Abstract
Climate change is driving extreme changes to the environment, posing substantial threats to global food security and bioenergy. Given the direct role of plant roots in mediating plant-environment interactions, engineering the form and function of root systems and their associated microbiota may mitigate these effects. Synthetic genetic circuits have enabled sophisticated control of gene expression in microbial systems for years and a surge of advances has heralded the extension of this approach to multicellular plant species. Targeting these tools to affect root structure, exudation, and microbe activity on root surfaces provide multiple strategies for the advancement of climate-ready crops.
Subject terms: Molecular engineering in plants, Plant physiology, Rhizobial symbiosis, Synthetic biology
Engineering the form and function of root systems and their associated microbiota could provide a means to mitigate adverse climate-driven effects. Here, the authors review the recent developments in plant and rhizobacterial synthetic biology and highlight engineering targets for applications in root systems and rhizosphere.
Introduction
In plants, the leap from genetic manipulation to genetic engineering will require knowledge of endogenous regulatory mechanisms and the establishment of precise molecular tools to modulate gene expression across time, space, and magnitude. Developing such control over gene regulation will unlock the potential of our crops to become more resilient in extreme weather and to rebalance carbon ratios between the atmosphere and global soils–the predominant terrestrial repository of carbon and de facto plant growth medium. Nonetheless, the absence of advanced genetic tools capable of reliably and predictably altering plant structure and functionality has impeded progress in this innovative field. In contrast, bacterial engineering benefits from a suite of well-characterized tools for both design and genetic manipulation. Naturally isolated root colonizing bacteria, known as rhizobacteria, can influence host plant immunity and development through the synthesis of plant hormones, and provide key services such as pathogen biocontrol and nutrient synthesis/solubilization. These traits can be transferred, tailored, and enhanced through synthetic biology approaches to create novel strains for improving crop resilience and soil carbon storage.
As they are the primary biological interface with soil, roots are a clear engineering target to sequester atmospheric carbon and fortify crops against stress. The functionality of roots is governed by the architectural layout of the root branches that dictates the extent of soil explored, the tissue types that express transporters to facilitate nutrient and water absorption, and the complex milieu of metabolites exuded that mediate interactions with soil microorganisms1. The integration of abiotic and biotic triggers on developmental and physiological responses allow for plasticity in root function that can manifest in diverse topologies, biochemical activity, and microbial profiles across individual plants, making each root system unique. Synthetic biology provides a potentially powerful approach to untangle and optimize the different processes that determine root system structure and function by establishing in vivo models where these relationships can be rigorously explored2,3.
Although we may not know all the consequences of modifying gene regulatory networks that control root system architecture, rhizodeposition, and root-microbe interactions, designing control over these processes is an important first step. This review first highlights plant genetic tools and tunable traits for designing roots with modified form, function, and rhizosphere interactions. Then, we discuss the process of engineering rhizobacteria as well as functions to target for improving crop performance in the face of climate change. From this exploration, we provide a holistic description of contexts in which a synthetic biology approach can be applied to plant root and microbiome engineering for improving crop resilience and sustainability.
Engineering form and function of roots
Engineering predictable patterns of gene expression in roots
Most projects aimed at engineering the form and function of root systems will likely begin with plans to control the expression of genes that affect the development and physiology of specific tissues or root types4,5. Modifying gene expression in plants using characterized promoters is commonplace and is facilitated by sourcing and testing promoter sequences with published empirical evidence of activity6,7. Previous studies have utilized tissue-specific expression datasets generated by Fluorescence Activated Cell Sorting (FACS), and more recently single-cell RNAseq, to identify source promoters from genes expressed with patterns of interest (e.g., root tissue-specific). While the use of such curated promoter parts (DNA sequences with defined functions that can be used as modules in a synthetic gene) is often sufficient to create reporter genes, the design specifications required to engineer a plant with a specific form/function often require much finer control of gene expression programs at both the spatial and magnitudinal level4. Recent work has expanded the tools available for designing specific patterns of gene expression by utilizing synthetic transcription factor-based and DNA recombination-based circuits.
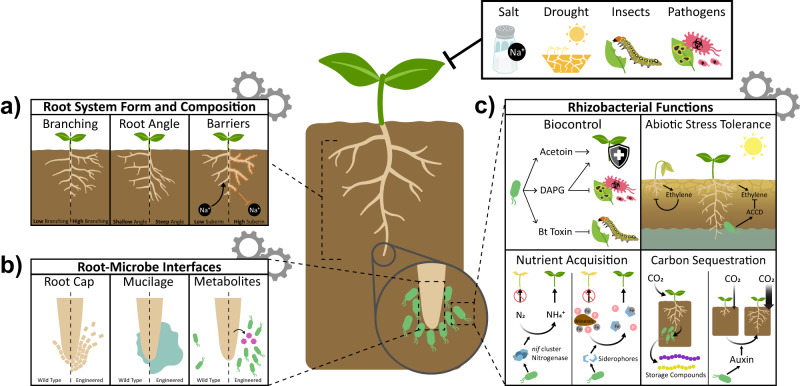
In Brophy et al., a large collection of sequences encoding DNA binding domains, sourced from various bacterial species8, was used to create synthetic transcription factors able to activate or repress expression of target genes in plants9,10. The well characterized DNA-binding specificity of these domains facilitated the construction of synthetic promoters responsive to the activity of the synthetic transcription factors. Screening this collection of transcription factors led to the identification of a subset that showed little cross reactivity, meaning they regulated expression predominantly through synthetic promoters that contained their cognate recognition sequence and not that of the other synthetic transcription factors tested (so-called orthogonality). The orthogonal nature of these synthetic transcription factor-promoter pairs facilitated the assembly of gene circuits capable of performing 14 different 2-input Boolean logic functions in tobacco transient assays. Boolean logic functions define the relationship between the activity state of inputs for the circuit and the circuit output. For example, an AND logic gate restricts the activity of the circuit to situations where both inputs to the circuit are in an ON state while activity is OFF when one or neither input is active. Successful circuit architectures were then ported into Arabidopsis, driven by native tissue-specific promoters, to create novel expression patterns based on the computed logic conferred by the specific circuit architecture. This system allows for the control of both the spatial pattern (Fig. 1) and expression level (Fig. 2a) of a downstream gene and utilizes a collection of parts with little sequence similarity, which limits the likelihood of gene silencing due to the presence of repetitive elements11.
Fig. 1. Two-input logic gates controlling tissue-specific gene expression in roots.
Native promoters such as the a SMB promoter (columella and lateral root cap) and b PIN4 promoter (columella and stele) can be used to drive tissue-specific expression of genes. By combining these promoters with synthetic activators and repressors, Brophy et al. generated circuits that can perform Boolean logic operations, creating novel patterns of gene expression not found in nature. c In the NOR gate, GFP is expressed only in tissues where both the PIN4 and SMB promoter are not active. d In the NIMPLY gate, GFP is expressed only where SMB is active, but PIN4 is not.
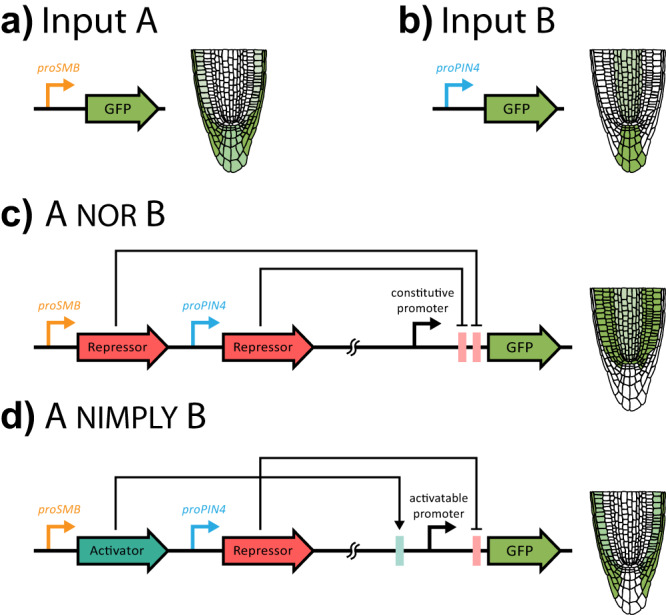
Fig. 2. Controlling expression through buffer gates and recombinase-based circuits.
Successful engineering of plant form will require fine control over gene expression, both in terms of magnitude and spatial patterning. a Circuits implementing buffer gates to tune expression of slr-1 (inhibits lateral root formation), allowed Brophy et al. to control the number of lateral roots formed. b Guiziou et al. utilized a serine integrase to create a circuit that permanently switches its output when the integrase is expressed, creating a form of cellular memory that records past gene activity.
In Lloyd et al., sequence-specific DNA recombinases were used, as an alternative approach, to trigger a stable change in synthetic genetic circuit architecture to permit or block the expression of a downstream reporter gene12. This system enables stable changes in gene expression since the circuit can be irreversibly modified by the recombinase. This irreversible nature may also allow the circuits to be especially sensitive to the expression level of the recombinase when traditional reporters prove insufficiently responsive. However, spurious activity of the synthetic gene circuit could occur if the promoter exhibits low-levels of expression outside of the domain of interest.
More recently, Guiziou et al. has demonstrated the utility of recombinases to record gene activity during lateral root development13. Here, serine integrases were used to flip a promoter element from a state that drives the expression of mTurquoise to an alternative state driving mScarlet expression (Fig. 2b). Input promoters were used to drive the expression of a serine integrase in the pericycle, which is a subpopulation of cells in the root that contributes founder cells for lateral root development. Successful implementation of the circuit led to mScarlet expression exclusively in lateral roots. In addition, combinatorial logic was introduced into these circuits by splitting the integrase coding sequence into two parts and fusing each half with the N or C-terminal half of an intein coding sequence (a splicing protein)14. Expression of the fusion proteins in the same cell allows for reconstitution of the integrase and recombination of the downstream reporter. Such combinatorial logic would allow for the recording function of the circuit to be triggered at a time of interest, or allow for greater tissue or condition specificity.
Together, these recent advances in synthetic gene circuits provide greater opportunities for researchers to tailor their synthetic gene architecture to the design specification of the target pathway. Transcription factor-based circuits enable analog or graded patterns of input promoter activity to be translated into graded patterns of circuit output, while recombinase-based systems generate digital (on/off) circuit outcomes that are stable over the long-term. Combination of these circuit modalities may allow for a wider spectrum of gene regulatory patterns to be engineered4.
Root architecture for water and nutrient uptake
Root systems in flowering plants are hierarchically branched structures1,15. Computational modeling of root system growth and physiology has revealed design rules that provide target goals for engineering16–19. In particular, work by the lab of Jonathan Lynch has established the steep, cheap, and deep ideotype for drought and nitrogen efficient agriculture20. This work has been heavily reviewed, but two traits (root system branching rate and gravity setpoint angle) are worth exploring here as their genetic basis has been more clearly defined and the application of synthetic gene circuits to modulate these traits is on the horizon (Fig. 3a). Furthermore, anatomical features of roots play prominent roles in defining their physiological interactions with soil water and nutrients21,22, and will be reviewed as an additional potential target for engineering.
Fig. 3. Tuning the rhizosphere through root and microbial engineering.
Future climate conditions will exacerbate abiotic (salt, drought, etc.) and biotic (pathogens and pests) stressors that negatively impact crop yield. Through synthetic biology, root form, function, and microbial interactions can be altered to create new crops better equipped to grow in these more challenging conditions. a Custom root system architectures can be created by changing branch rate and gravity setpoint angle, resulting in root systems more suited for water and nutrient acquisition. Modulating suberin deposition can limit the uptake of toxic sodium and metal ions, while insulating roots against nutrient loss. Each panel represents a trait to target for engineering. Left of the dashed line are roots resulting from decreasing the target trait. Right of the dashed line represents an increasing target trait. b Primary and lateral root apices are the main interfaces at which plants modify the local soil environment, and by extension the composition of their microbiome, through the process of rhizodeposition. Control over root cap shedding dynamics and mucilage release can potentially improve root penetration into soil and drought resistance. These features, as well as the release of certain sugars and other metabolites, are also attractive engineering targets for controlling the composition of the root microbiome. Each panel represents a trait to target for engineering. Left of the dashed line is the wild type condition. Right of the dashed line depicts an engineered root. c The plant root microbiome expands the genetic repertoire available to the plant, providing a plethora of beneficial functions to their host. The metabolic flexibility of bacteria allows for the potential engineering of a myriad of actuators to improve plant biotic and abiotic stress tolerance, nutrient acquisition, and carbon sequestration.
Root system size
Root system growth is heavily dependent on the formation of lateral root branches1,23,24, either from the primary root axis, or in the case of grasses and some eudicots, from branches that form from the base of the shoot15,25. In Brophy et al., the authors utilized synthetic gene circuits to tune the level of expression for a mutant signaling protein solitary root (slr)/iaa14–1 (Fig. 2a)10, which dominantly inhibits the transcriptional response to the auxin hormone26,27. In Arabidopsis, expression of slr/iaa14–1 from its native promoter causes a complete suppression of lateral root development as well as many other pleiotropic effects on shoot growth and root hair development10,27. To control these effects, expression of slr/iaa14–1 was limited to the pericycle tissue of roots, where lateral root primordia are induced, using the promoter proGATA2310. Quantitative tuning of lateral root development was then achieved using a buffer gate circuit architecture (Fig. 2a) where modifying the synthetic promoter driving slr/iaa14–1 expression affected its responsiveness to a synthetic transcription factor that was expressed from the GATA23 promoter. Reducing the number of cis-elements in the synthetic promoter and mutating these binding sites to reduce affinity for the transcription factor were both required to express slr/iaa14–1 at low enough levels to quantitatively affect lateral root number without blocking their development entirely. Similar approaches may be useful in grass species since dominant-negative AUX/IAA mutant genes have been shown to have similar effects on root branching28.
Modulating gravity setpoint angle
In addition to root system size, the placement of branches and their subsequent growth angle, with respect to gravity, can determine the relative efficiency that resources are captured from soil20. Root growth is guided by the response to gravity and the angle that root growth takes concerning the gravity vector is called the set point angle29,30. Lateral roots that grow more steeply will establish a deeper root system, while a more shallow orientation will increase exploration of soil closer to the surface. Several pathways have been identified that affect the angle of emergence and subsequent growth of lateral roots and some of this work has demonstrated adaptive value of genetic loci that promote deeper root systems under drought conditions in the field31–33. What is less clear is how effectively these pathways can be controlled to tune root system architecture. Loss of function in the newly discovered EGT2/WEEP genes leads to steeper branch angles in the roots of wheat, barley and Arabidopsis, suggesting a conserved function across flowering plants, making this an attractive pathway for engineering34,35. However, whether the expression level of these genes can affect gravity set point angle in a quantitative manner has not been tested. Overexpression of AtDRO1 in the TAC/LAZY family causes lateral roots to exhibit somewhat steeper growth trajectories33, however, like EGT2/WEEP, the effect is not specific to roots and gravity responses of axillary shoots are also affected. Surprisingly, only a few promoters have been directly tested for their ability to control gene expression exclusively in root or shoot tissues36–39. Establishing synthetic gene circuits that limit gene expression to above or below ground organ systems will be necessary to implement this engineering strategy, though this will be especially challenging in grasses where the majority of root biomass forms from shoots. Furthermore, manipulation of gravity set point angle in specific root types will likely be important for targeted improvements in root architecture and to prevent competition between roots of the same parent plant.
Modifying the selective uptake of solutes from soil
In roots, the cell-type specific pattern of cell wall modification enzymes and their regulators promote the formation of apoplastic (extracellular) barriers such as suberin and lignin21,40–42, which limit the passive movement of water and solutes in the tissue spaces outside of cells22,43. Engineering plants for sustainable agriculture will likely involve modifying these transport-associated pathways to enhance uptake of limiting nutrients, or to limit the uptake of toxic solutes, such as sodium and metal ions—conditions that are more likely to be present in degraded or marginal agricultural lands. Current research suggests that suberin is the more plastic apoplastic barrier component and hormone signaling pathways converge on the regulation of suberin biosynthesis genes in endodermal cells22. Furthermore, research into the patterning of the exodermal cell layer, which contains similar cell wall modifications as the endodermis and performs functions associated with drought and flooding tolerance, is also being explored and may lead to the ability to engineer transferable traits to new cell layers44. A better understanding of the different mechanisms that operate in the root to mediate communication and resource exchange with the rhizosphere, and its associated microbes, may also help leverage key root microbiome members to support plant and soil health.
Engineering the root-soil metabolic interface
Roots are a major soil interface for chemical exchange where diffusion, transport, new growth, and cell death render a revolving door of nutrient uptake and release. Across woody and herbaceous taxa, plants can deposit 10–90% of fixed carbon to the root system45,46. Thus, engineering plants to modulate the composition and quantity of these deposits may be a powerful approach to manipulating soil properties and a potential route for sequestering carbon below ground (Fig. 3b).
Hundreds of compounds are released from roots from long sugar polymers to central metabolites47,48. The mechanisms that regulate the production and spatial patterns of rhizodeposits are largely differentiated based on the molecular weight of their outputs. For the secretion of low molecular weight exudates, diffusive pores and active transporters vary in distribution and can localize to specific root regions, while mucilage, a hydrogel composed of a complex mixture of polymers and metabolites, is released by root cap tissues present at the root tip and linked to specific developmental stages of the tissue49,50. Studies suggest that the exudation profile of a plant root system is primarily determined by plant genotype. Natural variation in exudate quantity and profile is observed across species, cultivars, plant age, root types, root developmental zones, and growing environments51–54.
Root cap development and shedding dynamics
The root cap is a regenerative tissue that sheds cells into the soil to protect the root apical meristem. In most species, such as Pisum sativum (pea) and maize, root cap cells shed individually as living border cells, which survive in the soil for a brief period of time before undergoing programmed cell death55. In Arabidopsis thaliana, some other members of the Brassicaceae family (mustard, canola, and cabbage), and certain tree species, the entire outer root cap layer sheds as a single sheath of connected cells called border-like cells56–59. Given the range of root cap morphologies and developmental programs, engineering the size, shape, and nature of root cap clearance has the potential to affect root and root-microbe activity.
Rhizodeposition is largely mediated by non-dividing cells in the lateral root cap (LRC) and the columella. Lateral root cap cells undergo developmental programmed cell death (dPCD) and detach from the root via autolysis. In the columella, living cells are sloughed into the rhizosphere before undergoing dPCD60. These sacrificed root cap cells likely absorb mechanical shear as the roots drill through soil via new growth61. Thus, changes in root cap development could affect the capacity of the root to penetrate soil. In Arabidopsis, genetic regulation of root cap development has been partially deciphered. FEZ and SOMBRERO (SMB) are NAC-domain transcription factors and control the orientation of stem cell divisions and subsequent root cap daughter cell maturation, respectively. The fez mutant has fewer lateral root cap layers while the smb mutant produces an extra cell layer and has a root cap that extends past the meristematic zone. Because cells in the smb root cap mature more slowly, these plants also exhibit slower rates of root cap shedding. Consistently, mutations in these genes were found to impact penetration into gelled media62. To overcome soil density at greater depths, root cap engineering may need to be complemented with modifications of root hairs and lateral roots as penetration anchors, as well as changes to root angle, cell wall rigidity, and root diameter to ensure that new root architectures can be implemented in a range of soil types63.
Mucilage release
As a slimy matrix, mucilage provides lubrication to enable growing roots to penetrate the soil61,64. In contrast to small metabolites, root mucilage is highly hygroscopic and affects soil wetting properties65. Some major components of mucilage include pectin, cellulose, hemicellulose, and arabinogalactan proteins48,53. Roots modulate rhizodeposition according to environmental, pest, and nutrient signals. For example, mucilage release was observed to increase under moderate drought but decrease under severe drought66. Mucilage can vary in its chemical properties, which affects the way it interacts with ions present in the soil. Pectic mucilage in the halophyte (salt tolerant) Kosteletzkya virginica was found to sequester sodium ions across a range of tissues including the roots67. In a study of aluminum tolerance, mucilage from Melastoma malabathricum sequestered more cations than maize68.
Mucilage can serve as the sole carbon source for microbes and was found to stabilize the catalytic activity of soil microbes under drought conditions69,70. In maize, the mucilage of aerial brace roots supports colonization by nitrogen-fixing diazotrophs71. Thus, engineering roots to release different quantities or compositions of mucilage could be used to modify the physical, chemical, and biotic environment of the root. Currently, however, the few genes known to affect mucilage production and release also disrupts the development of the root cap, which performs many other physiological functions57. Further investigation into the downstream targets of root cap developmental regulators may uncover new genes for the engineering of this vital rhizodeposit.
Root metabolite release
Engineering exudation of root released metabolites is one strategy to promote the stable colonization of plants by commercial inoculants or attraction of natural beneficial bacteria72. In a study of Pseudomonas fluorescens root colonization, L-malate, but not D-malate, induced chemotaxis73. After confirming positive chemotaxis to a plant defense compound, 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA), Neal et al. observed lower levels of Pseudomonas putida on the roots of DIMBOA-deficient bx1 maize mutants74. Identifying specific chemoattractants and repellants released by roots or tuning host metabolism and incorporating novel microbe metabolic pathways are ways to manipulate community profile and root attachment. Understanding the impact of microbial chemoeffectors on plant host activity is also crucial to engineering sustained interactions in the rhizosphere. D-galactose released by cucumber roots is a strong chemoattractant for Bacillus velezensis SQR9. Roots exuded more galactose in response to SQR9, and supplementing the media with galactose increased B. velezensis root colonization75. This type of positive feedback in rhizosphere interactions has important implications for engineering persistent interactions between plants and microbes.
Engineering plant exudates can also enhance responses to pests and pathogens. Root exudates from knockdown lines of the gene ABC-C6 repelled two species of parasitic nematodes76. Supplementing roots with fractionated exudates determined that increased levels of hexadecaonic acid and pentadecane were the primary chemorepellants77. In a related study, root exudates from knockdown lines of the ethylene response genes ERF-E2 and ERF-E3 were more attractive to nematodes, while chemotaxis of Bacillus subtilis or Agrobacterium tumefaciens were not affected78. Warnock et al. applied exogenous dsRNAs to silence sugar transporters and observed reduced levels of exuded glucose, fructose, and sucrose, which inhibited chemotaxis of two nematode species79. The multifunctional nature of exuded metabolites highlights the potential power of synthetic circuits to spatiotemporally tune the activity of exudate transporters to reduce unwanted attractant activity while preserving the role these molecules play in supporting the broader microbiome.
Another approach in exudation engineering is to select for microbes that can use a rare substance as a sole carbon source. Opines are molecules rarely found outside of the crown gall tumors induced by Agrobacterium tumefaciens and the genes for opine catabolism are not widely distributed. In 1997, Savka and Farrand engineered opine-catabolism into P. fluorescens and observed higher densities of growth on transgenic opine-producing tobacco80. On Lotus japonicus roots engineered to produce opine, opine-catabolizing microbe species were more represented in the rhizosphere81. Engineering new rare carbon-source metabolism and exudation in roots could aid bioprospecting efforts to identify specialized root colonizing microbes capable of metabolizing these food sources.
Using rare compounds in rhizosphere engineering could also create orthogonal systems that reduce the effect of a complex plant-microbe metabolome. Rhizopines are inositol-derived molecules specially produced and catabolized by nitrogen-fixing rhizobia in root nodules. They are functionally similar to Agrobacterium opines82. In 2019, Geddes et al. observed luminescence from a rhizopine lux biosensor in rhizobia on inoculated Medicago truncatula and barley roots harboring a synthetic rhizopine biosynthesis pathway83. In the complex signaling environment of the rhizosphere, genetic circuitry using these rare compounds allows for unique inputs for targeted manipulation of bacterial activity. However, engineering non-endogenous metabolic pathways requires extensive knowledge of biosynthetic pathways of both the target and competing compounds and preferred/alternative substrates.
Engineering the microbial side of the rhizosphere
While plant synthetic biology approaches may one day usher in a generation of designer crops, the technical difficulties of plant transformation and genetic circuit design still limit the usability of these technologies outside of model plants. Bacteria, in comparison, benefit from fast generation times, predictive biophysical models, and broad host range techniques that have made rational design of genetic circuits possible, even in non-model species. The basic tools needed to deploy regulated circuits with functional outputs in plant beneficial rhizobacteria already exist, and may soon enable researchers to modulate plant function through their microbiome (Fig. 3c).
Designing and testing synthetic circuits in rhizobacteria
The successful function of a synthetic circuit depends heavily on its host chassis: the organism that houses and supports the designed circuitry. An ideal rhizobacterial chassis will be able to both accommodate heterologous protein/metabolite synthesis and robustly colonize the root. Root microbiome studies have identified a plethora of strains which both strongly colonize roots or have genetically tractable plant beneficial traits that can be mined for parts to use in engineering. Of these, root colonizing Pseudomonas spp. and Bacillus spp. are the most well studied and can be used to host a wide range of biosynthetic pathways. Alternatively, bacteria that are poor colonizers but possess other desirable features could be engineered for increased rhizosphere competence by altering traits such as biofilm formation, plant immunity evasion, and utilization of root exuded carbon84. The greatest challenge for any root colonizer will generally be competition with native soil microbiota which tend to quickly outcompete introduced strains, especially those hosting metabolically-costly machinery85. One solution is to use antibiotic producing chassis or engineering the expression of other biocontrol traits, in effect introducing a keystone species that carves out an ecological niche that impacts the rest of the microbiome. However, root association and community interactions are both complex polygenic traits and the underlying mechanisms for competitive colonization are not clearly understood, making engineering these features a non-trivial task. As an alternative to rational design, adaptive evolution can be used to generate strains with improved colonization in the lab. This technique has already been applied to evolve Pseudomonas and Bacillus strains with augmented colonization capabilities86,87.
The strength and expression pattern of different promoters and ribosome binding sites (RBS) can exhibit significant variability even between closely related strains88. Therefore, it is crucial to fine-tune these regulatory elements within a circuit to ensure functionality of its constituent genetic parts outside their original host contexts. Once designed, a genetic circuit must be empirically verified for activity in the chosen chassis, and will usually require optimization through multiple design-build-test-learn cycles.
While circuit designs are generally first assembled into self-replicating plasmid backbones, long-term functioning circuits are usually integrated into the genome of the target chassis to improve stability and remove the need for antibiotic selection. For precision engineering, CRISPR/Cas9 and its variants allow for specific editing of almost any site with a PAM sequence in a genome. Other technologies utilize modified ICEBs1 integrative and conjugative elements89 or recombineering90 to enable rapid genome integration of circuitry into a broad range of host strains.
Biotic stress tolerance
First identified in disease suppressive soils, rhizobacterial biocontrol strains that protect crops from insect pests and fungal/bacterial diseases offer attractive alternatives to environmentally damaging chemical pesticides. These strains can mediate plant resistance to pests and pathogens by modulating host immunity through induced systemic resistance (ISR), and/or by outcompeting, killing, or modulating attacking organisms. ISR protects plants against both disease and herbivory without major fitness costs91, but the mechanisms underlying its activation by rhizobacteria are not completely known and likely differ between plant/bacteria pairs. ISR activation in field plants is not ubiquitous, and requires ISR elicitor producing strains to pass a certain population threshold92. Thus, the ability to produce strong ISR elicitors at effective concentrations for host plants represents a prime target for engineering. A growing list of ISR elicitors, such as DAPG, 2,3-butanediol, and acetoin, have already enabled researchers to successfully engineer rhizobacteria with altered ISR activation capabilities93.
Better understood is the ability of certain biocontrol strains, in particular those of model biocontrol Pseudomonas and Bacillus species, to attenuate bacterial and fungal pathogens or insect herbivores. Pseudomonas spp. have been shown to protect a variety of plants by killing/inhibiting soil pathogens through the production of antibiotics (phenazines, pyoluteorin, pyrrolnitrin, 2,4-diacetylphloroglucinol/DAPG, etc), iron-chelating siderophores (pyoverdine and pyochelin), hydrogen cyanide, and toxin proteins94,95. Likewise, Bacillus spp. are known to produce iron siderophores and an array of antimicrobial/antifungal bacteriocins, lipopeptides, and polyketides96. Bacillus thuringiensis, which produces insect species specific Cry/Cyt toxin proteins, is the most widely used insect biocontrol strain and is already used in agricultural contexts worldwide for pest management. Outside of direct killing/inhibition, other rhizobacteria can modulate pathogen virulence via quorum quenching: the degradation of bacterial pheromones that mediate quorum sensing97. Using genome mining and comparative genomics tools, bacterial genomes can be analyzed to identify the genes and biosynthetic gene clusters underlying biocontrol effector synthesis98. Metabolic engineering can then be used to overproduce these targets in native contexts or port them to new chassis.
Abiotic stress tolerance
As climate change accelerates, crops will face abiotic stressors such as drought, high salinity, and extreme temperature with increasing severity and frequency. With this in mind, researchers have identified a plethora of rhizobacteria that confer abiotic stress tolerance to their host plants, either directly via the modification of the environment around the root, or indirectly by modulating host signaling and chemistry99. Rhizobacteria can alter soil chemistry around roots through the production of exopolysaccharide (EPS) matrices during biofilm formation, which increases water retention, improves soil macroporosity and adherence to the root, and restricts root uptake of Na+ ions and heavy metals100,101. Indirect mechanisms of increasing plant stress resistance involve modulating root architecture, growth inhibition, osmolyte accumulation, and ROS production in the host through the production/degradation of plant hormones (auxin/indole-3-acetic acid (IAA), 1-aminocyclopropane-1-carboxylic acid (ACC), cytokinins, abscisic acid (ABA))102, stress signals (trehalose, cadaverine)103,104, and other secondary metabolites (phenazine, 2,3-butanediol, etc.)105. It should be noted that the highly polygenic and pleiotropic nature of these resilience phenotypes can make it difficult to pinpoint specific mechanisms for engineering, though genetic determinants can still be identified in some cases. For example, several groups have altered EPS production and found that it is critical for the protective activity of Bacillus amyloliquefaciens, but whether EPS does so by altering soil chemistry, influencing plant stress responses, changing the colonization ability of rhizobacteria, or a combination of these factors is still unclear100,101. For the stress tolerance genetic actuators that have been identified, circuits for bacterial overexpression/heterologous expression of trehalose106, ACC deaminase107,108, and cytokinin109 successfully improved drought resistance in colonized plants. Using modern synthetic biology approaches, these circuits might be improved by adding the capability to sense and respond to environmental or host-derived stress signals or produce multiple synergistic tolerance actuators such as trehalose and ACC deaminase110.
Nutrient acquisition
Synthesis of ammonia for nitrogen fertilizer is energy intensive and generates significant greenhouse gas emissions111, making biological nitrogen fixation an attractive engineering target for improving the sustainability of non-legume crops. Minimal gene sets of the nitrogen fixation cluster (nif) are characterized for some species, but the complexity of nitrogenase assembly, the O2 sensitivity of its catalytic center, and relatively high fitness cost of hosting the pathway makes transfer of nif activity into non-native chassis difficult112. Engineering efforts have focused on optimization of protein stoichiometries113, reducing O2 sensitivity114, and altering downstream ammonia utilization/repression112,115, but have so far been unable to reconstitute native levels of nif activity.
Outside of N2 fixation, rhizobacteria can also improve acquisition of iron and phosphate, which are generally present in soil but not in bioavailable forms. Production of organic acids116, redox-active antibiotics117, and iron siderophores118 by rhizobacteria release and mobilize iron and phosphate ions from mineral complexes for uptake by plants. Rhizobacteria engineered to express heterologous organic acid operons demonstrated improved phosphate solubilization and promoted growth in rice119. Although heterologous siderophore expression has not yet been demonstrated in a rhizobacterial context, successful biosynthesis in other model gram-negative chassis suggests these same circuits can be applied to improve rhizosphere nutrient availability. In soil, a significant proportion of phosphate can also be bound in phytate, a plant-produced phosphate-storage molecule. Soil phytate is adsorbed to minerals, further decreasing phosphate bioavailability. Shulse et al. expressed 82 diverse phytases in 3 rhizobacterial chassis and identified 12 combinations that improved Arabidopsis growth via phosphate release from phytate120.
Carbon sequestration
Increasing the amount of carbon sequestered in soil can potentially offset a significant amount of anthropogenic carbon emissions, with the added benefit of improving soil quality121. Rhizobacteria are key players in this process, increasing soil organic matter (SOM) both directly through biomass accumulation and indirectly by influencing plant root growth122. A recent analysis by Tao et al. suggested that tipping microbial carbon usage to favor growth and byproduct formation over respiration is likely the most impactful strategy for increasing soil carbon sequestration123. This could be accomplished by using genetic circuits that increase the production of carbon rich storage compounds, such as polyhydroxyalkanoates, triacylglycerols, wax esters, and glycerol, or structural compounds such as bacterial cellulose124. An alternative strategy may involve increasing the amount of highly stable mineral-associated-organic-carbon in soil by elevating production of nitrogen rich carbon compounds that readily adsorb onto free mineral surfaces125. To avoid high fitness costs, any circuit will likely need to be regulated in response to carbon availability, so that synthesis of these compounds occurs only when excess carbon is available. In addition to storing carbon themselves, rhizobacteria can elevate plant carbon inputs to soil by altering root system size and architecture. Auxin/IAA producing rhizobacteria can increase primary root length and lateral root formation in plants126,127 and ACC deaminase producers can encourage root growth even under high stress conditions128, resulting in greater carbon input into soil as cellulose and other compounds.
Microbiome community engineering
Unreliable performance and stability in field conditions is the primary obstacle holding back beneficial rhizobacteria as a real-world solution for sustainable agriculture. While improving root colonization of individual strains can ameliorate this issue, the fact remains that environmental conditions and soil microbial consortia vary greatly from field to field129. To address this challenge, the next stage of rhizobacterial engineering will likely focus on microbial community engineering, controlling microbiome species composition or metagenome content to set the stage for optimal performance of synthetic circuits.
Approaches to community engineering can be generally split into bottom-up and top-down strategies. Bottom-up methods involve isolating and engineering individual strains to assemble into synthetic communities (SynComs)130,131. Simple SynComs have already demonstrated potential utility in the field, exhibiting greater stability and performance on plants compared to single-strain inoculations132. In contrast, the top-down approach aims to modify the metagenome of entire native communities, capturing as much natural diversity as possible while enhancing or removing target functions. Conjugation based tools XPORT89 and MAGIC133 utilize broad host range conjugative elements/vectors to first transform diverse species with engineered payloads and then utilize transposases to stably integrate circuits into genomes. Similarly, phages can be used to deliver and integrate circuitry into microbes. Although host range and payload size are more limited compared to conjugative tools, phages are well suited for targeting a single or small defined range of strains within a community134. Combining these tools with CRISPR payloads allows for gene level editing of the whole metagenome, potentially ‘deleting’ certain functions from an entire community135.
Future perspectives and conclusion
While recent advances in the application of synthetic biology approaches to plants has established a foundation of tools for future studies, there are several areas where substantial bottlenecks to progress remain. Adapting circuits to crop plants is challenging since few characterized tissue-specific or environment specific promoters have been directly tested. While single-cell transcriptomics and assessment of chromatin accessibility are becoming readily applicable approaches in any species with a sequenced genome136,137, testing the activity of sourced promoters still requires the production of transgenic plants, which slows the initial stages of circuit design. Hairy root transformation systems may be useful in this regard. However, the physiological relevance of these systems may have significant limitations in certain contexts138. Transient transformation in N. benthamiana has served as an efficient means of prototyping circuit designs10, but due to phylogenetic distance, this approach may have more limited utility when engineering monocots. Use of transient transfection assays in grasses will likely facilitate this transferability gap139. Beyond engineering a single organism, engineering plant-microbe interactions will benefit from the development of rapid gene expression systems that facilitate genetic manipulation of both the plant and the microbe. N. benthamiana has served as a useful system for studying plant-pathogen interactions140, and it will be interesting to explore whether molecular pathways associated with commensal or symbiotic plant-microbe interactions can be modeled and engineered in transiently transformed leaf tissues. Arbuscular mycorrhizal fungi (AMF) represent another promising target for genetic engineering as they form symbiotic relationships with most crop plants. Stable genetic transformation of AMF has not yet been shown141, but once this roadblock is overcome, genetic parts and circuits tested in other fungal phyla could potentially be ported for immediate use in AMF, unlocking a treasure trove of new chemistry for improving agriculture. Currently, deployment of transgenic microbes in the field is not allowed and significant effort will need to be made to establish a regulatory framework that allows for innovation while also protecting the environment and natural biodiversity142.
Despite these current limitations, new tools for plant synthetic biology have empowered researchers with the fine spatial and magnitudinal control over gene expression needed to predictably alter form and function in plants. As our understanding grows, we may soon see the first designer root systems and microbiomes engineered to both survive climate change through increased stress resistance and fight it by augmenting soil carbon sequestration. The curtain is opening on the next stage of crop engineering, and it will be exciting to see the elaborate choreography that synthetic biologists create between plants and microbes to achieve sustainable agriculture in the face of climate change.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We would like to thank members of the Dinneny lab for their insightful comments, which have greatly improved the work. Funding for work on plant synthetic biology research in the Dinneny lab is provided by the US Department of Energy Biological and Environmental Research Program grant DE-SC0023160 (J.R.D.). Support for C.J.R. and K.Y.S. provided by the US National Institutes of Health training grant 5 T32 GM007276. J.R.D. is a Chan Zuckerberg Biohub—San Francisco Investigator.
Author contributions
C.J.R., K.Y.S., and J.R.D. conceptualized, wrote and revised the manuscript.
Peer review
Peer review information
Nature Communications thanks Barney Geddes, Ryan Lister and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Carin J. Ragland, Kevin Y. Shih.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-45272-5.
References
- 1.Rellán-Álvarez R, Lobet G, Dinneny JR. Environmental control of root system biology. Annu. Rev. Plant Biol. 2016;67:619–642. doi: 10.1146/annurev-arplant-043015-111848. [DOI] [PubMed] [Google Scholar]
- 2.Brophy JAN. Toward synthetic plant development. Plant Physiol. 2022;188:738–748. doi: 10.1093/plphys/kiab568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brophy JAN, LaRue T, Dinneny JR. Understanding and engineering plant form. Semin. Cell Dev. Biol. 2017;79:68–77. doi: 10.1016/j.semcdb.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 4.Zhong V, Archibald BN, Brophy JAN. Transcriptional and post-transcriptional controls for tuning gene expression in plants. Curr. Opin. Plant Biol. 2023;71:102315. doi: 10.1016/j.pbi.2022.102315. [DOI] [PubMed] [Google Scholar]
- 5.Yaschenko AE, Fenech M, Mazzoni-Putman S, Alonso JM, Stepanova AN. Deciphering the molecular basis of tissue-specific gene expression in plants: can synthetic biology help. Curr. Opin. Plant Biol. 2022;68:102241. doi: 10.1016/j.pbi.2022.102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JY, et al. Transcriptional and posttranscriptional regulation of transcription factor expression in Arabidopsis roots. Proc. Natl Acad. Sci. USA. 2006;103:6055–6060. doi: 10.1073/pnas.0510607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquès-Bueno MDM, et al. A versatile multisite gateway-compatible promoter and transgenic line collection for cell type-specific functional genomics in Arabidopsis. Plant J. 2016;85:320–333. doi: 10.1111/tpj.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanton BC, et al. Genomic mining of prokaryotic repressors for orthogonal logic gates. Nat. Chem. Biol. 2014;10:99–105. doi: 10.1038/nchembio.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alamos S, Shih PM. Synthetic gene circuits take root. Science. 2022;377:711–712. doi: 10.1126/science.add6805. [DOI] [PubMed] [Google Scholar]
- 10.Brophy JAN, et al. Synthetic genetic circuits as a means of reprogramming plant roots. Science. 2022;377:747–751. doi: 10.1126/science.abo4326. [DOI] [PubMed] [Google Scholar]
- 11.Meyer P, Saedler H. Homology-dependent gene silencing in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:23–48. doi: 10.1146/annurev.arplant.47.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd JPB, et al. Synthetic memory circuits for stable cell reprogramming in plants. Nat. Biotechnol. 2022;40:1862–1872. doi: 10.1038/s41587-022-01383-2. [DOI] [PubMed] [Google Scholar]
- 13.Guiziou S, Maranas CJ, Chu JC, Nemhauser JL. An integrase toolbox to record gene-expression during plant development. Nat. Commun. 2023;14:1844. doi: 10.1038/s41467-023-37607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans TC, Jr, Xu M-Q, Pradhan S. Protein splicing elements and plants: from transgene containment to protein purification. Annu. Rev. Plant Biol. 2005;56:375–392. doi: 10.1146/annurev.arplant.56.032604.144242. [DOI] [PubMed] [Google Scholar]
- 15.G. Viana W, Scharwies JD, Dinneny JR. Deconstructing the root system of grasses through an exploration of development, anatomy and function. Plant Cell Environ. 2022;45:602–619. doi: 10.1111/pce.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch JP, Chimungu JG, Brown KM. Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. J. Exp. Bot. 2014;65:6155–6166. doi: 10.1093/jxb/eru162. [DOI] [PubMed] [Google Scholar]
- 17.Postma JA, Dathe A, Lynch JP. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014;166:590–602. doi: 10.1104/pp.113.233916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couvreur V, et al. Going with the flow: multiscale insights into the composite nature of water transport in roots. Plant Physiol. 2018;178:1689–1703. doi: 10.1104/pp.18.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobet G, Couvreur V, Meunier F, Javaux M, Draye X. Plant water uptake in drying soils. Plant Physiol. 2014;164:1619–1627. doi: 10.1104/pp.113.233486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch JP. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013;112:347–357. doi: 10.1093/aob/mcs293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla V, Barberon M. Building and breaking of a barrier: suberin plasticity and function in the endodermis. Curr. Opin. Plant Biol. 2021;64:102153. doi: 10.1016/j.pbi.2021.102153. [DOI] [PubMed] [Google Scholar]
- 22.Barberon M, et al. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell. 2016;164:447–459. doi: 10.1016/j.cell.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Yu P, Gutjahr C, Li C, Hochholdinger F. Genetic control of lateral root formation in cereals. Trends Plant Sci. 2016;21:951–961. doi: 10.1016/j.tplants.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Van Norman JM, Xuan W, Beeckman T, Benfey PN. To branch or not to branch: the role of pre-patterning in lateral root formation. Development. 2013;140:4301–4310. doi: 10.1242/dev.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omary M, et al. A conserved superlocus regulates above- and belowground root initiation. Science. 2022;375:eabf4368. doi: 10.1126/science.abf4368. [DOI] [PubMed] [Google Scholar]
- 26.Fukaki H, Nakao Y, Okushima Y, Theologis A, Tasaka M. Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J. 2005;44:382–395. doi: 10.1111/j.1365-313X.2005.02537.x. [DOI] [PubMed] [Google Scholar]
- 27.Fukaki H, Tameda S, Masuda H, Tasaka M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002;29:153–168. doi: 10.1046/j.0960-7412.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- 28.Woll K, et al. Isolation, characterization, and pericycle-specific transcriptome analyses of the novel maize lateral and seminal root initiation mutant rum1. Plant Physiol. 2005;139:1255–1267. doi: 10.1104/pp.105.067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Digby J, Firn RD. The gravitropic set-point angle (GSA): the identification of an important developmentally controlled variable governing plant architecture. Plant Cell Environ. 1995;18:1434–1440. doi: 10.1111/j.1365-3040.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 30.Roychoudhry S, Kepinski S. Analysis of gravitropic setpoint angle control in Arabidopsis. Methods Mol. Biol. 2015;1309:31–41. doi: 10.1007/978-1-4939-2697-8_4. [DOI] [PubMed] [Google Scholar]
- 31.Uga Y, et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013;45:1097–1102. doi: 10.1038/ng.2725. [DOI] [PubMed] [Google Scholar]
- 32.Yoshihara T, Spalding EP. LAZY genes mediate the effects of gravity on auxin gradients and plant architecture. Plant Physiol. 2017;175:959–969. doi: 10.1104/pp.17.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guseman JM, Webb K, Srinivasan C, Dardick C. DRO1 influences root system architecture in Arabidopsis and Prunus species. Plant J. 2017;89:1093–1105. doi: 10.1111/tpj.13470. [DOI] [PubMed] [Google Scholar]
- 34.Kirschner GK, et al. ENHANCED GRAVITROPISM 2 encodes a STERILE ALPHA MOTIF-containing protein that controls root growth angle in barley and wheat. Proc. Natl Acad. Sci. Usa. 2021;118:e2101526118. doi: 10.1073/pnas.2101526118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson JM, Kohler AR, Haus MJ, Hollender CA. Arabidopsis weep mutants exhibit narrow root angles. MicroPubl. Biol. 2022;2022:10. doi: 10.17912/micropub.biology.000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato Y, Tada Y. Comparative analysis of various root active promoters by evaluation of GUS expression in transgenic Arabidopsis. Plant Biotechnol. 2021;38:443–448. doi: 10.5511/plantbiotechnology.21.1011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vijaybhaskar V, Subbiah V, Kaur J, Vijayakumari P, Siddiqi I. Identification of a root-specific glycosyltransferase from Arabidopsis and characterization of its promoter. J. Biosci. 2008;33:185–193. doi: 10.1007/s12038-008-0036-5. [DOI] [PubMed] [Google Scholar]
- 38.Moisseyev G, et al. RGPDB: database of root-associated genes and promoters in maize, soybean, and sorghum. Database. 2020;2020:baaa038. doi: 10.1093/database/baaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner T, et al. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell. 2010;22:3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ursache R, et al. GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants. 2021;7:353–364. doi: 10.1038/s41477-021-00862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y, Rubio MC, Alassimone J, Geldner N. A mechanism for localized lignin deposition in the endodermis. Cell. 2013;153:402–412. doi: 10.1016/j.cell.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 42.Naseer S, et al. Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc. Natl Acad. Sci. Usa. 2012;109:10101–10106. doi: 10.1073/pnas.1205726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baxter I, et al. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet. 2009;5:e1000492. doi: 10.1371/journal.pgen.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kajala K, et al. Innovation, conservation, and repurposing of gene function in root cell type development. Cell. 2021;184:3333–3348.e19. doi: 10.1016/j.cell.2021.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Newman, E. I. In Ecological Interactions in Soil: Plants, Microbes and Animals Vol. 76, 107–121 (Blackwell Science Inc,1985).
- 46.Poorter H, et al. How does biomass distribution change with size and differ among species, an analysis for 1200 plant species from five continents. N. Phytol. 2015;208:736–749. doi: 10.1111/nph.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strehmel N, Böttcher C, Schmidt S, Scheel D. Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry. 2014;108:35–46. doi: 10.1016/j.phytochem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Amicucci MJ, et al. Strategy for structural elucidation of polysaccharides: elucidation of a maize mucilage that Harbors Diazotrophic bacteria. Anal. Chem. 2019;91:7254–7265. doi: 10.1021/acs.analchem.9b00789. [DOI] [PubMed] [Google Scholar]
- 49.Duan F, Giehl RFH, Geldner N, Salt DE, von Wirén N. Root zone-specific localization of AMTs determines ammonium transport pathways and nitrogen allocation to shoots. PLoS Biol. 2018;16:e2006024. doi: 10.1371/journal.pbio.2006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeda K, et al. Identification of periplasmic root-cap mucilage in developing columella cells of Arabidopsis thaliana. Plant Cell Physiol. 2019;60:1296–1303. doi: 10.1093/pcp/pcz047. [DOI] [PubMed] [Google Scholar]
- 51.McLaughlin S, Zhalnina K, Kosina S, Northen TR, Sasse J. The core metabolome and root exudation dynamics of three phylogenetically distinct plant species. Nat. Commun. 2023;14:1649. doi: 10.1038/s41467-023-37164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zickenrott I-M, Woche SK, Bachmann J, Ahmed MA, Vetterlein D. An efficient method for the collection of root mucilage from different plant species—A case study on the effect of mucilage on soil water repellency. J. Plant Nutr. Soil Sci. 2016;179:294–302. doi: 10.1002/jpln.201500511. [DOI] [Google Scholar]
- 53.Nazari M, et al. Mucilage polysaccharide composition and exudation in maize from contrasting climatic regions. Front. Plant Sci. 2020;11:587610. doi: 10.3389/fpls.2020.587610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaeger CH, 3rd, Lindow SE, Miller W, Clark E, Firestone MK. Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Appl. Environ. Microbiol. 1999;65:2685–2690. doi: 10.1128/AEM.65.6.2685-2690.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hawes MC, Gunawardena U, Miyasaka S, Zhao X. The role of root border cells in plant defense. Trends Plant Sci. 2000;5:128–133. doi: 10.1016/S1360-1385(00)01556-9. [DOI] [PubMed] [Google Scholar]
- 56.Hawes MC, Bengough G, Cassab G, Ponce G. Root caps and rhizosphere. J. Plant Growth Regul. 2002;21:352–367. doi: 10.1007/s00344-002-0035-y. [DOI] [Google Scholar]
- 57.Kumpf RP, Nowack MK. The root cap: a short story of life and death. J. Exp. Bot. 2015;66:5651–5662. doi: 10.1093/jxb/erv295. [DOI] [PubMed] [Google Scholar]
- 58.Endo I, Tange T, Osawa H. A cell-type-specific defect in border cell formation in the acacia mangium root cap developing an extraordinary sheath of sloughed-off cells. Ann. Bot. 2011;108:279–290. doi: 10.1093/aob/mcr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Driouich, A. et al. In Secretions and Exudates in Biological Systems (eds. Vivanco, J. M. & Baluška, F.) 91–107 (Springer Berlin Heidelberg, 2012).
- 60.Kumar N, Iyer-Pascuzzi AS. Shedding the last layer: mechanisms of root cap cell release. Plants. 2020;9:308. doi: 10.3390/plants9030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iijima M, Higuchi T, Barlow PW. Contribution of root cap mucilage and presence of an intact root cap in maize (Zea mays) to the reduction of soil mechanical impedance. Ann. Bot. 2004;94:473–477. doi: 10.1093/aob/mch166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roué J, et al. Root cap size and shape influence responses to the physical strength of the growth medium in Arabidopsis thaliana primary roots. J. Exp. Bot. 2020;71:126–137. doi: 10.1093/jxb/erz418. [DOI] [PubMed] [Google Scholar]
- 63.Tomobe H, et al. A mechanical theory of competition between plant root growth and soil pressure reveals a potential mechanism of root penetration. Sci. Rep. 2023;13:7473. doi: 10.1038/s41598-023-34025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosskopf, U., Uteau, D. & Peth, S. Effects of mucilage concentration at different water contents on mechanical stability and elasticity in a loamy and a sandy soil. Eur. J. Soil Sci. 10.1111/ejss.13189 (2022).
- 65.Ahmed MA, Kroener E, Holz M, Zarebanadkouki M, Carminati A. Mucilage exudation facilitates root water uptake in dry soils. Funct. Plant Biol. 2014;41:1129–1137. doi: 10.1071/FP13330. [DOI] [PubMed] [Google Scholar]
- 66.Preece C, Peñuelas J. Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil. 2016;409:1–17. doi: 10.1007/s11104-016-3090-z. [DOI] [Google Scholar]
- 67.Edmond Ghanem M, et al. Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: localization and composition in relation to salt stress. J. Plant Physiol. 2010;167:382–392. doi: 10.1016/j.jplph.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe T, Misawa S, Hiradate S, Osaki M. Characterization of root mucilage from Melastoma malabathricum, with emphasis on its roles in aluminum accumulation. N. Phytol. 2008;178:581–589. doi: 10.1111/j.1469-8137.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- 69.Knee EM, et al. Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Mol. Plant. Microbe Interact. 2001;14:775–784. doi: 10.1094/MPMI.2001.14.6.775. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed MA, et al. Soil microorganisms exhibit enzymatic and priming response to root mucilage under drought. Soil Biol. Biochem. 2018;116:410–418. doi: 10.1016/j.soilbio.2017.10.041. [DOI] [Google Scholar]
- 71.Pankievicz VCS, et al. Nitrogen fixation and mucilage production on maize aerial roots is controlled by aerial root development and border cell functions. Front. Plant Sci. 2022;13:977056. doi: 10.3389/fpls.2022.977056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng H, et al. Chemotaxis of beneficial rhizobacteria to root exudates: the first step towards root-microbe Rhizosphere interactions. Int. J. Mol. Sci. 2021;22:6655. doi: 10.3390/ijms22136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oku S, Komatsu A, Nakashimada Y, Tajima T, Kato J. Identification of Pseudomonas fluorescens chemotaxis sensory proteins for malate, succinate, and fumarate, and their involvement in root colonization. Microbes Environ. 2014;29:413–419. doi: 10.1264/jsme2.ME14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neal AL, Ahmad S, Gordon-Weeks R, Ton J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS One. 2012;7:e35498. doi: 10.1371/journal.pone.0035498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, et al. Induced root-secreted D-galactose functions as a chemoattractant and enhances the biofilm formation of Bacillus velezensis SQR9 in an McpA-dependent manner. Appl. Microbiol. Biotechnol. 2020;104:785–797. doi: 10.1007/s00253-019-10265-8. [DOI] [PubMed] [Google Scholar]
- 76.Cox DE, et al. ABC transporter genes ABC-C6 and ABC-G33 alter plant-microbe-parasite interactions in the rhizosphere. Sci. Rep. 2019;9:19899. doi: 10.1038/s41598-019-56493-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun, C., Li, Q., Han, L., Chen, X. & Zhang, F. The effects of allelochemicals from root exudates of Flaveria bidentis on two Bacillus species. Front. Plant Sci.13, 10.3389/fpls.2022.1001208 (2022). [DOI] [PMC free article] [PubMed]
- 78.Dyer S, et al. Ethylene Response Factor (ERF) genes modulate plant root exudate composition and the attraction of plant parasitic nematodes. Int. J. Parasitol. 2019;49:999–1003. doi: 10.1016/j.ijpara.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Warnock ND, et al. Exogenous RNA interference exposes contrasting roles for sugar exudation in host-finding by plant pathogens. Int. J. Parasitol. 2016;46:473–477. doi: 10.1016/j.ijpara.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Savka MA, Farrand SK. Modification of rhizobacterial populations by engineering bacterium utilization of a novel plant-produced resource. Nat. Biotechnol. 1997;15:363–368. doi: 10.1038/nbt0497-363. [DOI] [PubMed] [Google Scholar]
- 81.Oger PM, Mansouri H, Nesme X, Dessaux Y. Engineering root exudation of lotus toward the production of two novel carbon compounds leads to the selection of distinct microbial populations in the rhizosphere. Microb. Ecol. 2004;47:96–103. doi: 10.1007/s00248-003-2012-9. [DOI] [PubMed] [Google Scholar]
- 82.Savka, M. A. et al. In Molecular Microbial Ecology of the Rhizosphere. 1145–1161 (John Wiley & Sons, Inc., 2013).
- 83.Geddes BA, et al. Engineering transkingdom signalling in plants to control gene expression in rhizosphere bacteria. Nat. Commun. 2019;10:3430. doi: 10.1038/s41467-019-10882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cole BJ, et al. Genome-wide identification of bacterial plant colonization genes. PLoS Biol. 2017;15:e2002860. doi: 10.1371/journal.pbio.2002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Björklöf K, Sen R, Jørgensen KS. Maintenance and impacts of an inoculated mer/luc-tagged Pseudomonas fluorescens on microbial communities in birch rhizospheres developed on humus and peat. Microb. Ecol. 2003;45:39–52. doi: 10.1007/s00248-002-2018-8. [DOI] [PubMed] [Google Scholar]
- 86.Li E, et al. Experimental-evolution-driven identification of Arabidopsis Rhizosphere competence genes in Pseudomonas protegens. MBio. 2021;12:e0092721. doi: 10.1128/mBio.00927-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nordgaard M, et al. Experimental evolution of Bacillus subtilis on Arabidopsis thaliana roots reveals fast adaptation and improved root colonization. iScience. 2022;25:104406. doi: 10.1016/j.isci.2022.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tas H, Grozinger L, Stoof R, de Lorenzo V, Goñi-Moreno Á. Contextual dependencies expand the re-usability of genetic inverters. Nat. Commun. 2021;12:355. doi: 10.1038/s41467-020-20656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brophy JAN, et al. Engineered integrative and conjugative elements for efficient and inducible DNA transfer to undomesticated bacteria. Nat. Microbiol. 2018;3:1043–1053. doi: 10.1038/s41564-018-0216-5. [DOI] [PubMed] [Google Scholar]
- 90.Ke J, et al. CRAGE-CRISPR facilitates rapid activation of secondary metabolite biosynthetic gene clusters in bacteria. Cell Chem. Biol. 2022;29:696–710.e4. doi: 10.1016/j.chembiol.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 91.Haney CH, et al. Rhizosphere-associated Pseudomonas induce systemic resistance to herbivores at the cost of susceptibility to bacterial pathogens. Mol. Ecol. 2018;27:1833–1847. doi: 10.1111/mec.14400. [DOI] [PubMed] [Google Scholar]
- 92.Raaijmakers JM, et al. Dose-response relationships in biological control of fusarium wilt of radish by Pseudomonas spp. Phytopathology. 1995;85:1075–1081. doi: 10.1094/Phyto-85-1075. [DOI] [Google Scholar]
- 93.Peng G, et al. Engineering Bacillus velezensis with high production of acetoin primes strong induced systemic resistance in Arabidopsis thaliana. Microbiol. Res. 2019;227:126297. doi: 10.1016/j.micres.2019.126297. [DOI] [PubMed] [Google Scholar]
- 94.Purtschert-Montenegro G, et al. Pseudomonas putida mediates bacterial killing, biofilm invasion and biocontrol with a type IVB secretion system. Nat. Microbiol. 2022;7:1547–1557. doi: 10.1038/s41564-022-01209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu F, et al. Characteristics of biological control and mechanisms of Pseudomonas chlororaphis zm-1 against peanut stem rot. BMC Microbiol. 2022;22:9. doi: 10.1186/s12866-021-02420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shahid, I. et al. Profiling of metabolites of Bacillus spp. and their application in sustainable plant growth promotion and biocontrol. Front. Sustain. Food Syst.10.3389/fsufs.2021.605195 (2021).
- 97.Dong YH, Xu JL, Li XZ, Zhang LH. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl Acad. Sci. USA. 2000;97:3526–3531. doi: 10.1073/pnas.97.7.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeuchi K, Someya N, Morohoshi T. Genome-mining approaches for the evaluation of Pseudomonas protegens and related strains isolated from the rhizosphere in Japan. Physiol. Mol. Plant Pathol. 2023;125:101981. doi: 10.1016/j.pmpp.2023.101981. [DOI] [Google Scholar]
- 99.Ahmad HM, et al. Plant growth-promoting rhizobacteria eliminate the effect of drought stress in plants: a review. Front. Plant Sci. 2022;13:875774. doi: 10.3389/fpls.2022.875774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu X, et al. Epsc involved in the encoding of exopolysaccharides produced by bacillus amyloliquefaciens FZB42 act to boost the drought tolerance of Arabidopsis thaliana. Int. J. Mol. Sci. 2018;19:3795. doi: 10.3390/ijms19123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang D-C, et al. Biofilms positively contribute to bacillus amyloliquefaciens 54-induced Drought tolerance in tomato plants. Int. J. Mol. Sci. 2019;20:6271. doi: 10.3390/ijms20246271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orozco-Mosqueda MDC, Santoyo G, Glick BR. Recent avances in the bacterial phytohormone modulation of plant growth. Plants. 2023;12:606. doi: 10.3390/plants12030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cassán F, et al. Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur. J. Soil Biol. 2009;45:12–19. doi: 10.1016/j.ejsobi.2008.08.003. [DOI] [Google Scholar]
- 104.Vílchez JI, García-Fontana C, Román-Naranjo D, González-López J, Manzanera M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016;7:1577. doi: 10.3389/fmicb.2016.01577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cho S-M, Kim YH, Anderson AJ, Kim YC. Nitric oxide and hydrogen peroxide production are involved in systemic drought tolerance induced by 2 R,3R-butanediol in Arabidopsis thaliana. Plant Pathol. J. 2013;29:427–434. doi: 10.5423/PPJ.OA.07.2013.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodríguez-Salazar J, Suárez R, Caballero-Mellado J, Iturriaga G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiol. Lett. 2009;296:52–59. doi: 10.1111/j.1574-6968.2009.01614.x. [DOI] [PubMed] [Google Scholar]
- 107.Wang C, Knill E, Glick BR, Défago G. Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can. J. Microbiol. 2000;46:898–907. doi: 10.1139/w00-071. [DOI] [PubMed] [Google Scholar]
- 108.Liu Y, Cao L, Tan H, Zhang R. Surface display of ACC deaminase on endophytic enterobacteriaceae strains to increase saline resistance of host rice sprouts by regulating plant ethylene synthesis. Microb. Cell Fact. 2017;16:214. doi: 10.1186/s12934-017-0831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu J, Li X-L, Luo L. Effects of engineered sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress. Appl. Environ. Microbiol. 2012;78:8056–8061. doi: 10.1128/AEM.01276-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orozco-Mosqueda MDC, et al. The production of ACC deaminase and trehalose by the plant growth promoting bacterium pseudomonas sp. UW4 synergistically protect tomato plants against salt stress. Front. Microbiol. 2019;10:1392. doi: 10.3389/fmicb.2019.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Menegat S, Ledo A, Tirado R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022;12:14490. doi: 10.1038/s41598-022-18773-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ryu M-H, et al. Control of nitrogen fixation in bacteria that associate with cereals. Nat. Microbiol. 2020;5:314–330. doi: 10.1038/s41564-019-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smanski MJ, et al. Functional optimization of gene clusters by combinatorial design and assembly. Nat. Biotechnol. 2014;32:1241–1249. doi: 10.1038/nbt.3063. [DOI] [PubMed] [Google Scholar]
- 114.Takimoto R, et al. A critical role of an oxygen-responsive gene for aerobic nitrogenase activity in Azotobacter vinelandii and its application to Escherichia coli. Sci. Rep. 2022;12:4182. doi: 10.1038/s41598-022-08007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schnabel T, Sattely E. Engineering posttranslational regulation of glutamine synthetase for controllable ammonia production in the plant symbiont Azospirillum brasilense. Appl. Environ. Microbiol. 2021;87:e0058221. doi: 10.1128/AEM.00582-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oteino N, et al. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McRose DL, Newman DK. Redox-active antibiotics enhance phosphorus bioavailability. Science. 2021;371:1033–1037. doi: 10.1126/science.abd1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Trapet P, et al. The Pseudomonas fluorescens siderophore pyoverdine weakens arabidopsis thaliana defense in favor of growth in iron-deficient conditions. Plant Physiol. 2016;171:675–693. doi: 10.1104/pp.15.01537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wagh J, et al. Inoculation of genetically modified endophytic herbaspirillum seropedicae Z67 endowed with gluconic and 2-ketogluconic acid secretion, confers beneficial effects on rice (Oriza sativa) plants. Plant Soil. 2016;409:51–64. doi: 10.1007/s11104-016-2937-7. [DOI] [Google Scholar]
- 120.Shulse CN, et al. Engineered root bacteria release plant-available phosphate from phytate. Appl. Environ. Microbiol. 2019;85:e01210–19. doi: 10.1128/AEM.01210-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paustian, K., Larson, E., Kent, J., Marx, E. & Swan, A. Soil C sequestration as a biological negative emission strategy. Front. Clim.10.3389/fclim.2019.00008 (2019).
- 122.Ludwig M, et al. Microbial contribution to SOM quantity and quality in density fractions of temperate arable soils. Soil Biol. Biochem. 2015;81:311–322. doi: 10.1016/j.soilbio.2014.12.002. [DOI] [Google Scholar]
- 123.Tao F, et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature. 2023;618:981–985. doi: 10.1038/s41586-023-06042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mason-Jones K, Breidenbach A, Dyckmans J, Banfield CC, Dippold MA. Intracellular carbon storage by microorganisms is an overlooked pathway of biomass growth. Nat. Commun. 2023;14:2240. doi: 10.1038/s41467-023-37713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kopittke PM, et al. Soil organic matter is stabilized by organo-mineral associations through two key processes: the role of the carbon to nitrogen ratio. Geoderma. 2020;357:113974. doi: 10.1016/j.geoderma.2019.113974. [DOI] [Google Scholar]
- 126.Zúñiga A, et al. An engineered device for indoleacetic acid production under quorum sensing signals enables cupriavidus pinatubonensis JMP134 To stimulate plant growth. ACS Synth. Biol. 2018;7:1519–1527. doi: 10.1021/acssynbio.8b00002. [DOI] [PubMed] [Google Scholar]
- 127.Gilbert S, et al. Auxin-producing bacteria from duckweeds have different colonization patterns and effects on plant morphology. Plants. 2022;11:721. doi: 10.3390/plants11060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Orozco-Mosqueda MDC, Glick BR, Santoyo G. ACC deaminase in plant growth-promoting bacteria (PGPB): an efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020;235:126439. doi: 10.1016/j.micres.2020.126439. [DOI] [PubMed] [Google Scholar]
- 129.Edwards J, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl Acad. Sci. USA. 2015;112:E911–E920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bai Y, et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature. 2015;528:364–369. doi: 10.1038/nature16192. [DOI] [PubMed] [Google Scholar]
- 131.Lozano GL, et al. Introducing THOR, a model microbiome for genetic dissection of community behavior. MBio. 2019;10:e02846–18. doi: 10.1128/mBio.02846-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ali M, Ahmad Z, Ashraf MF, Dong W. Maize endophytic microbial-communities revealed by removing PCR and 16 S rRNA sequencing and their synthetic applications to suppress maize banded leaf and sheath blight. Microbiol. Res. 2021;242:126639. doi: 10.1016/j.micres.2020.126639. [DOI] [PubMed] [Google Scholar]
- 133.Ronda C, Chen SP, Cabral V, Yaung SJ, Wang HH. Metagenomic engineering of the mammalian gut microbiome in situ. Nat. Methods. 2019;16:167–170. doi: 10.1038/s41592-018-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ando H, Lemire S, Pires DP, Lu TK. Engineering modular viral scaffolds for targeted bacterial population editing. Cell Syst. 2015;1:187–196. doi: 10.1016/j.cels.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rubin BE, et al. Species- and site-specific genome editing in complex bacterial communities. Nat. Microbiol. 2022;7:34–47. doi: 10.1038/s41564-021-01014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guillotin B, et al. A pan-grass transcriptome reveals patterns of cellular divergence in crops. Nature. 2023;617:785–791. doi: 10.1038/s41586-023-06053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marand AP, Chen Z, Gallavotti A, Schmitz RJ. A cis-regulatory atlas in maize at single-cell resolution. Cell. 2021;184:3041–3055.e21. doi: 10.1016/j.cell.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 138.Ron M, et al. Hairy root transformation using agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Physiol. 2014;166:455–469. doi: 10.1104/pp.114.239392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coy MR, Abbitt SE, Frank MJ. Protoplast isolation and transfection in maize. Methods Mol. Biol. 2022;2464:91–104. doi: 10.1007/978-1-0716-2164-6_7. [DOI] [PubMed] [Google Scholar]
- 140.Goodin MM, Zaitlin D, Naidu RA, Lommel SA. Nicotiana benthamiana: its history and future as a model for plant-pathogen interactions. Mol. Plant. Microbe Interact. 2008;21:1015–1026. doi: 10.1094/MPMI-21-8-1015. [DOI] [PubMed] [Google Scholar]
- 141.Helber N, Requena N. Expression of the fluorescence markers DsRed and GFP fused to a nuclear localization signal in the arbuscular mycorrhizal fungus glomus intraradices. N. Phytol. 2008;177:537–548. doi: 10.1111/j.1469-8137.2007.02257.x. [DOI] [PubMed] [Google Scholar]
- 142.Stirling F, Silver PA. Controlling the Implementation of transgenic microbes: are we ready for what synthetic Biology sas to offer. Mol. Cell. 2020;78:614–623. doi: 10.1016/j.molcel.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.