Summary
Stroke prevention is central to the management of patients with atrial fibrillation (AF) which has moved towards a more holistic or integrative care approach. The published evidence suggests that management of AF patients following such a holistic approach based on the Atrial fibrillation Better Care (ABC) pathway is associated with a lower risk of stroke and adverse events. Risk assessment, re-assessment and use of direct oral anticoagulants (DOACs) are important for stroke prevention in AF. The stroke and bleeding risks of AF patients are not static and should be re-assessed regularly. Bleeding risk assessment is to address and mitigate modifiable bleeding risk factors, and to identify high bleeding risk patients for early review and follow-up. Well-controlled comorbidities and healthy lifestyles also play an important role to achieve a better clinical outcome. Digital health solutions are increasingly relevant in the diagnosis and management of patients with AF, with the potential to improve stroke prevention. In this review, we provide an update on stroke prevention in AF, including importance of holistic management, risk assessment/re-assessment, and stroke prevention for special AF populations. Evidence-based and structured management of AF patients would reduce the risk of stroke and other adverse events.
Keywords: Atrial fibrillation, Stroke risk, Bleeding risk, ABC pathway, Oral anticoagulants
Introduction
Atrial fibrillation (AF) is the commonest heart rhythm disorder, leading to an increased risk of stroke and mortality, as well as hospitalisation from heart failure. Strokes associated with AF are associated with greater mortality and disability, and lower rates of discharge to own homes, leading to much focus on stroke prevention as one of main pillars of AF management.1,2
Key messages.
-
•
The ABC (“A" Avoid stroke with Anticoagulation; “B" Better symptoms control; “C" Cardiovascular risk factors and comorbidities management) pathway is a simple and structured holistic approach for patients with atrial fibrillation (AF) which would result in a lower risk of ischaemic stroke and better clinical outcomes.
-
•
The stroke risk represented by the CHA2DS2-VASc score of AF patients is not static and should be re-assessed regularly (4–6 months after the index evaluation in European Society of Cardiology guidelines; at least every year and every 4 months if possible in Asia Pacific Heart Rhythm Society guidelines).
-
•
Bleeding risk assessment is to address and mitigate modifiable bleeding risk factors, and to identify high bleeding risk patients for early review and follow-up.
-
•
Direct oral anticoagulants (DOACs) are generally the preferred choice over warfarin for stroke prevention in AF, while the importance of appropriate dosing of DOACs should be emphasized since underdosing could be a reason for breakthrough stroke.
-
•
In addition to oral anticoagulants (OACs), early rhythm control and well managements of cardiovascular risk factors and comorbidities could also improve clinical outcomes of AF patients.
-
•
Factor XI inhibitors are “novel” OACs which may potentially provide new choice for stroke prevention in AF once their efficacy and safety are proved by ongoing phase III trials.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed with the search terms “atrial fibrillation”, “stroke prevention”, “oral anticoagulant”, and “bleeding” from 2000 until May, 2023. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
The risk of stroke in AF is not homogenous, and depends on the presence or absence of various stroke risk factors. The more common and validated stroke risk factors have been used to formulate stroke risk stratification scores, to aid clinical decision making and the use of stroke prevention therapies.3 Previous studies have looked at the risk of stroke in relation to the presence or absence of a particular stroke risk factor. However, what we do increasingly realise is that patients with AF very often have more than one comorbidity, so-called multimorbidity, which substantially increases the risk of stroke and thromboembolism as well as other adverse complications.4,5
Recent studies have tried to focus on these clinically complex patients. Proietti et al.5 reported on the long-term relationship between AF multimorbidity (as defined by the Charlson Comorbidity Index, CCI) and the use of anticoagulant therapies. Between 2002 and 2014, there was a gradual increase in mean CCI in the non-AF cohort, which is unsurprising given the increasingly mean age of the general population as well as the improved care of patients with acute cardiac conditions, such as myocardial infarction. In contrast, the patients with AF had a much steeper rise in their CCI between 2002 and 2014, and 18% of the overall AF cohort had a high multimorbidity (CCI ≥4). This increased CCI translated to higher risk of complications such as stroke, major bleeding and all-cause mortality. Despite their high risk nature, there was an inverse relationship between CCI and use of oral anticoagulants (OACs) to reduce the risk of stroke. Hence, there was a major treatment gap despite recognising that these patients are at substantially high risk of adverse cardiac events.
Apart from multimorbidity, AF patients also had a higher frailty.6 Frail AF patients were associated with a higher risk of all-cause death (odds ratio [OR] 5.56, 95% confidence interval [CI] 3.46–8.94), ischaemic stroke (OR 1.59, 95% CI 1.00–2.52), and bleeding (OR 1.64, 95% CI 1.11–2.41), when compared to robust individuals. Despite being on OACs, there remains a residue risk of adverse events, and therefore, the current AF management has moved towards a much more holistic or integrative care. In this review, we provide an update on stroke prevention in AF, including importance of holistic management, risk assessment/re-assessment, and stroke prevention strategy for special AF populations.
A holistic approach to risk assessment and management of atrial fibrillation
The steps for a holistic approach to risk assessment and management of AF patients includes the consideration of stroke prevention where appropriate, patient-centred and symptom-directed decisions on rate or rhythm control therapy, and finally, the addressing of cardiovascular risk factors, comorbidities and lifestyle modifications.7 This patient pathway or patient journey highlights the necessity of managing AF in a holistic manner, whether the patient is managed by the general practitioner, internal medicine, stroke medicine or the cardiologist.
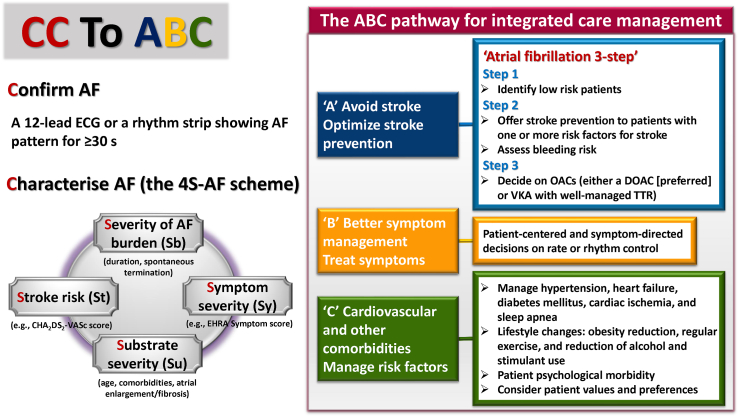
This is summarised in the European guidelines as the CC to ABC (Fig. 1).8 The CC stands for firstly to Confirm AF, then to Characterise AF, the latter by using the 4S-AF scheme.10 The “ABC (Atrial fibrillation Better Care)” represents an integrated approach to improve management in AF based on 3 pillars: “A" Avoid stroke with Anticoagulation; “B" Better symptoms control; “C" Cardiovascular risk factors and comorbidities management.
Fig. 1.
CC to ABC—the standard of diagnosis, evaluation and integrated care management of AF. AF = atrial fibrillation; DOACs = direct oral anticoagulants; ECG = electrocardiogram; VKA = vitamin-K antagonist; OACs = oral anticoagulants; TTR = time in therapeutic range. The figure was redrawn and modified from 2020 ESC AF guidelines and 2021 focused update consensus guidelines of the APHRS on stroke prevention in atrial fibrillation.8,9
Confirm AF
The diagnosis of AF could be made by a standard 12-lead ECG recording or a single-lead ECG tracing of ≥30s reviewed by the physicians.11 Digital health solutions with smart wearables are more and more commonly adopted in the daily practice which could provide the opportunity to screen for and diagnose AF and have been employed on a mass population screening in large studies such as the Apple Heart Study,12 the Huawei Heart Study13 and the Fitbit Heart Study.14 These smart wearables can be divided into those based on photoplethysmogram (PPG) signals and those which provide a single lead ECG to detect AF.15 Importantly, although more and more smart wearables could detect AF based on PPG, the diagnosis of AF cannot be made solely based on the notifications.13,15 Indeed, the Apple Heart study demonstrated that the positive predictive value of AF based on PPG-based notification of irregular heart beat was only around 0.84.12 Therefore, an ECG strip showing AF confirmed by the physician is still required.
A potential application of smart wearables is to detect or diagnose AF for stroke survivors.16,17 The CRYSTAL AF trial demonstrated that AF was detected in 12.4% of patients by 12 months after the implantation of insertable cardiac monitor.18 With the improving technology, smart wearables may be able to replace the role of insertable cardiac monitor for patients who could operate the smartwatch well in the near future.
Characterise AF (the 4S-AF scheme)
The 4S-AF scheme10 comprises four domains: stroke risk (St), symptoms (Sy), severity of AF burden (Sb), and substrate (Su).
Stroke risk is assessed by the CHA2DS2-VASc score; Symptom severity by the EHRA symptom score; Severity of AF burden, classified as paroxysmal, persistent or permanent in nature, with detailed information about durations/frequencies of AF episodes if available; and Substrate severity as reflected by age, structural heart disease and comorbidities. Also, imaging modalities could be used to demonstrate atrial fibrosis and diagnose atrial cardiomyopathy.10
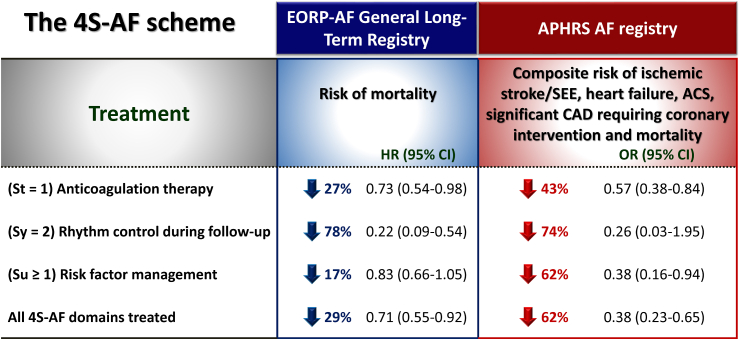
Various studies have shown that appropriate characterisations of AF patients according to the 4S-AF scheme followed by corresponding managements were associated with improved clinical outcomes (Fig. 2).19, 20, 21, 22 Patients with all 4S-AF domains treated were associated with a 29% lower risk of mortality in EORP-AF registry, and a 62% lower risk of composite outcome of ischaemic stroke/systemic embolic events (SEEs), heart failure, acute coronary syndrome, significant coronary artery disease requiring coronary intervention and mortality in APHRS registry.19,20 For Sb domain, the AF burden should ideally be assessed via continuous monitoring, such as the atrial lead of the pacemaker or insertable cardiac monitor. Prior study showed significant interactions between the AF duration and CHA2DS2-VASc score regarding the risk of ischaemic stroke/SEE.23 The stroke/SEE rates were low in patients with a CHA2DS2-VASc score of 0–1 regardless of device-detected AF duration. However, stroke risk crossed an actionable threshold defined as >1%/year in patients with a CHA2DS2-VASc score of 2 with >23.5 h of AF, those with a CHA2DS2-VASc score of 3–4 with >6 min of AF, and patients with a CHA2DS2-VASc score ≥5 even with no AF.23 Whether information of AF burden could further guide the stroke prevention strategy in AF patients with an “intermediate” CHA2DS2-VASc score deserves further studies.
Fig. 2.
Effects of treatments according to 4S-AF scheme. Data in this figure were adapted from the papers by Ding et al.19 and Chao et al.20
The atrial fibrillation better care (ABC) pathway
The ABC pathway was proposed as a simple strategy that streamlines primary and secondary care management of patients with AF.7 The ABC pathway allows early intervention in a simple and structured holistic approach that is applicable whether the patient is managed in primary or secondary care, by cardiologists or non-cardiologists. This issue is important since several previous studies demonstrated that AF patients diagnosed or cared by cardiologist had a better outcome compared to non-cardiologist, probably owing to a higher rate of appropriate stroke prevention with OACs and better rate control or rhythm control treatments.24, 25, 26 Actually, nurse-led care of patients with AF is superior to usual care provided by a cardiologist in terms of cardiovascular hospitalizations and cardiovascular mortality,27 which emphasized the importance of multidisciplinary AF management including specialist nurses as part of holistic AF care.
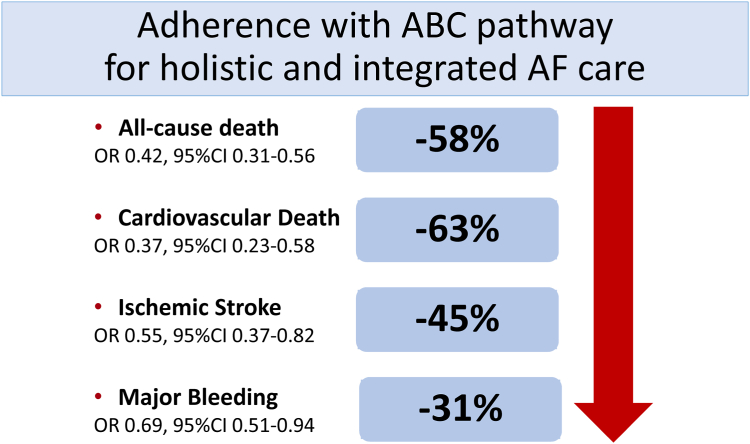
The evidence-based ABC pathway has now been investigated in various retrospective analyses, prospective cohorts and post-hoc analyses of trial cohorts,28,29 as well as one published prospective randomized controlled trial (RCT), the mAFA-II trial.30 In a systematic review and meta-analysis of over 285,000 patients, adherence or compliance with the ABC pathway was present in 21%, which translated to a 58% reduction in all-cause death, 63% reduction in cardiovascular death, a 45% reduction in ischaemic stroke, and a 31% reduction in major bleeding (Fig. 3).29 Ongoing prospective RCTs with the ABC pathway such as the Horizon Europe funded AFFIRMO programme31 and the MIRACLE-AF (ClinicalTrials.gov NCT04622514) will provide further trial evidence.
Fig. 3.
Impacts of adherence to the ABC pathway on clinical outcomes in AF patients. The figure was redraw and modified from the paper by Romiti et al.29
“A” domain of the ABC pathway
The A domain in the ABC pathway includes 3 steps for stroke prevention in AF (Fig. 1).
Step 1: Identify low risk patients. Step 2: Offer stroke prevention to patients with one or more risk factors for stroke. Assess bleeding risk. Step 3: Decide on OACs (either a direct oral anticoagulant [DOAC] [preferred] or vitamin K antagonist [VKA] with well-managed time in the therapeutic range [TTR])
Current concepts in stroke risk assessment
Many attempts over the years have tried to identify better ways to define or identify the high risk patients who could be targeted for oral anticoagulant therapy. Currently, the recommended scoring scheme for stroke risk assessment has shifted from the CHADS2 to CHA2DS2-VASc score (Table 1), aiming at facilitating decision-making for stroke prevention. There are several issues which should be clarified regarding the use of the scoring scheme.
Table 1.
CHA2DS2-VASc and HAS-BLED scores.
| CHA2DS2-VASc score–Risk factors and definitions | Point awarded | |
|---|---|---|
| C |
Congestive heart failure Clinical HF, or objective evidence of moderate to severe LV dysfunction, or HCM |
1 |
| H | Hypertension or on antihypertensive therapy | 1 |
| A | Age 75 years or older | 2 |
| D |
Diabetes mellitus Treatment with oral hypoglycemic drugs and/or insulin or fasting blood glucose >125 mg/dL (7 mmol/L) |
1 |
| S |
Stroke Previous stroke, TIA, or thromboembolism |
2 |
| V |
Vascular disease Angiographically significant CAD, previous myocardial infarction, PAD, or aortic plaque |
1 |
| A | Age 65–74 years | 1 |
| Sc | Sex category (female) | 1 |
| Maximum score | 9 | |
| HAS-BLED score–Risk factors and definitions | ||
| H |
Uncontrolled hypertension SBP >160 mmHg |
1 |
| A |
Abnormal renal and/or hepatic function Dialysis, transplant, serum creatinine >200 mmol/L, cirrhosis, bilirubin >x 2 upper limit of normal, AST/ALT/ALP >3 x upper limit of normal |
1 point for each |
| S |
Stroke Previous ischaemic or hemorrhagic stroke |
1 |
| B |
Bleeding history or predisposition Previous major haemorrhage or anemia or severe thrombocytopenia |
1 |
| L |
Labile INR TTR < 60% in patient receiving VKA |
1 |
| E |
Elderly Aged > 65 years or extreme frailty |
1 |
| D |
Drugs or excessive alcohol drinking Concomitant use of antiplatelet or NSAID; and/or excessive alcohol per week |
1 point for each |
| Maximum score | 9 | |
The table was based on 2020 ESC AF guidelines.8
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CAD = coronary artery disease; HCM = hypertrophic cardiomyopathy; HF = heart failure; INR = international normalized ratio; LV = left ventricle; NSAID = non-steroidal anti-inflammatory drug; PAD peripheral artery disease; SBP = systolic blood pressure; TIA = transient ischaemic attack; TTR = time in therapeutic range; VKA = vitamin K antagonist.
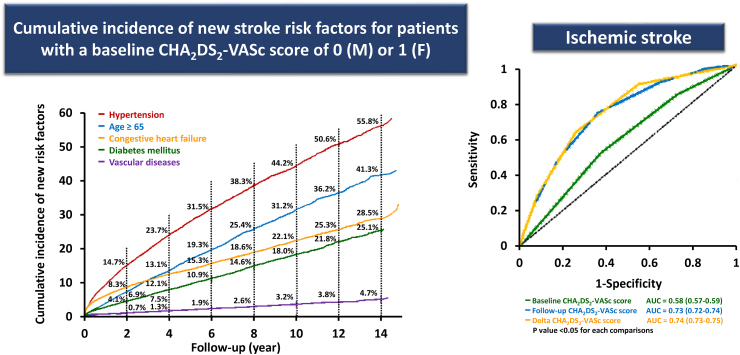
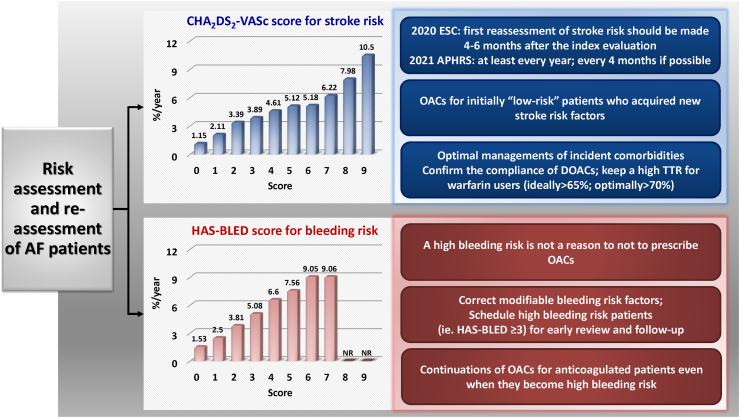
First, the impact of a particular risk factor is very often defined at baseline and used to predict the risk of stroke remotely, such as five or ten years later. There is increasing evidence showing that the risk is not static and actually is dynamic depending on ageing, acquirements of incident comorbidities as well as being altered by polypharmacy and risk factor management.32 Hypertension is the most commonly acquired new stroke risk component, followed by age≥65, heart failure, diabetes mellitus and vascular diseases (Fig. 4).33 Both follow-up CHA2DS2-VASc and delta-CHA2DS2-VASc scores (the difference between the baseline and follow-up scores) had better predictive values compared to the baseline CHA2DS2-VASc score assessed by C-statistics34 (Fig. 4). For patients with an initial CHA2DS2-VASc score of 0 (males 1) or 1 (females), around 11.2% of men and 11.1% of women would have a CHA2DS2-VASc score of at least 1 (men) or 2 (women) at 6 months after incident AF.34 The 2020 ESC AF guidelines recommended that the first reassessment of stroke risk should be made 4–6 months after the index evaluation, while the 2021 APHRS recommended that the stroke risk of AF patients should be re-assessed at least every year and every 4 months if possible.8,9 This is particularly important for initially low-risk patients who did not receive OACs since OACs should be prescribed once their CHA2DS2-VASc scores increased. Even for patients who were already under anticoagulation, the compliance and adherence of OACs should be further emphasized because an increase in the CHA2DS2-VASc score would result in a higher stroke risk.
Fig. 4.
Incident stroke risk factors and the predictive accuracies of baseline, follow-up and delta CHA2DS2-VASc scores. AUC = area under the curve; F = female; M = male. The figure was redrawn, and data were adapted from the papers by Chao et al.33,34
With the availability of sophisticated tools like artificial intelligence and machine learning, the stroke risk stratification can currently account for changing multimorbidity and the dynamic nature of risk factors which would be helpful in the decision-making process for risk assessment.35,36 Even then, clinical risk scores only have modest predictive values for identifying high risk patients. There were some complicated scoring schemes proposed, either by considering more clinical risk factors or including biomarkers (whether urine, blood or imaging) which may show statistically significant but actually very marginal change of the c-index. It should be emphasized that statistical significance is not the same as clinical significance, which needs to be balanced against practical application in busy clinical scenarios. Currently, the role of stroke scoring scheme is to identify patients at low stroke risk and stroke prevention therapies are not necessary.1,2
Does female sex matter? Using CHA2DS2-VASc or CHA2DS2-VA
Female sex is a stroke risk modifier rather than a stroke risk factor per se.37 In patients with 1 or more stroke risk factors, being women adds to the stroke risks compared to men.37,38
In a meta-analysis of published studies, women are associated with a higher risk of all-cause mortality (relative risk 1.12, 95% CI 1.07–1.17), cardiovascular mortality (1.93, 1.44–2.60), stroke (1.99, 1.46–2.71), cardiac events (1.55, 1.15–2.08), and heart failure (1.16, 1.07–1.27) compared to men.39 Importantly, AF women not only have an increased risk of stroke but also experience more severe strokes compared to men.40 In the Tasmanian AF Study (TAFS), female sex was an independent negative predictor of OAC prescriptions (OR 0.83; 95% CI 0.69–0.99).41 The recognition and awareness of AF women having other risk factors as a high risk population may overcome the gap of OAC use.
There were few studies actually validating the use of CHA2DS2-VA (i.e., excluding the female sex criterion, putatively for ‘simplicity’ reasons) as an alternative to CHA2DS2-VASc score.42,43 As mentioned above, these studies showed only a very marginal improvement of predictive accuracy assessed by c-index (still around or even lower than 0.7). Also, there were several methodological limitations. For example, the analyses were performed based on baseline risk factors without accounting for dynamic changes and the assessments only focused on non-anticoagulated patients at baseline without considering the OAC initiation during the follow up. Therefore, the CHA2DS2-VASc rather than CHA2DS2-VA scores should be the preferred scoring scheme for stroke risk assessment.
Patients with a CHA2DS2-VASc score of 1 (men) or 2 (women)
The default setting for stroke prevention is to provide OACs (preferred DOACs) for every AF patient unless they are low risk for stroke (a CHA2DS2-VASc score of 0 for men or 1 for women). Based on this concept, the international guidelines recommended no antithrombotic therapy for AF patients with a CHA2DS2-VASc score of 0 (men) or 1 (women), and OACs should be prescribed for those with a CHA2DS2-VASc score ≥2 (men) or ≥3 (women). However, whether OACs should be prescribed for AF patients with single stroke risk factor beyond gender, that is score 1 for men or 2 for women, is less clear as such patients only represented a small subgroup of the RCTs and other evidence comes from observational cohorts suggesting that the net clinical benefit remains in favour of DOACs.44, 45, 46, 47
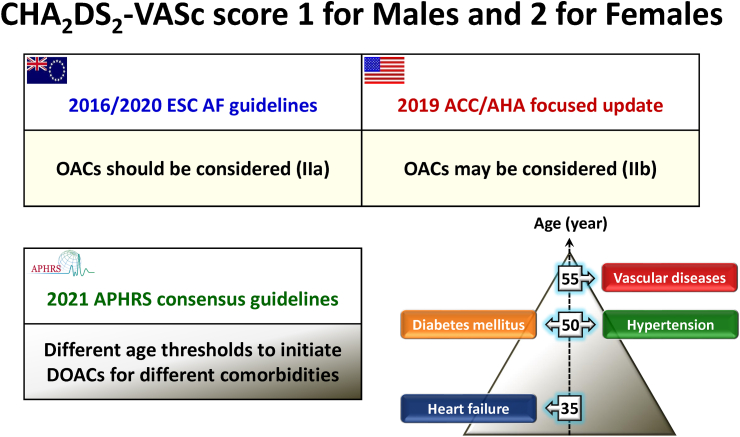
Although the ESC guidelines recommended the use of OACs as a class IIa recommendation for this population,8 it was only listed as a class IIb recommendation in 2019 American College of Cardiology (ACC) and American Heart Association (AHA) guidelines.48 Different from ESC and ACC/AHA, the APHRS guidelines9 provided a detailed recommendation about different age thresholds to initiate DOACs for patients with different single comorbidity (Fig. 5). Based on the tipping point of initiations of DOACs set at a stroke risk of 0.9%/year without OACs, the age thresholds were 35 years for heart failure, 50 for hypertension or diabetes, and 55 for vascular diseases.49 Individual discussion and shared decision making is needed, and consideration of AF burden may also be factored into the discussion.
Fig. 5.
Recommendations about stroke prevention in ESC, ACC/AHA and APHRS guidelines. ACC/AHA = American College of Cardiology/American Heart Association; APHRS = Asia Pacific Heart Rhythm Society; ESC = European Society of Cardiology; DOACs = direct oral anticoagulants; OACs = oral anticoagulants.
Bleeding risk assessment
Like stroke risk, bleeding risk is also highly dynamic, and is an interaction between non-modifiable and modifiable bleeding risk factors.50 Importantly, bleeding risk assessment is to address and mitigate modifiable bleeding risk factors, and to identify high bleeding risk patients for early review and follow-up.50,51
There are various bleeding risk scores that have been published.51 The systematic review and evidence appraisal concluded that the HAS-BLED score (Table 1) provided the best prediction for bleeding risks.52 Although some complex clinical scores and biomarker-based bleeding risk schemes were proposed, there is little evidence showing that they could confer added advantage in the real world practice. Again, a statistical significance is not the same as significant practical application.
The HAS-BLED score is recommended in guidelines and has been tested in the prospective mAFA-II trial which was a prospective cluster randomised trial where patients were managed in usual care or intervention clusters. In the intervention group, a mobile health (mHealth) app with ABC pathway implemented was adopted, and the HAS-BLED score was used to identify and mitigate modifiable bleeding risk factors. What this trial showed was that in the usual care arm, the major bleeding rate at 12 months was 4.3%, while in the intervention arm, the major bleed rate at 12 months was 2.1%.53 In the usual care arm, the use of OACs (either VKAs or DOACs) declined from 58.8% to 34.4% by 12 months. In contrast, oral anticoagulation uptake increased from 63.4% to 70.2% in the intervention arm whereby bleeding risk factors were well managed for high bleeding risk patients.53
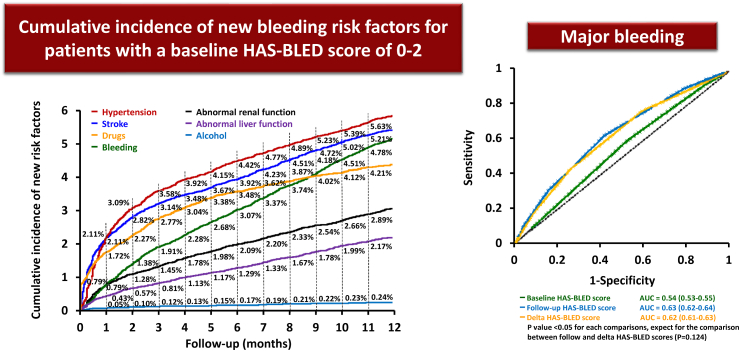
The same as stroke risk, the HAS-BLED score would change over time with the hypertension being as the most commonly incident bleeding risk factor54 (Fig. 6). Also, the accuracies of the follow-up or delta HAS-BLED scores in the prediction of major bleeding were significantly higher than that of the baseline HAS-BLED score55 (Fig. 6). A frequently encountered clinical scenario is the increment of the HAS-BLED score of AF patients who were already under OACs. In a recent report, around 22.2% of anticoagulated AF patients who had a baseline HAS-BLED score of 0–2 wound have an increasing HAS-BLED score to ≥3 at 1 year.54 Patients who kept on OACs even after their HAS-BLED scores increased to ≥3 were associated with a lower risk of ischaemic stroke (hazard ratio [HR] 0.60; 95% CI 0.53–0.69), major bleeding (HR 0.78; 95% CI 0.67–0.91), all-cause mortality (HR 0.88; 95% CI 0.79–0.97), and any adverse events (HR 0.75; 95% CI 0.68–0.82). Therefore, for patients who were initially or become high bleeding risk, the evidence suggests that we should try to correct modifiable bleeding risk factors and schedule them for early review and follow-up, rather than withholding or discontinuing OACs (Fig. 7).
Fig. 6.
Incident bleeding risk factors and the predictive accuracies of baseline, follow-up and delta HAS-BLED scores. AUC = area under the curve. The figure was redrawn, and data were adapted from the papers by Chao et al.54,55
Fig. 7.
Risk assessment and re-assessment in AF. AF = atrial fibrillation; DOACs = direct oral anticoagulants; NR = not reported; OACs = oral anticoagulants; TTR = time in therapeutic range. The figure was redrawn, and data were adapted from the papers by Chao et al.9,56,57
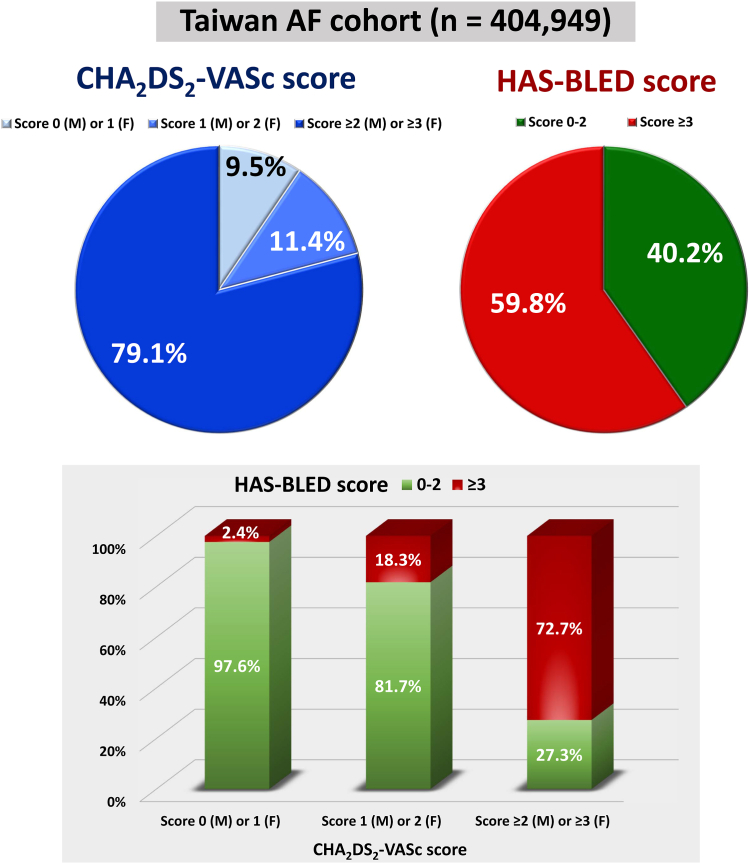
Of note, the CHA2DS2-VASc and HAS-BLED schemes share some risk factor components, and therefore, they would co-distribute to some degree.58 Overall, only around 10% of AF patients had a CHA2DS2-VASc score of 0 (males) or 1 (females), and 40.2% had a low risk of bleeding (HAS-BLED score 0–2) (Fig. 8). Most importantly, although these 2 scoring schemes were correlated, there were not exchangeable. For AF patients with a single stroke risk factor (CHA2DS2-VASc score of 1 for males or 2 for females), still around 18.3% of them were at high bleeding risk (HAS-BLED score ≥3).58 Even among these patients, the use of OACs was associated with a lower risk of composite adverse events of ischaemic stroke, intracranial haemorrhage (ICH), or mortality (4.19/100 person-years vs. 5.22/100 person-years, HR 0.78; 95% CI 0.62–0.99).58 Therefore, a high bleeding risk is not a reason to not to prescribe OACs, as recommended in Fig. 7.
Fig. 8.
Distribution and co-distribution of CHA2DS2-VASc and HAS-BLED score. AF = atrial fibrillation; F = female; M = male. Data in this figure were adapted from the paper by Chao et al.58
Obviously, stroke and bleeding risk assessments are the central part of these 3 steps. Most international guidelines recommended the use of CHA2DS2-VASc and HAS-BLED scores to assess the risks of stroke and bleeding for AF patients, respectively. The risks of ischaemic stroke (non-anticoagulated) and major bleeding (anticoagulated) of different CHA2DS2-VASc and HAS-BLED scores, for example, are shown in Fig. 7.56,57
Evidence for warfarin and DOACs for stroke prevention in AF
In a meta-analysis of the historical trials, Hart et al.59 reported that when compared with the control, adjusted-dose warfarin (6 trials, 2900 participants) and antiplatelet agents (8 trials, 4876 participants) reduced stroke by 64% (95% CI 49%–74%) and 22% (95% CI 6%–35%), respectively. Mortality was also reduced by warfarin, but not antiplatelet therapy, when compared to control. Adjusted-dose warfarin was substantially more efficacious than antiplatelet therapy (relative risk reduction 39%; 95% CI 22%–52%) (12 trials, 12,963 participants).
The introduction of DOACs has significantly increased the appropriate prescription rates of OACs and improved the clinical outcomes of AF patients, even in the elderly.60,61 In a meta-analysis of the phase 3 trials of the DOACs, Ruff et al.62 reported that DOACs significantly reduced stroke or systemic embolic events by 19% compared with warfarin (RR 0.81, 95% CI 0.73–0.91), mainly driven by a reduction in haemorrhagic stroke (RR 0.49, 95% CI 0.38–0.64). DOACs also significantly reduced all-cause mortality (RR 0.90, 95% CI 0.85–0.95) and ICH (RR 0.48, 95% CI 0.39–0.59), but increased gastrointestinal bleeding (RR 1.25, 95% CI 1.01–1.55).
In a recent report from the COMBINE AF (A Collaboration Between Multiple Institutions to Better Investigate Non-Vitamin K Antagonist Oral Anticoagulant Use in Atrial Fibrillation) database, which includes all patients randomized in the 4 pivotal trials of DOACs versus warfarin in AF, standard-dose DOACs reduced the risk of stroke/SEE by 19%, mortality by 8%, intracranial haemorrhage (ICH) by 55% compared to warfarin.63 The advantages of DOACs over warfarin have also been reported in different real-world cohorts.64,65 In a recent study which focused on 8007 AF patients staying well on warfarin for a median of around 7 years without any events of ischaemic stroke or ICH, patients who shifted from warfarin to DOACs were associated with a lower risk of ischaemic stroke and major bleeding.66
The contra-indications of DOACs include AF patients with mechanical heart valves or moderate to severe mitral stenosis (MS). In the RE-ALIGN trial, dabigatran resulted in a higher risk of bleeding and thromboembolic events compared to warfarin in patients (around 23% had AF) who underwent mechanical aortic- or mitral-valve replacement.67 For AF patients with MS, there were no randomized trials to compare DOACs and warfarin until the INVICTUS trial was published.68 Although the INVICTUS trial enrolled AF patients with rheumatic heart diseases rather than specifically targeting for MS, more than 80% of the study population had moderate to severe MS, defined as a valve area of less than 2.0 cm2. The results demonstrated that rivaroxaban had a higher risk of composite adverse events of stroke, SEE, myocardial infarction or death from vascular or unknown causes (HR 1.25, 95% CI 1.10–1.41).68 The better outcomes in warfarin group may be due to the better care of warfarin users since the study was not a double-blind one.
Importance of appropriate dosing of DOACs
Although frequent and routine monitoring of anticoagulant effect is not required for DOACs, the DOACs should be prescribed at the appropriate dosing according to the dosing criteria defined in the randomized trials. However, in the daily practice, clinical physicians would prescribe DOACs at the dosing not on-labelling, so-called “off-label or in-appropriate dosing”, for AF patients. In the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) study, around 13% of patients received DOACs at an inappropriate dosing (underdosing in 9.4% and overdosing in 3.4%).69 The off-label dosing rate is higher in Asia, as high as 32% (27% underdosing and 5% overdosing).70 Regional differences about the inappropriate dosing rates of DOACs were clearly observed in the recent report from global ETNA-AF program.71 For 26,823 AF patients treated with edoxaban, the overall in-appropriate dosing rate was 17.3% which seems to be higher in South Korea/Taiwan (29.2%), followed by Europe (17.0%) and Japan (14.9%).71 In a recent meta-analysis, off-label underdosing of DOACs does not reduce bleeding outcomes, and was associated with an increased risk of all-cause mortality (HR 1.28, 95% CI 1.10–1.49).72 Among patients who experienced ischaemic stroke under OACs (breakthrough stroke), insufficient anticoagulation was identified as the possible cause in 31.7% of patients,73 and prescriptions of off-label low-dose DOACs were observed in 30.3% of DOAC users.74 These reports highlight the importance of prescribing the correct DOAC dose to improve clinical outcomes in patients with AF.
Left atrial appendage occlusion or exclusion
Left atrial appendage occlusion (LAAO) is proposed to be an interventional solution for AF patients at high stroke risk who are deemed unable (actually not absolutely contraindicated) or unwilling to take long-term OAC, based on the presumption that much of the clot occurs in the left atrial appendage and therefore LAAO would reduce the risk of subsequent stroke. Nonetheless, the risk of stroke in AF is a systemic issue with the presence of a prothrombotic state which may not be easily prevented via “local” treatment. In addition, even in the historical trials, vascular disease such as the presence of complex aortic plaque on the descending aorta was an independent predictor of the risk of ischaemic stroke.75
The trials comparing LAAO to warfarin have tended to show a reduction in the composite outcome, although ischaemic stroke tended to be higher compared to warfarin, but major bleeding was more common in warfarin users (Table 2).76, 77, 78 Ongoing trials comparing LAAO versus DOACs suggest that the overall net clinical outcome was in favour of LAAO, but the trials have been limited by relatively small numbers in comparison to the huge phase 3 RCTs of DOACs.
Table 2.
RCTs of LAAO—main findings.
| Trial | Designa and cohort size | Main findings |
|---|---|---|
| PROTECT-AF76 | Watchman (n = 463) vs. Warfarin (n = 244) |
|
| PREVAIL77 | Watchman (n = 269) vs. Warfarin (n = 138) | |
| PRAGUE-1778 | LAAO device (n = 201) vs. DOAC (n = 201) |
|
PROTECT-AF, WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients With Atrial Fibrillation; PREVAIL, Evaluation of the WATCHMAN Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long term Warfarin Therapy; PRAGUE, Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in Atrial Fibrillation; CrI, Credible Interval; RR, Rate Ratio; sHR, Subdistribution Hazard Ratio; CI, Confidence interval. DOAC = direct oral anticoagulants.
All RCTs were noninferiority trials.
Stroke, systemic embolism, and cardiovascular/unexplained death.
Stroke or systemic embolism >7 days after randomization.
Stroke, transient ischaemic attack, systemic embolism, cardiovascular death, major or non-major clinically relevant bleeding, or procedure/device-related complications.
In the LAAOS-III trial that enrolled participants with AF who had undergone cardiac surgery, the risk of ischaemic stroke or SSE was lower with concomitant LAAO performed during the surgery than without it.79 Stroke or SEE occurred in 4.8% in the occlusion group and 7.0% in the no-occlusion group (HR 0.67; 95% CI 0.53–0.85). Perioperative bleeding, heart failure, or death was not significantly different between the trial groups.
Stroke prevention with the B component of the ABC pathway
While the use of OAC is essential to the A component of the ABC pathway, adequate management of the B and C components is complementary and additive to optimal stroke prevention in patients with AF and one or more stroke risk factors.7
The “B” component refers to better symptom control with individualized patient-centred and symptom-directed decisions on rate and rhythm control. The historical AF trials comparing the strategy of rhythm versus rate control in ‘all comers’ did not show any significant difference between these 2 strategies regarding the reduction of stroke in AF patients.80 However, in the recent EAST-AFNET4 trial (Early treatment of Atrial fibrillation for Stroke prevention Trial), which compared early rhythm control versus usual care in patients with AF diagnosed within 1 year before enrolment, the primary outcome (a composite of death from cardiovascular causes, stroke, or hospitalization with worsening of heart failure or acute coronary syndrome) was reduced by 21% in favour of early rhythm control (HR 0.79, 95% CI 0.66–0.94).81 This primary outcome reduction was driven by a reduction in cardiovascular death from 1.3% to 1.0% (HR 0.72, 95% CI 0.52–0.98) and a reduction in stroke from 0.9% to 0.6% (HR 0.65, 95% CI 0.44–0.97). Even in the secondary stroke prevention subgroup of the EAST- AFNET4 trial, there was benefit clearly in favour of early rhythm control, as shown in a prespecified exploratory analysis of 217 patients with a history of prior stroke randomized to early rhythm control (n = 110) or usual care (n = 107).82
All patients randomized to early rhythm control arm in the EAST-AFNET4 trial underwent rhythm control using mostly antiarrhythmic drugs or, less frequently, catheter ablation for AF (8% at baseline and 19.4% at 2-year follow-up), while patients in the usual care arm received a rhythm control therapy if symptomatic despite rate control therapy.81 Treatment of concomitant cardiovascular conditions and guideline-adherent OAC were mandated in all EAST-AFNET4 patients, and in-person follow-up visits at 1 and 2 years after randomization were combined with a mailed questionnaire follow-up at 6-month intervals during the trial. In addition, patients in the early rhythm control arm were actively engaged with transmitting to the study team a patient-operated single-lead electrocardiogram twice per week and when symptomatic during the trial duration.
Depending on the data source, the proportion of EAST-AFNET4-eligible patients in real-world cohorts varies from 34.0% to 72.9% (Table 3).83, 84, 85, 86, 87, 88, 89, 90 Translating the EAST-AFNET4 trial to real-world practice has yielded inconsistent results (Table 3, [A]). The largest real-world study of early rhythm control in AF published to date, the nationwide cohort study from Taiwan (n = 301,064 patients with first-diagnosed AF), suggested that early rhythm control does reduce the risk of stroke, heart failure, mortality, and acute myocardial infarction and overall cardiovascular events when compared to usual care, but most of the benefit was in the first three months and also in the younger patients with less comorbidities or structural heart disease (P int <0.001).84
Table 3.
Real-world studies and RCT sub-analyses comparing early rhythm control versus usual care in patients with AF.
| (A) Real-world studies | ||||
|---|---|---|---|---|
| Study/Year | Cohort size (N) | Data source | EAST-eligiblea patients % (N) | Main findings (The EAST-AFNET4 primary outcome, unless otherwise specified) |
| Kim D et al., 2021 | 22,635 | Korean NHI dataset | NR |
|
| ||||
| Kim D et al.83 2022 | 54,216 | Korean NHI dataset | 69.3% (37,557) |
|
| Chao TF et al.84 2022 | 301,064 | Taiwan NHI dataset | NR |
|
| Proetti M et al.85 2022 | 10,707 | ESC-EORP AF Gen LT prospective registry | 34.0% (3774) | HR 0.99; 95% CI 0.65–1.52 |
| Dickow J et al.86 2022 | 109,739 | A US administrative database | 72.9% (79,948) |
|
| ||||
| Kany S et al.87 2022 | 35,526 | UK Biobank dataset | 61.9% (22,003) | HR 0.87; 95% CI 0.72–1.04 |
| (B) RCT or RCT sub-study | ||||
| Study | Cohort size (N) | Source | Eligible | Main findings |
| Park J et al.89 The RAFAS Trial, 2022 | 300 Acute stroke and newly diagnosed AF |
RCT | NA |
|
| Yang E et al.90 An AFFIRM sub-study, 2021 | 2526 | RCT | NA |
|
EAST, Early treatment of Atrial fibrillation for Stroke prevention Trial; NHI, National Health Insurance; NR, Not reported; HR, Hazard Ratio; aHR, adjusted Hazard Ratio; AMI, Acute myocardial infarction; ESC-EORP AF Gen LT, European Society of Cardiology - EURObservational Research Programme AF General Long-Term; US, United States; RCT, Randomized Controlled Trial; RAFAS, Risks and benefits of urgent rhythm control of Atrial Fibrillation in patients with Acute Stroke; NA, Not applicable; IS, ischemic stroke; AFFIRM, Atrial Fibrillation Follow-up Investigation of Rhythm management.
EAST-AFNET4 eligibility criteria: adults (≥18 years of age) who had early AF (i.e., AF diagnosed ≤12 months before enrolment) and who were older than 75 years of age, had had a previous transient ischaemic attack or stroke, or met two of the following criteria: age greater than 65 years, female sex, heart failure, hypertension, diabetes mellitus, severe coronary artery disease, chronic kidney disease (Modification of Diet in Renal Disease stage 3 or 4 [glomerular filtration rate, 15–59 ml per minute per 1.73 m2 of body-surface area]), and left ventricular hypertrophy (diastolic septal wall width, >15 mm).
The relevance of optimal timing of rhythm control treatment initiation (i.e., within or more than 1 year after the diagnosis of AF) for outcome risk reduction has also been shown in a Korean nationwide cohort study88,91 (Table 3, [A]). However, early rhythm control strategy in patients with acute stroke and newly documented AF was not significantly associated with reduction in recurrent stroke in an RCT,89 and a secondary analysis of the randomized AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm management) trial showed no significant stroke, cardiovascular hospitalization or mortality between rate or rhythm control among study participants with first-diagnosed AF within 6 months of study enrolment90 (Table 3, [B]). Thus, it remains unclear whether the benefits of early rhythm control shown in the EAST-AFNET4 trial can be generalized to patients receiving rhythm control treatment later after the diagnosis of AF.
While the effects of early rhythm control were consistent across various subgroups in the EAST-AFNET4 main trial report,81 a pre-specified EAST-AFNET4 sub-analysis showed a significant early rhythm control benefit in patients with a greater comorbidity burden (i.e., a CHA2DS2-VASc score ≥ 4) but not in those with a CHA2DS2-VASc score <4 (P interaction = 0.037).92 A recent real-world dataset analysis yielded similar results,93 whereas another real-world study suggested that most of the benefit from early rhythm control would be seen in younger patients with less comorbidity or structural heart disease.84
Clearly, it is not a ‘one size fits all’ in terms of reducing the risk of stroke. Actually, early rhythm control therapy was delivered on top of high oral anticoagulation rates and high use of rate control in both arms. The clinical benefits were achieved using variable treatment patterns of antiarrhythmic drugs and AF ablation, applied within guideline recommendations, rather than a simplified and specific rhythm control strategy.94
Stroke prevention with the C component of the ABC pathway
Adverse atrial remodelling in patients with AF is promoted by AF itself, as well as the underlying cardiovascular or other comorbidity, risk factors and unhealthy lifestyle. Conceptually, early rhythm control intervention to prevent the AF-associated remodelling might reduce the risk of adverse cardiovascular events including stroke, but comprehensive comorbidity management and lifestyle optimisation (i.e., the C component of the ABC pathway) is also important for the reduction of AF burden and may complement OAC for stroke prevention in AF.95,96
This is clearly evident, for example, with respect to the importance of blood pressure (BP) control with optimal targets of 120–129 mmHg for systolic BP and <80 mmHg for diastolic BP to reduce the risk of adverse cardiovascular events, including stroke, bleeding, ICH, and hospitalisation from heart failure.97,98 Hypertension burden is also closely related to the risk of other AF-associated complications such as dementia,99 and visit-to-visit BP variability as a surrogate measure of the quality of BP control has been associated with worse outcomes in AF patients.
Comorbidities and cardiovascular risk factors are not independent, but interact with each other thus further potentiating substrate remodelling, AF progression and adverse cardiovascular outcomes.100 Hence, the interventions focusing on a single condition in a multimorbid patient might be less beneficial than a comprehensive management. In the RACE 3 (Routine vs. Aggressive risk factor driven upstream rhythm Control for prevention of Early atrial fibrillation in heart failure) RCT, for example, a structured intervention addressing the underlying conditions in patients with persistent AF and mild or moderate heart failure significantly improved maintenance of sinus rhythm and reduced total AF burden.101 Although RACE 3 trial was not designed and powered to investigate the difference of stroke, a lower AF burden may be associated with a lower risk of stroke and the concept has been mentioned in the 2020 ESC AF guidelines.8,23 Similarly, body weight reduction together with intensive risk factor management resulted in a reduction in AF burden and severity,102 whereas RCTs focusing on single condition management failed to show a significant difference (Table 4).103, 104, 105, 106, 107, 108, 109 Also, clustering of unhealthy lifestyles (i.e., poor exercise, regular smoking, regular drinking) in AF patients leads to a higher risk of major adverse cardiovascular events including ischaemic stroke, whereas healthy lifestyle behaviours was associated with a significant reduction in the risk of ischaemic stroke.96 Good adherence to comorbidity and risk factor management is necessary to achieve optimal effects. Notably, in the RACE 3 trial, the superiority of targeted therapy in sinus rhythm maintenance could not be preserved at 5-year follow-up,110 which underscores the importance of persistent efforts towards optimal comorbidity and risk factor control.
Table 4.
RCTs of risk factor modification to reduce AF recurrence and burden.
| Study | Cohort | Cohort size | Intervention/duration | Outcome |
|---|---|---|---|---|
| Abed et al.102 2013 | Symptomatic paroxysmal or persistent AF | N = 150 | Weight loss, intensive blood pressure reduction, prescribed exercise regime, OSA therapy with CPAP, alcohol abstinence or reduction, T2DM control, smoking cessation/15 months | Reduction in AF episodes and symptoms |
| RACE 3101 2018 | Persistent AF and HF | N = 245 | Weight loss, intensive blood pressure reduction, prescribed exercise regime/12 months | Reduction in AF recurrence and burden |
| SORT-AF103 2021 | Paroxysmal or persistent AF undergoing AF ablation | N = 133 | Weight loss, prescribed exercise regime/12 months | No difference in AF recurrence |
| Voskoboinik et al.104 2021 | Symptomatic paroxysmal or persistent AF | N = 140 | Alcohol abstinence or reduction/6 months | Reduction in AF recurrence |
| SMAC-AF105 2017 | BP > 130/180 mmHg undergoing AF ablation | N = 184 | Intense blood pressure reduction | No difference in AF recurrence |
| Malmo et al.106 2016 | Symptomatic paroxysmal or persistent AF | N = 51 | Prescribed exercise regime/3 months | Reduction in AF burden and symptoms |
| Caples et al.107 2019 | Persistent AF and AHI > 5 | N = 25 | OSA therapy with CPAP/12 months | No difference in AF recurrence |
| Traaen et al.108 2021 | Paroxysmal AF and AHI > 15 | N = 109 | OSA therapy with CPAP/5 months | No difference in AF burden |
| Hunt et al.109 2022 | Paroxysmal AF and AHI > 15 | N = 83 | OSA therapy with CPAP/12 months | No difference in AF recurrence |
AF, Atrial fibrillation; OSA, Obstructive sleep apnoea; CPAP, Continuous positive airway pressure; T2DM, Type 2 Diabetes mellitus; HF, Heart failure; AHI, Apnoea—hypopnoea Index.
The linkage of mobile health solutions and telemedicine could be helpful for risk factor management such as BP control. Such a digital health intervention in AF has been prospectively tested in the mAFA-II trial and found to be associated with reduction in adverse clinical events, including stroke.30 One ongoing trial in Europe is undertaken as part of the AFFIRMO programme,31 where AF patients with multimorbidity are being managed according to an ABC pathway in a cluster randomised trial, and digital health solutions will be engaged in the management of elderly multimorbid patients within the EHRA-PATHS research project.111
Special situations
Associated vascular diseases
Patients with AF may have associated vascular diseases, and they may present with an acute coronary syndrome requiring a percutaneous coronary intervention (PCI)/stenting. Such patients require an initial course of triple therapy (i.e., OAC, a P2Y12 inhibitor and aspirin), which duration (1–4 weeks) should be tailored according to individual patient bleeding and ischaemic risks (in patients at particularly high bleeding risk, the aspirin may be omitted from the start).8,112 Thereafter, a double therapy (OAC plus a P2Y12 inhibitor, preferably clopidogrel) should be continued for up to 12 months, followed by OAC alone.8
The paradigm shift towards shortening of the combined antithrombotic therapy in patients with AF undergoing primary or elective PCI/stenting is based on the evidence from five safety RCTs113, 114, 115, 116, 117 showing that: i) OAC plus a P2Y12 inhibitor (mostly clopidogrel) is associated with significantly lower risk of bleeding complications compared with triple therapy and ii) DOACs are associated with significantly less bleeding compared to VKA in any combined antithrombotic therapy regimen. While none of these RCTs was powered to assess ischaemic outcomes, several meta-analyses of the RCT suggested that omitting aspirin very early after an acute coronary syndrome with PCI/stenting might be associated with some increase in the ischaemic risk, thus supporting individualised treatment decision-making.118,119
Some AF patients with ischaemic strokes have associated large vessel diseases such as carotid disease that can be managed with OAC monotherapy, as there is no impact on recurrent stroke or mortality, but a substantial increase in the risk of major bleeding including ICH by the use of combination therapy.120
Elderly
The elderly patients with AF represent a management problem, as there still appears to be substantial undertreatment of these patients in clinical practice. Clinical trial data support the proactive stroke prevention of elderly AF patients. The BAFTA trial showed that AF patients aged ≥75 benefited from warfarin compared to aspirin, in terms of reducing the risk of the primary outcome.121 In the ELDERCARE-AF trial of very elderly Japanese DOAC-eligible patients with AF who were not appropriate candidates for standard doses of OAC, edoxaban 15 mg once-daily was superior to placebo in preventing stroke or SSE and did not result in a significantly higher incidence of major bleeding.122
In a large study of patients with AF aged ≥80 years, the proportion of anticoagulated patients increased over recent years (mostly due to increasing uptake of DOACs) but the substantial proportion of these patients (56.4%) remain non-anticoagulated.123 The use of DOACs compared to no OAC was particularly associated with a reduction in stroke, mortality and dementia. With the use of warfarin, there was no difference in stroke but a reduction in mortality and dementia was observed. When a DOAC was compared to warfarin, there was a clear advantage of DOAC for the reduction of stroke, mortality and dementia with less bleeding (Table 5).123
Table 5.
Comparisons of no OAC, Warfarin and DOAC in patients with AF aged ≥80 years.123
| Outcome | Comparison |
|||||
|---|---|---|---|---|---|---|
| DOAC vs. no OAC |
Warfarin vs. no OAC |
DOAC vs. Warfarin |
||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Dementia | 0.68 | 0.65–0.71 | 0.76 | 0.73–0.79 | 0.90 | 0.86–0.93 |
| All-cause mortality | 0.49 | 0.48–0.50 | 0.67 | 0.66–0.68 | 0.74 | 0.72–0.76 |
| Ischaemic stroke | 0.87 | 0.83–0.91 | 0.99 | 0.95–1.04 | 0.86 | 0.82–0.90 |
| Major bleeding | 1.08 | 1.05–1.11 | 1.05 | 1.03–1.07 | 0.88 | 0.85–0.90 |
DOAC, direct oral anticoagulant; OAC, Oral anticoagulant; HR, Hazard Ratio; CI, Confidence Interval.
In another observational cohort of extreme elderly patients (age >90) with high risk of bleeding (a history of ICH, gastrointestinal bleeding, or chronic kidney disease), compared with no OAC, warfarin was associated with a higher risk of the composite endpoint (HR 1.16; 95% CI 1.05–1.29), whereas DOACs were associated with a lower risk (HR 0.76; 95% CI 0.70–0.83).124 Beneficial effects of OAC (particularly DOACs) has also been observed in several real-world studies.125 Importantly, when using a DOAC in elderly patients with AF, adequate dosing as per the drug label should be chosen to avoid unnecessary complications.126
Atrial high rate episode
Another question of concern is what to do with atrial high-rate episodes (AHRE) detected by cardiac implantable electronic devices (CIED) in patients without clinically diagnosed AF.8 Indeed, subclinical AF is a challenging paradigm, especially since the burden of AF is very often heterogenous and varying from period to period. Furthermore, many studies have shown that an episode of AHRE is not temporarily associated with the thromboembolic events. Two clinical trials, the NOAH (NCT02618577) and ARTESiA (NCT01938248), are conducted to investigate the use of a DOAC compared to control in terms of managing these patients with AHRE on CIED. Just recently, the results of NOAH-AFNET 6 were published, showing that anticoagulation with edoxaban did not significantly reduce the incidence of a composite of cardiovascular death, stroke, or SSE as compared with placebo, but it led to a higher incidence of a composite of death or major bleeding.127 The results of ARTESiA trial would provide more insights related to the managements of patients with AHREs.
AF ablation
For AF patients undergoing AF ablations, DOACs are at least as effective and safe as warfarin during the peri-ablation period.128 The important question is whether OACs should be continued after AF ablation for patients without evidence of AF recurrence after the blanking period. Based on current guidelines, the use of OACs or not after AF ablation should be determined according to patients’ CHA2DS2-VASc scores rather than AF status under the concern that a considerable proportion of recurrent asymptomatic AF would not be detected but may still result in stroke.8,9 However, these recommendations are mainly expert opinions and not supported by randomized trials. Results of several trials, such as OCEAN (NCT02168829) and ALONE AF (NCT04432220), may be able to answer this question in the future.
Breakthrough stroke
Even on OACs, some patients would still suffer from ischaemic stroke. The annual risk of breakthrough stroke in DOAC arms of 4 pivotal trials are as the following: 1.34% in dabigatran 110 mg, 0.92% in dabigatran 150 mg, 1.7% in rivaroxaban, 0.97% in apixaban, 1.25% in edoxaban (60/30 mg) and 1.77% in edoxaban (30/15 mg).129 Patients with AF and ischaemic stroke while on OAC are at increased risk of recurrent ischaemic stroke (7% at 1 year) and death (12.4% at 3 months).130 Several studies reported the associations between the changes of OACs after stroke but showed different results.131, 132, 133 The study based on Taiwan insurance database demonstrated that DOAC-switch was associated with a lower risk of major cardiovascular events compared with the DOAC-same group (HR 0.78, 95% CI, 0.62–0.99).133 However, another study from Hong Kong showed that DOAC-switch was associated with an increased risk of recurrent ischaemic stroke (HR 1.62, 95% CI 1.25–2.11) compared with DOAC-same.132 In the DOAC-same group, adjunctive antiplatelet agent was not associated with a reduced risk of recurrent ischaemic stroke.132 In the pooled analysis of 7 prospective cohort studies showed that OAC-change was not associated with decreased risk of stroke (HR 1.2, 95% CI 0.7–2.1) compared with OAC-unchanged.131 These diverse results may suggest that the detailed reasons underlying the breakthrough stroke are multifactorial, including insufficient anticoagulation (suboptimal INR level or underdosing of DOACs), competing mechanism not related to AF (such as large artery atherosclerosis, small vessel disease, patent foramen ovale/atrial septal defect, etc.), advanced atrial dysfunction or left ventricular systolic dysfunction, etc.73,129 Comprehensive evaluations and shared decision making are necessary to determine the subsequent stroke prevention strategy.
Survivors of ICH
Survivors of ICH had a higher risk of both subsequent stroke and ICH events,134 and use of OAC or not is a difficult issue. In the Start or STop Anticoagulants Randomised Trial (SoSTART), 203 AF patients were randomly assigned at a median of 115 days (interquartile range 49–265) after ICH (101 were assigned to start and 102 to avoid OAC). After a median follow-up of 1.2 years, starting oral anticoagulation was not non-inferior to avoiding oral anticoagulation: eight (8%) of 101 in the start group versus four (4%) of 102 in the avoid group had ICH recurrences (HR 2.42, 95% CI 0.72–8.09).135 In another Apixaban versus no anticoagulation after anticoagulation-associated intracerebral haemorrhage in patients with atrial fibrillation (APACHE-AF) trial which recruited 101 patients at a median of 46 days (interquartile range 21–74) after ICH, non-fatal stroke or vascular death occurred in 13 (26%) participants allocated to apixaban (annual event rate 12.6%, 95% CI 6.7–21.5) and in 12 (24%) allocated to avoid anticoagulation (11.9%, 95% CI 6.2–20.8; HR 1.05, 95% CI 0.48–2.31) without significant difference (p = 0.90).136 Since these 2 trials were underpowered to answer this issue, further large-scale randomized trials are necessary.
End-stage renal disease
Stroke prevention in AF patients with end-stage renal disease (ESRD) is challenging due to the lacking of high-quality data. There were three published randomized trials (2 for apixaban and 1 for rivaroxaban) comparing the efficacy and safety of DOACs and warfarin among AF patients with ESRD undergoing hemodialysis.137, 138, 139 Apixaban at the dosing either following the criteria defined in the ARISTOTLE trial or fixed 2.5 mg bid showed similar bleeding risk to warfarin,138,139 while rivaroxaban (10 mg/day) significantly reduced the risk of life-threatening and major bleeding compared to warfarin.137 However, all these 3 trials were limited by the small sample size and the high premature/permanent discontinuation rate of anticoagulation, and more data are required to understand this issue.
Factor XI inhibitors
Factor XI inhibitors are “novel” OACs which may potentially provide new choice for stroke prevention in AF. The results of the phase II study, the PACIFIC-AF trial, showed that the FXIa inhibitor asundexian at doses of 20 mg and 50 mg once daily resulted in lower rates of bleeding compared with standard dosing of apixaban, with near-complete in-vivo FXIa inhibition.140 Currently, the phase III trials of asundexian (OCEANIC-AF; NCT05643573) and milvexian (LIBREXIA-AF; NCT05757869) are ongoing.
Conclusion
Evidence-based management of AF patients following the ABC integrated care pathway has been associated with a lower risk of stroke and other adverse events. Risk assessment, re-assessment and use of DOACs are central to stroke prevention in AF. Well-controlled comorbidities and healthy lifestyles also plan an important role to achieve a better outcome. Digital health solutions are increasingly relevant in the diagnosis and management of patients with AF, with the potential to improve stroke prevention.
Contributors
Tze-Fan Chao and Tatjana S. Potpara performed the literature review and wrote a first draft of the paper. Gregory Y.H. Lip provided extensive input into subsequent versions of the paper.
Declaration of interests
All authors declare that they have no conflicts of interest.
References
- 1.Chiang C.E., Chao T.F., Choi E.K., et al. Stroke prevention in atrial fibrillation: a scientific statement of JACC: Asia (Part 1) JACC Asia. 2022;2(4):395–411. doi: 10.1016/j.jacasi.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang C.E., Chao T.F., Choi E.K., et al. Stroke prevention in atrial fibrillation: a scientific statement of JACC: Asia (Part 2) JACC Asia. 2022;2(5):519–537. doi: 10.1016/j.jacasi.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane D.A., Lip G.Y.H. Stroke and bleeding risk stratification in atrial fibrillation: a critical appraisal. Eur Heart J Suppl. 2020;22(Suppl O):O14–O27. doi: 10.1093/eurheartj/suaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proietti M., Esteve-Pastor M.A., Rivera-Caravaca J.M., et al. Relationship between multimorbidity and outcomes in atrial fibrillation. Exp Gerontol. 2021;153 doi: 10.1016/j.exger.2021.111482. [DOI] [PubMed] [Google Scholar]
- 5.Proietti M., Marzona I., Vannini T., et al. Long-term relationship between atrial fibrillation, multimorbidity and oral anticoagulant drug use. Mayo Clin Proc. 2019;94(12):2427–2436. doi: 10.1016/j.mayocp.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Proietti M., Romiti G.F., Raparelli V., et al. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: a systematic review and meta-analysis of 1,187,000 patients. Ageing Res Rev. 2022;79 doi: 10.1016/j.arr.2022.101652. [DOI] [PubMed] [Google Scholar]
- 7.Lip G.Y.H. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14(11):627–628. doi: 10.1038/nrcardio.2017.153. [DOI] [PubMed] [Google Scholar]
- 8.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 9.Chao T.F., Joung B., Takahashi Y., et al. 2021 Focused update consensus guidelines of the Asia pacific heart rhythm society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemostasis. 2022;122(1):20–47. doi: 10.1055/s-0041-1739411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potpara T.S., Lip G.Y.H., Blomstrom-Lundqvist C., et al. The 4S-AF scheme (stroke risk; symptoms; severity of burden; substrate): a novel approach to in-depth characterization (rather than classification) of atrial fibrillation. Thromb Haemostasis. 2021;121(3):270–278. doi: 10.1055/s-0040-1716408. [DOI] [PubMed] [Google Scholar]
- 11.Chan N.Y., Orchard J., Agbayani M.J., et al. 2021 Asia Pacific Heart Rhythm Society (APHRS) practice guidance on atrial fibrillation screening. J Arrhythm. 2022;38(1):31–49. doi: 10.1002/joa3.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez M.V., Mahaffey K.W., Hedlin H., et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y., Wang H., Zhang H., et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74(19):2365–2375. doi: 10.1016/j.jacc.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Lubitz S.A., Faranesh A.Z., Selvaggi C., et al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit heart study. Circulation. 2022;146(19):1415–1424. doi: 10.1161/CIRCULATIONAHA.122.060291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svennberg E., Tjong F., Goette A., et al. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace. 2022;24(6):979–1005. doi: 10.1093/europace/euac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding E.Y., CastanedaAvila M., Tran K.V., et al. Usability of a smartwatch for atrial fibrillation detection in older adults after stroke. Cardiovasc Digit Health J. 2022;3(3):126–135. doi: 10.1016/j.cvdhj.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul T.J., Tran K.V., Mehawej J., et al. Anxiety, patient activation, and quality of life among stroke survivors prescribed smartwatches for atrial fibrillation monitoring. Cardiovasc Digit Health J. 2023;4(4):118–125. doi: 10.1016/j.cvdhj.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanna T., Diener H.C., Passman R.S., et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 19.Ding W.Y., Proietti M., Boriani G., et al. Clinical utility and prognostic implications of the novel 4S-AF scheme to characterize and evaluate patients with atrial fibrillation: a report from ESC-EHRA EORP-AF Long-Term General Registry. Europace. 2022;24(5):721–728. doi: 10.1093/europace/euab280. [DOI] [PubMed] [Google Scholar]
- 20.Chao T.F., Tse H.F., Teo W.S., et al. Clinical utility and prognostic implications of the 4S-AF scheme: report from Asia pacific heart rhythm society atrial fibrillation registry. Eur J Clin Invest. 2022;52(10) doi: 10.1111/eci.13825. [DOI] [PubMed] [Google Scholar]
- 21.Malavasi V.L., Vitolo M., Colella J., et al. Rhythm- or rate-control strategies according to 4S-AF characterization scheme and long-term outcomes in atrial fibrillation patients: the FAMo (Fibrillazione Atriale in Modena) cohort. Intern Emerg Med. 2022;17(4):1001–1012. doi: 10.1007/s11739-021-02890-x. [DOI] [PubMed] [Google Scholar]
- 22.Rivera-Caravaca J.M., Piot O., Roldan-Rabadan I., et al. Characterization of atrial fibrillation in real-world patients: testing the 4S-AF scheme in the Spanish and French cohorts of the EORP-AF long-term general registry. Europace. 2022;24(2):202–210. doi: 10.1093/europace/euab202. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan R.M., Koehler J., Ziegler P.D., Sarkar S., Zweibel S., Passman R.S. Stroke risk as a function of atrial fibrillation duration and CHA(2)DS(2)-VASc score. Circulation. 2019;140(20):1639–1646. doi: 10.1161/CIRCULATIONAHA.119.041303. [DOI] [PubMed] [Google Scholar]
- 24.Turakhia M.P., Hoang D.D., Xu X., et al. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: the Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J. 2013;165(1):93–101.e1. doi: 10.1016/j.ahj.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 25.O'Neal W.T., Claxton J.S., Sandesara P.B., et al. Provider specialty, anticoagulation, and stroke risk in patients with atrial fibrillation and cancer. J Am Coll Cardiol. 2018;72(16):1913–1922. doi: 10.1016/j.jacc.2018.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S.H., Chao T.F., Chan Y.H., Liao J.N., Chen S.A. Clinical outcomes of patients with incident atrial fibrillation diagnosed by cardiologists compared to non-cardiologists. Eur J Intern Med. 2023;112:140–142. doi: 10.1016/j.ejim.2023.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks J.M., de Wit R., Crijns H.J., et al. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33(21):2692–2699. doi: 10.1093/eurheartj/ehs071. [DOI] [PubMed] [Google Scholar]
- 28.Stevens D., Harrison S.L., Kolamunnage-Dona R., Lip G.Y.H., Lane D.A. The Atrial Fibrillation Better Care pathway for managing atrial fibrillation: a review. Europace. 2021;23(10):1511–1527. doi: 10.1093/europace/euab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romiti G.F., Pastori D., Rivera-Caravaca J.M., et al. Adherence to the 'atrial fibrillation better care' pathway in patients with atrial fibrillation: impact on clinical outcomes-A systematic review and meta-analysis of 285,000 patients. Thromb Haemostasis. 2022;122(3):406–414. doi: 10.1055/a-1515-9630. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y., Lane D.A., Wang L., et al. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol. 2020;75(13):1523–1534. doi: 10.1016/j.jacc.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 31.Johnsen S.P., Proietti M., Maggioni A.P., Lip G.Y.H. A multinational European network to implement integrated care in elderly multimorbid atrial fibrillation patients: the AFFIRMO Consortium. Eur Heart J. 2022;43(31):2916–2918. doi: 10.1093/eurheartj/ehac265. [DOI] [PubMed] [Google Scholar]
- 32.Domek M., Gumprecht J., Mazurek M., Chao T.F., Lip G.Y.H. Should we judge stroke risk by static or dynamic risk scores? A focus on the dynamic nature of stroke and bleeding risks in patients with atrial fibrillation. J Cardiovasc Pharmacol. 2019;74(6):491–498. doi: 10.1097/FJC.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 33.Chao T.F., Liao J.N., Tuan T.C., et al. Incident Co-morbidities in patients with atrial fibrillation initially with a CHA2DS2-VASc score of 0 (males) or 1 (females): implications for reassessment of stroke risk in initially 'low-risk' patients. Thromb Haemostasis. 2019;119(7):1162–1170. doi: 10.1055/s-0039-1683933. [DOI] [PubMed] [Google Scholar]
- 34.Chao T.F., Lip G.Y.H., Liu C.J., et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71(2):122–132. doi: 10.1016/j.jacc.2017.10.085. [DOI] [PubMed] [Google Scholar]
- 35.Lip G.Y.H., Genaidy A., Tran G., Marroquin P., Estes C., Sloop S. Improving stroke risk prediction in the general population: a comparative assessment of common clinical rules, a new multimorbid index, and machine-learning-based algorithms. Thromb Haemostasis. 2022;122(1):142–150. doi: 10.1055/a-1467-2993. [DOI] [PubMed] [Google Scholar]
- 36.Lip G.Y.H., Tran G., Genaidy A., Marroquin P., Estes C., Landsheft J. Improving dynamic stroke risk prediction in non-anticoagulated patients with and without atrial fibrillation: comparing common clinical risk scores and machine learning algorithms. Eur Heart J Qual Care Clin Outcomes. 2022;8(5):548–556. doi: 10.1093/ehjqcco/qcab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen P.B., Skjoth F., Overvad T.F., Larsen T.B., Lip G.Y.H. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA(2)DS(2)-VA score rather than CHA(2)DS(2)-VASc? Circulation. 2018;137(8):832–840. doi: 10.1161/CIRCULATIONAHA.117.029081. [DOI] [PubMed] [Google Scholar]
- 38.Friberg L., Benson L., Rosenqvist M., Lip G.Y. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344 doi: 10.1136/bmj.e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emdin C.A., Wong C.X., Hsiao A.J., et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang C., Seyfang L., Ferrari J., et al. Do women with atrial fibrillation experience more severe strokes? Results from the Austrian stroke unit registry. Stroke. 2017;48(3):778–780. doi: 10.1161/STROKEAHA.116.015900. [DOI] [PubMed] [Google Scholar]
- 41.Pilcher S.M., Alamneh E.A., Chalmers L., Bereznicki L.R. The tasmanian atrial fibrillation study (TAFS): differences in stroke prevention according to sex. Ann Pharmacother. 2020;54(9):837–845. doi: 10.1177/1060028020904969. [DOI] [PubMed] [Google Scholar]
- 42.Tomita H., Okumura K., Inoue H., et al. Validation of risk scoring system excluding female sex from CHA2DS2-VASc in Japanese patients with nonvalvular atrial fibrillation–subanalysis of the J-RHYTHM registry. Circ J. 2015;79(8):1719–1726. doi: 10.1253/circj.CJ-15-0095. [DOI] [PubMed] [Google Scholar]
- 43.Choi S.Y., Kim M.H., Kim H.B., et al. Validation of the CHA2DS2-VA score (excluding female sex) in nonvalvular atrial fibrillation patients: a nationwide population-based study. J Clin Med. 2022;11(7):1823. doi: 10.3390/jcm11071823. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Chao T.F., Liu C.J., Wang K.L., et al. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65(7):635–642. doi: 10.1016/j.jacc.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Fauchier L., Clementy N., Bisson A., et al. Should atrial fibrillation patients with only 1 nongender-related CHA2DS2-VASc risk factor Be anticoagulated? Stroke. 2016;47(7):1831–1836. doi: 10.1161/STROKEAHA.116.013253. [DOI] [PubMed] [Google Scholar]
- 46.Lip G.Y., Skjoth F., Rasmussen L.H., Larsen T.B. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65(14):1385–1394. doi: 10.1016/j.jacc.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 47.Lip G.Y., Skjoth F., Rasmussen L.H., Nielsen P.B., Larsen T.B. Net clinical benefit for oral anticoagulation, aspirin, or No therapy in nonvalvular atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) J Am Coll Cardiol. 2015;66(4):488–490. doi: 10.1016/j.jacc.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 48.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 49.Chao T.F., Lip G.Y.H., Lin Y.J., et al. Age threshold for the use of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation: insights into the optimal assessment of age and incident comorbidities. Eur Heart J. 2019;40(19):1504–1514. doi: 10.1093/eurheartj/ehy837. [DOI] [PubMed] [Google Scholar]
- 50.Lip G.Y., Lane D.A. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemostasis. 2016;14(9):1711–1714. doi: 10.1111/jth.13386. [DOI] [PubMed] [Google Scholar]
- 51.Gorog D.A., Gue Y.X., Chao T.F., et al. Assessment and mitigation of bleeding risk in atrial fibrillation and venous thromboembolism: executive summary of a European and asia-pacific expert consensus paper. Thromb Haemostasis. 2022;122(10):1625–1652. doi: 10.1055/s-0042-1750385. [DOI] [PubMed] [Google Scholar]
- 52.Borre E.D., Goode A., Raitz G., et al. Predicting thromboembolic and bleeding event risk in patients with non-valvular atrial fibrillation: a systematic review. Thromb Haemostasis. 2018;118(12):2171–2187. doi: 10.1055/s-0038-1675400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo Y., Lane D.A., Chen Y., Lip G.Y.H., mAF-App II Trial investigators Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA-II randomized trial. Am J Med. 2020;133(10):1195–1202.e2. doi: 10.1016/j.amjmed.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Chao T.F., Chan Y.H., Chiang C.E., et al. Continuation or discontinuation of oral anticoagulants after HAS-BLED scores increase in patients with atrial fibrillation. Clin Res Cardiol. 2022;111(1):23–33. doi: 10.1007/s00392-021-01816-z. [DOI] [PubMed] [Google Scholar]
- 55.Chao T.F., Lip G.Y.H., Lin Y.J., et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemostasis. 2018;118(4):768–777. doi: 10.1055/s-0038-1636534. [DOI] [PubMed] [Google Scholar]
- 56.Chao T.F., Liu C.J., Tuan T.C., et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: which scoring system should be used for Asians? Heart Rhythm. 2016;13(1):46–53. doi: 10.1016/j.hrthm.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Chao T.F., Lip G.Y.H., Lin Y.J., et al. Major bleeding and intracranial hemorrhage risk prediction in patients with atrial fibrillation: attention to modifiable bleeding risk factors or use of a bleeding risk stratification score? A nationwide cohort study. Int J Cardiol. 2018;254:157–161. doi: 10.1016/j.ijcard.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 58.Chao T.F., Chan Y.H., Tuan T.C., et al. Should oral anticoagulants still be prescribed to patients with atrial fibrillation with a single stroke risk factor but at high bleeding risk? A nationwide cohort study. Eur Heart J Qual Care Clin Outcomes. 2022;8(5):588–595. doi: 10.1093/ehjqcco/qcab050. [DOI] [PubMed] [Google Scholar]
- 59.Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 60.Chao T.F., Chiang C.E., Lin Y.J., et al. Evolving changes of the use of oral anticoagulants and outcomes in patients with newly diagnosed atrial fibrillation in taiwan. Circulation. 2018;138(14):1485–1487. doi: 10.1161/CIRCULATIONAHA.118.036046. [DOI] [PubMed] [Google Scholar]
- 61.Cheng W.H., Chiang C.E., Lin Y.J., et al. Non-vitamin K antagonist oral anticoagulants in elderly (>/=85 years) patients with newly diagnosed atrial fibrillation: changing clinical practice and outcomes for stroke prevention in a nationwide cohort study. Mayo Clin Proc. 2021;96(1):52–65. doi: 10.1016/j.mayocp.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 62.Ruff C.T., Giugliano R.P., Braunwald E., et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2013;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 63.Carnicelli A.P., Hong H., Connolly S.J., et al. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145(4):242–255. doi: 10.1161/CIRCULATIONAHA.121.056355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larsen T.B., Skjoth F., Nielsen P.B., Kjaeldgaard J.N., Lip G.Y. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chao T.F., Chiang C.E., Liao J.N., Chen T.J., Lip G.Y.H., Chen S.A. Comparing the effectiveness and safety of nonvitamin K antagonist oral anticoagulants and warfarin in elderly asian patients with atrial fibrillation: a nationwide cohort study. Chest. 2020;157(5):1266–1277. doi: 10.1016/j.chest.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 66.Liu S.H., Chao T.F., Chan Y.H., et al. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients without previous oral anticoagulants or stable under warfarin: a nationwide cohort study. Europace. 2023;25(5) doi: 10.1093/europace/euad120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eikelboom J.W., Connolly S.J., Brueckmann M., et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 68.Connolly S.J., Karthikeyan G., Ntsekhe M., et al. Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med. 2022;387(11):978–988. doi: 10.1056/NEJMoa2209051. [DOI] [PubMed] [Google Scholar]
- 69.Steinberg B.A., Shrader P., Thomas L., et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol. 2016;68(24):2597–2604. doi: 10.1016/j.jacc.2016.09.966. [DOI] [PubMed] [Google Scholar]
- 70.Chan Y.H., Chao T.F., Chen S.W., et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm. 2020;17(12):2102–2110. doi: 10.1016/j.hrthm.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 71.Chao T.F., Unverdorben M., Kirchhof P., et al. Prescribing patterns and outcomes of edoxaban in atrial fibrillation: one-year data from the global ETNA-AF program. J Clin Med. 2023;12(5) doi: 10.3390/jcm12051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caso V., de Groot J.R., Sanmartin Fernandez M., et al. Outcomes and drivers of inappropriate dosing of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation: a systematic review and meta-analysis. Heart. 2023;109(3):178–185. doi: 10.1136/heartjnl-2022-321114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polymeris A.A., Meinel T.R., Oehler H., et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry. 2022;93(6):588–598. doi: 10.1136/jnnp-2021-328391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paciaroni M., Caso V., Agnelli G., et al. Recurrent ischemic stroke and bleeding in patients with atrial fibrillation who suffered an acute stroke while on treatment with nonvitamin K antagonist oral anticoagulants: the RENO-EXTEND study. Stroke. 2022;53(8):2620–2627. doi: 10.1161/STROKEAHA.121.038239. [DOI] [PubMed] [Google Scholar]
- 75.Blackshear J.L., Pearce L.A., Hart R.G., et al. Aortic plaque in atrial fibrillation: prevalence, predictors, and thromboembolic implications. Stroke. 1999;30(4):834–840. doi: 10.1161/01.str.30.4.834. [DOI] [PubMed] [Google Scholar]
- 76.Reddy V.Y., Doshi S.K., Kar S., et al. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70(24):2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 77.Holmes D.R., Jr., Doshi S.K., Kar S., et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65(24):2614–2623. doi: 10.1016/j.jacc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 78.Osmancik P., Herman D., Neuzil P., et al. 4-Year outcomes after left atrial appendage closure versus nonwarfarin oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2022;79(1):1–14. doi: 10.1016/j.jacc.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 79.Whitlock R.P., Belley-Cote E.P., Paparella D., et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384(22):2081–2091. doi: 10.1056/NEJMoa2101897. [DOI] [PubMed] [Google Scholar]
- 80.Caldeira D., David C., Sampaio C. Rate versus rhythm control in atrial fibrillation and clinical outcomes: updated systematic review and meta-analysis of randomized controlled trials. Archives of cardiovascular diseases. 2012;105(4):226–238. doi: 10.1016/j.acvd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Kirchhof P., Camm A.J., Goette A., et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 82.Jensen M., Suling A., Metzner A., et al. Early rhythm-control therapy for atrial fibrillation in patients with a history of stroke: a subgroup analysis of the EAST-AFNET 4 trial. Lancet Neurol. 2023;22(1):45–54. doi: 10.1016/S1474-4422(22)00436-7. [DOI] [PubMed] [Google Scholar]
- 83.Kim D., Yang P.S., You S.C., et al. Early rhythm control therapy for atrial fibrillation in low-risk patients: a nationwide propensity score-weighted study. Ann Intern Med. 2022;175(10):1356–1365. doi: 10.7326/M21-4798. [DOI] [PubMed] [Google Scholar]
- 84.Chao T.F., Chan Y.H., Chiang C.E., et al. Early rhythm control and the risks of ischemic stroke, heart failure, mortality, and adverse events when performed early (<3 Months): a nationwide cohort study of newly diagnosed patients with atrial fibrillation. Thromb Haemostasis. 2022;122(11):1899–1910. doi: 10.1055/a-1807-0336. [DOI] [PubMed] [Google Scholar]
- 85.Proietti M., Vitolo M., Harrison S.L., et al. Real-world applicability and impact of early rhythm control for European patients with atrial fibrillation: a report from the ESC-EHRA EORP-AF Long-Term General Registry. Clin Res Cardiol: official journal of the German Cardiac Society. 2022;111(1):70–84. doi: 10.1007/s00392-021-01914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dickow J., Kirchhof P., Van Houten H.K., et al. Generalizability of the EAST-AFNET 4 trial: assessing outcomes of early rhythm-control therapy in patients with atrial fibrillation. J Am Heart Assoc. 2022;11(11) doi: 10.1161/JAHA.121.024214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kany S., Cardoso V.R., Bravo L., et al. Eligibility for early rhythm control in patients with atrial fibrillation in the UK Biobank. Heart. 2022;108(23):1873–1880. doi: 10.1136/heartjnl-2022-321196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim D., Yang P.S., You S.C., et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ. 2021;373:n991. doi: 10.1136/bmj.n991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park J., Shim J., Lee J.M., et al. Risks and benefits of early rhythm control in patients with acute strokes and atrial fibrillation: a multicenter, prospective, randomized study (the RAFAS trial) J Am Heart Assoc. 2022;11(3) doi: 10.1161/JAHA.121.023391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang E., Tang O., Metkus T., et al. The role of timing in treatment of atrial fibrillation: an AFFIRM substudy. Heart Rhythm. 2021;18(5):674–681. doi: 10.1016/j.hrthm.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 91.Kim D., Yang P.S., You S.C., et al. Comparative effectiveness of early rhythm control versus rate control for cardiovascular outcomes in patients with atrial fibrillation. J Am Heart Assoc. 2021;10(24) doi: 10.1161/JAHA.121.023055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rillig A., Borof K., Breithardt G., et al. Early rhythm control in patients with atrial fibrillation and high comorbidity burden. Circulation. 2022;146(11):836–847. doi: 10.1161/CIRCULATIONAHA.122.060274. [DOI] [PubMed] [Google Scholar]
- 93.Dickow J., Kany S., Roth Cardoso V., et al. Outcomes of early rhythm control therapy in patients with atrial fibrillation and a high comorbidity burden in large real-world cohorts. Circ Arrhythm Electrophysiol. 2023;16 doi: 10.1161/CIRCEP.122.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Metzner A., Suling A., Brandes A., et al. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: a detailed analysis of treatment patterns in the EAST - AFNET 4 trial. Europace. 2022;24(4):552–564. doi: 10.1093/europace/euab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Proietti M., Romiti G.F., Olshansky B., Lane D.A., Lip G.Y.H. Comprehensive management with the ABC (atrial fibrillation better care) pathway in clinically complex patients with atrial fibrillation: a post hoc ancillary analysis from the AFFIRM trial. J Am Heart Assoc. 2020;9(10) doi: 10.1161/JAHA.119.014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elliott A.D., Middeldorp M.E., Van Gelder I.C., Albert C.M., Sanders P. Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. 2023;20(6):404–417. doi: 10.1038/s41569-022-00820-8. [DOI] [PubMed] [Google Scholar]
- 97.Kim D., Yang P.S., Kim T.H., et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(11):1233–1245. doi: 10.1016/j.jacc.2018.05.076. [DOI] [PubMed] [Google Scholar]
- 98.Krittayaphong R., Winijkul A., Methavigul K., Lip G.Y.H. Impact of achieving blood pressure targets and high time in therapeutic range on clinical outcomes in patients with atrial fibrillation adherent to the atrial fibrillation better care pathway: a report from the COOL-AF registry. J Am Heart Assoc. 2023;12(3) doi: 10.1161/JAHA.122.028463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung H., Yang P.S., Kim D., et al. Associations of hypertension burden on subsequent dementia: a population-based cohort study. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-91923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lau D.H., Nattel S., Kalman J.M., Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136(6):583–596. doi: 10.1161/CIRCULATIONAHA.116.023163. [DOI] [PubMed] [Google Scholar]
- 101.Rienstra M., Hobbelt A.H., Alings M., et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39(32):2987–2996. doi: 10.1093/eurheartj/ehx739. [DOI] [PubMed] [Google Scholar]
- 102.Abed H.S., Wittert G.A., Leong D.P., et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310(19):2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 103.Gessler N., Willems S., Steven D., et al. Supervised Obesity Reduction Trial for AF ablation patients: results from the SORT-AF trial. Europace. 2021;23(10):1548–1558. doi: 10.1093/europace/euab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Voskoboinik A., Kalman J.M., De Silva A., et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20–28. doi: 10.1056/NEJMoa1817591. [DOI] [PubMed] [Google Scholar]
- 105.Parkash R., Wells G.A., Sapp J.L., et al. Effect of aggressive blood pressure control on the recurrence of atrial fibrillation after catheter ablation: a randomized, open-label clinical trial (SMAC-AF [substrate modification with aggressive blood pressure control]) Circulation. 2017;135(19):1788–1798. doi: 10.1161/CIRCULATIONAHA.116.026230. [DOI] [PubMed] [Google Scholar]
- 106.Malmo V., Nes B.M., Amundsen B.H., et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133(5):466–473. doi: 10.1161/CIRCULATIONAHA.115.018220. [DOI] [PubMed] [Google Scholar]
- 107.Caples S.M., Mansukhani M.P., Friedman P.A., Somers V.K. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol. 2019;278:133–136. doi: 10.1016/j.ijcard.2018.11.100. [DOI] [PubMed] [Google Scholar]
- 108.Traaen G.M., Aakeroy L., Hunt T.E., et al. Effect of continuous positive airway pressure on arrhythmia in atrial fibrillation and sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2021;204(5):573–582. doi: 10.1164/rccm.202011-4133OC. [DOI] [PubMed] [Google Scholar]
- 109.Hunt T.E., Traaen G.M., Aakeroy L., et al. Effect of continuous positive airway pressure therapy on recurrence of atrial fibrillation after pulmonary vein isolation in patients with obstructive sleep apnea: a randomized controlled trial. Heart Rhythm. 2022;19(9):1433–1441. doi: 10.1016/j.hrthm.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 110.Nguyen B.O., Crijns H., Tijssen J.G.P., et al. Long-term outcome of targeted therapy of underlying conditions in patients with early persistent atrial fibrillation and heart failure: data of the RACE 3 trial. Europace. 2022;24(6):910–920. doi: 10.1093/europace/euab270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heidbuchel H., Van Gelder I.C., Desteghe L. ESC and EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: the Horizon 2020 EHRA-PATHS project. Eur Heart J. 2022;43(15):1450–1452. doi: 10.1093/eurheartj/ehab672. [DOI] [PubMed] [Google Scholar]
- 112.De Caterina R., Agewall S., Andreotti F., et al. Great Debate: triple antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting should be limited to 1 week. Eur Heart J. 2022;43(37):3512–3527. doi: 10.1093/eurheartj/ehac294. [DOI] [PubMed] [Google Scholar]
- 113.Dewilde W.J., Oirbans T., Verheugt F.W., et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 114.Gibson C.M., Mehran R., Bode C., et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 115.Cannon C.P., Bhatt D.L., Oldgren J., et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(16):1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 116.Lopes R.D., Heizer G., Aronson R., et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 117.Vranckx P., Valgimigli M., Eckardt L., et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394(10206):1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 118.Potpara T.S., Mujovic N., Proietti M., et al. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. Europace. 2020;22(1):33–46. doi: 10.1093/europace/euz259. [DOI] [PubMed] [Google Scholar]
- 119.Gargiulo G., Goette A., Tijssen J., et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019;40(46):3757–3767. doi: 10.1093/eurheartj/ehz732. [DOI] [PubMed] [Google Scholar]
- 120.Harrison S.L., Buckley B.J.R., Lane D.A., et al. Antiplatelet agents and oral anticoagulant use in patients with atrial fibrillation and carotid artery disease after first-time ischaemic stroke. Cardiovasc Drugs Ther. 2023 doi: 10.1007/s10557-023-07433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mant J., Hobbs F.D., Fletcher K., et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 122.Okumura K., Akao M., Yoshida T., et al. Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med. 2020;383(18):1735–1745. doi: 10.1056/NEJMoa2012883. [DOI] [PubMed] [Google Scholar]
- 123.Harrison S.L., Buckley B.J.R., Ritchie L.A., et al. Oral anticoagulants and outcomes in adults >/=80 years with atrial fibrillation: a global federated health network analysis. J Am Geriatr Soc. 2022;70(8):2386–2392. doi: 10.1111/jgs.17884. [DOI] [PubMed] [Google Scholar]
- 124.Chao T.F., Chiang C.E., Chan Y.H., et al. Oral anticoagulants in extremely-high-risk, very elderly (>90 years) patients with atrial fibrillation. Heart Rhythm. 2021;18(6):871–877. doi: 10.1016/j.hrthm.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 125.Potpara T.S., Mujovic N., Lip G.Y.H. Challenges in stroke prevention among very elderly patients with atrial fibrillation: discerning facts from prejudices. Europace. 2020;22(2):173–176. doi: 10.1093/europace/euz344. [DOI] [PubMed] [Google Scholar]
- 126.Eikelboom J.W., Weitz J.I. Direct oral anticoagulants in the very elderly. Thromb Haemostasis. 2023;123(4):377–379. doi: 10.1055/a-2021-8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kirchhof P., Toennis T., Goette A., et al. Anticoagulation with edoxaban in patients with atrial high-rate episodes. N Engl J Med. 2023;389(13):1167–1179. doi: 10.1056/NEJMoa2303062. [DOI] [PubMed] [Google Scholar]
- 128.Chao T.F., Joung B., Takahashi Y., et al. 2021 Focused update of the 2017 consensus guidelines of the Asia Pacific Heart Rhythm Society (APHRS) on stroke prevention in atrial fibrillation. J Arrhythm. 2021;37(6):1389–1426. doi: 10.1002/joa3.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galea R., Seiffge D., Raber L. Atrial fibrillation and ischemic stroke despite oral anticoagulation. J Clin Med. 2023;12(18) doi: 10.3390/jcm12185784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Benz A.P., Hohnloser S.H., Eikelboom J.W., et al. Outcomes of patients with atrial fibrillation and ischemic stroke while on oral anticoagulation. Eur Heart J. 2023;44(20):1807–1814. doi: 10.1093/eurheartj/ehad200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seiffge D.J., De Marchis G.M., Koga M., et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87(5):677–687. doi: 10.1002/ana.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ip Y.M.B., Lau K.K., Ko H., et al. Association of alternative anticoagulation strategies and outcomes in patients with ischemic stroke while taking a direct oral anticoagulant. Neurology. 2023;101(4):e358–e369. doi: 10.1212/WNL.0000000000207422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hsieh M.T., Liu C.H., Lin S.H., et al. Recurrent ischemic stroke and cardiovascular outcomes in patients with atrial fibrillation with ischemic stroke despite direct oral anticoagulants. Stroke. 2023;54(4):e145–e146. doi: 10.1161/STROKEAHA.122.041197. [DOI] [PubMed] [Google Scholar]
- 134.Chao T.F., Liu C.J., Liao J.N., et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133(16):1540–1547. doi: 10.1161/CIRCULATIONAHA.115.019794. [DOI] [PubMed] [Google Scholar]
- 135.So S.C. Effects of oral anticoagulation for atrial fibrillation after spontaneous intracranial haemorrhage in the UK: a randomised, open-label, assessor-masked, pilot-phase, non-inferiority trial. Lancet Neurol. 2021;20(10):842–853. doi: 10.1016/S1474-4422(21)00264-7. [DOI] [PubMed] [Google Scholar]
- 136.Schreuder F., van Nieuwenhuizen K.M., Hofmeijer J., et al. Apixaban versus no anticoagulation after anticoagulation-associated intracerebral haemorrhage in patients with atrial fibrillation in the Netherlands (APACHE-AF): a randomised, open-label, phase 2 trial. Lancet Neurol. 2021;20(11):907–916. doi: 10.1016/S1474-4422(21)00298-2. [DOI] [PubMed] [Google Scholar]
- 137.De Vriese A.S., Caluwe R., Van Der Meersch H., De Boeck K., De Bacquer D. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. 2021;32(6):1474–1483. doi: 10.1681/ASN.2020111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pokorney S.D., Chertow G.M., Al-Khalidi H.R., et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146(23):1735–1745. doi: 10.1161/CIRCULATIONAHA.121.054990. [DOI] [PubMed] [Google Scholar]
- 139.Reinecke H., Engelbertz C., Bauersachs R., et al. A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation. 2023;147(4):296–309. doi: 10.1161/CIRCULATIONAHA.122.062779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piccini J.P., Caso V., Connolly S.J., et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet. 2022;399(10333):1383–1390. doi: 10.1016/S0140-6736(22)00456-1. [DOI] [PubMed] [Google Scholar]