Summary
Atrial fibrillation (AF) is often associated with limiting symptoms, and with significant impairment in quality of life. As such, treatment strategies aimed at symptom control form an important pillar of AF management. Such treatments include a wide variety of drugs and interventions, including, increasingly, catheter ablation. These strategies can be utilised either singly or in combination, to improve and restore quality of life for patients, and this review covers the current evidence base underpinning their use. In this Review, we discuss the pros and cons of rate vs. rhythm control, while offering practical tips to non-specialists on how to utilise various treatments and counsel patients about all relevant treatment options. These include antiarrhythmic and rate control medications, as well as interventions such as cardioversion, catheter ablation, and pace-and-ablate.
Keywords: Atrial fibrillation, Rate control, Rhythm control, Catheter ablation
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed with the search terms “atrial fibrillation”, “symptom relief”, “rate control”, and “rhythm control” from 1995 until April 2023. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this review.
Key Messages.
-
•
Atrial Fibrillation is often associated with limiting symptoms and with significant impairment in quality of life.
-
•
Ventricular rate control with drugs may sometimes be sufficient to improve symptoms, especially in the elderly and/or people with limited mobility.
-
•
Rhythm control options include cardioversion, anti-arrhythmic drugs, and catheter ablation.
-
•
Catheter ablation has been shown to the intervention with the largest impact on symptoms and quality of life, and is increasingly used earlier in management.
Introduction
Atrial fibrillation (AF), whilst sometimes asymptomatic, may cause significant symptoms in many patients. These include fatigue, breathlessness, palpitations and pre-syncope. The “B” component of the guideline-recommended Atrial Fibrillation Better Care (“ABC”) pathway relates to “Better Symptom Control”.1 In this part of the clinical series on AF, we discuss the management AF symptom burden.
When deciding how to manage a patient’s symptoms, first, underlying risk factors and comorbidities possibly associated with symptoms warrant identification and start of treatment, if indicated. Second, it is necessary to consider a ‘rate control’ and/or ‘rhythm control’ strategy. The former means accepting the state of recurrent episodes of AF, or the presence of permanent AF and focussing on controlling the heart rate. The latter means attempting to restore and maintain sinus rhythm. Either strategy carries benefits and risks, which we will discuss here.
Haemodynamics and symptomatology of atrial fibrillation
AF has two main haemodynamic consequences that contribute to symptom burden and impaired quality of life.2 Firstly, AF leads to failure of normal functioning of the atria as reservoirs, conduits and pumping chambers. Secondly, rapidly conducting atrial impulses result in a fast and irregular ventricular rate, causing palpitations. In the long term, this may cause or exacerbate heart failure, and may induce microcirculatory flow abnormalities, resulting in ischaemia.3
These haemodynamic consequences together lead to a reduction of cardiac output by 20–30%4 and are causally related to AF symptomatology. Those patients who are more dependent on atrial contribution to stroke volume—for instance, patients with impaired left ventricular function (heart failure with reduced ejection fraction [HFrEF]) or impaired diastolic function (hypertrophic cardiomyopathy or heart failure with preserved ejection fraction [HFpEF])—may experience higher symptom burden especially breathlessness, fatigue and rarely presyncope/syncope. AF is now the most common cardiovascular cause for urgent hospital admission in the western world. Rate and/or rhythm control strategies are pivotal in reducing AF-related symptoms.1,5 It is important to make a clear distinction between symptom burden and arrhythmia burden: the latter indicates the proportion of time a patient spends in AF, as assessed by either implantable ECG recorders or prolonged ambulatory monitoring.5 Whilst there is often close correlation between AF burden and symptom burden, this may not always be the case.6
Acute management of atrial fibrillation
The choice of therapy will depend on patient characteristics, presence of heart failure and other comorbidities, haemodynamic stability and patient preference. It is critical to remember that AF may be a consequence of another disease process, such as sepsis, ischemia or heart failure exacerbation. In this setting, treatment should be directed at the underlying cause, and AF should be treated with initially a focus on rate control. In those who are haemodynamically unstable as a result of AF, emergency rhythm control with direct current cardioversion (DCCV) should be considered.5
In acute-onset AF without haemodynamic compromise, some favour cardioversion—either electrical or pharmacological—whilst others favour rate control. Acute rate control has a rapid onset of action, dose-dependent effects, and is generally safe.7, 8, 9, 10, 11 Acute cardioversion is more controversial. Although usually successful, studies show that AF spontaneously terminates within 48 h of onset in up to 70% of cases.12 For this reason, acute rate control with early follow-up and, if necessary, referral for non-urgent cardioversion may be preferable.
Shared decision-making is important, as some patients with severe symptoms may prefer upfront cardioversion, whilst others may prefer the delayed approach. Appropriate anticoagulation should always be instituted, irrespective of the strategy chosen and irrespective of the pattern of AF depending on the CHA2DS2Vasc score.
Rhythm control of atrial fibrillation
The goal of rhythm control is to restore and maintain sinus rhythm, which may improve symptoms and quality of life. This approach may involve a combination of approaches, including cardioversion, use of antiarrhythmic drugs (AADs) and catheter ablation.5
Pursuing restoration of sinus rhythm is best evidenced for those with symptomatic, ideally paroxysmal, AF. For such individuals, rhythm control carries a class 1A recommendation for improving symptoms and quality of life in international guidelines.5
The benefit of a rhythm control strategy beyond symptom improvement is less well established, and is an area of ongoing research. It is recognised that AF is a progressive disease,13 and progression from paroxysmal to persistent and permanent AF confers a worse prognosis.14 Over time, atrial enlargement results in mitral and tricuspid annular stretch, leading to significant AV valvular regurgitation. This, along with deterioration of left ventricular function secondary to fast and/or irregular rates, can lead to heart failure.
Furthermore, AF is associated with cognitive decline and dementia, due to a mixture of cerebral hypoperfusion, silent infarcts and microbleeds.15 Several studies show improvements in cerebral blood flow and reduction in cognitive decline associated with AF rhythm control.15,16
In contrast to the older AFFIRM and AF-CHF trials, the more recent EAST AF-NET 4 trial indicated that early rhythm-control therapy was associated with a lower risk of adverse cardiovascular outcomes in patients with recent AF (diagnosis ≤1 year ago) and cardiovascular conditions.17, 18, 19 Hence early rhythm control is emerging as a prognostically beneficial approach.5,17,20
Although the evidence base remains incomplete, some factors suggest that first line rhythm control may be appropriate for patients. Those with recent onset, paroxysmal AF, particularly with adverse symptoms, are most likely to benefit. However, apparently asymptomatic patients may also benefit from a trial of sinus rhythm restoration, as this may bring out unrecognised symptoms.5,21
It should be recognised that not all patients will be suitable for a rhythm control approach. For example, it may be difficult to restore and maintain sinus rhythm in a morbidly obese patient, where an initial focus on lifestyle modification may be more appropriate.
Rhythm control—therapeutic options
The options for restoring sinus rhythm include, in isolation or in combination, electrical cardioversion, AADs and catheter ablation.
AADs approximately double the likelihood of maintaining sinus rhythm compared with no rhythm-control therapy.20 Several AADs are available, with different mechanisms of action and varying efficacy and safety profiles. These medications are typically classified according to their mechanism of action (Vaughan-Williams classification) although many act through several mechanisms.22 The choice of AAD for rhythm control on AF should be individualised based on factors such as underlying cardiac disease, comorbidities, age, drug interactions, local availability of interventions, and treatment preferences.
It should be kept in mind that meta-analyses on AADs use (even those published in recent years) mainly include trials performed 2–4 decades ago.23 A more cautious and rational use of AADs is appropriate in current practice for patients with structural heart disease. This is reflected in the recent EAST AF-NET 4 trial clearly indicating a benefit on hard clinical endpoints with rhythm control, which was mainly constituted of AADs use.17
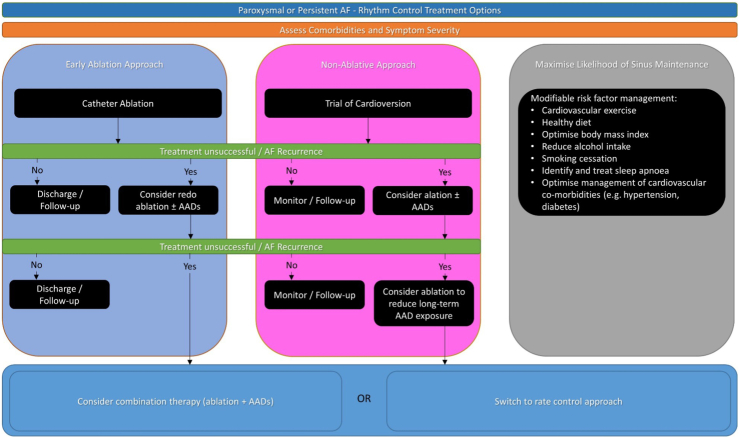
In general, AADs tend to be more toxic than rate control drugs, and long-term exposure may not be desirable for the majority of patients. Careful monitoring, along with patient education, should be undertaken. Catheter ablation offers an effective alternative to restore sinus rhythm, taking a small up-front risk of an invasive procedure while potentially removing the long-term adverse effects of AAD exposure. The decision on which therapy to pursue should, as always, be individualised along with shared decision making. An approach to rhythm control is summarised in Fig. 1.
Fig. 1.
Rhythm control of atrial fibrillation.
Cardioversion
Acute pharmacological cardioversion, for example with intravenous flecainide or vernakalant, may be an option in some individuals. This carries the benefit of not needing the deep sedation required for DCCV, but does carry a risk of medication-related side effects. An advantage of using intravenous flecainide is that, if successful and well-tolerated, it may be prescribed for subsequent pill-in-the-pocket oral use. It may also be contraindicated in some patients, such as those with significant LV dysfunction and or prolongation of the QT-interval. In the acute setting, clinicians should consider a wait and see approach as discussed earlier. In the event of haemodynamically unstable arrhythmia, emergency DCCV should be considered.
For those with persistent AF, elective DCCV is beneficial for assessment of symptoms in sinus rhythm. Those experiencing a great improvement, followed by a relapse when AF recurs, are most likely to benefit from an on-going rhythm control strategy. This also allows assessment of unnoticed symptoms, as some patients unconsciously adapt to their AF without realising that they are more breathless or fatigued than they were previously.
For elective outpatient DCCV, pre-treatment with AADs is an option, as this increases the chance of success and is generally well tolerated in the short-term.24 Maintenance of sinus rhythm by 3 months post-DCCV is associated with a significant improvement in quality of life.25
However, not every patient will be suitable for cardioversion. Some may have contraindications to drugs or DCCV. Others may simply prefer not to undergo cardioversion. Importantly, some patients may have adverse features that, despite being eligible, render success unlikely. Some of these are non-modifiable, such as age and sex, however others—including hypertension, obesity and alcohol excess—should be carefully addressed before if cardioversion—or indeed rhythm control as a whole—is planned.26
If AF recurs after cardioversion, repeat cardioversion may be considered.5 Alternatively, referral for catheter ablation may be appropriate if significant symptom benefit was observed during sinus rhythm.
Cardioversion is a very safe procedure overall, but strict anticoagulation pre- and post-procedure must be adhered to in order to minimise the risk of procedure-related stroke.5
Class Ic AADs—flecainide and propafenone
The class Ic AADs, which block sodium channels, are commonly utilised for rhythm control of AF. Due to their negative inotropic and pro-arrhythmic ventricular effects, they are contraindicated in HRrEF and ischaemic heart disease.22,23 However, they are highly effective for AF patients with structurally normal hearts.23,27,28 As these drugs block sodium channels, they must be avoided in those with sodium channelopathies such as Brugada syndrome.
In a meta-analysis of RCTs comparing flecainide or propafenone to placebo or no treatment, the risk of AF recurrence was significantly lower with Class Ic AADs (odds ratio [OR] 0.36, 95% confidence interval [CI] 0.28–0.45).23
The concurrent existence of atrial flutter (AFL) alongside AF should be considered when prescribing flecainide or propafenone. Class Ic drugs may organise AF into AFL, whilst also slowing the atrial rate enough to facilitate 1:1 AV conduction with very rapid heart rates and potentially haemodynamic instability.27 For this reason, they are preferably prescribed alongside beta-blockers or calcium channel blockers.
In suitable patients—for example, younger patients with structurally normal hearts and infrequent paroxysms of AF, class Ic drugs, particularly flecainide, can be utilised as a ‘pill-in-the-pocket’ approach after proven safety and efficacy in-hospital. This means that the patient avoids chronic administration and simply takes a dose when their AF occurs. This approach is generally successful and reduces episode duration, as well as hospitalisation.27
Class III AADs—amiodarone and dronedarone
Class III AADs, which block potassium channels, are less proarrhythmic and are highly effective in maintaining sinus rhythm.23 Amiodarone is considered the most effective AAD and is widely utilised in clinical practice.5 This is in part due to its pleiotropic effects—whilst considered a class III drug due to predominant potassium blockade, it also affects sodium, alpha, beta and calcium channels. However, it has well-recognised side-effects, and long-term use may be associated with thyroid dysfunction, liver toxicity and lung fibrosis.
In general, amiodarone is therefore preferred for short-term use. As mentioned above, it may be used as pre-treatment for DCCV to improve success rates. It may also be useful to supress AF-related symptoms whilst a patient awaits more definitive treatment with catheter ablation. Long-term use may be beneficial in selected patients, such as elderly frail people with symptomatic AF who would not be suitable for alternative therapies. In such situations, the benefits of maintaining sinus rhythm likely outweigh the risks of long-term extra cardiac side effects.
Dronedarone was developed as an alternative to amiodarone, specifically lacking the iodine affinity and lipophilicity of its predecessor.29 Dronedarone demonstrated efficacy, as well as a reduction in hard endpoints such as hospitalisation and death in large RCTs.29 However, it is less effective than amiodarone,30 and it is contraindicated in heart failure, due to an observed increase in adverse clinical outcomes trials,29,31 which limits its applicability in real-world practice. It may be effective for appropriately selected patients.32
Sotalol
Sotalol is a Class II (beta-blocker) drug by definition, but has additional class III effects at doses >160 mg daily5 and is effective at maintaining sinus rhythm in RCTs.23 As Sotalol lacks the pleiotropic effects of amiodarone, it may result in QT-prolongation with associated ventricular pro-arrhythmia, and is contraindicated in several conditions such as HFrEF, asthma and significant renal failure.5 Sotalol has been associated several years ago with an increased mortality signal,23,33 hence it is generally utilised with caution after other AADs have failed or are contraindicated.
In an RCT comparing amiodarone to sotalol and placebo in patients with persistent AF, the rate of conversion to sinus rhythm was significantly higher in the amiodarone and sotalol groups than with placebo (27% vs. 24% vs. 1%, p < 0.001).34 Amiodarone was superior to sotalol for maintaining sinus rhythm, but both drugs had similar efficacy in patients with ischemic heart disease.
Catheter ablation
Catheter ablation has emerged as a cornerstone of rhythm control therapy for AF. It has long been recognised that ectopy from the pulmonary veins (PVs) is frequently the underlying trigger of AF. Preventing these triggers from entering the left atrium (LA), by pulmonary vein isolation (PVI), is highly efficacious in preventing AF if the atria are not severely remodelled. Indeed, evidence shows that catheter ablation outperforms AADs, including amiodarone.35, 36, 37, 38, 39, 40
Ablation carries up-front procedural risks, such as vascular injury, tamponade and stroke—some of which may be life-threatening. However, the overall safety profile of ablation procedures is well demonstrated, and the benefit of withdrawing potentially toxic AADs post-procedure generally outweighs such risks. A 2021 meta-analysis demonstrated the superiority of ablation vs. AADs for suppressing arrhythmia (RR 0.64; p < 0.01), with an associated reduction in healthcare utilisation and similar safety outcomes.41
A recent study including over 37,000 patients from 2008 to 2017 demonstrated the improving safety profile of catheter ablation.42 Across this timeframe, overall complications decreased from 7.5% to 5%, with 30-day mortality occurring in less than 1 in 850 patients. It is expected that this trend will have continued into the modern era; indeed, a recently published pooled analysis showed a decrease in complications from 5.3% to 3.8% from 2013 to 2022.43
Furthermore, the recent advent of Pulsed Field Ablation (PFA) may be the most important advance in the field of AF ablation in recent decades. PFA allows rapid isolation of the pulmonary veins, with a superior safety profile, since it is highly cardioselective.44, 45, 46 The MANIFEST-PF survey found that PFA procedures take around an hour on average, with no incidences of oesophageal or phrenic nerve injury across 1758 patients and a major complication rate of just 1.6%.45
Catheter ablation has been proven to result in significant improvements in quality of life (QOL).47 Data from the VISTAX study in patients with paroxysmal AF showed a large improvement in AFEQT score (a validated AF-related quality of life questionnaire) from 61.3 to 87.2 (p < 0.001).6 The same study also showed a substantial improvement in 4 of 5 domains of the EQ-5D-5L questionnaire. Furthermore, QOL improvement was inversely related to the residual AF burden following ablation, arguing against a significant placebo effect. In the setting of heart failure, significant quality of life benefits have also been demonstrated.48
Aside from controlling symptoms, ablation may also provide prognostic benefit. This particularly may apply to certain patients with heart failure.49 A recent meta-analysis including 3598 HFrEF patients compared medical (rate or rhythm) therapy against catheter ablation.50 Ablation resulted in a significant improvement in all-cause mortality (OR 0.51; p = 0.0003), rehospitalisation (OR 0.44; p = 0.003), and a trend towards reduced stroke risk (OR 0.59; p = 0.27). LVEF also improved more in the ablation arm (+6.8%; p = 0.0004), as did quality of life. Where heart failure is caused by a rapid irregular ventricular rhythm—so-called tachycardiomyopathy—international guidelines recommend catheter ablation as first-line therapy.5
Overall, catheter ablation has become significantly more successful and safe procedure, with an increasing role as first-line therapy for AF in paroxysmal AF. There is still room for improvement, however. Success rates are lower for persistent AF than in paroxysmal AF. This is largely because, as AF progresses, atrial remodelling, i.e., atrial cardiomyopathy, worsens and non-PV triggers may become more prevalent, setting the stage for AF to persist. The evidence supporting ablation of non-PV triggers or creating extra lines is variable51—clearly, the bulk of benefit is obtained by isolating the PVs in atria without severe atrial cardiomyopathy. It is hoped that on-going research into ablation will find ways to improve outcomes in such settings. Furthermore, as ablation is an invasive procedure, some patients may prefer to avoid this and opt for tablet-based therapy instead. This emphasises the importance of shared decision making.
Rate control of atrial fibrillation
Long term rate control may be pursued in many patients, especially where rhythm control is contraindicated, considered futile or has proved to be unsuccessful. In many cases effective rate control is sufficient to improve AF-related symptoms.5 Rate control is easy to institute and manage, and is associated with a low risk of treatment-related adverse events and AF-related hospital admissions.2
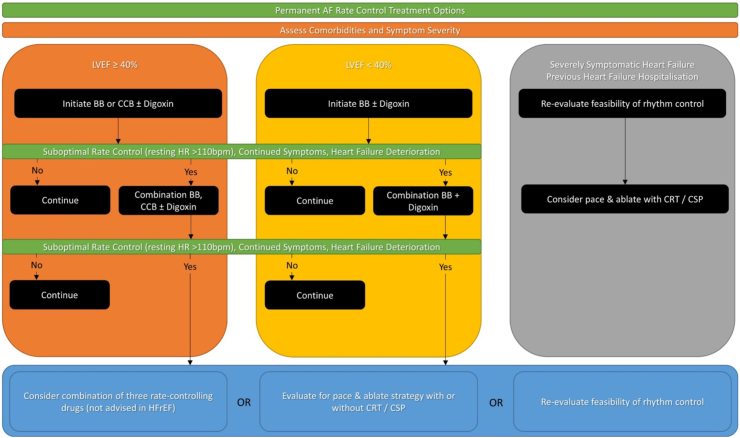
Long-term rate control therapy may be used as an adjunct to rhythm control, or as sole treatment strategy in those who did not require restoration of sinus rhythm, those who fail rhythm control, and those where the risks of a rhythm control strategy outweigh the benefits.2 Periodic evaluation and reassessment is required in order to optimise symptom management (Fig. 2).
Fig. 2.
Rate control of atrial fibrillation.
Rate control as first line therapy
Rate control can be instituted as first choice treatment. This may be appropriate in patients who are unlikely to benefit from sinus rhythm restoration—for example, elderly sedentary patients with minimal AF-related symptoms. Alternatively, this strategy may be appropriate if rhythm control is unlikely to work—for example, patients with longstanding persistent AF and severe atrial enlargement.
Target heart rate
In the RACE II trial in patients with permanent AF, there was no difference between strict and lenient heart rate control for a composite of clinical events, NYHA class or hospitalisation (strict: target heart rate <80 beats per minute (bpm) at rest and <110 bpm during moderate exercise; lenient: target heart rate <110 bpm).52,53 Similar results were found in post-hoc analysis of AFFIRM and RACE.54
In many patients, lenient rate control is a good starting point. However, when symptoms remain or heart failure is suspected, stricter rate control targets are needed. It is also important to consider the potential effects of dysregulated cerebral blood flow, which may increase the risk of dementia in patients with AF.55
Rate control—therapeutic options
Pharmacological rate control options include beta-blockers, rate-limiting calcium channel blockers (CCBs), digoxin or combination therapy. Some antiarrhythmic drugs also have rate-limiting properties (e.g., amiodarone, dronedarone, sotalol) but are generally instituted for rhythm control purposes.2 For this reason, addition of a rate control drug may not be needed when using amiodarone, dronedarone, sotalol for rhythm control. The choice of rate control drugs depends on symptom burden, presence of comorbidities (e.g., heart failure) and potential side-effects and interactions.
Non-pharmacological rate control can also be achieved with the “pace-and-ablate” strategy, consisting of implantation of a permanent pacemaker followed by atrioventricular node ablation.
Beta-blockers
Beta-blockers antagonise sympathetic beta1-receptor activity in the atrioventricular node and thus slow the ventricular rate.2 Beta-blockers are most frequently used as rate-controlling agents due to their rapid onset of action, dose-dependent effect and safety profile.7
Beta-blockers are also beneficial in patients with HFrEF,56 which frequently co-exists with AF; however, the prognostic benefit of beta-blockers in HFrEF seems less apparent or even absent in patients with AF.57,58 Beta-blockers may, however, be preferable in HFrEF over other options, due to the negative inotropic effects of drugs such as verapamil, and potential adverse effects of digoxin.59, 60, 61
In selected patients who are haemodynamically unstable or with severely impaired LVEF, intravenous esmolol can be considered.62
Rate-limiting calcium channel blockers
Verapamil and diltiazem are non-dihydropyridine CCBs. They function by increasing the refractory period of the atrioventricular node, thus slowing conduction and thereby heart rate.2 Because of their rapid onset of action and dose-dependent effect,7, 8, 9, 10, 11 verapamil and diltiazem can be used in both acute and long-term settings.
CCBs provide effective rate control, potentially even more so than beta-blockers.63 Due to their differing mechanisms, they may be a useful option for those experiencing side effects from beta-blockers.64 Verapamil and diltiazem may also lead to better exercise capacity than beta-blockers, and may be therefore a better option in physically active patients.65,66 However, verapamil is contraindicated in HFrEF due to negative inotropic effects.5
Digoxin
Digoxin is a cardiac glycoside that inhibits the sodium-potassium ATPase and augments parasympathetic tone. Presumably through tonic increase in parasympathetic activity, digoxin reduces atrioventricular conductance.2 Digoxin may not be as effective when patients have a high sympathetic drive (i.e., during physical activity, or in critically ill patients). In selected patients who are haemodynamically unstable or with severely impaired LVEF, intravenous digoxin can be used. Digoxin is renally excreted, has a narrow therapeutic range and some common drug interactions (e.g., with verapamil or antibiotics). Therefore, careful use with periodic evaluation and monitoring of serum levels is suggested.
In RCTs, there is no association between the use of digoxin and any increase or decrease in all-cause mortality, and lower dosages of digoxin may be associated with improved prognosis.67 In the open-label RATE-AF (Rate Control Therapy Evaluation in Permanent Atrial Fibrillation) trial in patients with symptomatic permanent AF, there was no difference in patient-reported quality of life outcomes at 6 months between low-dose digoxin and bisoprolol, but fewer adverse effects were reported with digoxin, and a greater improvement in modified EHRA and NYHA classes was seen with digoxin.68
Combined pharmacologic therapies for rate control
Combinations of different rate controlling drugs are indicated only when needed to achieve the target heart rate or to better control symptoms, though studies assessing every possible combination are lacking. Most often combination therapy with digoxin is used in both acute and long-term settings.69 Careful follow-up to avoid bradycardia is advised. Combining beta-blockers with CCBs should only be performed in secondary care with careful monitoring given the high risk of severe bradycardia or hypotension.
Pace-and-ablate
Permanent pacemaker implant in combination with ablation of the atrioventricular node has the advantage of controlling and regularising heart rate in patients with AF. Whilst this reduces the ventricular consequences of AF, the atria are left unchanged and so the lack of atrial transport persists. Furthermore, ventricular dyssynchrony caused by ventricular pacing may have an adverse impact on stroke volume in some patients.70
The procedure is relatively simple and has a low complication rate and low medium-term mortality risk.71, 72, 73, 74 The pace-and-ablate strategy improves AF-related symptoms and most often does not worsen LV function.75 On the contrary, it may improve LVEF in patients with tachycardiomyopathy.76, 77, 78
However, most pace-and-ablate studies include only older patients, and long-term follow-up data are lacking. In particular, concerns about potential cumulative lifelong complications associated with indwelling hardware need to be carefully considered. Therefore, for younger patients, this is only an option if all other approaches, including rhythm control, have been carefully considered and/or exhausted.
The choice of device (right ventricular, biventricular or conduction system pacing) is dependent on patient characteristics, presence of heart failure and LVEF.79, 80, 81 In patient with pre-existing biventricular or conduction system pacing devices for heart failure, 100% pacing is preferable to maximise benefit.82 AF reduces effective pacing in this setting, and atrioventricular node ablation, as compared with pharmacological rate control, has been associated with improvements in all-cause mortality, cardiovascular mortality, and NYHA class.49,83
Before undertaking pace-and-ablate, it is worth giving consideration to rhythm control, especially in those with HFrEF. It may be that patients in whom sinus rhythm can feasibly be restored and maintained may have better outcomes than those undergoing pace-and-ablate.49,84
Table 1 summarises the various approaches to both rate and rhythm control discussed in this review.
Table 1.
Therapeutic options for rate and rhythm control of atrial fibrillation.
| Therapy | Rhythm | Rate | Comments |
|---|---|---|---|
| Beta-blockers | ✓ | Often first line, good safety profile. Caution in asthma and acute heart failure. | |
| Non-DHP calcium-channel blockers | ✓ | Contraindicated in HFrEF. | |
| Digoxin | ✓ | Narrow therapeutic range—monitor serum levels. | |
| Class I AADs | ✓ | Monitor QRS (class IC) or QT-interval (class IA). Contraindicated in HFrEF. Consider pill-in-the-pocket approach in selected patients. | |
| Class III AADs | ✓ | ✓ | Avoid in long-term due to potentially toxic side effects, especially for amiodarone. Useful for pre-treatment of DCCV. Monitor QT interval. |
| Cardioversion | ✓ | Generally safe. Consider in persistent AF to assess symptom status in sinus rhythm and guide further management. | |
| Catheter ablation | ✓ | The most effective rhythm control approach. Especially beneficial in heart failure/tachycardiomyopathy. Note upfront procedural risks. Patient preference is very important. | |
| Pace & ablate | ✓ | Generally last line therapy, but essentially guarantees rate control and ventricular regularity. Renders patient dependent upon pacemaker—hence ideally avoid in younger patients due to infection risk with repeated box changes. |
AAD, anti-arrhythmic drug; AF, atrial fibrillation; DCCV, direct current cardioversion; DHP, dihydropyridine; HFrEF, heart failure with reduced ejection fraction.
Conclusion
AF may cause adverse symptoms and haemodynamic compromise due to loss of atrial transport and rapid, irregular ventricular contraction. Treatment of AF-related symptoms consists of rate and/or rhythm control. In general, rhythm control is best applied to healthier, paroxysmal AF patients with significant symptom burden, and those with heart failure. In multi co-morbid patients with low likelihood of long-term sinus rhythm maintenance, rate control may be more appropriate.
Decisions on which strategy to pursue, and how to achieve the intended goals, should be made alongside the patient, taking into account co-morbidities, structural cardiac abnormalities, symptomatology, haemodynamic status and patient preference.
Contributors
All 4 authors (DG, MR, IvG, LF) contributed to this manuscript. DG prepared the Rhythm control section, and collated the different sections to prepare the manuscript. MR and IvG prepared the Rate control section and reviewed the collated manuscript, LF prepared the section on drugs and hybrid techniques and reviewed the collated manuscript.
Declaration of interests
DG reports: institutional research grants from Biosense Webster, Boston Scientific and Medtronic, and speaker fees from Boston Scientific.
References
- 1.Lip G.Y.H. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14(11):627–628. doi: 10.1038/nrcardio.2017.153. http://www.nature.com/articles/nrcardio.2017.153 Available from: [DOI] [PubMed] [Google Scholar]
- 2.Van Gelder I.C., Rienstra M., Crijns H.J.G.M., Olshansky B. Rate control in atrial fibrillation. Lancet. 2016;388(10046):818–828. doi: 10.1016/S0140-6736(16)31258-2. [DOI] [PubMed] [Google Scholar]
- 3.Goette A., Bukowska A., Dobrev D., et al. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur Heart J. 2009;30(11):1411–1420. doi: 10.1093/eurheartj/ehp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anselmino M., Scarsoglio S., Ridolfi L., De Ferrari G.M., Saglietto A. Insights from computational modeling on the potential hemodynamic effects of sinus rhythm versus atrial fibrillation. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.844275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the Europe. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. https://academic.oup.com/eurheartj/article-pdf/42/5/373/36414945/ehaa612.pdf Available from: [DOI] [PubMed] [Google Scholar]
- 6.Gupta D., Vijgen J., De Potter T., et al. Quality of life and healthcare utilisation improvements after atrial fibrillation ablation. Heart. 2021;107(16):1296–1302. doi: 10.1136/heartjnl-2020-318676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheuermeyer F.X., Grafstein E., Stenstrom R., et al. Safety and efficiency of calcium channel blockers versus beta-blockers for rate control in patients with atrial fibrillation and no acute underlying medical illness. Acad Emerg Med. 2013;20(3):222–230. doi: 10.1111/acem.12091. [DOI] [PubMed] [Google Scholar]
- 8.Tisdale J.E., Padhi I.D., Goldberg A.D., et al. A randomized, double-blind comparison of intravenous diltiazem and digoxin for atrial fibrillation after coronary artery bypass surgery. Am Heart J. 1998;135(5 Pt 1):739–747. doi: 10.1016/s0002-8703(98)70031-6. [DOI] [PubMed] [Google Scholar]
- 9.Schreck D.M., Rivera A.R., Tricarico V.J. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Ann Emerg Med. 1997;29(1):135–140. doi: 10.1016/s0196-0644(97)70319-6. [DOI] [PubMed] [Google Scholar]
- 10.Segal J.B., McNamara R.L., Miller M.R., et al. The evidence regarding the drugs used for ventricular rate control. J Fam Pract. 2000;49(1):47–59. [PubMed] [Google Scholar]
- 11.Siu C.W., Lau C.P., Lee W.L., Lam K.F., Tse H.F. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37(7):2174–2179. doi: 10.1097/CCM.0b013e3181a02f56. (quiz 2180) [DOI] [PubMed] [Google Scholar]
- 12.Pluymaekers N.A.H.A., Dudink E.A.M.P., Luermans J.G.L.M., et al. Early or delayed cardioversion in recent-onset atrial fibrillation. N Engl J Med. 2019;380(16):1499–1508. doi: 10.1056/NEJMoa1900353. http://www.ncbi.nlm.nih.gov/pubmed/30883054 Available from: [DOI] [PubMed] [Google Scholar]
- 13.Dudink E.A.M.P., Erküner Ö., Berg J., et al. The influence of progression of atrial fibrillation on quality of life: a report from the Euro Heart Survey. Europace. 2018;20(6):929–934. doi: 10.1093/europace/eux217. [DOI] [PubMed] [Google Scholar]
- 14.de Vos C.B., Pisters R., Nieuwlaat R., et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55(8):725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Calvert P., Gupta D., Lip G.Y.H. The neurocognitive effects of atrial fibrillation: benefits of the ABC pathway. Eur Heart J Cardiovasc Pharmacother. 2023;9(5):413–420. doi: 10.1093/ehjcvp/pvad021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunch T.J., Crandall B.G., Weiss J.P., et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22(8):839–845. doi: 10.1111/j.1540-8167.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhof P., Camm A.J., Goette A., et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 18.Wyse D.G., Waldo A.L., DiMarco J.P., et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 19.Roy D., Talajic M., Nattel S., et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 20.Camm A.J., Naccarelli G.V., Mittal S., et al. The increasing role of rhythm control in patients with atrial fibrillation: JACC State-of-the-art review. J Am Coll Cardiol. 2022;79(19):1932–1948. doi: 10.1016/j.jacc.2022.03.337. [DOI] [PubMed] [Google Scholar]
- 21.Arnar D.O., Mairesse G.H., Boriani G., et al. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS) Europace. 2019;21(6):844–845. doi: 10.1093/europace/euz046. [DOI] [PubMed] [Google Scholar]
- 22.Piccini J.P., Fauchier L. Rhythm control in atrial fibrillation. Lancet. 2016;388(10046):829–840. doi: 10.1016/S0140-6736(16)31277-6. [DOI] [PubMed] [Google Scholar]
- 23.Valembois L., Audureau E., Takeda A., Jarzebowski W., Belmin J., Lafuente-Lafuente C. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9(9) doi: 10.1002/14651858.CD005049.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Um K.J., McIntyre W.F., Healey J.S., et al. Pre- and post-treatment with amiodarone for elective electrical cardioversion of atrial fibrillation: a systematic review and meta-analysis. Europace. 2019;21(6):856–863. doi: 10.1093/europace/euy310. [DOI] [PubMed] [Google Scholar]
- 25.Sandhu R.K., Smigorowsky M., Lockwood E., Savu A., Kaul P., McAlister F.A. Impact of electrical cardioversion on quality of life for the treatment of atrial fibrillation. Can J Cardiol. 2017;33(4):450–455. doi: 10.1016/j.cjca.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Ecker V., Knoery C., Rushworth G., et al. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin Cardiol. 2018;41(6):862–870. doi: 10.1002/clc.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Echt D.S., Ruskin J.N. Use of flecainide for the treatment of atrial fibrillation. Am J Cardiol. 2020;125(7):1123–1133. doi: 10.1016/j.amjcard.2019.12.041. [DOI] [PubMed] [Google Scholar]
- 28.Sestito A., Molina E. Atrial fibrillation and the pharmacological treatment: the role of propafenone. Eur Rev Med Pharmacol Sci. 2012;16(2):242–253. [PubMed] [Google Scholar]
- 29.Heijman J., Hohnloser S.H., Camm A.J. Antiarrhythmic drugs for atrial fibrillation: lessons from the past and opportunities for the future. Europace. 2021;23(23 Suppl 2):ii14–ii22. doi: 10.1093/europace/euaa426. [DOI] [PubMed] [Google Scholar]
- 30.Le Heuzey J.Y., De Ferrari G.M., Radzik D., Santini M., Zhu J., Davy J.M. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21(6):597–605. doi: 10.1111/j.1540-8167.2010.01764.x. [DOI] [PubMed] [Google Scholar]
- 31.Køber L., Torp-Pedersen C., McMurray J.J.V., et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358(25):2678–2687. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 32.Khan M.H., Rochlani Y., Aronow W.S. Efficacy and safety of dronedarone in the treatment of patients with atrial fibrillation. Expert Opin Drug Saf. 2017;16(12):1407–1412. doi: 10.1080/14740338.2017.1387246. [DOI] [PubMed] [Google Scholar]
- 33.Pratt C.M., Camm A.J., Cooper W., et al. Mortality in the survival with ORal D-sotalol (SWORD) trial: why did patients die? Am J Cardiol. 1998;81(7):869–876. doi: 10.1016/s0002-9149(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 34.Singh B.N., Singh S.N., Reda D.J., et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352(18):1861–1872. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 35.Di Biase L., Mohanty P., Mohanty S., et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. http://www.ncbi.nlm.nih.gov/pubmed/27029350 Available from: [DOI] [PubMed] [Google Scholar]
- 36.Roufeida Belgaid D. Ablation vs. amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device. Cardiovasc Disord Med. 2016;1(3) doi: 10.15761/CDM.1000115. [DOI] [Google Scholar]
- 37.Kuniss M., Pavlovic N., Velagic V., et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. EP Europace. 2021;23(7):1033–1041. doi: 10.1093/europace/euab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poole J.E., Bahnson T.D., Monahan K.H., et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol. 2020;75(25):3105–3118. doi: 10.1016/j.jacc.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turagam M.K., Musikantow D., Whang W., et al. Assessment of catheter ablation or antiarrhythmic drugs for first-line therapy of atrial fibrillation. JAMA Cardiol. 2021;6(6):697. doi: 10.1001/jamacardio.2021.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razzack A.A., Lak H.M., Pothuru S., et al. Efficacy and safety of catheter ablation vs antiarrhythmic drugs as initial therapy for management of symptomatic paroxysmal atrial fibrillation: a meta-analysis. Rev Cardiovasc Med. 2022;23(3):112. doi: 10.31083/j.rcm2303112. [DOI] [PubMed] [Google Scholar]
- 41.Imberti J.F., Ding W.Y., Kotalczyk A., et al. Catheter ablation as first-line treatment for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Heart. 2021;107(20):1630–1636. doi: 10.1136/heartjnl-2021-319496. [DOI] [PubMed] [Google Scholar]
- 42.Ngo L., Ali A., Ganesan A., Woodman R., Adams R., Ranasinghe I. Ten-year trends in mortality and complications following catheter ablation of atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2022;8(4):398–408. doi: 10.1093/ehjqcco/qcab102. [DOI] [PubMed] [Google Scholar]
- 43.Benali K., Khairy P., Hammache N., et al. Procedure-related complications of catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2023;81(21):2089–2099. doi: 10.1016/j.jacc.2023.03.418. [DOI] [PubMed] [Google Scholar]
- 44.Reddy V.Y., Dukkipati S.R., Neuzil P., et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021;7(5):614–627. doi: 10.1016/j.jacep.2021.02.014. http://www.ncbi.nlm.nih.gov/pubmed/33933412 Available from: [DOI] [PubMed] [Google Scholar]
- 45.Ekanem E., Reddy V.Y., Schmidt B., et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF) EP Europace. 2022;24(8):1256–1266. doi: 10.1093/europace/euac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt B., Bordignon S., Tohoku S., et al. 5S study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol. 2022;15(6) doi: 10.1161/CIRCEP.121.010817. [DOI] [PubMed] [Google Scholar]
- 47.Mark D.B., Anstrom K.J., Sheng S., et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1275–1285. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parkash R., Wells G.A., Rouleau J., et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation. 2022;145(23):1693–1704. doi: 10.1161/CIRCULATIONAHA.121.057095. [DOI] [PubMed] [Google Scholar]
- 49.Calvert P., Farinha J.M., Gupta D., Kahn M., Proietti R., Lip G.Y.H. A comparison of medical therapy and ablation for atrial fibrillation in patients with heart failure. Expert Rev Cardiovasc Ther. 2022;20(3):169–183. doi: 10.1080/14779072.2022.2050695. [DOI] [PubMed] [Google Scholar]
- 50.Chen S., Pürerfellner H., Meyer C., et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J. 2020;41(30):2863–2873. doi: 10.1093/eurheartj/ehz443. http://www.ncbi.nlm.nih.gov/pubmed/31298266 Available from: [DOI] [PubMed] [Google Scholar]
- 51.Calvert P., Lip G.Y.H., Gupta D. Radiofrequency catheter ablation of atrial fibrillation: a review of techniques. Trends Cardiovasc Med. 2022;33(7):405–415. doi: 10.1016/j.tcm.2022.04.002. http://www.ncbi.nlm.nih.gov/pubmed/35421538 Available from: [DOI] [PubMed] [Google Scholar]
- 52.Groenveld H.F., Crijns H.J.G.M., Van den Berg M.P., et al. The effect of rate control on quality of life in patients with permanent atrial fibrillation: data from the RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation II) study. J Am Coll Cardiol. 2011;58(17):1795–1803. doi: 10.1016/j.jacc.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 53.Van Gelder I.C., Groenveld H.F., Crijns H.J.G.M., et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362(15):1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 54.Van Gelder I.C., Wyse D.G., Chandler M.L., et al. Does intensity of rate-control influence outcome in atrial fibrillation? An analysis of pooled data from the RACE and AFFIRM studies. Europace. 2006;8(11):935–942. doi: 10.1093/europace/eul106. [DOI] [PubMed] [Google Scholar]
- 55.Papanastasiou C.A., Theochari C.A., Zareifopoulos N., et al. Atrial fibrillation is associated with cognitive impairment, all-cause dementia, vascular dementia, and Alzheimer’s disease: a systematic review and meta-analysis. J Gen Intern Med. 2021;36(10):3122–3135. doi: 10.1007/s11606-021-06954-8. http://www.ncbi.nlm.nih.gov/pubmed/34244959 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. https://academic.oup.com/eurheartj/article/42/36/3599/6358045 Available from: [DOI] [PubMed] [Google Scholar]
- 57.Kotecha D., Holmes J., Krum H., et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384(9961):2235–2243. doi: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 58.Rienstra M., Damman K., Mulder B.A., Van Gelder I.C., McMurray J.J.V., Van Veldhuisen D.J. Beta-blockers and outcome in heart failure and atrial fibrillation: a meta-analysis. JACC Heart Fail. 2013;1(1):21–28. doi: 10.1016/j.jchf.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Washam J.B., Stevens S.R., Lokhnygina Y., et al. Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the rivaroxaban once daily oral direct factor xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF) Lancet. 2015;385(9985):2363–2370. doi: 10.1016/S0140-6736(14)61836-5. [DOI] [PubMed] [Google Scholar]
- 60.Osman M.H., Farrag E., Selim M., Osman M.S., Hasanine A., Selim A. Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wassertheil-Smoller S., McGinn A.P., Martin L., Rodriguez B.L., Stefanick M.L., Perez M. The associations of atrial fibrillation with the risks of incident invasive breast and colorectal cancer. Am J Epidemiol. 2017;185(5):372–384. doi: 10.1093/aje/kww185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imamura T., Kinugawa K. Novel rate control strategy with landiolol in patients with cardiac dysfunction and atrial fibrillation. ESC Heart Fail. 2020;7(5):2208–2213. doi: 10.1002/ehf2.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulimoen S.R., Enger S., Carlson J., et al. Comparison of four single-drug regimens on ventricular rate and arrhythmia-related symptoms in patients with permanent atrial fibrillation. Am J Cardiol. 2013;111(2):225–230. doi: 10.1016/j.amjcard.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Koldenhof T., Wijtvliet P.E.P.J., Pluymaekers N.A.H.A., et al. Rate control drugs differ in the prevention of progression of atrial fibrillation. Europace. 2022;24(3):384–389. doi: 10.1093/europace/euab191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Figulla H.R., Gietzen F., Zeymer U., et al. Diltiazem improves cardiac function and exercise capacity in patients with idiopathic dilated cardiomyopathy. Results of the diltiazem in dilated cardiomyopathy trial. Circulation. 1996;94(3):346–352. doi: 10.1161/01.cir.94.3.346. [DOI] [PubMed] [Google Scholar]
- 66.Ulimoen S.R., Enger S., Pripp A.H., et al. Calcium channel blockers improve exercise capacity and reduce N-terminal Pro-B-type natriuretic peptide levels compared with beta-blockers in patients with permanent atrial fibrillation. Eur Heart J. 2014;35(8):517–524. doi: 10.1093/eurheartj/eht429. [DOI] [PubMed] [Google Scholar]
- 67.Ziff O.J., Lane D.A., Samra M., et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351 doi: 10.1136/bmj.h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kotecha D., Bunting K.V., Gill S.K., et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA. 2020;324(24):2497–2508. doi: 10.1001/jama.2020.23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darby A.E., Dimarco J.P. Management of atrial fibrillation in patients with structural heart disease. Circulation. 2012;125(7):945–957. doi: 10.1161/CIRCULATIONAHA.111.019935. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt M., Brömsen J., Herholz C., et al. Evidence of left ventricular dyssynchrony resulting from right ventricular pacing in patients with severely depressed left ventricular ejection fraction. EP Europace. 2007;9(1):34–40. doi: 10.1093/europace/eul131. [DOI] [PubMed] [Google Scholar]
- 71.Wang R.X., Lee H.C., Hodge D.O., et al. Effect of pacing method on risk of sudden death after atrioventricular node ablation and pacemaker implantation in patients with atrial fibrillation. Heart Rhythm. 2013;10(5):696–701. doi: 10.1016/j.hrthm.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 72.Queiroga A., Marshall H.J., Clune M., Gammage M.D. Ablate and pace revisited: long term survival and predictors of permanent atrial fibrillation. Heart. 2003;89(9):1035–1038. doi: 10.1136/heart.89.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geelen P., Brugada J., Andries E., Brugada P. Ventricular fibrillation and sudden death after radiofrequency catheter ablation of the atrioventricular junction. Pacing Clin Electrophysiol. 1997;20(2 Pt 1):343–348. doi: 10.1111/j.1540-8159.1997.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 74.Lim K.T., Davis M.J.E., Powell A., et al. Ablate and pace strategy for atrial fibrillation: long-term outcome of AIRCRAFT trial. Europace. 2007;9(7):498–505. doi: 10.1093/europace/eum091. [DOI] [PubMed] [Google Scholar]
- 75.Chatterjee N.A., Upadhyay G.A., Ellenbogen K.A., McAlister F.A., Choudhry N.K., Singh J.P. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis and systematic review. Circ Arrhythm Electrophysiol. 2012;5(1):68–76. doi: 10.1161/CIRCEP.111.967810. [DOI] [PubMed] [Google Scholar]
- 76.Ozcan C., Jahangir A., Friedman P.A., et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N Engl J Med. 2001;344(14):1043–1051. doi: 10.1056/NEJM200104053441403. [DOI] [PubMed] [Google Scholar]
- 77.Bradley D.J., Shen W.K. Overview of management of atrial fibrillation in symptomatic elderly patients: pharmacologic therapy versus AV node ablation. Clin Pharmacol Ther. 2007;81(2):284–287. doi: 10.1038/sj.clpt.6100062. [DOI] [PubMed] [Google Scholar]
- 78.Wood M.A., Brown-Mahoney C., Kay G.N., Ellenbogen K.A. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation. 2000;101(10):1138–1144. doi: 10.1161/01.cir.101.10.1138. [DOI] [PubMed] [Google Scholar]
- 79.Chatterjee N.A., Upadhyay G.A., Ellenbogen K.A., Hayes D.L., Singh J.P. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis of biventricular vs. right ventricular pacing mode. Eur J Heart Fail. 2012;14(6):661–667. doi: 10.1093/eurjhf/hfs036. [DOI] [PubMed] [Google Scholar]
- 80.Brignole M., Pokushalov E., Pentimalli F., et al. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. 2018;39(45):3999–4008. doi: 10.1093/eurheartj/ehy555. [DOI] [PubMed] [Google Scholar]
- 81.Brignole M., Pentimalli F., Palmisano P., et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J. 2021;42(46):4731–4739. doi: 10.1093/eurheartj/ehab569/6358077. [DOI] [PubMed] [Google Scholar]
- 82.Glikson M., Nielsen J.C., Kronborg M.B., et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42(35):3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 83.Ganesan A.N., Brooks A.G., Roberts-Thomson K.C., Lau D.H., Kalman J.M., Sanders P. Role of AV nodal ablation in cardiac resynchronization in patients with coexistent atrial fibrillation and heart failure a systematic review. J Am Coll Cardiol. 2012;59(8):719–726. doi: 10.1016/j.jacc.2011.10.891. http://www.ncbi.nlm.nih.gov/pubmed/22340263 Available from: [DOI] [PubMed] [Google Scholar]
- 84.Khan M.N., Jaïs P., Cummings J., et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778–1785. doi: 10.1056/NEJMoa0708234. http://www.ncbi.nlm.nih.gov/pubmed/18946063 Available from: [DOI] [PubMed] [Google Scholar]