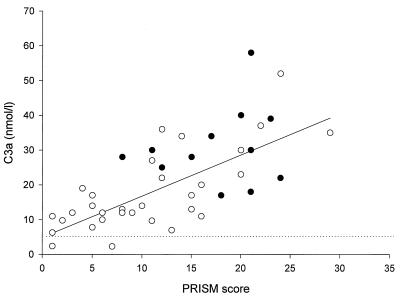
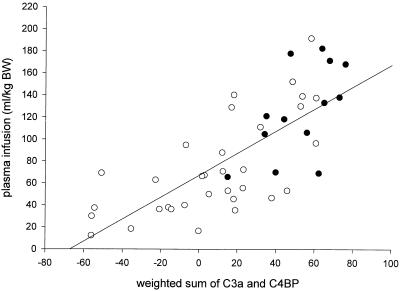
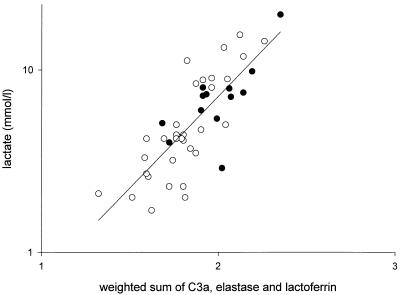
IL-6, IL-8, and CRP levels were closely correlated with all parameters for the severity of disease (Table 3). This was also observed for the complement activation products. The strongest correlation appeared to be that between the levels of the complement activation products and the PRISM score (Fig. 3) or the total amount of plasma infused. Of the neutrophil degranulation products, lactoferrin correlated with four of five severity-of-disease parameters; there was a strong correlation between elastase and the arterial lactate levels (r = 0.59; P < 0.001). Multiple regression analysis of the various categories of variables showed that the levels of C3a (P < 0.001) and C4BP (P < 0.001) were independently related to the total amount of plasma infused; Fig. 4 represents the relation (r = 0.77; P < 0.001) between the total amount of plasma infused and the weighted sum (using the regression coefficients as the weight) of C3a and C4BP [73 × (log10C3a) − 1.0 × C4BP]. Similar statistical analyses showed that the levels of C3a (P < 0.05), elastase (P = 0.007), and lactoferrin (P = 0.001) were independently related to the arterial lactate levels. Figure 5 represents the relation (r = 0.78; P < 0.001) between the lactate levels and the weighted sum of C3a, elastase, and lactoferrin [0.142 × (log10C3a) + 0.280 × (log10elastase) + 0.257 × (log10 lactoferrin)].
TABLE 3.
Correlation coefficients between clinical and inflammatory parametersa
| Parameter | Correlation with:
|
||||
|---|---|---|---|---|---|
| PRISM | Lact | Time | WBC | Inf | |
| IL-6 | 0.41 | 0.43 | −0.52 | −0.7 | 0.62 |
| IL-8 | 0.36 | 0.45 | −0.46 | −0.7 | 0.59 |
| CRP | −0.34 | −0.3 | 0.42 | 0.43 | −0.41 |
| sPLA2 | 0.36 | ||||
| Elastase | 0.59 | ||||
| Lactoferrin | 0.29 | 0.68 | −0.33 | 0.37 | |
| C3a | 0.69 | 0.46 | −0.51 | −0.4 | 0.75 |
| C3b/c | 0.53 | 0.53 | −0.39 | −0.4 | 0.57 |
| C3-CRP | 0.39 | ||||
| C4b/c | 0.46 | 0.33 | −0.29 | 0.38 | |
| C4-CRP | |||||
| fC1-In | |||||
| iC1-Inf | |||||
| C4BP | −0.32 | 0.36 | −0.43 | ||
Values are Spearman rank correlation coefficients; only significant (P < 0.05) values are presented. Lact, arterial lactate; Time, duration of petechiae; WBC, leukocyte count; Inf, total amount of plasma infused.
FIG. 3.
C3a levels on admission versus PRISM score in surviving (open circles) and nonsurviving (solid circles) patients. The dotted line represents the upper level of normal. The solid line represents the least-squares regression line for all data points (r = 0.69; P < 0.001).
FIG. 4.
Total amount of plasma infused (in milliliters per kilogram of body weight [BW]) versus the weighted sum of the initial levels of C3a and C4BP [73.1 × (log10C3a) − 1.0 × C4BP] in surviving (open circles) and nonsurviving (solid circles) patients. The line represents the least-squares regression line for all data points (r = 0.77; P < 0.001).
FIG. 5.
Arterial lactate (in millimoles per liter) on admission versus the weighted sum of the initial levels of C3a, elastase, and lactoferrin [0.142 × (log10C3a) + 0.280 × (log10elastase) + 0.257 × (log10lactoferrin)] in surviving (open circles) and nonsurviving (solid circles) patients. The line represents the least-squares regression line for all data points (r = 0.78; P < 0.001).
Relations among inflammatory parameters.
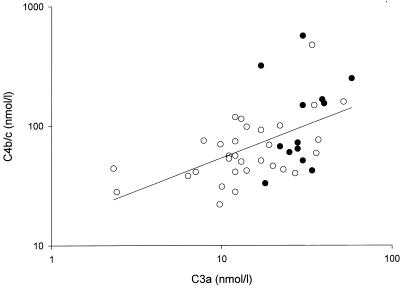
The levels of the complement activation products C3a and C3b/c correlated positively with those of IL-6 and IL-8 and negatively with that of CRP (Table 4). The levels of complement-CRP complexes showed an inverse correlation pattern: the correlation of these markers of CRP-dependent complement activation with interleukins was negative, whereas that with CRP was positive. The levels of C4b/c, representing the activation of the classical pathway of complement, did not correlate with that of IL-6, IL-8, or CRP. However, these levels showed a strong correlation with C3a (Fig. 6) and C3b/c levels, as well as with the levels of C4-CRP complexes, indicating that a substantial part of the activation of the complement system had occurred through the classical pathway, probably via CRP. C3- and C4-CRP complexes both closely correlated with IL-6 and IL-8, CRP, lactoferrin, and C4BP. The fC1-Inh levels correlated with levels of the other complement regulation protein, C4BP. The levels of iC1-Inh correlated with levels of sPLA2. Finally, the levels of elastase and lactoferrin correlated well with those of IL-6 and IL-8 (Table 4).
TABLE 4.
Relations among inflammatory mediatorsa
| Parameter | Correlation with:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | sPLA2 | Elastase | Lactoferrin | C3a | C3b/c | C3-CRP | C4b/c | C4-CRP | fC1-In | iC1-In | C4BP | |
| IL-6 | −0.68 | 0.4 | 0.56 | 0.58 | 0.59 | −0.56 | −0.53 | −0.33 | ||||
| IL-8 | −0.61 | 0.41 | 0.59 | 0.56 | 0.65 | −0.57 | −0.52 | −0.3 | ||||
| CRP | 0.32 | −0.3 | −0.36 | −0.3 | 0.64 | 0.55 | 0.36 | |||||
| sPLA2 | 0.32 | 0.3 | 0.41 | |||||||||
| Elastase | 0.66 | 0.5 | 0.3 | |||||||||
| Lactoferrin | 0.3 | 0.66 | 0.39 | 0.53 | −0.47 | −0.37 | ||||||
| C3a | −0.36 | −0.3 | 0.39 | 0.72 | 0.58 | 0.35 | ||||||
| C3b/c | −0.3 | 0.5 | 0.53 | 0.72 | 0.5 | |||||||
| C3-CRP | 0.64 | −0.5 | 0.91 | 0.67 | ||||||||
| C4b/c | 0.3 | 0.58 | 0.5 | 0.38 | ||||||||
| C4-CRP | 0.55 | −0.4 | 0.91 | 0.38 | 0.6 | |||||||
| fC1-Inf | 0.52 | |||||||||||
| iC1-Inf | 0.4 | 0.39 | 0.32 | |||||||||
| C4BP | 0.36 | 0.67 | 0.6 | 0.52 | ||||||||
Values are Spearman rank correlation coefficients; only significant (P < 0.05) values are presented.
FIG. 6.
Initial C4b/c values versus C3a levels in surviving (open circles) and nonsurviving (solid circles) patients (for all patients, r = 0.58 and P < 0.001).
Time course of laboratory parameters.
Table 2 presents the time course of the different inflammatory variables. Levels of CRP and sPLA2 remained elevated until 72 h after admission and were not different in survivors and nonsurvivors, except for the CRP levels on admission. The levels of the complement activation products C3a and C4b/c were still elevated at 72 h, while those of C3b/c were already normalized at 24 h. In 50% of the patients, C3a and C4bc were higher at 24 h than on admission. The course of complement-CRP complexes was remarkable in that the levels were higher in survivors and the levels observed on days 1 and 3 were higher than those on admission. Elastase levels decreased until 72 h after admission but remained elevated. At 24 h, the levels were different in survivors and nonsurvivors. At 72 h, the levels in surviving patients were still elevated, except in two patients. The levels of lactoferrin decreased more rapidly: at 24 h, the levels were within the normal range in 50% of the survivors. The levels of fC1-Inh increased gradually until 72 h after admission. At that time 50% of the survivors had levels above the upper level of normal. There was a more rapid decrease in levels of iC1-Inh: at 24 h, 50% of both survivors and nonsurvivors had levels within normal limits. C4BP levels increased gradually over time, although at 72 h there was a wide range (73 to 224%) of plasma levels.
DISCUSSION
In this study of children with septic shock and purpura a high degree of complement activation was observed, which closely correlated with mortality, severity of disease, and degree of capillary leakage, as estimated by the amount of plasma infused. A substantial part of this complement activation was through the classical pathway, as can be deduced from the close relation between C4 and C3 activation products (C4b/c and C3a or C3b/c). This activation persisted for days, and in some patients it even increased after admission. The complement system can be activated through the classical pathway by antigen-antibody complexes, through the alternative pathway by bacteria or LPS, and through the mannan binding protein pathway. As the majority of SMD patients will probably have low or no titers of antibodies, the prevailing view is that it is mainly the alternative pathway that contributes to complement activation in this disease. Recently Brandtzaeg and colleagues measured activation products of both the alternative and the classical pathways in 20 patients with systemic meningococcal disease (4). Activation of C4 was not different in patients with shock and those without shock, nor did they find a correlation between C4 and C3 activation products. They concluded that the classical pathway contributed only slightly to total complement activation in patients with shock. In our patients, the level of C4b/c significantly correlated with that of C3b/c or C3a, indicating that at least part of the C3 was activated via the classical pathway. Since we included only patients with shock, the difference from the results of Brandtzaeg et al. may have been caused by the larger number of children with severe disease in our study. Accordingly, levels of activated C3 correlated better with severity indices than those of activated C4 (Table 2), implying that the latter correlation may have been missed with a smaller number of patients.
Assuming that levels of antibodies against meningococci are low in patients with SMD, one can raise the question of which mechanism the classical pathway was activated through. CRP can activate the classical pathway upon binding to the phospholipid phosphatidylcholine, and it has been suggested that this may occur particularly in the presence of the enzyme sPLA2, another acute-phase protein (11). We measured CRP-complement complexes as indicators of CRP-mediated complement activation (34) and also assessed the relationship between CRP levels and complement activation parameters to address the question of whether CRP was involved in the observed complement activation. CRP levels were negatively correlated with those of the complement activation products C3a and C3b/c, and there was a negative relation between C3-CRP levels and survival (Fig. 2). Hence, the contribution of CRP-mediated activation to the total complement activation was probably minor. On the other hand, the correlation between C4b/c levels and those of C4-CRP complexes was significant, indicating that at least a substantial part of the classical pathway activation had occurred via CRP (Table 4). CRP levels also positively correlated with the duration of petechiae, suggesting a shorter disease course in the patients with lower CRP levels. CRP levels were lower in nonsurvivors than in survivors; at 24 h the levels had further increased in both groups, but there was no longer a difference between the groups. Thus, very likely the difference between the CRP levels of surviving and nonsurviving patients on admission reflected the fact that the nonsurvivors were admitted earlier in their disease course. Accordingly, levels of sPLA2, which increase somewhat earlier during an acute-phase reaction (25), were not different in survivors and nonsurvivors.
Activation of the classical pathway of the complement system is regulated by, among other things, the plasma proteins C1-Inh and C4BP. C1-Inh inhibits activated C1, whereas C4BP inactivates C4b by acting as a cofactor for the cleavage of C4b by factor I. Both C4BP and C1-Inh are acute-phase proteins, and during severe infections their levels in plasma are increased. In our patients the levels of C4BP were decreased and were negatively correlated with outcome and severity of disease (as shown by PRISM and total plasma infused) and positively correlated with the level of C1-Inh. The decreased levels of C4BP may be explained by leakage to the extravascular space, suppression of its synthesis by tumor necrosis factor alpha (23), binding to serum amyloid protein P (27), or binding to bacterial surface proteins (18). To what extent these or other mechanisms accounted for the decreased levels of C4BP in our patients is difficult to say. Yet this decrease is remarkable, considering the acute-phase behavior of C4BP, and together with the relatively low levels of C1-Inh, it may indicate a poor inhibition of the classical pathway. Furthermore, decreased levels of C4BP may have implications for the coagulation system: low levels of C4BP lead to relatively higher levels of free protein S, which may be of benefit during septic shock (16, 29).
C1-Inh is the only known inhibitor in plasma of activated C1 and is the major inhibitor of activated factor XII and kallikrein of the contact pathway of coagulation. Inhibition of these so-called target proteinases by C1-Inh leads to the formation of proteinase–C1-Inh complexes. The formation of these complexes is accompanied by the generation of iC1-Inh species. These species may also result from inactivation by other endogenous (e.g., neutrophilic elastase) or exogenous, i.e., bacterial, proteinases. C1-Inh in plasma may thus exist in three forms: fC1-Inh, iC1-Inh, and C1-Inh complexed to a proteinase (20). In our patients the levels of fC1-Inh were decreased or normal, which, considering its acute-phase behavior, suggests a relative deficiency of this inhibitor and hence a diminished regulation of the complement and contact system, with subsequent release of biologically active peptides. The time course of the levels of fC1-Inh suggested consumption of this inhibitor, in particular during the early phase of the disease. High levels of iC1-Inh have been observed in experimental septic shock in baboons (6) as well as in patients with vascular leakage syndrome during IL-2 therapy (13). Our data do not allow conclusions regarding the mechanism of the generation of iC1-Inh in our patients.
Neutrophils have been implicated as important mediators of vascular injury (2, 26, 31) by the release of toxic oxygen species and lysosomal proteinases, such as elastase, upon stimulation by a large variety of agonists. In addition, elastase may facilitate activation of the complement, coagulation, and fibrinolytic systems by inactivating the major inhibitors of these cascade systems (7, 21). The levels of elastase and lactoferrin in our patients were similar to those found in adult patients with sepsis (21). The levels correlated with outcome, as well as with lactate levels (Fig. 6). Hence, activation and degranulation of neutrophils may contribute to tissue hypoxia and capillary leakage, for example, by plugging capillaries. Elastase and lactoferrin were both also correlated with complement activation products and IL-6 and IL-8, suggesting a cooperative effect of cytokines and complement in the process of neutrophil adherence and degranulation.
The hallmark of an acute inflammatory reaction is accumulation of WBC and increased permeability of vessels to macromolecules, which leads to edema. The tissue edema that results from increased capillary permeability and protein-rich fluid leakage leads to tissue injury and finally organ dysfunction (32). Alterations in microcirculatory permeability can occur through a chain of events that includes disruption of the glycocalix (the negatively charged surface coat of the endothelial cells), activation of specific receptors (adhesion molecules), generation of an intracellular signal via a second messenger (e.g., cyclic AMP, Ca2+, or protein kinase C), cytoskeletal changes, and subsequent alteration of the geometry of the interendothelial junctions (19, 32). During sepsis the presence of (endo)toxins and primary mediators like tumor necrosis factor alpha and IL-1 directly and indirectly leads to activation of endothelial cells; induces secondary mediators like IL-2, IL-4, IL-8, gamma interferon, histamine, and bradykinin; results in metabolism of arachidonic acid to form leukotrienes, thromboxane, platelet-activating factor, and prostaglandins; and induces the formation of thrombin. An activated complement cascade results in vascular abnormalities and neutrophil activation, by which neutrophil-induced damage may occur after degranulation, aggregation, and adherence to the endothelium. Almost all of these agents have direct effects on the vascular endothelium and cause alterations in endothelial permeability, though the specific mechanisms for most of them remain to be elucidated. To prove a causal role in the pathogenesis of capillary leakage, studies of complement inhibitors are necessary. Initial experience with C1-Inh in capillary leakage syndrome induced by IL-2 (13) or following bone marrow transplantation (22) indeed seems to support a role for complement in the pathogenesis of this syndrome. Individuals with inherited component deficiencies have a markedly increased risk of acquiring systemic meningococcal infections and may experience recurrent episodes of these. A striking finding in individuals with late complement component deficiencies compared with normal persons is the low mortality rate associated with meningococcal disease (8). It is therefore tempting to speculate that complement plays a dual role in the pathogenesis of meningococcal sepsis: on the one hand it contributes to the defense against meningococci; on the other hand, in patients suffering from SMD, excessive activation (in particular via mechanisms not triggered by the microorganisms themselves) may contribute to tissue damage and a complicated disease course.
In conclusion, excessive activation of the complement system was demonstrated in children with septic shock and purpura; the activation had occurred in part via the classical pathway and was related to outcome, severity of disease, and extent of capillary leakage. Further studies, for example, focusing on the effects of the therapeutical administration of C1 esterase inhibitor, may reveal whether this activation contributes to a detrimental disease course.
REFERENCES
- 1.Beatty D W, Ryder C R, Heese H D. Complement abnormalities during an epidemic of group B meningococcal infection in children. Clin Exp Immunol. 1986;64:465–470. [PMC free article] [PubMed] [Google Scholar]
- 2.Bone R C. Inhibitors of complement and neutrophils: a critical evaluation of their role in the treatment of sepsis. Crit Care Med. 1992;20:891–898. [PubMed] [Google Scholar]
- 3.Brandtzaeg P. Systemic meningococcal disease: clinical pictures and pathophysiological background. Rev Med Microbiol. 1996;7:63–72. [Google Scholar]
- 4.Brandtzaeg P, Hogasen K, Kierulf P, Mollnes T E. The excessive complement activation in fulminant meningococcal septicemia is predominantly caused by alternative pathway activation. J Infect Dis. 1996;173:647–655. doi: 10.1093/infdis/173.3.647. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P B, Mollnes T E, Kierulf P. Complement activation and endotoxin levels in systemic meningococcal disease. J Infect Dis. 1989;160:58–65. doi: 10.1093/infdis/160.1.58. [DOI] [PubMed] [Google Scholar]
- 6.de Boer J P, Creasey A A, Chang A, Roem D, Eerenberg A J M, Hack C E, Taylor F B., Jr Activation of the complement system in baboons challenged with live Escherichia coli: correlation with mortality and evidence for a biphasic activation patterns. Infect Immun. 1993;61:4293–4301. doi: 10.1128/iai.61.10.4293-4301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer J P, Wolbink G J, Thijs L G, Baars J W, Wagstaff J, Hack C E. Interplay of complement and cytokines in the pathogenesis of septic shock. Immunopharmacology. 1992;24:135–148. doi: 10.1016/0162-3109(92)90019-9. [DOI] [PubMed] [Google Scholar]
- 8.Densen P. Interaction of complement with Neisseria meningitidis and Neisseria gonorrhoeae. Clin Microbiol Rev. 1989;2:S11–S17. doi: 10.1128/cmr.2.suppl.s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickneite G. Influence of C1-inhibitor on inflammation, edema and shock. Behring Inst Mitt. 1993;93:299–305. [PubMed] [Google Scholar]
- 10.Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Rev. 1993;12:295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- 11.Gronroos J M, Kuttila K, Nevalainen T J. Group II phospholipase A2 in serum in critically ill surgical patients. Crit Care Med. 1994;22:956–959. doi: 10.1097/00003246-199406000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Hack C E, Nuijens J H, Felt-Bersma R J, Schreuder W O, Eerenberg-Belmer A J, Paardekooper J, Bronsveld W, Thijs L G. Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am J Med. 1989;86:20–26. doi: 10.1016/0002-9343(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 13.Hack C E, Ogilvie A C, Eisele B, Jansen P M, Wagstaff J, Thijs L G. Initial studies on the administration of C1-esterase inhibitor to patients with septic shock or with a vascular leak syndrome induced by interleukin-2 therapy. Prog Clin Biol Res. 1994;388:335–357. [PubMed] [Google Scholar]
- 14.Hack C E, Paardekooper J, Eerenberg A J, Navis G O, Nijsten M W, Thijs L G, Nuijens J H. A modified competitive inhibition radioimmunoassay for the detection of C3a. Use of 125I-C3 instead of 125I-C3a. J Immunol Methods. 1988;108:77–84. doi: 10.1016/0022-1759(88)90405-x. [DOI] [PubMed] [Google Scholar]
- 14a.Hazelzet, J. A., R. Kornelisse, G. van Mierlo, K. van Joosten, R. de Groot, and C. Hack. 1997. Complement activation in children with septic shock and purpura: classical or alternative pathway. Shock 7(Suppl. 1):74.
- 14b.Hazelzet, J. A., R. Kornelisse, G. van Mierlo, E. van der Voort, R. de Groot, and C. Hack. 1997. The importance of C1-inhibitor in children with septic shock and purpura. Shock 7(Suppl. 2):12.
- 15.Hazelzet J A, Risseeuw-Appel I M, Kornelisse R F, Hop W C J, Dekker I, Joosten K F M, de Groot R, Hack C E. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thromb Haemostasis. 1996;76:932–938. [PubMed] [Google Scholar]
- 16.Hesselvik J, Malm J, Dahlbäck B, Blombäck M. Protein C, protein S and C4b-binding protein in severe infection and septic shock. Thromb Haemostasis. 1991;65:126–129. [PubMed] [Google Scholar]
- 17.Hogasen K, Michaelsen T, Mellbye O J, Bjune G. Low prevalence of complement deficiencies among patients with meningococcal disease in Norway. Scand J Immunol. 1993;37:487–489. doi: 10.1111/j.1365-3083.1993.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnsson E, Thern A, Dahlback B, Heden L O, Wikstrom M, Lindahl G. A highly variable region in members of the streptococcal M protein family binds the human complement regulator C4BP. J Immunol. 1996;157:3021–3029. [PubMed] [Google Scholar]
- 19.Lampugnani M G, Caveda L, Breviario F, Del Maschio A, Dejana E. Endothelial cell-to-cell junctions. Structural characteristics and functional role in the regulation of vascular permeability and leukocyte extravasation. Bailliere’s Clin Haematol. 1993;6:539–558. doi: 10.1016/s0950-3536(05)80187-8. [DOI] [PubMed] [Google Scholar]
- 20.Nuijens J, Eerenberg-Belmer A, Huijbregts C, Schreuder W, Felt-Bersma R, Abbink J, Thijs L, Hack C. Proteolytic inactivation of plasma C1 inhibitor in sepsis. J Clin Invest. 1989;84:443–450. doi: 10.1172/JCI114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuijens J H, Abbink J J, Wachtfogel Y T, Colman R W, Eerenberg A J, Dors D, Kamp A J, Strack van Schijndel R J, Thijs L G, Hack C E. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med. 1992;119:159–168. [PubMed] [Google Scholar]
- 22.Nurnberger W, Heying R, Burdach S, Gobel U. C1 esterase inhibitor concentrate for capillary leakage syndrome following bone marrow transplantation. Ann Hematol. 1997;75:95–101. doi: 10.1007/s002770050321. [DOI] [PubMed] [Google Scholar]
- 23.Phillips D J, Novinger M S, Evatt B L, Hooper W C. TNF-alpha suppresses IL-1 alpha and IL-6 upregulation of C4b-binding protein in HepG-2 hepatoma cells. Thromb Res. 1996;81:307–314. doi: 10.1016/0049-3848(96)00002-3. [DOI] [PubMed] [Google Scholar]
- 24.Pollack M M, Ruttimann U E, Getson P R. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16:1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Purzanski W, Wilmore D W, Suffredini A, Martich G D, Hoffman A G, Browning J L, Stefanski E, Sternby B, Vadas P. Hyperphospholipasemia A2 in human volunteers challenged with intravenous endotoxin. Inflammation. 1992;16:561–570. doi: 10.1007/BF00918980. [DOI] [PubMed] [Google Scholar]
- 26.Smedly L A, Tonnesen M G, Sandhaus R A, Haslett C, Guthrie L A, Johnston R B, Jr, Henson P M, Worthen G S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986;77:1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen I J, Nielsen E H, Andersen O, Danielsen B, Svehag S E. Binding of complement proteins C1q and C4bp to serum amyloid P component (SAP) in solid contra liquid phase. Scand J Immunol. 1996;44:401–407. doi: 10.1046/j.1365-3083.1996.d01-326.x. [DOI] [PubMed] [Google Scholar]
- 28.Sternberg J. A rate nephelometer for measuring specific proteins by immunoprecipitation reaction. Clin Chem. 1977;23:1456–1464. [PubMed] [Google Scholar]
- 29.Taylor F B, Jr, Chang A, Ferrell G, Mather T, Catlett R, Blick K, Esmon C T. C4b-binding protein exacerbates the host response to E. coli. Blood. 1991;78:357–363. [PubMed] [Google Scholar]
- 30.Thijs L, Hack C. Role of the complement cascade in severe sepsis. In: Lamy M, Thijs L, editors. Mediators of sepsis. Berlin, Germany: Springer; 1992. pp. 78–98. [Google Scholar]
- 31.Vedder N B, Winn R K, Rice C L, Harlan J M. Neutrophil-mediated vascular injury in shock and multiple organ failure. Prog Clin Biol Res. 1989;299:181–191. [PubMed] [Google Scholar]
- 32.Wang X, Andersson R. The role of endothelial cells in the systemic inflammatory response syndrome and multiple system organ failure. Eur J Surg. 1995;161:703–713. [PubMed] [Google Scholar]
- 33.Wolbink G J, Bollen J, Baars J W, ten Berge R J, Swaak A J, Paardekooper J, Hack C E. Application of a monoclonal antibody against a neoepitope on activated C4 in an ELISA for the quantification of complement activation via the classical pathway. J Immunol Methods. 1993;163:67–76. doi: 10.1016/0022-1759(93)90240-8. [DOI] [PubMed] [Google Scholar]
- 34.Wolbink G J, Brouwer M C, Buysmann S, ten Berge I J, Hack C E. CRP-mediated activation of complement in vivo: assessment by measuring circulating complement-C-reactive protein complexes. J Immunol. 1996;157:473–479. [PubMed] [Google Scholar]
- 35.Wolbink G J, Schalkwijk C, Baars J, Wagstaff W J, Van den Bosch H, Hack C E. Therapy with interleukin-2 induces the systemic release of phospholipase-A2. Cancer Immunol Immunother. 1995;41:287–292. doi: 10.1007/BF01517216. [DOI] [PMC free article] [PubMed] [Google Scholar]