Abstract
Introduction
Women with a prior stillbirth or a history of recurrent first trimester miscarriages are at increased risk of adverse pregnancy outcomes. However, little is known about the impact of a second trimester pregnancy loss on subsequent pregnancy outcome. This review investigated if second trimester miscarriage or termination for medical reason or fetal anomaly (TFMR/TOPFA) is associated with future adverse pregnancy outcomes.
Material and methods
A systematic review of observational studies was conducted. Eligible studies included women with a history of a second trimester miscarriage or termination for medical reasons and their pregnancy outcomes in the subsequent pregnancy. Where comparative studies were identified, studies which compared subsequent pregnancy outcomes for women with and without a history of second trimester loss or TFMR/TOPFA were included. The primary outcome was livebirth, and secondary outcomes included: miscarriage (first and second trimester), termination of pregnancy, fetal growth restriction, cesarean section, preterm birth, pre‐eclampsia, antepartum hemorrhage, stillbirth and neonatal death. Studies were excluded if exposure was nonmedical termination or if related to twins or higher multiple pregnancies. Electronic searches were conducted using the online databases (MEDLINE, Embase, PubMed and The Cochrane Library) and searches were last updated on June 16, 2023. Risk of bias was assessed using the Newcastle‐Ottawa scale. Where possible, meta‐analysis was undertaken. PROSPERO registration: CRD42023375033.
Results
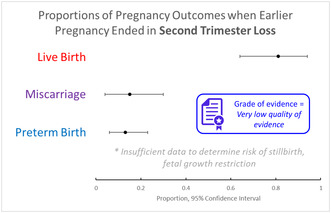
Ten studies were included, reporting on 12 004 subsequent pregnancies after a second trimester pregnancy miscarriage. No studies were found on outcomes after second trimester TFMR/TOPFA. Overall, available data were of “very low quality” using GRADE assessment. Meta‐analysis of cohort studies generated estimated outcome frequencies for women with a previous second trimester loss as follows: live birth 81% (95% CI: 64–94), miscarriage 15% (95% CI: 4–30, preterm birth 13% [95% CI: 6–23]).The pooled odds ratio for preterm birth in subsequent pregnancy after second trimester loss in case–control studies was OR 4.52 (95% CI: 3.03–6.74).
Conclusions
Very low certainty evidence suggests there may be an increased risk of preterm birth in a subsequent pregnancy after a late miscarriage. However, evidence is limited. Larger, higher quality cohort studies are needed to investigate this potential association.
Keywords: late miscarriage/spontaneous abortion, second trimester miscarriage/spontaneous abortion, subsequent pregnancy outcomes, termination of pregnancy for fetal anomaly, termination of pregnancy for medical reason or fetal anomaly
Very low certainty evidence suggests there may be an increased risk of preterm birth after a late miscarriage. However, evidence is very limited, and this association requires further study using larger cohort studies.
Abbreviations
- TFMR
termination for medical reason
- TOPFA
termination of pregnancy for fetal anomaly
Key message.
The impact of second trimester miscarriage on subsequent pregnancy outcomes is largely unknown. Existing evidence suggests preterm birth risk is higher, but high‐quality research is urgently needed. There were no studies addressing pregnancy after second trimester termination for medical/fetal reasons.
1. INTRODUCTION
Having a history of recurrent first trimester miscarriages or a prior stillbirth significantly increases the risk of adverse outcomes in subsequent pregnancies, including preterm birth, neonatal death and pre‐eclampsia. 1 , 2 Lamont et al. 3 found that women with a previous stillbirth are almost five times more likely to have a stillbirth in subsequent pregnancy, compared with women with a previous live birth (odds ratio [OR] 4.77, 95% confidence interval [CI]: 3.70–6.15). Similarly, Wu et al. 4 found women with recurrent first trimester miscarriages are at increased risk of preterm birth (OR 1.60 [95% CI: 1.45–1.78]). It is also recognized that the more first trimester miscarriages a woman has the greater the risk of subsequent miscarriage and even having a single first trimester miscarriage significantly increased the risk of subsequent miscarriage. 5 , 6 However, the impact of a previous second trimester pregnancy loss (miscarriage or termination for medical reasons or fetal anomaly [TFMR/TOPFA]) on future pregnancies is less certain.
Second trimester miscarriage, also known as late miscarriage, occurs in approximately 1–2 in 100 pregnancies. 7 , 8 It is defined as a spontaneous pregnancy loss which occurs from 13 to 23 + 6 weeks’ gestation in the UK. Second trimester miscarriage accounts for around 15% of all miscarriages. 9 After a second trimester miscarriage many couples are understandably anxious that they will be at greater risk of another miscarriage or other adverse pregnancy outcomes in future. In addition, in the UK around 5000 wanted pregnancies are terminated for fetal or medical reasons annually with the majority undergoing termination in the second trimester. 10 Couples may be equally anxious about the risks to future pregnancy after undergoing second trimester termination for medical/fetal reasons.
There are several etiologies of second trimester loss, including chromosomal abnormality, infection, congenital birth defects, cervical insufficiency, placental and uterine pathologies. 11 We hypothesized that there may also be shared pathophysiology amongst pregnancy loss at any gestation and that there may be an increased risk of adverse pregnancy outcomes in future pregnancies after second trimester miscarriage or TFMR/TOPFA. Giving birth in the second trimester has been hypothesized as akin to a very preterm birth, whether miscarriage occurs spontaneously, or methods are used to induce cervical dilatation and labor in the second trimester for an ultrasound diagnosed miscarriage or a planned TFMR/TOPFA. We know that women with a prior preterm birth are known to be at greater risk of preterm birth in future pregnancies. 12 Women with a prior first trimester miscarriage are also at greater risk of preterm birth. 3 Therefore, we hypothesized that women who have a second trimester miscarriage or TFMR/TOPFA may be at increased risk of preterm birth as well as at greater risk of subsequent pregnancy loss, including second trimester miscarriage in their subsequent pregnancies. Knowledge of any such increased risks is vital to plan appropriate clinical care pathways in pregnancies after second trimester pregnancy loss. This systematic review of observational studies aimed to answer the research question: “Does a second trimester pregnancy loss (miscarriage or TFMR/TOPFA) lead to an increased risk of adverse perinatal and obstetric risks in the subsequent pregnancy?”. Prior systematic reviews 13 , 14 , 15 have highlighted an association with termination or uterine evacuative procedures and subsequent risk of preterm birth but, to the best of our knowledge, none have focused solely on spontaneous second trimester miscarriage or termination for TFMR/TOPFA.
2. MATERIAL AND METHODS
A systematic review of observational studies was conducted. Literature searches were performed using online research databases (MEDLINE, Embase, PubMed and The Cochrane Library) employing a search strategy developed a priori. The search strategy was initially developed in MEDLINE and adapted for each database thereafter. The following search terms, including synonyms, were used: second trimester miscarriage, late miscarriage, pregnancy loss, termination for medical reason, termination for fetal anomaly, pregnancy outcomes, subsequent or next pregnancy. MeSH terms, Boolean operators and truncation were utilized. There were no language or date restrictions, nor other limits used. Reference lists of included studies were hand‐searched for any additional papers. Searches were last updated on June 16, 2023. Where appropriate, attempts were made to contact authors for further information with a single reminder sent where the initial request unanswered. Where only an abstract was available, and no further data were available by contacting authors, studies were excluded. PROSPERO registration was approved a priori (PROSPERO ID CRD420233750033).
For studies to be eligible for inclusion, the population was defined as women with a least two singleton pregnancies. For cohort studies the exposure was defined as a history of second trimester loss or TFMR/TOPFA, and in comparative studies, outcomes had to be compared to women without a history of second trimester loss of TFMR/TOPFA. Cases were defined as women with a history of prior second trimester miscarriage or termination for medical reason and controls were women without a history of second trimester loss. Cross‐sectional and ecological studies were included where women with previous second trimester miscarriage or termination for medical reason and their subsequent pregnancy outcomes were studied. The primary outcome was livebirth in the subsequent pregnancy. The secondary outcomes included miscarriage, termination of pregnancy, preterm birth, fetal growth restriction, cesarean section, pre‐eclampsia, antepartum hemorrhage, stillbirth, neonatal death, neonatal unit admission and mode of birth. Studies were excluded if they included twins or higher multiple pregnancies, or if pregnancies were terminated for nonmedical reasons. Studies which investigated the exposure of prior recurrent pregnancy losses, whether late or early miscarriages, were excluded.
Data were extracted from eligible papers using a standardized form, developed specifically for this review. Information was extracted on study design, methods used, outcomes and findings by two independent researchers. Any disagreements were settled by discussion with the remaining two reviewers. Raw data published or supplied by the authors were used. If raw data were not available, proportions were calculated from percentages or from any charts using Plot Digitizer. 16 Risk of bias was assessed using the Newcastle‐Ottawa scale for case–control and cohort studies. 17 This was completed independently for each study by two reviewers, with any disagreements being resolved by discussion with remaining authors. The quality, appropriateness and certainty of the evidence and was assessed using the GRADE approach. 18 MOOSE guidelines were followed for this review. 19
2.1. Statistical analysis
Primary data analysis and data aggregation were performed on all studies deemed eligible for inclusion. Where appropriate, Stata Version 14 (College Station, TX) was used to carry out meta‐analysis. The Metaprop and Metan commands were used to generate forest plots. 20 Random‐effects models were used to produce summary odds ratios and 95% confidence intervals, where there were two or more case–control or cohort studies. Data from individual studies were pooled using the Mantel–Haenszel method. I2 was used to estimate statistical heterogeneity and was classified according to established criteria. 21 A p‐value of <0.05 was considered statistically significant. Funnel plots were planned to be used to assess risk of publication bias where at least five studies were included for each research question. However, this could not be conducted with the evidence available. We planned to undertake a sensitivity analysis but there were an insufficient number of included studies to complete this.
3. RESULTS
The PRISMA flow chart outlines the results of the literature searches (Figure 1). Twenty‐nine full text studies were assessed for eligibility, and all were published in English. Ten studies were deemed eligible for inclusion (Table 1). 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Each of the included studies considered second trimester miscarriage only, and no studies were found which studied pregnancy outcomes after second trimester termination for TFMR/TOPFA. Two papers met the eligibility criteria, but they were abstracts only 32 , 33 and were excluded. Both authors were contacted but no further information was available therefore they were excluded.
FIGURE 1.
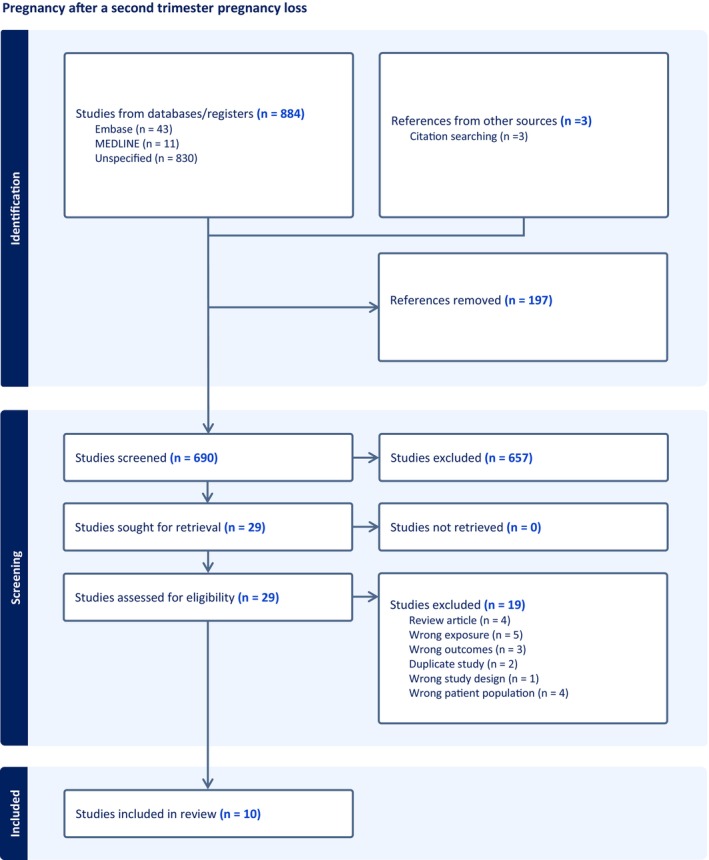
PRISMA flow chart summarizing the results of the search strategy.
TABLE 1.
Summary of the characteristics of the studies included in this review.
| Study | Location | Design | Population | Outcomes reported | Findings |
|---|---|---|---|---|---|
| Cheung et al. (2023) 22 | Queen Mary Hospital, The University of Hong Kong, Hong Kong | Retrospective cohort study | Women with a subsequent pregnancy after a second trimester loss (n = 64). | Live birth, term delivery, preterm birth and second trimester miscarriage in subsequent pregnancy. | The risk of second trimester loss in subsequent pregnancy is increased for women having a loss due to rupture of membranes or an inevitable miscarriage, compared to women having a silent miscarriage. |
| Edlow et al. (2007) 23 | University Hospital of Pennsylvania, USA |
Retrospective case–control study |
Comparison between three groups – (1) women with a history of second trimester loss (n = 38), (2) women with a history of spontaneous preterm birth (n = 76), (3) women with a history of full‐term birth (n = 76). | Second trimester loss and spontaneous preterm birth in subsequent pregnancies. | Women with a previous second trimester loss are more at risk of preterm birth or second trimester loss in subsequent pregnancy. |
| Goldenberg et al. (1993) 24 | Department of Obstetrics and Gynecology, The University of Alabama at Birmingham, USA | Retrospective case–control study | Comparison between three groups – (1) women with a previous second trimester loss (n = 95), (2) women who had a previous delivery at 25–36 weeks (n = 515), (3) women who had a previous term delivery (n = 2781). | Preterm birth (delivery before 37 weeks and before 34 weeks given separately), neonatal death and stillbirth. | Women with a previous second trimester loss are at a higher risk of preterm birth, neonatal death and stillbirth in subsequent pregnancy. The strongest association with adverse outcomes in subsequent pregnancy is index loss between 19–22 weeks. |
| Joubert et al. (2022) 25 | Academic center in Paris, France | Retrospective cohort study | n = 78 women having subsequent pregnancies after a second trimester loss. | Term delivery, recurrent second trimester miscarriage and preterm birth in the subsequent pregnancy. | 64.1% of subsequent pregnancies ended in term delivery, 21.8% resulted in recurrent second trimester loss and 14.1% in preterm birth. |
| Linehan et al. (2019) 26 | Tertiary referral center in University College Cork, Ireland | Prospective cohort study | n = 110 women with a second trimester miscarriage, having a subsequent pregnancy. | Term birth, first and second trimester miscarriage, preterm birth, neonatal death and cesarean section in subsequent pregnancy. | Women with a previous second trimester loss have a higher risk of preterm birth and recurrent second trimester loss. |
| Puyenbroek & Stolte (1983) 27 | Free University Hospital of Amsterdam | Case control study | n = 28 women having a second trimester miscarriage, compared to a control group of n = 56. | Preterm birth. | There is a correlation between spontaneous abortion and second trimester loss in subsequent pregnancy. |
| Roberts et al. (2016) 28 | New South Wales, Australia | Retrospective cohort study | n = 4290 women having subsequent pregnancies immediately after a second trimester loss. | Preterm birth and miscarriage in subsequent pregnancy. | No clear statement of outcomes in subsequent pregnancy. |
| Sneider et al. (2016) 29 | Denmark | Prospective cohort study | n = 6194 women who had a previous first second trimester miscarriage, followed by miscarriage or delivery >16 weeks in their subsequent pregnancy. | Recurrence rate of second trimester loss or preterm birth in subsequent pregnancy. | Recurrence rate of second trimester loss varies depending on the cause, with cervical insufficiency having the highest recurrence rate. |
| Yang et al. (2023) 30 | Center for Reproductive Medicine of Shandong University, China | Case–control study | n = 1072 women with a previous second trimester loss after a first treatment cycle of IVF, having a subsequent pregnancy. Control group of n = 4219 women having an IVF, followed by subsequent pregnancy. | Live birth, first trimester (“early”) miscarriage, second trimester (“late”) miscarriage and preterm birth. | Women having a first cycle of IVF with an unexplained second trimester loss, or second trimester loss due to cervical causes, had an increased risk of miscarriage and lower rates of live births in subsequent pregnancy. |
| Yusuf et al. (2023) 31 | Perinatal History Clinic, London, UK | Retrospective cohort study | n = 35 women with a previous second trimester loss and a subsequent pregnancy. | Live birth and adverse outcomes in subsequent pregnancy. Adverse outcomes include pre‐eclampsia, preterm birth, stillbirth, fetal growth restriction. | 4/5 women had a healthy subsequent pregnancy after a second trimester loss. |
Included study characteristics are shown in Table 1. Eligible studies included a total of 12 004 subsequent pregnancies. Studies were conducted from 1983 to2023. Six of the studies were cohort studies, and four were case–control studies. One study did not provide any relevant data for analysis. All of the included studies were conducted in high or higher middle‐income countries (Figure 2). There was considerable variation in sample sizes, with the number of subsequent pregnancies varying from 35 to 6194. There was variation in the populations of women that were identified. For example, Roberts et al. 28 identified a population of women who had a subsequent loss immediately after their index second trimester loss. In addition, Sneider et al. 29 only included women who had a second trimester loss in their first pregnancy. Yang et al. 30 only included women having a second trimester loss after a first cycle of IVF treatment.
FIGURE 2.
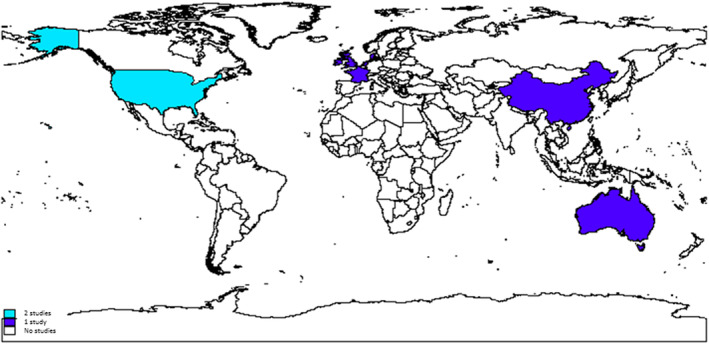
Map showing the location of included studies. Light blue indicates countries where two studies were conducted and the dark blue where one study was conducted.
The case control studies were generally of low quality, and several studies had no control data (Table 2). Included cohort studies were similarly overall low quality and none had an unexposed group (Table 3). Included studies were all deemed at significant risk of bias. All data were assessed to be of a “very low quality” using the GRADE method, particularly due to a reliance on comparatively small cohort studies, as all were observational, all had high risk of bias and significant heterogeneity between studies.
TABLE 2.
Risk of bias assessment of the case control studies in this review, showing stars awarded for each domain.
TABLE 3.
Risk of bias assessment of the cohort studies in this review, showing stars awarded for each domain.
For the cohort studies, there were three outcomes which were deemed appropriate for meta‐analysis, as two or more studies provided data on these outcomes ‐ live birth, preterm birth and miscarriage in subsequent pregnancy. The pooled proportion for preterm birth (95% CI: 6%–16%) and miscarriage (95% CI: 4%–30%) in the next pregnancy was 13% in women with a prior second trimester miscarriage. Two case control studies provided estimates for the odds of preterm birth in subsequent pregnancy. The pooled odds ratio (OR) was 4.52 with a 95% CI of 3.03–6.07 (Figure 3). These data are similar to that of the preterm birth from the meta‐analysis of cohort studies, with a pooled estimate of 13%, and a range of 6%–16%. Yang et al. 30 conducted a case–control study, which specifically compared data from women who had conceived via in vitro fertilization (IVF) who had a prior second trimester loss, compared to women who had conceived using IVF, but with no prior second trimester loss. The live birth rate was significantly lower in women who had a second trimester loss (51.8% vs. 69.7%, OR 0.46, 95% CI: 0.41, 0.54) and the second trimester loss was higher (9.0% vs 3.5%, OR 2.69, 95% CI: 2.06, 3.51). 30 However, the rate of preterm birth was unchanged in that study (11.0% vs. 9.7%, OR 1.15, 95% CI: 0.93, 1.44). 30 Table 4 demonstrates other adverse pregnancy outcomes studied, however, there were insufficient data to conduct further statistical analyses.
FIGURE 3.
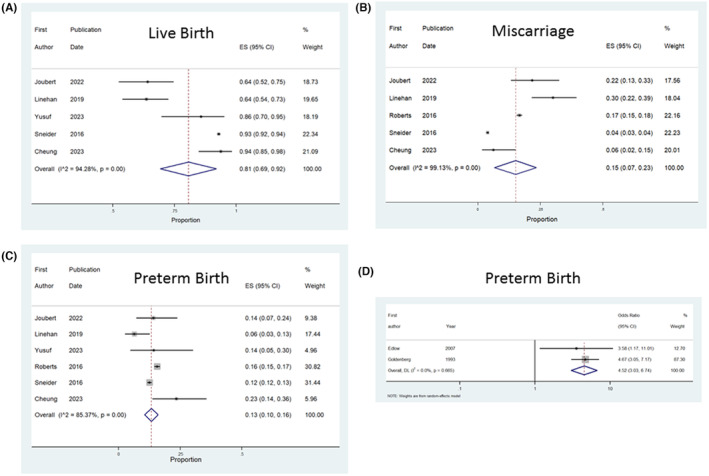
Forest plots showing the results of meta‐analysis as follows. (A) proportion of live births in subsequent pregnancy in cohort studies, (B) proportion of miscarriages in subsequent pregnancy in cohort studies, (C) proportion of preterm births in subsequent pregnancy in cohort studies and (D) odds of preterm birth in subsequent pregnancy in case control studies.
TABLE 4.
Summary of the data provided by studies on various outcomes featured in the criteria of this review. Percentages are rounded to the first decimal place.
| Authors, year | Total participants (n) | Live birth n (%) | Fetal growth restriction n (%) | Miscarriage n (%) | Cesarean birth n (%) | Neonatal death n (%) | Pre‐eclampsia n (%) | Preterm birth n (%) | Stillbirth n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Cheung et al. (2023) 22 | 64 | 60 (93.8) | NR | 4 (6.3) | NR | NR | NR | 15 (23.4) | NR |
| Edlow et al. (2007) 23 | 30 | NR | NR | 8 (27.0) | NR | NR | NR | 10 (33.3) | NR |
| Goldenberg et al. (1993) 24 | 95 | NR | NR | NR | NR | 5.7 (6.0) | NR | 37 (39.0) | 5 (5.0) |
| Joubert et al. (2022) 25 | 78 | 50 (64.1) | NR | 17 (21.8) | NR | NR | NR | 11 (14.1) | NR |
| Linehan et al. (2019) 26 | 110 | 70 (63.6) | NR | 33 (30.0) | 34 (30.9) | 1 (0.9) | NR | 7 (6.4) | NR |
| Puyenbroek & Stolte (1983) 27 | 28 | NR | NR | NR | NR | NR | NR | NR | NR |
| Roberts et al. (2016) 28 | 4290 | NR | NR | 712 (16.6) | NR | NR | NR | 673 (15.7) | NR |
| Sneider et al. (2016) 29 | 6194 | 5756 (92.6) | NR | 244 (3.9) | 419 (6.8) | NR | 149 (2.4) | 773 (12.5) | NR |
| Yang et al. (2023) 30 | 1072 | 556 (51.8) | NR | 189 (17.6) | NR | NR | NR | 118 (11.0) | NR |
| Yusuf et al. (2023) 31 | 35 | 30 (85.7) | 1 (2.9) | NR | NR | NR | 3 (8.6) | 5 (14.3) | NR |
Note: Outcomes are not always mutually exclusive.
Abbreviation: NR, not reported.
4. DISCUSSION
To the authors' knowledge, this is the first systematic review to investigate the impact of second trimester miscarriage or TMFR/TOPFA on subsequent pregnancy outcomes. The data from eligible studies indicate that there is a lower proportion of live births than expected, alongside an increase in preterm birth and miscarriage in subsequent pregnancies, following a second trimester miscarriage; however, findings should be interpreted with caution as evidence was very low quality, at risk of bias with significant clinical and statistical heterogeneity. Overall, there were very limited studies published which investigated the impact of a second trimester miscarriage on subsequent pregnancy outcomes meaning there were insufficient data to conclude whether there is an increase in any of the other subsequent pregnancy outcomes. No studies were found which specifically investigated the effect of having a TFMR/TOPFA on subsequent pregnancy outcome.
A strength of this review was the adherence to a registered protocol developed a priori and the use of a thorough search strategy. Authors were contacted for additional data where appropriate. Two independent reviewers conducted the screening and extraction of data, with discussion of each study with each of the study authors. However, there were several limitations to this review. In some cases, raw data were not available, so they were derived from percentages or graphs. Statistical analysis was only performed on two of the several listed secondary outcomes, due to a lack of published studies and data available. Moreover, the data that were available were of a “very low quality”, as assessed by the GRADE approach thus our findings of any associations should be interpreted with caution. We did not search gray literature for unpublished studies therefore this is a limitation. We included all observational studies including those without comparator groups which affects the quality of results. We were specifically interested in studies which addressed second trimester miscarriage or TFMR/TOPFA. However, we acknowledge due to differing gestational ages used to define viability across the world that some studies may have been excluded which may have included gestations 20–24 weeks labeled as preterm births and this is a limitation of our review. There was significant clinical and statistical heterogeneity between studies. The population sizes of some studies were small, with three studies reporting less than 40 women included. Inclusion criteria and the populations of women differed between studies. All of the studies were conducted in high‐ or middle‐income countries, suggesting any findings may not be generalizable (Figure 2) globally. Given the potential for disparity in prevalence of adverse pregnancy outcomes, miscarriage and preterm birth in lower income countries, this highlights a substantial gap in the literature.
With a background risk of preterm birth of approximately 6% in the UK and approximately 10% in the USA, 6 the pooled proportions in our review, and the limited evidence from case–control studies suggest the incidence of preterm birth may be elevated in women with a prior late miscarriage compared to background risk. 34 , 35 , 36 Furthermore, Yang et al. 30 reported a similar trend when only considering second‐trimester losses after IVF conceptions. In addition, prior systematic reviews which considered specifically a history or prior termination or uterine evacuation or curettage surgical procedures and subsequent risk of cervical insufficiency 13 , 14 , 15 report an increased subsequent risk of preterm birth, which is in keeping with the evidence presented in this review. Of note, our findings must be interpreted with caution due to the low‐quality evidence when specifically, second trimester miscarriage only was considered.
An association between second‐trimester loss and preterm birth is consistent with previous research on stillbirth and miscarriage which suggests that an increased risk of preterm birth in subsequent pregnancy after first and third trimester pregnancy losses. 1 , 2 , 37 Egerup et al. 6 suggest that women with prior pregnancy loss including a small number of women with prior late miscarriage were at greater risk of subsequent secondary recurrent pregnancy loss. And given previous evidence highly suggestive that women with a prior stillbirth are much more likely to have another stillbirth, 3 we highlight that similar research is needed for second trimester miscarriage and TFMR/TOPFA. Oliver‐Williams et al. 37 found that any prior miscarriage up to 24 weeks' gestation appeared to predispose to a higher risk of preterm birth, but using Scottish national data the authors were unable to differentiate first or second trimester gestational age at time of initial miscarriage.
It is plausible that preterm birth may be increased following late miscarriage given the evidence for such an association after late termination of pregnancy in the second trimester, 13 a prior preterm birth 12 and after recurrent first trimester losses. 4 , 6 The risk may relate to premature cervical dilatation or the abnormal initiation of labor as well as iatrogenic procedures performed. 13 This review provides some evidence that such a hypothesis may be correct, but highlights significant paucity of evidence to confirm or refute whether there is a significant risk of preterm birth after second trimester miscarriage.
Given the potential psychological impact of being labeled high risk for women, the costs to health services of instigating preterm birth surveillance, including cervical length transvaginal scanning, we believe high quality research specifically addressing the risk of preterm birth and other adverse outcomes after second trimester loss is needed. Many hospitals may already offer enhanced care in the next pregnancy after a late miscarriage or TFMR/TOPFA; however, this may not be universal thus high quality evidence to confirm or refute an association would likely benefit couples, health care providers and health services as well as the wider research community to better understand the impact of second trimester pregnancy loss and ensuring appropriate antenatal care in future. Therefore, it is vital high quality cohort studies using routinely collected or prospectively collected data are conducted to address whether prior second trimester loss is associated with adverse pregnancy outcome in subsequent pregnancies.
5. CONCLUSION
Whilst not definitive, available very low certainty evidence suggests there may be an increased risk of preterm birth in a subsequent pregnancy after a late miscarriage. Larger cohort studies are needed to investigate this association, which if confirmed suggests that women with second trimester pregnancy miscarriage may require additional surveillance in future pregnancies. In addition, further studies are needed to investigate subsequent pregnancy outcomes after a TFMR/TOPFA.
AUTHOR CONTRIBUTIONS
AH and AW developed the idea, AW wrote and registered the protocol on PROSPERO. BM undertook the literature searches. AH and AW screened titles, abstracts and full text articles. KP and DP extracted all data and performed the initial data analysis. AH undertook meta‐analysis. KP wrote the first draft of the manuscript. All authors contributed to the writing of each draft of the manuscript and have approved the final submitted version.
FUNDING INFORMATION
Funding provided by the University of Manchester and Tommy's charity.
CONFLICT OF INTEREST STATEMENT
The authors declare there are no conflicts of interest.
Supporting information
Table S1.
Patel K, Pirie D, Heazell AEP, Morgan B, Woolner A. Subsequent pregnancy outcomes after second trimester miscarriage or termination for medical/fetal reason: A systematic review and meta‐analysis of observational studies. Acta Obstet Gynecol Scand. 2024;103:413‐422. doi: 10.1111/aogs.14731
REFERENCES
- 1. Black M, Shetty A, Bhattacharya S. Obstetric outcomes subsequent to intrauterine death in the first pregnancy. BJOG. 2007;115(2):269‐274. [DOI] [PubMed] [Google Scholar]
- 2. Field K, Murphy DJ. Perinatal outcomes in a subsequent pregnancy among women who have experienced recurrent miscarriage: a retrospective cohort study. Hum Reprod. 2015;30(5):1239‐1245. [DOI] [PubMed] [Google Scholar]
- 3. Lamont K, Scott NW, Jones GT, Bhattacharya S. Risk of recurrent stillbirth: systematic review and meta‐analysis. BMJ. 2015;350(8014):h3080. [DOI] [PubMed] [Google Scholar]
- 4. Wu CQ, Nichols K, Carwana M, Cormier N, Maratta C. Preterm birth after recurrent pregnancy loss: a systematic review and meta‐analysis. Fertil Steril. 2022;117(4):811‐819. [DOI] [PubMed] [Google Scholar]
- 5. Bhattacharya S, Townend J, Bhattacharya S. Recurrent miscarriage: are three miscarriages one too many? Analysis of a Scottish population‐based database of 151,021 pregnancies. Eur J Obstet Gynecol Reprod Biol. 2010;150(1):24‐27. [DOI] [PubMed] [Google Scholar]
- 6. Egerup P, Kolte AM, Larsen EC, Krog M, Nielsen HS, Christiansen OB. Recurrent pregnancy loss: what is the impact of consecutive versus non‐consecutive losses? Hum Reprod. 2016;31(11):2428‐2434. [DOI] [PubMed] [Google Scholar]
- 7. Miscarriage statistics . Tommy's. Available from: https://www.tommys.org/baby‐loss‐support/miscarriage‐information‐and‐support/miscarriage‐statistics#general
- 8. Smith L, Dickens J, Bender Atik R, Bevan C, Fisher J, Hinton L. Parents' experiences of care following the loss of a baby at the margins between miscarriage, stillbirth and neonatal death: a UK qualitative study. BJOG. 2020;127(7):868‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Church E, Mulbagal K, Tomlinson J, Abdusamad K. Ensuring optimal management for families who experience a second trimester pregnancy loss. NHS England 2017. Available from: https://www.england.nhs.uk/north/wp‐content/uploads/sites/5/2018/05/STPL_Guideline_Final_March_2017_v1.0.pdf
- 10. Break the silence around TFMR . Antenatal Results and Choices (ARC). Available from: https://www.arc‐uk.org/for‐parents/break‐the‐silence‐around‐tfmr/
- 11. Dukhovny S, Zutshi P, Abbott JF. Recurrent second trimester pregnancy loss: evaluation and management. Curr Opin Endocrinol Diabetes Obes. 2009;16(6):451‐458. [DOI] [PubMed] [Google Scholar]
- 12. Tingleff T, Vikanes Å, Räisänen S, Sandvik L, Murzakanova G, Laine K. Risk of preterm birth in relation to history of preterm birth: a population‐based registry study of 213 335 women in Norway. BJOG. 2022;129(6):900‐907. [DOI] [PubMed] [Google Scholar]
- 13. Brittain JJ, Wahl SE, Strauss JF, et al. Prior spontaneous or induced abortion is a risk factor for cervical dysfunction in pregnant women: a systematic review and meta‐analysis. Reprod Sci. 2023;13(30):2025‐2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemmers M, Verschoor MAC, Hooker AB, et al. Dilatation and curettage increases the risk of subsequent preterm birth: a systematic review and meta‐analysis. Hum Reprod. 2016;31:34‐45. [DOI] [PubMed] [Google Scholar]
- 15. Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2016;214(5):572‐591. doi: 10.1016/j.ajog.2015.12.044 [DOI] [PubMed] [Google Scholar]
- 16. PlotDigitizer: Extract Data from Graph Image Online . PlotDigitizer. Available from: https://plotdigitizer.com/
- 17. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. The Ottawa Hospital. 2021. Available from. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 18. Schunemann H, Higgins J, Vist G, et al. Chapter 14: Completing “Summary of findings” tables and grading the certainty of the evidence. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 2022. Available from: https://training.cochrane.org/handbook/current/chapter‐14
- 19. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. Jama. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 20. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health. 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deeks J, Higgins J, Altman D. Chapter 10: Analysing data and undertaking meta‐analyses. Cochrane handbook for systematic reviews of interventions version 6.3. 2022. Available from: https://training.cochrane.org/handbook
- 22. Cheung KW, Seto MTY, Wang W, Mok YK, Cheung VYT. Clinical presentation, investigation, underlying causes, and subsequent pregnancy outcomes among different phenotypes of second trimester miscarriage. J Obstet Gynaecol Res. 2023;49(2):539‐547. [DOI] [PubMed] [Google Scholar]
- 23. Edlow AG, Srinivas SK, Elovitz MA. Second‐trimester loss and subsequent pregnancy outcomes: what is the real risk? Am J Obstet Gynecol. 2007;197(6):581.e1‐581.e6. [DOI] [PubMed] [Google Scholar]
- 24. Goldenberg RL, Mayberry SK, Copper RL, Dubard MB, Hauth JC. Pregnancy outcome following a second trimester loss. Obstet Gynecol. 1993;81(3):444‐446. [PubMed] [Google Scholar]
- 25. Joubert M, Sibiude J, Bounan S, Mandelbrot L. Mid‐trimester miscarriage and subsequent pregnancy outcomes: the role of cervical insufficiency in a cohort of 175 cases. J Matern Fetal Neonatal Med. 2021;35(24):4698‐4703. [DOI] [PubMed] [Google Scholar]
- 26. Linehan LA, Morris AG, Meaney S, O'Donoghue K. Subsequent pregnancy outcomes following second trimester miscarriage—a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2019;237:198‐203. [DOI] [PubMed] [Google Scholar]
- 27. Puyenbroek JI, Stolte LAM. The relationship between spontaneous and induced abortion and the occurrence of second‐trimester abortion in subsequent pregnancies. Eur J Obstet Gynecol Reprod Biol. 1983;14(5):299‐309. [DOI] [PubMed] [Google Scholar]
- 28. Roberts CL, Algert CS, Ford JB, Nippita TA, Morris JM. Association between interpregnancy interval and the risk of recurrent loss after a midtrimester loss. Human Reprod. 2016;31(12):2834‐2840. [DOI] [PubMed] [Google Scholar]
- 29. Sneider K, Christiansen OB, Sundtoft IB, Langhoff‐Roos J. Recurrence of second trimester miscarriage and extreme preterm delivery at 16‐27 weeks of gestation with a focus on cervical insufficiency and prophylactic cerclage. Acta Obstet Gynecol Scand. 2016;95(12):1383‐1390. [DOI] [PubMed] [Google Scholar]
- 30. Yang L, Ni T, Huang Y, et al. Impact of a previous late miscarriage on subsequent pregnancy outcomes: a retrospective cohort study over 10 years. Int J Gynaecol Obstet. 2023;00:1‐8. [DOI] [PubMed] [Google Scholar]
- 31. Yusuf H, Stokes J, Wattar BHA, Petrie A, Whitten SM, Siassakos D. Chance of healthy versus adverse outcome in subsequent pregnancy after previous loss beyond 16 weeks: data from a specialized follow‐up clinic. J Matern Fetal Neonatal Med. 2023;36(1):2165062. [DOI] [PubMed] [Google Scholar]
- 32. Sato Y, Nobuhiro H, Hachisuga N, Sakai A, Kido S, Kato K. The 72nd annual congress of the Japan Society of Obstetrics and Gynecology. J Obstet Gynaecol Res. 2020;46(8):1579‐1622. [DOI] [PubMed] [Google Scholar]
- 33. Pennell C, White S, White C, Andreasson E, White M, Jacobsson B. Concurrent abstracts session one Monday 23rd may 1330‐1500. J Paediatr Child Health. 2016;52(S2):16‐28. [Google Scholar]
- 34. Preterm labour and birth . NICE 2016. Available from: https://www.nice.org.uk/guidance/qs135/resources/preterm‐labour‐and‐birth‐pdf‐75545420722117
- 35. Births in Scotland Year Ending 31 March 2021 . Public Health Scotland. 2021. Available from: https://publichealthscotland.scot/publications/births‐in‐scotland/births‐in‐scottish‐hospitals‐year‐ending‐31‐march‐2021/
- 36. U.S. Department of Health & Human Services . Preterm Birth. 2020. Available from: www.cdc.gov, https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm#:~:text=Preterm%20birth%20is%20when%20a
- 37. Oliver‐Williams C, Fleming M, Wood A, Smith G. Previous miscarriage and the subsequent risk of preterm birth in Scotland, 1980‐2008: a historical cohort study. BJOG. 2015;122(11):1525‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


