Abstract
It is thought that lipopolysaccharide (LPS) from gram-negative bacteria contributes significantly to the pathogenesis of septic shock. In vitro studies to address the mechanisms involved in this process have often investigated human monocytes or mouse macrophages, since these cells produce many of the mediators found in septic patients. Targeting of these mediators, especially tumor necrosis factor alpha (TNF-α), has been pursued as a means of reducing mortality in sepsis. Two experimental approaches were designed to test the assumption that in vitro studies with macrophages accurately predict in vivo mechanisms of LPS pathogenesis. In the first approach, advantage was taken of the fact that on consecutive days after injection of thioglycolate into mice, increased numbers of macrophages could be harvested from the peritoneum. These cells manifested markedly enhanced levels of in vitro TNF-α, interleukin 6 (IL-6), and nitric oxide production in response to LPS. In d-galactosamine-sensitized mice, however, thioglycolate treatment significantly decreased mortality due to LPS, as well as levels of circulating TNF-α and IL-6. Anti-TNF-α treatment confirmed this cytokine’s role in the observed lethality. In a second experimental approach, we compared the mouse macrophage-stimulating potencies of different LPS preparations with their lethalities to mice. In these studies, the in vitro macrophage-stimulating profiles presented by rough-LPS and smooth-LPS preparations were the reverse of their relative lethal potencies in vivo. In conclusion, peritoneal macrophages appear not to be the major cells responsible for the overall host response during endotoxic shock. These findings underscore the importance of verifying the correlation of in vivo systems with in vitro systems when attributing specific functions to a cell type.
Infections caused by gram-negative bacteria constitute one of the major causes of the sepsis syndrome, characterized by hypotension, tachycardia, tachypnea, disseminated intravascular coagulation, and multiple organ system failure. The mortality rate for septic shock patients continues to be unacceptably high, in spite of therapeutic intervention and rigorous supportive care (5, 23). In the last several years, many studies have focused on efforts to define the pathogenic mechanisms responsible for the inflammatory response that results in the sepsis syndrome. Many laboratories have contributed to the development of the concept that lipopolysaccharide (LPS), a major constituent of the outer cell membrane in gram-negative bacteria, is an important contributing factor to the pathogenesis of the sepsis syndrome. In this respect, it has been established that the administration of LPS to experimental animals or human volunteers will reproduce many of the proinflammatory and pathophysiological responses seen in patients with septic shock (7, 29).
Both human monocytes and mouse macrophages have been extensively utilized as prototypic cells involved in the pathogenesis of sepsis in vitro. Most studies have shown that the interaction of LPS with these cells induces the release of several cytokines, including tumor necrosis factor (TNF), interleukin 1 (IL-1), IL-6, IL-8, and gamma interferon (IFN-γ), as well as other inflammatory mediators such as prostaglandins, leukotrienes, platelet activation factor, and nitrogen and oxygen intermediates. Most of these immunological mediators present multiple biologic effects, play a critical role in inflammation and immune responses, and have been recognized as key mediators in the pathogenesis of infectious diseases and, more particularly, the pathophysiologic alterations observed in endotoxic shock (6, 26, 30, 37, 40, 41, 43). Potential roles for TNF-α, IL-1, IL-4, IL-6, IL-8, IL-10, and IFN-γ have been suggested by the results of in vivo studies of bacteremia and septic shock caused by both gram-positive and gram-negative bacteria. Also, those studies have provided evidence that immunotherapeutic intervention strategies that abrogate the biological activities of many of these mediators can have a significant protective effect against the lethal effects of LPS (4, 12, 16).
Over the past years, a number of laboratories investigating the events leading to endotoxic shock have pointed to TNF-α as a main mediator of the sepsis syndrome, as evidenced by an increase of this cytokine upon LPS administration to experimental animals and human volunteers, as well as during clinical sepsis (7, 20). In experimental animals, sensitization to the lethal effects of LPS by d-galactosamine (d-GalN) also parallels a marked sensitization to toxicity caused by TNF-α (14, 15), making this model particularly amenable to study of the role of TNF-α in sepsis. Additionally, immunotherapeutic intervention to abrogate the biological activity of TNF-α has been established as a means to protect against the lethal effects of endotoxin in animal models but has proven more problematic in clinical trials for the treatment of sepsis (1, 10, 28). The information provided by all these studies, however, remains controversial, depending on the experimental approach being utilized, and there are clearly a number of studies that suggest strongly that TNF alone cannot fully explain the lethal effect of LPS. In this regard, it has been reported that TNF serum levels do not correlate with mortality (33, 36). Also, whereas treatment of mice with LPS to elicit TNF production, or with exogenous TNF, confers protection against subsequent cecal ligation and puncture (8) or against bacterial challenge in granulocytopenic mice (3), elimination of TNF has variable effects on survival in cases of endotoxemia (9, 11, 32). Endotoxic LPS can also stimulate inflammatory cells to produce other proinflammatory cytokines that are often detected in the sera of patients with sepsis due to gram-negative bacteria. A number of recent reports have suggested that it is not TNF-α alone, but rather the balance of pro- versus anti-inflammatory cytokines, that dictates the severity and lethality of murine sepsis (17, 42).
The purpose of the studies reported here was to test the assumption that in vitro studies to evaluate the LPS-phenotypic response of mouse macrophages would accurately predict in vivo mechanisms of LPS pathogenesis. Therefore, we have carried out experiments in vitro to assess macrophage responses to LPS, and in parallel, we have used a mouse model of hypersensitivity to LPS induced by d-GalN treatment to assess in vivo lethality. Our experiments have demonstrated that macrophages harvested from the peritoneum on consecutive days after the injection of thioglycolate manifested temporally enhanced in vitro production of TNF-α, IL-6, and nitric oxide in response to LPS. In the in vivo mouse model of d-GalN-induced sensitization to LPS, however, thioglycolate treatment induced a significant decrease in mortality due to LPS, as well as in levels of circulating TNF-α and IL-6. The central role of TNF-α in the observed lethality was, nevertheless, confirmed by treatment of mice with anti-TNF-α antibody (Ab). As an alternative method to explore the question, we have used normal and d-GalN-treated mice that have been administered various doses of two LPS preparations known to differ significantly in their capacities to induce TNF-α production in mouse macrophages in vitro. Our results support the idea that mouse peritoneal macrophages are not major contributors to the host cytokine responses during endotoxin-induced injury in vivo. These results underscore the importance of verifying potential correlations of in vivo systems with in vitro systems when attributing specific functions to a given cell type.
MATERIALS AND METHODS
Animals.
Female C3HeB/FeJ mice, 6 to 10 weeks of age, were purchased from Jackson Laboratories (Bar Harbor, Maine), housed for at least 1 week in laminar-flow isolation units in the Kansas University Medical Center vivarium (which is accredited by the American Association for Accreditation of Laboratory Animal Use) under alternate dark-light cycles, and fed pellet chow ad libitum.
Reagents.
R60 (Ra) rough LPS from Salmonella minnesota (R-LPS) or smooth LPS from Escherichia coli 0111:B4 (S-LPS), containing less than 1.5% protein (wt/wt), were purchased from List Biological Laboratories (Campbell, Calif.). The LPS preparations were solubilized in pyrogen-free, sterile, distilled H2O to make stock solutions of 1 mg/ml. The stock solutions were sonicated for 3 min (W385 sonicator; Heat System Ultrasonic Inc., Farmingdale, N.Y.) and appropriately diluted immediately before use. d-GalN (Sigma Chemical Co., St. Louis, Mo.) sodium salts were freshly prepared in phosphate-buffered saline (PBS) just prior to use. Brewer thioglycolate, containing less than 0.1 endotoxin unit of LPS, as determined by a Limulus amebocyte assay, was purchased from Difco Laboratories, Detroit, Mich.
In vivo lethality studies.
Groups of mice were injected via the intraperitoneal route with various doses of LPS. For the d-GalN sensitization model, mice were injected with 0.4 ml of a solution of 20 mg of d-GalN in PBS/25 g of body weight, or with an equivalent volume of vehicle, containing increasing amounts of LPS. Some mice were injected with thioglycolate 5 days prior to the LPS challenge, as previously described. Mortality was assessed after 14 h of LPS administration in the d-GalN model, or after 48 h in the normal mouse model, as described previously (38). For the analysis of cytokines in serum, mice were bled via retro-orbital sinus puncture at 1 and 4 h after LPS injection. When mice were passively immunized against TNF-α, 6.75 × 104 neutralizing units of anti-TNF-α Ab (the kind gift of Roderick McCallum) per mouse was injected via the intraperitoneal route 4 h prior to endotoxin challenge, as described previously (21).
Macrophage isolation and stimulation.
Exudate macrophages were obtained by peritoneal lavage with 10 ml of sterile culture medium on consecutive days after intraperitoneal injection of 1.5 ml of 4% Brewer thioglycolate. Viability and total cell counts were assessed by staining with trypan blue. Differential counts were performed on cytospins stained with Diff-Quick solution. Total cells were suspended in RPMI 1640 medium containing 0.3% NaHCO3 (Sigma Chemical Co.), 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (JRH Biosciences, Lenexa, Kans.) and seeded at 0.5 × 106 to 2 × 106 macrophages/well (in 24- or 6-well culture plates; Costar, Cambridge, Mass.). After 2 to 3 h of incubation at 37°C and 5% CO2, nonadherent cells were removed by two washes with culture medium, and adherent cells were stimulated with the indicated concentrations of LPS. Following incubation at 37°C in 5% CO2 for 18 h, culture supernatants were collected and assayed for TNF-α and IL-6 as described below (2). Cell attachment was assessed by staining of total versus adherent cells with 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl-tetrazolium bromide (MTT; Sigma). Absorbance at 570 nm (A570) was measured with an MR5000 microplate reader (Dynatech Laboratories, Chantilly, Va.). Alternatively, total numbers of cells previous to and after adhesion were quantitated with a cell counter (2M; Coulter Electronics, Ltd., Hialeah, Fla.) after cell lysis and nuclear release.
TNF-α bioassay.
The amount of TNF-α present in culture supernatants was quantified by assessing the extent of killing of the murine cell line L929 (2, 45). Briefly, cells were seeded into 96-well plates at 4 × 105/well in RPMI 1640 complete medium supplemented with a 10% fetal bovine serum-derived product (FCS; HyClone Laboratories, Logan, Utah). After overnight incubation at 37°C in 5% CO2, new medium containing 5 μg of actinomycin D (Merck & Co., West Point, Pa.)/ml was used to replace the original medium. After an additional 2 h of incubation, macrophage culture supernatants were added to triplicate wells and serially diluted. The viability of L929 cells at 18 h was determined by the MTT incorporation assay as described above. TNF-α amounts were calculated by comparison with a recombinant mouse TNF-α (Genzyme, Cambridge, Mass.) standard assayed in each plate. TNF-α levels in plasma were also determined, by an enzyme-linked immunosorbent assay (ELISA) protocol described below for IL-6, by using anti-TNF-α Abs from Pharmingen (San Diego, Calif.).
IL-6 determination.
The presence of IL-6 amounts in macrophage culture supernatants was determined by ELISA (2, 45). Briefly, 96-well Immulon 1 microtiter plates (Dynatech) were coated overnight at 4°C with 100 μl of a 1-μg/ml solution of rat anti-mouse IL-6 monoclonal Ab (MAb; Pharmingen)/well. After the plates were washed and blocked for 2 h with 200 μl of PBS–10% FCS/well, samples or standard (recombinant mouse IL-6, 5 μg/ml; Genzyme) in PBS–10% FCS was added in triplicate and diluted into the plates, which were further incubated for 2 h at 37°C. After being washed again, plates were incubated for 45 min with 100 μl of biotinylated anti-IL-6 MAb (1 μg/ml in PBS–10% FCS)/well. Plates were washed, and 100 μl of avidin-peroxidase conjugate (Pierce, Rockford, Ill.)/well was added. After 30 min of incubation and a wash, 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB)-peroxidase substrate system (1:1; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added. The reaction was terminated by the addition of 100 μl of 1 M phosphoric acid/well, and A450 was determined with a Dynatech MR5000 reader.
Analysis of NO.
NO production in culture supernatants was assessed by measuring the amount of nitrite, a metabolic product of NO (2, 45). Briefly, supernatants in triplicate were mixed 1:1 with Griess reagent [0.1% N-(1-naphthyl)ethylenediamine dihydrochloride–1% sulfanilamide in 5% H2PO4 (1:1, vol/vol); Sigma] in 96-well microtiter plates, and A570 was measured with a Dynatech MR5000 microplate reader.
Statistical analysis.
Data from in vitro experiments are expressed as means and compared statistically by Student’s t test. Mortality data were analyzed by Fisher’s exact test. Cumulative data and 50% lethal doses (LD50) were determined according to the method of Reed and Muench (31). Levels of significance were determined by using the Epistat statistical package (T. Gustafson, Round Rock, Tex.) or Sigmaplot software (Jandel Co., San Rafael, Calif.) with a personal computer. P values less than 0.05 were considered statistically significant.
RESULTS
Differential activation of macrophages at different days after thioglycolate injection.
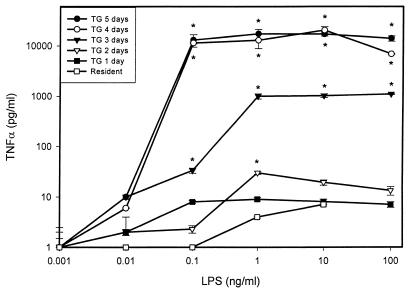
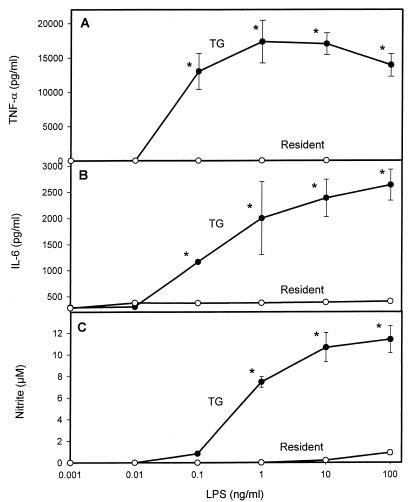
Mouse macrophages exposed to LPS in vitro demonstrate differential functional responses. In order to evaluate the in vitro response of host cells to LPS, we compared the production of TNF-α, nitric oxide, and IL-6 by mouse macrophages isolated from the peritoneal cavities of mice at different times following the injection of the inflammatory stimulus thioglycolate. As expected, the number of macrophages that could be harvested from the peritoneum increased with the number of days after thioglycolate injection. As shown by the data in Table 1, after 5 days of exposure to thioglycolate, the yield of total cells, as well as that of total macrophages, from the mouse peritoneum had increased by almost 1 order of magnitude. Also, as revealed by differential staining, the macrophages presented more characteristics of activation, such as increased volume, shape changes, and “foaming,” at increasing days after thioglycolate injection (data not shown). In addition to the increase in cell number, those macrophages harvested from the peritoneum on consecutive days after thioglycolate injection showed markedly increased levels of in vitro TNF-α production upon activation with R-LPS (Fig. 1). As can readily be observed from the data presented in Fig. 2, the same degree of significant enhancement by thioglycolate of activation with R-LPS at day 5 posttreatment was observed when IL-6 and nitric oxide were analyzed in the same cell culture supernatants.
TABLE 1.
Time course of the effects of intraperitoneal treatment with thioglycolate on the numbers of cells harvested from the peritoneal cavity in micea
| No. of days after thioglycolate treatment | 106 Cells (avg ± SEM)b harvested/mouse
|
Level of cell adhesionc | |||||
|---|---|---|---|---|---|---|---|
| Totald | Lymphocytese | Monocytes/macrophages | Neutrophils | Eosinophils | Mast cells | ||
| 0 | 1.62 ± 0.04 | 0.55 ± 0.01 | 0.91 ± 0.02 | 0.04 ± 0.00 | 0.00 ± 0.00 | 0.12 ± 0.00 | 46.7 |
| 1 | 10.12 ± 1.01 | 0.71 ± 0.08 | 0.78 ± 0.09 | 7.81 ± 1.00 | 0.81 ± 0.09 | 0.04 ± 0.01 | 55.5 |
| 2 | 11.64 ± 1.00 | 0.14 ± 0.01 | 9.15 ± 0.68 | 2.28 ± 0.30 | 1.34 ± 0.10 | 0.06 ± 0.00 | 67.28 |
| 3 | 11.59 ± 0.10 | 0.60 ± 0.01 | 9.27 ± 0.10 | 1.5 ± 0.02 | 1.19 ± 0.02 | 0.07 ± 0.00 | 94.37 |
| 4 | 10.71 ± 0.09 | 0.83 ± 0.01 | 8.33 ± 0.07 | 0.46 ± 0.00 | 1.03 ± 0.01 | 0.05 ± 0.00 | 93.98 |
| 5 | 12.93 ± 0.50 | 0.46 ± 0.02 | 11.71 ± 0.49 | 0.36 ± 0.03 | 0.33 ± 0.01 | 0.07 ± 0.00 | 93.40 |
Cells were harvested from the peritoneum by lavage at increasing days after thioglycolate injection.
From four mice.
Estimated by incorporation of MTT in adherent cells versus total number of cells.
Total cells were counted after staining with trypan blue.
Differential counts were performed on cytospins stained with Diff-Quick.
FIG. 1.
LPS-stimulated TNF-α production by mouse macrophages harvested at different days after thioglycolate (TG) injection. Peritoneal exudate was harvested at various times following thioglycolate administration and treated in vitro with increasing concentrations of R-LPS. Supernatants were assessed for the presence of TNF-α after 18 h. TNF-α was quantitated as the extent of cytotoxicity for the fibroblast cell line L929 as described in Materials and Methods. Results represent averages of triplicate determinations ± standard errors of the means (SEM) from one representative experiment, repeated four times under equivalent conditions. ∗, P < 0.05 (by Student’s t test) with respect to the group treated with LPS alone.
FIG. 2.
Effects of thioglycolate on mediator secretion by LPS-stimulated mouse macrophages. Cells were harvested at 0 (resident) or 5 (TG) days after thioglycolate injection. TNF-α (A), IL-6 (B), and nitric oxide (C) production after 18 h of R-LPS stimulation was analyzed in supernatants as described in Materials and Methods. ∗, P < 0.05 (by Student’s t test) in comparison to the day 0 group.
LPS-induced mortality in thioglycolate-pretreated mice.
In order to determine if there was a correlation between the in vitro observations of R-LPS-induced TNF-α production by peritoneal macrophages and in vivo induction of circulating levels of TNF-α, we used the model of d-GalN sensitization to LPS. This experimental model has been extensively investigated as a sensitive means by which to assess the toxic effects of TNF-α (14, 15). Therefore, the extent of contribution to lethality of an increase in TNF-α production by peritoneal cells from mice pretreated with thioglycolate might then be reflected in a differential increment of sensitivity to LPS in this d-GalN model. For these studies, therefore, either thioglycolate or sterile saline was administered to mice via the intraperitoneal route. Five days later, all mice were given d-GalN and increasing doses of R-LPS. The mortality of the animals was then recorded after LPS challenge, and the results are depicted in Fig. 3. As previously demonstrated by Galanos et al. (14, 15), the administration of otherwise nonlethal nanogram quantities of LPS together with d-GalN induced high mortality rates after 12 to 14 h of challenge in both groups of animals. No further mortality was detected over a period of 48 h. In contrast to the expected results, however, the administration of thioglycolate 5 days earlier actually diminished, rather than enhanced, the rate of mortality induced by R-LPS in the same model of d-GalN sensitization.
FIG. 3.
LPS-induced mortality in thioglycolate (TG)-pretreated mice. Mice were pretreated either with thioglycolate or with saline. Five days later, all animals received graded doses of R-LPS and 20 mg of d-GalN/g of body weight. Lethality was recorded 14 h after R-LPS challenge in d-GalN-sensitized mice. Cumulative data were depicted according to Reed and Muench’s method (31). ∗, statistically significant (P < 0.05 by Fisher’s exact test) compared with the respective R-LPS dose in thioglycolate-treated animals. The number of mice in each group is given in parentheses.
Circulating cytokines induced by LPS in thioglycolate-pretreated animals.
By using the same mouse model of sensitization to LPS and TNF-α, the next series of experiments was performed in order to determine the actual level of production of TNF-α in vivo. The presence of this cytokine was therefore assessed in serum collected 1.5 or 4 h after R-LPS challenge from mice treated with thioglycolate or with saline as a control 5 days previously. As can be seen from the data depicted in Fig. 4A, the administration of R-LPS induced readily detectable circulating levels of TNF-α above background at 1.5 h post-LPS administration. The levels of TNF-α were significantly decreased in all animals at 4 h compared to the 1.5-h levels (data not shown). In contrast to what might be expected from the in vitro data, however, but in accordance with the data presented in Fig. 3, mice pretreated with thioglycolate manifested lower levels of TNF-α in circulation in response to R-LPS challenge than mice pretreated with sterile saline. Additionally, the levels of IL-6 in circulation showed patterns similar to those observed for TNF-α (Fig. 4B).
FIG. 4.
Effects of thioglycolate (TG) pretreatment on circulating TNF-α (A) or IL-6 (B) levels induced by LPS in d-GalN-sensitized mice. Mice were treated as described in the text, and cytokine levels in serum collected 1.5 h after R-LPS challenge were determined by ELISA. Cytokine levels in sera collected 4 h after stimulation were below the detection limit. Results from one representative experiment, repeated five times, are depicted.
These data would indicate that there is a correlation between the ability of LPS to induce TNF-α in vivo and lethality. In fact, when circulating levels of TNF-α and IL-6 at 1.5 h induced by R-LPS (Fig. 4) were analyzed as functions of lethality (Fig. 3), high degrees of correlation were obtained (r = 0.79 and r = 0.68, respectively), both in animals treated with thioglycolate and in those given saline. However, this correlation does not extend to the ability of peritoneal macrophages to respond in vitro to LPS stimulation by producing this cytokine (compare with Fig. 2).
Effect of targeting TNF-α as a means to decrease LPS-induced mortality in thioglycolate-pretreated mice.
Efforts to decrease the mortality induced by LPS by targeting some of the mediators thought to be involved in shock, such as TNF-α, have been attempted in a number of published studies. Therefore, in the next series of experiments, we administered TNF-α-neutralizing Abs in the d-GalN model of sensitization to LPS. We anticipated that if, in fact, less total TNF-α was being generated in the thioglycolate-pretreated animals, then less anti-TNF-α Ab should be required to provide protection. As shown by the data in Fig. 5, the administration of increasing doses of anti-TNF-α Ab significantly decreased the mortality rates induced by 5 μg of R-LPS/kg in both saline- and thioglycolate-pretreated d-GalN-sensitized mice. Of interest, and as predicted, animals pretreated with thioglycolate required significantly less TNF-α-neutralizing Ab to decrease mortality rates than did control, saline-treated mice.
FIG. 5.
Effects of anti-TNF-α Ab treatment on LPS-induced mortality in thioglycolate (TG)- versus saline-pretreated, d-GalN-sensitized mice (n = 8 per group). Various amounts of anti-TNF-α neutralizing Abs were administered to mice 3 h before the administration of d-GalN plus R-LPS (5 μg/kg). Mortality was recorded after 14 h, and cumulative data were depicted according to the method described by Reed and Muench (31). ∗, P < 0.05 (by Fisher’s exact test).
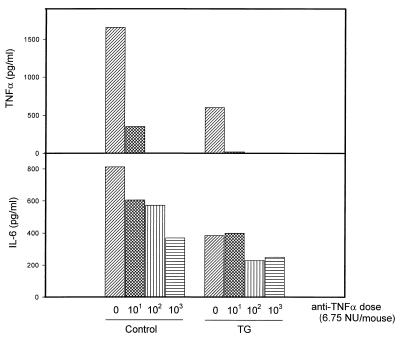
Using the same protocol, we further investigated the effects of administering anti-TNF-α Ab on cytokine production induced by LPS in vivo (Fig. 6). As has been previously demonstrated, the administration of 5 μg of R-LPS/kg induced the synthesis of levels of both TNF-α and IL-6 that were readily detectable in circulation at 1.5 h. Also as previously shown (Fig. 4), treatment of the animals with thioglycolate 5 days previously diminished their abilities to produce IL-6 and TNF-α in response to LPS. The administration of anti-TNF-α neutralizing Ab prior to the LPS challenge abrogated the detection of TNF-α in circulation and significantly diminished the production of IL-6 in vivo. The latter finding should not be totally surprising, given the enhancing role that TNF-α plays in secretion (34). As was found earlier in the mortality studies, the doses of anti-TNF-α neutralizing Ab required to diminish the production of both cytokines were lower in animals previously treated with thioglycolate than in control mice.
FIG. 6.
Effect of anti TNF-α Ab on TNF-α and IL-6 production induced by LPS in d-GalN-sensitized mice pretreated with thioglycolate (TG) or saline (control). Mice were treated as described in the text, and cytokine levels in serum were evaluated by ELISA. Results from one representative experiment, repeated five times with similar results, are depicted.
Differential mortality induced by different preparations of LPS in normal versus d-GalN-sensitized mice.
Published reports from a number of laboratories, including studies from our own laboratory, have clearly shown that different preparations of LPS can induce different cytokine responses in macrophages in vitro. In this respect, for example, results from our laboratory have shown that S-LPS from E. coli was significantly less active in stimulating macrophages to produce TNF-α than R-LPS from S. minnesota (Fig. 7A, partially reproduced from reference 45). This observation, therefore, provides an alternative approach to testing whether the differences observed in assays of the in vitro response of peritoneal macrophages to LPS can accurately reflect in vivo LPS-mediated mortality. Therefore, we compared the mortality rates induced in mice by these two preparations of R- and S-chemotype LPS in order to test directly the concept that an increased level of TNF-α production in vitro induced by R-LPS in comparison to S-LPS should be reflected by higher sensitivity to R-LPS than to S-LPS in the d-GalN sensitization model in vivo. The lethal effects of both LPS chemotypes were then analyzed, and the mortality results after challenge are depicted in Fig. 7B. Contrary to what might be expected from the in vitro data presented in Fig. 7A, the LD50 of S-LPS was approximately sixfold lower than that of R-LPS (Fig. 7B, left panel). In normal mice, however, the two chemotypes of LPS induced virtually indistinguishable levels of mortality as functions of the LPS dose (Fig. 7B, right panel). Of interest, however, even though the LD50 were superimposable in normal mice (Fig. 7B, right panel), the temporal profile of lethality response induced by R-LPS was significantly delayed with respect to that induced by S-LPS (data not shown).
FIG. 7.
(A) In vitro TNF-α response induced by S-LPS or R-LPS (Ra-LPS) in peritoneal-exudate macrophages. TNF-α was quantitated by cytotoxicity toward L929 cells, as described above. Results are averages of triplicate determinations ± SEM from one representative experiment (partially reproduced from reference 45) with permission of the publisher). (B) (Left) Mortality rates induced by different preparations of LPS (S-LPS and Ra-LPS) in d-GalN-sensitized mice. Animal mortality was recorded 14 h after challenge, and cumulative data were depicted according to the method described by Reed and Muench (31). ∗, P < 0.05 by Fisher’s exact test. (Right) Mortality rates induced by S-LPS and Ra-LPS in normal mice, 24 h after LPS challenge.
DISCUSSION
Over the last several decades, many laboratories have carried out experiments designed to elucidate the mechanisms involved in the activation of host cells induced by LPS during inflammatory processes. A number of these studies have contributed to the identification of a series of proinflammatory mediators that have been proposed as key effector molecules in the pathogenesis of septic shock. TNF-α is now thought to play a major role in several, but not all, experimental models of sepsis, including LPS lethality in mice. Here we report several experimental approaches that have been designed to evaluate the participation of LPS-induced TNF-α produced by macrophages in the development and pathogenesis of septic shock.
Surprisingly, our results have demonstrated a potential lack of correlation between results obtained from in vitro experiments and those obtained from in vivo experiments. We initially observed that exudate macrophages isolated from the peritoneums of thioglycolate-treated mice showed a significantly increased capacity to produce TNF-α, IL-6, and nitric oxide in response to LPS in comparison to resident macrophages. It has been observed previously that thioglycolate-elicited macrophages could produce larger amounts of lymphocyte-activating factor than resident cells (34). It could be hypothesized that the difference observed between elicited and resident macrophages could be due to different cell compositions. Even when an increased proportion of macrophages could be obtained at increasing days after thioglycolate injection, this difference could not account for the differences observed in cell response. This observation that resident macrophages are not as responsive to LPS for the production of these inflammatory mediators as thioglycolate-elicited macrophages could be confirmed by a recent report showing that resident macrophage responsiveness is, in part, induced by adherence to plastic (22). In this respect, the fact that resident exudate cells showed a lower level of adherence to the plastic, due to a higher proportion of lymphocytes, could not account for the decrease of several orders of magnitude in the response to LPS in comparison with elicited macrophages. Opposite results, however, were observed in our d-GalN sensitization model, in which animals became markedly more sensitive to the lethal effect of LPS due to a higher degree of sensitivity to TNF toxicity. The results of the in vivo experiments showed a decreased mortality rate in d-GalN-sensitized animals pretreated with thioglycolate, correlating with a decreased amount of TNF in circulation. These results, therefore, could lead to the idea that macrophage-derived TNF-α might not be the crucial factor contributing to death in septic shock. The participation of TNF-α, however, would appear to be significant, since our experiments for which results are shown in Fig. 2 demonstrated, in accordance with several other reports, that the administration of Abs neutralizing TNF-α significantly diminished the rate of mortality induced by LPS in these animals. Moreover, this effect seems not to be specific for LPS, since it has been reported that anti-TNF Ab also protects GalN-sensitized mice from shock induced by staphylococcal enterotoxin B or toxic shock syndrome toxin 1 (24, 27). Thus, these data would contribute information on the precise role of LPS-macrophage interactions as pivotal events in the generation of TNF-α, leading to the development of the systemic inflammatory response syndrome and septic shock.
This is not to say, however, that macrophage-derived TNF-α cannot contribute to TNF-α-mediated lethality in the d-GalN mouse model. In this respect, one need only consider the seminal studies of Galanos and coworkers showing that high sensitivity to the lethal effects of LPS was achieved for d-GalN-treated mice of the C3H/HeJ (nonresponder to LPS) strain by transfer of macrophages from the C3H/HeN (LPS responder) strain (13). Nevertheless, the more global concept that peritoneal macrophages might serve as the primary source of cells producing TNF-α in the LPS-challenged experimental animals does not appear to be fully validated, and other organs, such as the liver, with a high proportion of Kupffer cells, could readily serve as likely sources of TNF-α observed in circulation after the injection of LPS into mice (18). Recent studies by Kumins et al. (23) showing that partial hepatectomy reduces the level of LPS-induced circulating TNF would certainly be supportive of such views, although some previous studies suggested that splenic macrophages would not constitute the main source of TNF (35).
It has been described that preexposure of cells or animal to low doses of endotoxin induces an altered pattern of mediator production in response to a subsequent LPS challenge, a phenomenon known as endotoxin tolerance (46). In this context, a possible explanation for our in vivo results would be that animals exposed for several days to the irritant stimulus thioglycolate manifested a “desensitization”-like state when challenged with LPS, thus showing lower levels of TNF-α secretion into the bloodstream than nonstimulated control mice. While the total levels of circulating mediators such as TNF-α participating in vivo might not necessarily be reflected by results obtained in vitro, this phenomenon would not explain the fact that exudate macrophages did not manifest a tolerant phenotype when examined for LPS responses in vitro. Rather, these cells were activated towards higher in vitro responses to LPS by the thioglycolate treatment. Also, in a completely different system evaluated in parallel, R-LPS and S-LPS preparations manifested stimulating activities in vitro that were not reflective of their lethal potencies in vivo. Similar differences in the potencies of R-LPS and S-LPS to induce TNF in vitro have been reported by us and others (19, 45). However, this second example of a lack of correlation between in vitro and in vivo activities of LPS, in which no preliminary treatment was administered to the mice prior to LPS challenge, would also argue against a desensitization hypothesis as fully explaining these findings. Another possible explanation of our findings would be that the inflammatory response elicited by the thioglycolate contributes to an intraperitoneal accumulation of inhibitory molecules such as soluble CD14 (sCD14) that could neutralize LPS toxicity. It has been reported that the preadministration of sCD14 partially reduced mortality due to LPS, but it did not prevent the shock symptoms and liver injury, nor did it affect the secretion of proinflammatory cytokines in normal or galactosamine-sensitized mice (39). This would be in opposition to our results, where we have observed a decrease in levels of LPS-induced circulatory TNF-α and IL-6 in thioglycolate-pretreated mice.
In conclusion, our results suggest that peritoneal macrophages appear not to be the major contributing cells in overall host response during endotoxic shock. Even when this observation could be due to the systems under evaluation, the data reported here confirm several contradictions between the results obtained from in vitro and in vivo systems of evaluation of LPS activation. Similarly, evidence showing that circulating cytokine levels participating in in vivo lethality might not necessarily parallel in vitro results has been reported recently (44). Our findings might, therefore, allow the paradigm of TNF-α production by peritoneal macrophages as the main event involved in the pathogenesis of septic shock to be challenged. Finally, these results underscore the importance of verifying the correlation of in vivo and in vitro systems when attributing specific functions to a specific cell type(s) and/or to a given inflammatory mediator(s).
ACKNOWLEDGMENTS
This work was supported by grants from the National Cancer Institute (PO1 CA54474 and R37-AI23447) and an unrestricted medical grant from Merck and Co. C.R.A. was a scholar of the Kansas Health Foundation.
We acknowledge Roderick McCallum with gratitude for his kind gift of anti-TNF-α Ab. We thank James Goss of List Biologicals for helpful assistance and Kathy Rode for help in the preparation of the final form of the manuscript.
REFERENCES
- 1.Abraham E, Wunderink R, Silverman H, Perl T M, Nasraway S, Levy H, Bone R, Wenzel R P, Balk R, Allred R, et al. Efficacy and safety of monoclonal antibodies to tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha Mab sepsis group. JAMA. 1997;273:934–941. [PubMed] [Google Scholar]
- 2.Amura C R, Chen L C, Hirohashi N, Lei M G, Morrison D C. Two functionally independent pathways for the LPS-dependent activation of mouse peritoneal macrophages. J Immunol. 1997;159:5079–5083. [PubMed] [Google Scholar]
- 3.Amura C R, Fontan P A, Sanjuan N, Sordelli D O. The effect of treatment with interleukin-1 and tumor necrosis factor on Pseudomonas aeruginosa lung infection in a granulocytopenic mouse model. Clin Immunol Immunopathol. 1994;73:261–266. doi: 10.1006/clin.1994.1196. [DOI] [PubMed] [Google Scholar]
- 4.Beutler B, Milsark I W, Cerami A. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effects of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 5.Bone R C. Sepsis syndrome. New insights into its pathogenesis and treatment. Infect Dis Clin N Am. 1991;5:793–805. [PubMed] [Google Scholar]
- 6.Bucklin S E, Russell S W, Morrison D C. Participation of IFN-gamma in the pathogenesis of LPS lethality. Prog Clin Biol Res. 1994;388:399–406. [PubMed] [Google Scholar]
- 7.Cannon J G. Endotoxin and cytokine responses in human volunteers. In: Ryan J L, Morrison D C, editors. Bacterial endotoxic lipopolysaccharides. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 312–326. [Google Scholar]
- 8.Echtenacher B, Hültner L, Männel D N. Cellular and molecular mechanisms of TNF protection in septic peritonitis. J Inflamm. 1996;47:85–89. [PubMed] [Google Scholar]
- 9.Eskandari M K, Bolgos G, Miller C, Nguyen D T, DeForge L E, Remick D G. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 10.Fiedler V B, Loof I, Sander E, Voehringer V, Galanos C, Fournel M A. Monoclonal antibody to tumor necrosis factor-alpha prevents lethal endotoxin sepsis in adult rhesus monkeys. J Lab Clin Med. 1992;120:574–588. [PubMed] [Google Scholar]
- 11.Franks A K, Kujawa K I, Yaffe L J. Experimental elimination of tumor necrosis factor in low-dose endotoxin models has variable effects on survival. Infect Immun. 1991;59:2609–2614. doi: 10.1128/iai.59.8.2609-2614.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freudenberg M A, Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freudenberg M A, Keppler D, Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986;51:891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galanos C, Freudenberg M A, Salamao R, Mossman H, Kumazawa Y. Galactosamine-induced sensitization to the lethal effects of endotoxin. In: Ryan J L, Morrison D C, editors. Bacterial endotoxic lipopolysaccharides. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 75–104. [Google Scholar]
- 16.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glauser M P, Heumann D, Baumgartner J D, Cohen J. Pathogenesis and potential stratagies for prevention and treatment of septic shock: an update. Clin Infect Dis. 1994;18:S205–S216. doi: 10.1093/clinids/18.supplement_2.s205. [DOI] [PubMed] [Google Scholar]
- 18.Harbrecht B G, Wang S C, Simmons R L, Billiar T R. Cyclic GMP and guanylate cyclase mediate lipopolysaccharide-induced Kupffer cell tumor necrosis factor-alpha synthesis. J Leukoc Biol. 1995;57:297–302. doi: 10.1002/jlb.57.2.297. [DOI] [PubMed] [Google Scholar]
- 19.Henricson B E, Perera P Y, Qureshi N, Takayama K, Vogel S N. Rhodopseudomonas sphaeroides lipid A derivatives block in vitro induction of tumor necrosis factor and endotoxin tolerance by smooth lipopolysaccharide and monophosphoryl lipid A. Infect Immun. 1992;60:4285–4290. doi: 10.1128/iai.60.10.4285-4290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hesse D G, Tracey K J, Fong Y, Manogue K R, Palladino M A, Jr, Cerami A, Shires G T, Lowry S F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988;166:147–153. [PubMed] [Google Scholar]
- 21.Hill M R, McCallum R E. Identification of tumor necrosis factor as a transcriptional regulator of the phosphoenolpyruvate carboxykinase gene following endotoxin treatment of mice. Infect Immun. 1992;60:4040–4050. doi: 10.1128/iai.60.10.4040-4050.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodge-Dufour J, Noble P W, Horton M R, Bao C, Wysoka M, Burdick M D, Strieter R M, Trinchieri G, Puré E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–2500. [PubMed] [Google Scholar]
- 23.Kumins N H, Hunt J, Gamelli R L, Filkins J P. Partial hepatectomy reduces the endotoxin-induced peak circulating level of tumor necrosis factor in rats. Shock. 1996;5:385–388. doi: 10.1097/00024382-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Miethke T, Duschek K, Wahl C, Heeg K, Wagner H. Pathogenesis of the toxic shock syndrome: T cell mediated lethal shock caused by the superantigen TSST-1. Eur J Immunol. 1993;23:1494–1500. doi: 10.1002/eji.1830230715. [DOI] [PubMed] [Google Scholar]
- 25.Morrison D C, Danner R L, Dinarello C A, Munford R S, Natanson C, Pollack M, Spitzer J A, Ulevitch R J, Vogel S N, McSweegan E. Bacterial endotoxins and pathogenesis of Gram-negative infections: current status and future directions. J Endotoxin Res. 1994;1:71–83. [Google Scholar]
- 26.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 27.Nagaki M, Muto Y, Ohnishi H, Yasuda S, Sano K, Naito T, Maeda T, Yamada T, Moriwaki H. Hepatic injury and lethal shock in galactosamine-sensitized mice induced by the superantigen staphylococcal enterotoxin B. Gastroenterology. 1994;106:450–458. doi: 10.1016/0016-5085(94)90604-1. [DOI] [PubMed] [Google Scholar]
- 28.Natanson C, Hoffman W D, Suffredini A F, Eichacker P Q, Danner R L. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994;120:771–787. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- 29.Raetz C R, Ulevitch R J, Wright S D, Sibley C H, Ding A, Nathan C F. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 1991;5:2652–2660. doi: 10.1096/fasebj.5.12.1916089. [DOI] [PubMed] [Google Scholar]
- 30.Redl H, Schalag G, Bahrami S, Schade U, Ceska M, Stutz P. Plasma neutrophil-activating peptide-1/interleukin-8 and neutrophil elastase in a primate bacteremia model. J Infect Dis. 1991;164:383–388. doi: 10.1093/infdis/164.2.383. [DOI] [PubMed] [Google Scholar]
- 31.Reed L J, Muench H A. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 32.Remick D, Manohar P, Bolgos G, Rodriguez J, Moldawer L, Wollenberg G K. Blockade of tumor necrosis factor reduced lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4:89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Rigato O, Ujvari S, Castelo A, Salomao R. Tumor necrosis factor alpha (TNF-α) and sepsis: evidence for a role in host defense. Infection. 1996;24:314–318. doi: 10.1007/BF01743367. [DOI] [PubMed] [Google Scholar]
- 34.Rosenstreich D L, Vogel S N, Jacques A, Wahl L M, Scher I, Mergenhagen S E. Differential endotoxin sensitivity of lymphocytes and macrophages from mice with an X-linked defect in B cell maturation. J Immunol. 1978;121:685–690. [PubMed] [Google Scholar]
- 35.Salkowski C A, Neta R, Wynn T A, Strassmann G, van Rooijen N, Vogel S N. Effect of liposome-mediated macrophage depletion on LPS-induced cytokine gene expression and radioprotection. J Immunol. 1995;155:3168–3179. [PubMed] [Google Scholar]
- 36.Sánchez-Cantú L, Rode H N, Yun T J, Christou N V. Tumor necrosis factor alone does not explain the lethal effect of lipopolysaccharide. Arch Surg. 1991;126:231–235. doi: 10.1001/archsurg.1991.01410260121017. [DOI] [PubMed] [Google Scholar]
- 37.Shalaby M R, Halgunste J, Haugen O A, Aarset H, Aarden L, Waage A. Cytokine-associated tissue injury and lethality in mice: a comparative study. Clin Immunol Immunopathol. 1992;61:69–82. doi: 10.1016/s0090-1229(06)80008-5. [DOI] [PubMed] [Google Scholar]
- 38.Silverstein R, Norimatsu M, Morrison D C. Fundamental differences during Gram-positive versus Gram-negative sepsis become apparent during bacterial challenge of d-galactosamine-treated mice. J Endotoxin Res. 1997;4:173–181. [Google Scholar]
- 39.Stelter F, Witt S, Furll B, Jack R S, Hartung T, Schutt C. Different efficacy of soluble CD14 treatment in high- and low-dose LPS models. Eur J Clin Investig. 1998;28:205–213. doi: 10.1046/j.1365-2362.1998.00264.x. [DOI] [PubMed] [Google Scholar]
- 40.Tracey K J, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 41.Ulich T R, Guo K Z, Remick D, del Castillo J, Yin S M. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991;146:2316–2323. [PubMed] [Google Scholar]
- 42.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Ba Z F, Chaudry I H. Mechanism of hepatocellular dysfunction during early sepsis. Key role of increased gene expression and release of proinflammatory cytokines tumor necrosis factor and interleukin-6. Arch Surg. 1997;132:364–369. doi: 10.1001/archsurg.1997.01430280038005. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Peterson J W, Nielsen D W, Klimpel G R. Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol. 1997;159:4868–4878. [PubMed] [Google Scholar]
- 45.Zhang X, Morrison D C. Lipopolysaccharide structure-function relationship in activation versus reprogramming of mouse peritoneal macrophages. J Leukoc Biol. 1993;54:444–450. doi: 10.1002/jlb.54.5.444. [DOI] [PubMed] [Google Scholar]
- 46.Zuckerman S H, Evans G F. Endotoxin tolerance: in vivo regulation of tumor necrosis factor and interleukin-1 synthesis is at the transcriptional level. Cell Immunol. 1992;140:513–519. doi: 10.1016/0008-8749(92)90216-c. [DOI] [PubMed] [Google Scholar]